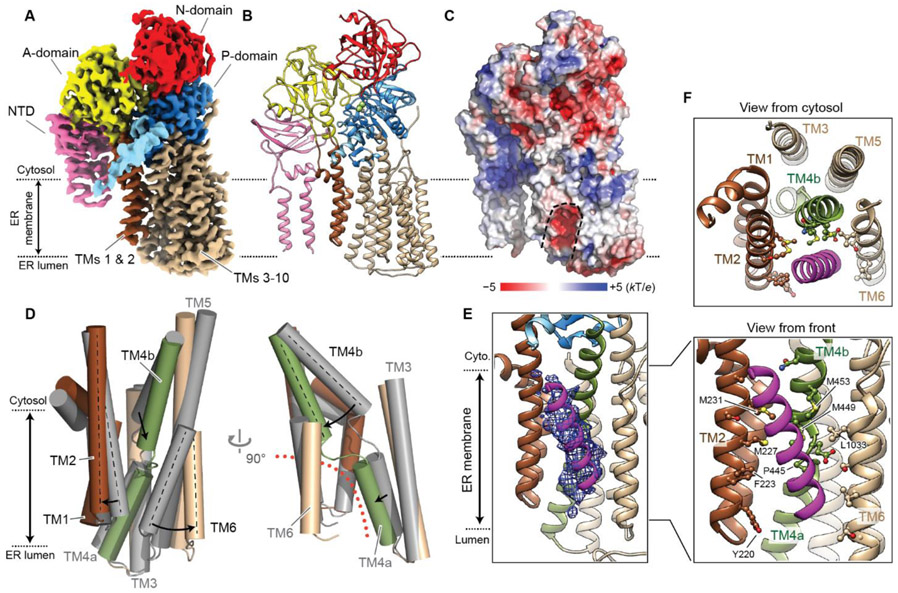
Fig. 4. Structure of Spf1 in an outward-open conformation.
(A and B) 3.3-Å-resolution cryo-EM reconstruction (A) and atomic model (B) of the BeF3-bound Spf1. Shown are the front views. We note that unlike the inward-open structure, TMa and TMb are flexible in this conformation. TMa and TMb were modeled as polyalanine helices based on the apo structure. (C) A heatmap of surface electrostatics. Substrate-binding cavity is outlined with a dashed line. (D) Conformational changes of TMs 1–6 from the inward-open (apo; gray) to outward-open (BeF3-bound; colored) states. Left, a front view; right, a side view. The outward-open substrate-binding pocket is indicated by an orange dotted line. (E) The density of copurified putative substrate (magenta mesh; 5-Å-lowpass-filtered) fitted with a poly-alanine helix model (also see fig. S20A). (F) As in E, but with amino-acid sidechains of Spf1 facing the substrate density shown in a ball-and-stick representation. Top, a cytosolic view; bottom, a front view.