Abstract
Background
The severity and mortality from COVID-19 infection vary among populations. The aim of this study was to determine the prevalence and predictors of mortality among patients hospitalized with COVID-19 infection in a tertiary care hospital in Oman.
Methods
We conducted a retrospective study using database that included: demographic, clinical characteristics, laboratory parameters, medications and clinical outcomes of all patients hospitalized in Royal Hospital, Muscat, Oman, between March 12, 2020 and December 1st 2020. Univariate and multivariate logistic regression was performed to investigate the relationship between each variable and the risk of death of COVID-19 infected patients.
Results
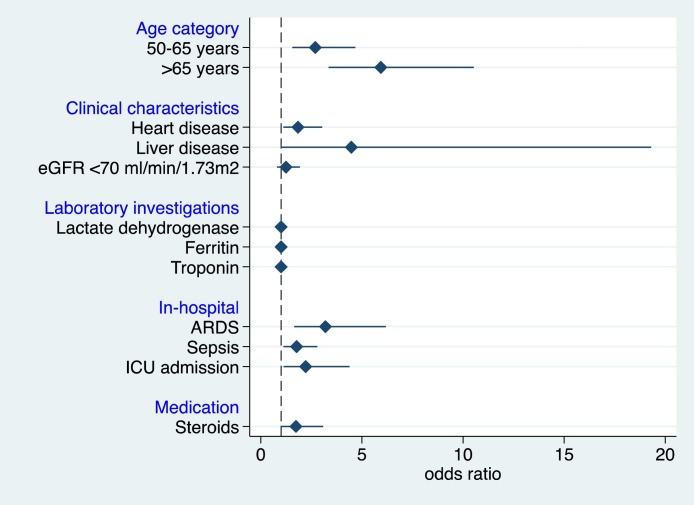
In total,1002 patients with COVID-19 infection with mean age of the cohort was 54 ± 16 years (65% (n = 650) male) were included, with an overall and intensive care unit (ICU) mortalities of 26% (n = 257) and 42% (n = 199/473), respectively. The prevalence of ICU admission was 47% (n = 473) and the need for mechanical ventilation was 41% (n = 413). The overall length of stay in the ICU was 13 (9–21) days. Adjusting for other factors in the model, the multivariable logistic regression demonstrated that in-hospital mortality in admitted COVID-19 patients was associated with old age (p < 0.001), heart diseases (adjusted odds ratio (aOR), 1.84; 95% confidence interval (CI): 1.11–3.03; p = 0.018), liver diseases (aOR, 4.48; 95% CI: 1.04–19.3; p = 0.044), those with higher ferritin levels (aOR, 1.00; 95% CI: 1.00–1.00; p = 0.006), acute respiratory distress syndrome (ARDS) (aOR, 3.20; 95% CI: 1.65–6.18; p = 0.001), sepsis (aOR, 1.77; 95% CI: 1.12–2.80; p = 0.022), and those that had ICU admission (aOR, 2.22; 95% CI: 1.12–4.38; p = 0.022).
Conclusion
In this cohort, mortality in hospitalized COVID-19 patients was high and was associated with advanced age, heart diseases, liver disease, high ferritin, ARDS, sepsis and ICU admission. These high-risk groups should be prioritized for COVID-19 vaccinations.
Keywords: SARS-CoV-2, COVID-19, Heart disease, Liver diseases, Ferritin, ARDS, Sepsis, Intensive care unit, Mortality
Introduction
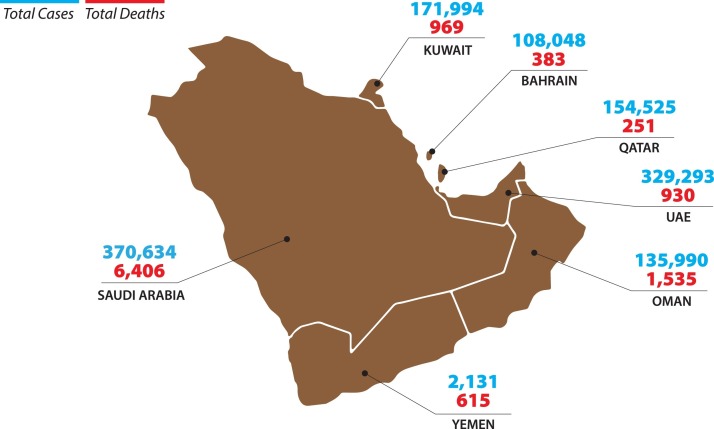
Since the first case of coronavirus disease 2019 (COVID-19) was confirmed in China in December 2019, more than 106,828,187 million individuals have become ill, and more than 2,330,403 have died [1]. In Oman, the first case of COVID-19 was diagnosed on February 24th 2020 [2], [3]. Since then, the total number of cumulative cases has reached 135,990 as of February 2021 and 1535 reported deaths (Fig. 1 ) with a case fatality rate of 0.03% [4].
Fig. 1.
Total confirmed COVID-19 Cases and Deaths in Oman and the Gulf Cooperation Council Countries.
COVID-19 pandemic causes exceptional challenge to humanity worldwide due to important knowledge gaps in understanding the patterns of transmission, disease phenotypes, genetic susceptibility, risk factors associated with mortality, and lack of effective therapies [2], [3]. Despite its tremendous impact, there is a paucity of research on the epidemiology and clinical outcomes of patients with COVID-19 in the Eastern Mediterranean Region (EMRO). Most of the available research on prevalence of death and their association with epidemiological risk factors has emerged from China, European Union and the USA [5], [6], [7], [8]. The risk factors for mortality greatly vary among populations that differ in demographic, clinical characteristic and the resilience of the health care system ultimately affecting the outcomes.
Several risk factors have been associated with COVID-19 disease severity and death such as gender [5], [6], [7], [8], [9], hypertension [5], [8], [9], [14], [15], diabetes [5], [8], [9], [14], [15], smoking [10], [11], [12], [13] and cardiovascular disease [15], [16], [17]. Other factor such as cerebrovascular diseases, CD3 + CD8+ T-cells ⩽75 cells μL−1 and cardiac troponin I ⩾0.05 ng mL−1 has been associated with predictors for mortality of COVID-19 pneumonia patients [6]. Most of the countries in the EMRO region have the highest epidemiologic transition over the past 50 years that outpaced their health care systems. The health of the population is being influenced by large burden of non-communicable chronic diseases, especially diabetes, heart diseases, hypertension and chronic kidney diseases (CKD) [18], [19], [20], [21], [22], [23]. All are potential risk factors for mortality in patients with COVID-19 infection.
Our study aims to determine the prevalence as well as the demographic and clinical characteristics, treatment patterns, and risk factors associated with in-hospital mortality among hospitalized COVID-19 patients in Oman. Identification of groups at risk for in-hospital mortality will help in stratifying and prioritizing high risk populations for COVID-19 vaccinations.
Methods
Study design and data source
We conducted a retrospective study to address the study aim using Royal Hospital (RH) COVID-19 registry database. The RH is the main tertiary hospital designated nationally for the care of the COVID-19 patients. The database contains demographic, clinical characteristics, laboratory parameters, medications and clinical outcomes of all patients hospitalized in the RH between 12 March and December 1st 2020.
Study variables
The demographic characteristics examined were age, gender and nationality. Hospital visit information such as: type of admission, length of stay (LOS) and discharge disposition, were assessed. The clinical characteristics included baseline comorbidities such as diabetes mellitus, hypertension, dyslipidemia, respiratory diseases and heart diseases. In addition, acute complications were assessed such as acute respiratory failure, acute respiratory distress syndrome (ARDS), sepsis, acute kidney failure and acute liver damage. The laboratory parameters included: estimated glomerular filtration rate (eGFR), white blood cell (WBC) count, absolute lymphocyte count (ALC), C-reactive protein (CRP); lactate dehydrogenase (LDH); alanine transaminase (ALT); ferritin, D-dimer, troponin, corrected calcium and vitamin D levels. Pharmacological therapies included steroids, tocilizumab, convalescent plasma (CP) and therapeutic plasma exchange (TPE), used for the target patient population were examined.
Clinical outcomes
Clinical outcomes assessed included in-hospital mortality as well as demographic and clinical factors associated with mortality of admitted COVID-19 patients in Oman. In-hospital mortality was defined as percentage of patients with COVID-19 who died in the hospital.
Statistical analysis
Descriptive statistics were used to describe the data. For categorical variables, frequencies and percentages were reported. Differences between groups were analyzed using Pearson's χ 2 tests. For continuous variables, mean and standard deviation was used to present the data while analyses were performed using Student's t-test. Abnormal distributed variables were summarized using median and interquartile range and analyzed using Wilcoxon-Mann–Whitney test.
The association between in-hospital mortality and various predictors was evaluated using multivariable logistic regression model utilizing the simultaneous method. Variables with p-values >0.5 were eventually removed from the model. The variables initially included age, gender, diabetes mellitus, hypertension, heart disease, liver disease, eGFR, WBC count, ALC, CRP, LDH, ALT, ferritin, D-dimer, troponin, bilateral infiltrates, ARDS, sepsis, ICU admission; LOS, TPE, CP, tocilizumab and steroids use. The goodness-of-fit of the multivariable logistic models were examined using the Hosmer & Lemeshow goodness-of-fit statistic. The discriminatory power of the logistic models was assessed by the area under the receiver operating characteristics (ROC) curve also known as the C-index. An a priori two-tailed level of significance was set at 0.05. Statistical analyses were conducted using STATA version 16.1 (STATA Corporation, College Station, TX, USA).
Results
The study included a total of 1002 admitted COVID-19 patients. The overall mean age of the cohort was 54 ± 16 years with the majority in the <50 years age group (40%; n = 405), 65% (n = 650) were males and 76% (n = 759) were Omani citizen. The three most prevalent co-morbidities included hypertension (50%; n = 498), diabetes mellitus (48%; n = 482) and heart diseases (19%; n = 186). A total of 42% (n = 425) and 19%; (n = 192) of the hospitalized patients had ARDS, 41% (n = 413) were intubated, and sepsis, respectively. Forty seven percent (n = 473) of the patients were admitted to ICU with an overall median length of hospital stay of 8 days (4–15). The three most prominent management strategies included the use of steroids (65%; n = 649), CP (39%; n = 392), and tocilizumab (32%; n = 325).
Patients with the age group ≥65 years were more likely to be associated with in-hospital mortality than those who were aged 50–64 years (51% vs. 32%; p < 0.001) as well as those that were <50 years of age (51% vs. 17%; p < 0.001). In-hospital mortality was also associated in those that had diabetes mellitus (62% vs. 43%; p < 0.001), hypertension (63% vs. 45%; p < 0.001), heart disease (30% vs. 15%; p < 0.001), respiratory disease (15% vs. 9.4%; p = 0.01), liver disease (3.9% vs. 1.2%; p = 0.007) and those with eGFR of <60 ml/min/1.73 m2 (43% vs. 27%; p < 0.001). Those that died in hospital had higher WBC count (8.6 vs. 6.6 × 109 L–1; p < 0.001), CRP (122 vs. 100 mg/dL; p = 0.02), LDH (511 vs. 404 U/L; p < 0.001), ferritin (983 vs. 708 ng/mL; p < 0.001), D-dimer (2.32 vs. 1.02 ng/mL; p < 0.001), and troponin (53 vs. 14 ng/mL; p < 0.001) levels. However, those who died had lower ALC levels (0.8 vs. 1.0 × 109 L–1; p < 0.001).
The COVID-19 patients that died in hospital were more likely to have bilateral infiltrates in their lungs (94% vs. 81%; p < 0.001), ARDS (77% vs. 31%; p < 0.001), more likely to be intubated (78% vs. 28%; p < 0.001), sepsis (40% vs. 12%; p < 0.001), ICU admission (77% vs. 37%; p < 0.001), and longer overall median length of hospital (11 vs. 7 days; p < 0.001). They were also more likely to be managed with CP (47% vs. 36%; p = 0.001), tocilizumab (51% vs. 26%; p < 0.001) and steroids (84% vs. 58%; p < 0.001). Other demographic and clinical characteristics are found in Table 1 .
Table 1.
Demographic and clinical characteristics of the admitted COVID-19 cohort stratified by mortality.
| Characteristics, n (%) unless specified otherwise | All (n = 1002) | Died |
p-Value | |
|---|---|---|---|---|
| No (n = 745) | Yes (n = 257) | |||
| Demographic | ||||
| Age, mean ± SD, years | 54 ± 16 | 51 ± 16 | 63 ± 14 | <0.001 |
| Age categories | ||||
| <50 years | 405 (40%) | 362 (49%) | 43 (17%) | |
| 50–64 years | 309 (31%) | 226 (30%) | 83 (32%) | <0.001 |
| ≥65 years | 288 (29%) | 157 (21%) | 131 (51%) | |
| Male gender | 650 (65%) | 477 (64%) | 173 (67%) | 0.341 |
| Omani | 759 (76%) | 556 (75%) | 203 (79%) | 0.16 |
| Clinical | ||||
| Diabetes mellitus | 482 (48%) | 323 (43%) | 159 (62%) | <0.001 |
| Hypertension | 498 (50%) | 335 (45%) | 163 (63%) | <0.001 |
| Heart disease | 186 (19%) | 109 (15%) | 77 (30%) | <0.001 |
| Respiratory disease | 109 (11%) | 70 (9.4%) | 39 (15%) | 0.01 |
| Liver disease | 19 (1.9%) | 9 (1.2%) | 10 (3.9%) | 0.007 |
| eGFR < 70 ml/min/1.73 m2 | 311 (31%) | 200 (27%) | 111 (43%) | <0.001 |
| Laboratory, median [IQR] | ||||
| WBC count, ×109 L−1 [996/1002] | 7 (4.8–11) | 6.6 (4.6–10) | 8.6 (6.0–14) | <0.001 |
| ALC count, ×109 L−1 [998/1002] | 0.9 (0.6–1.4) | 1 (0.7–1.4) | 0.8 (0.6–1.2) | <0.001 |
| CRP, mg/dL [843/1002] | 104 (45–180) | 100 (43–172) | 122 (55–202) | 0.02 |
| LDH, U L−1 [919/1002] | 426 (321–571) | 404 (309–536) | 511 (371–733) | <0.001 |
| Ferritin, ng/mL [924/1002] | 774 (345–1552) | 708 (305–1433) | 983 (501–1924) | <0.001 |
| ALT, IU L−1 [959/1002] | 36 (21–64) | 36 (21–62) | 36 (20–70) | 0.767 |
| D-dimer, ng/mL [703/1002] | 1.19 (0.61–3.66) | 1.02 (0.55–2.73) | 2.32 (0.98–8.04) | <0.001 |
| Troponin, ng L−1 [728/1002] | 19 (9–70) | 14 (7–44) | 53 (18–173) | <0.001 |
| Vitamin D, ng/mL [240/1002] | 63 (47–85) | 63 (47–85) | 62 (50–79) | 0.799 |
| Hospital | ||||
| Bilateral infiltrates | 847 (85%) | 605 (81%) | 242 (94%) | <0.001 |
| ARDS | 425 (42%) | 228 (31%) | 197 (77%) | <0.001 |
| Intubation | 413 (41%) | 212 (28%) | 201 (78%) | <0.001 |
| Sepsis | 192 (19%) | 89 (12%) | 103 (40%) | <0.001 |
| ICU admission | 473 (47%) | 274 (37%) | 199 (77%) | <0.001 |
| LOS, median [IQR], days | 8 (4–15) | 7 (3–14) | 11 (6–17) | <0.001 |
| Management | ||||
| Plasma exchange | 62 (6.2%) | 43 (5.8%) | 19 (7.4%) | 0.352 |
| Convalescent plasma | 392 (39%) | 270 (36%) | 122 (47%) | 0.001 |
| Tocilizumab | 325 (32%) | 194 (26%) | 131 (51%) | <0.001 |
| Steroids | 649 (65%) | 433 (58%) | 216 (84%) | <0.001 |
SD, standard deviation; eGFR, estimated glomerular filtration rate (n = 1000); IQR, interquartile range; WBC, white blood cell; ALC, absolute lymphocyte count; CRP, C-reactive protein; LDH, lactate dehydrogenase; ALT, alanine transaminase; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; LOS, length of stay.
Adjusting for other factors in the multivariate model (Fig. 2 ), the multiple logistic regression demonstrated that in-hospital mortality in admitted COVID-19 patients was associated with old age (p < 0.001), those with heart diseases (adjusted odds ratio (aOR), 1.84; 95% confidence interval (CI): 1.11–3.03; p = 0.018), liver diseases (aOR, 4.48; 95% CI: 1.04–19.3; p = 0.044), those with higher ferritin levels (aOR, 1.00; 95% CI: 1.00–1.00; p = 0.006), ARDS (aOR, 3.20; 95% CI: 1.65–6.18; p = 0.001), sepsis (aOR, 1.77; 95% CI: 1.12–2.80; p = 0.022), and those that had ICU admission (aOR, 2.22; 95% CI: 1.12–4.38; p = 0.022).
Fig. 2.
Adjusted odds ratios of the predictors of mortality of admitted COVID-19 patients (N = 1002). eGFR, estimated glomerular filtration rate; ARDS, ARDS, acute respiratory distress syndrome; ICU, intensive care unit. For age category, age <50 years was the reference group. The Hosmer and Lemeshow p-value was 0.953 while the c-statistic was 0.84, denoting good model fit.
Discussion
The present study showed that hospitalized patients with COVID-19 were relatively young, two thirds were male and three quarters were citizens. Diabetes was a major comorbidity in half of the patients. Additionally, hypertension and heart diseases were present in half and one fifth of the study population, respectively. Almost half of the hospitalized patients with COVID-19 were admitted to the ICU and managed mainly with steroids (65%), CP (39%) and tocilizumab (32%). Mortality was high at staggering around 50% among elderly (≥65 years) and middle-aged patients with strong association with various non-communicable diseases (hypertension and diabetes mellitus) including low eGFR. The high mortality was associated with various abnormal laboratory parameters including WBC, CRP, LDH, D-dimer, troponin and ferritin. In addition, mortality was associated with various clinical and radiological respiratory abnormalities including ARDS and bilateral infiltrates.
Multiple factors potentially contributed to the rapid transmissibility of COVID-19 infection. Early into the pandemic, the World Health Organization (WHO) estimated the basic reproduction number for a contagious disease (R0) for SARS-CoV-2 to be 1.4–2.5. This subsequently increased to R0 of 3.28 suggesting that every index case could potentially infect about three others [24]. Furthermore, an infected person can transmit the virus to others both before they show symptoms and when they are symptomatic [25]. Most COVID-19 transmission appears to be due to exposure to the respiratory droplets and aerosols of an infected person. However, recent research indicates detection of SARS-CoV-2 RNA in other body fluids suggesting the possibility of other routes of transmission, such as bloodborne, urinary, and gastrointestinal tract and indicate its ease of spread [24].
The overall risk factors and treatment patterns associated with in-hospital mortality among patients treated in hospitals across EMRO remain largely unknown. Few studies, either surveillance data with minimal clinical information or small cohorts, have been conducted early into the pandemic in the region on predictors of mortality. However, the results have been inconsistent [26], [27], [28], [29], [30]. In a study from Kingdom of Saudi Arabia (KSA) on 89 patients (4.27%) who died, the clinical predictors of death were obesity, history of smoking and diabetes mellitus [26]. In a cohort of 1096 patients from Kuwait recruited from February to April 2020, mortality predictors were asthma, smoking and elevated procalcitonin levels [27]. In another study from KSA conducted between March and May 2020 on 352 critical ill patients on predictors of 28-day mortality, the mortality rate was 32.1%. Multivariate regression analysis showed that older age, active smoking, pulmonary embolism, decreased SpO2/FiO2 ratio, and increased lactate and D-dimers were mortality predictors [28].
We have conducted a retrospective study including 1002 patients admitted to one of the main tertiary care hospitals in the country representing 10% of all COVID-19 related hospitalization in the country. In a multivariant analysis, in-hospital mortality in admitted COVID-19 patients was associated with advanced age, heart diseases, liver diseases, high ferritin, ARDS, sepsis and ICU admission.
In the USA, a national study from 592 hospitals of 64,781 patients with confirmed COVID-19 who were discharged between April 1 and May 31, 2020, showed that 19.4% of patients with COVID-19 required care in ICU; 15.9% of patients received invasive mechanical ventilation; and 20.3% of patients died [31]. In the current study, 47% of patients with COVID-19 required care in the ICU, 41% of patients received invasive mechanical ventilation and 26.0% of patients died with ICU mortality reaching 42%. The in-hospital mortality rate estimated in this study was similar to a retrospective cohort from Kuwait involving 103 ICU patients where the fatality rate was 43.7%; 85.5% were males and 38% of the patients had more than two comorbidities. Pre-existing hypertension, moderate/severe ARDS, lymphocyte counts <0.5, albumin <22, procalcitonin >0.2, D-dimer >1200 and the need for continuous renal replacement therapy were significantly associated with mortality [30].
The mortality rate in our study is higher than what was reported in a previous study by Richardson et al., as well as the prevalence of ICU admissions (47% vs. 19.4%) and invasive mechanical ventilation use (41% vs. 15.9%) [32]. An explanation could be that our hospital was designated for patients with severe and critical COVID-19 pneumonia. Furthermore, patients were sometimes transferred late in their illness to our hospital due to delays in diagnosis. Lack of effective antivirals, increase hospital volume and inadequate adherence to standard supportive therapy, might have also contributed to the poor clinical outcomes in some patients. Timing of the different therapies is probably essential for the successful response. Inhibition of viral proliferation in early stage of COVID-19 with antivirals could prevent subsequent severe complications and improve the clinical outcome In contrast, patients with critical COVID-19 would benefit from anti-inflammatory therapy to treat cytokine release syndrome; the main cause of multi-organ failure and death [33]. A number of therapies has been recently advocated as potentially effective in the management of COVID-19 infection such as remdesivir, steroids, IL-6 inhibitors and anticoagulation [34], [35], [36]. However, supporting evidence is weak for modalities such as convalescent plasma, ivermectin, lopinavir/ritonavir, and interferons [37], [38]. Well-designed clinical trials are required to investigate the safety and efficacy of ivermectin and other agents such as traditional Chinese herbs, vitamin D and C.
In this cohort, the in-hospital mortality was highest among COVID-19 infected patients with age group 65 and above as more than half of the patients in this age category died. Previous studies in macaques inoculated with SARS-CoV have shown that age-dependent defects in immune cells results in a robust increase in expression of genes associated with inflammation leading to decrease in type I interferon beta, affecting the ability to suppress the virus and consequently to poor outcomes [39]. This association between age and mortality is consistent with prior reports [31], [32], [40]. Similarly, in the USA [31], patients aged 65 years and older disproportionally accounted for more than 75% of all in-hospital deaths. The odds of death were 16.2 times higher in patients aged 80 years or older than among those aged 18 to 34 years. Furthermore, mortality rate in the age group 65–79 years was 39.2%. Male patients had 18% greater odds of death than female patients. In our study, no difference in mortality rate based on gender was observed.
Among baseline comorbidities, similar to a large multicenter cohort of 1305 hospitalized COVID-19 patients in Michigan, USA [17], where hypertension, diabetes mellitus and CKD were the most common comorbidities, the three most prevalent co-morbidities in the current study were also hypertension, diabetes mellitus and CKD. However, when adjusted among various confounders in the multivariant analysis, only liver disease and heart diseases were associated with significant in-hospital mortality (aOR of 4.48, 95%CI: 1.11–3.03, p = 0.018 and aOR of 1.84, 95%CI: 1.04–19.3, p = 0.044, respectively).
Low survival rates have been associated with myocardial infarction, congestive heart failure, cerebrovascular disease, respiratory disease, dementia, diabetes, any malignant neoplasm metastatic solid tumour, hypertension and hyperlipidemia [41], [42], [43]. In addition, increasing number of comorbidities have been associated with lower survival [17]. In this cohort, compared to survivors, non-survivors significantly had a lower lymphocyte count but a higher count of WBC and high CRP in univariate analysis. These findings are consistent with the previous research [28], [30]. Moreover, the laboratory parameters predictors of high mortality were ferritin, LDH, D-dimer and troponin. These parameters are indicative of cytokine storm and cardiac involvement, both have been associated with increased mortality in several other studies [6], [43], [44].
Increased high-sensitivity cardiac troponin I during COVID-19 hospitalization was found in more than half of those who died in several reports [45], [46], [47]. In addition, patients admitted with pneumonia had increased coagulation activity, marked by increased D-dimer concentrations [40], [48]. Increased mortality rate has been reported at D-dimer levels above 1, mainly through ischaemia and thrombosis induced by the systemic pro-inflammatory cytokine [49], [50], [51], [52]. Furthermore, angiotensin converting enzyme 2 receptor, the receptor for SARS-CoV-2, is expressed on myocytes and vascular endothelial cells [53], [54] so there is at least theoretical potential possibility of direct cardiac involvement by the virus.
Among COVID-19 related complications mortality was higher in patient with ARDS, ICU admission and sepsis. Similarly, in a retrospective, multicenter cohort study from Wuhan, China, describing 191 laboratory-confirmed COVID-19 patients who had died by Jan 31, 2020, sepsis was the most frequently observed complication, followed by respiratory failure, ARDS, heart failure and septic shock [15]. This was also reported in USA national data [31], as the most common acute complication was acute respiratory failure (30.8%; n = 19,960), followed by acute kidney failure (18.8%; n = 12,181) and sepsis (18.6%; n = 12,039). Sepsis has been a common complication, which might be directly caused by SARS-CoV-2 infection as several reports indicated low prevalence of bacterial co-infection [55], [56], [57]. Further research is needed to investigate the pathogenesis of sepsis in COVID-19 illness and the potential role of immunomodulating therapies in sepsis.
The median LOS in the current study was 8 (4–15) days while in those that were admitted to ICU, their median LOS was longer, at 13 (9–21) days. The median total hospital LOS in the current study was longer than what was previously reported 5 (2–10) days from US national data [31], [32].
In order to reduce COVID-19 mortality and in the absence of effective therapeutics and shortage in vaccines availability that can shorten or eliminate infectivity, successful control of SARS-CoV-2 continues to rely on early detection and isolation of symptomatic cases and reducing the risk of transmission in pre-symptomatic stage. This could only be accomplished through adopting public health measures such as mask wearing, social distancing, cough antiquate, hand hygiene; and strategic testing of contacts and populations at high risk of exposing others. High quality research is needed to clarify the roles of the different transmission routes in order to suppress SARS-CoV-2 transmission and prevent morbidity and mortality. Accelerating the development of effective and safe vaccines against COVID-19 and ensuring fair and equitable distribution of the vaccines for all countries are important measures to combat the virus.
This study has several limitations. First, not all laboratory parameters were collected, in particularly, in patients with moderate COVID-19 illness, which potentially could underestimate their role. Second, hospital registry database did not include many other important clinical details such as other clinical co-morbidities including malignancy, procedures, and use of medications such as antivirals, antimalaria, supplements, NSAIDs and ACE inhibitors that could have affected the outcomes. Third, due to the nature of observational studies, although adjusted analysis was performed to control for patient and clinical characteristics, we could not detect causal relationships between the assessed medications and in-hospital mortality. Third, the in-hospital mortality rate reported in this study was estimated in hospitalized patients, who had severe and critical COVID-19; thus, it might not reflect the mortality rate in all patients with COVID-19. The overall mortality rate of COVID-19 is likely to be lower when the mild or moderate cases are taken into account.
Conclusion
In this cohort study of hospitalized patients with COVID-19 infection, COVID-19 was associated with high ICU admission and in-hospital mortality rates. The in-hospital mortality was associated with older age, liver disease, heart disease, ARDS, sepsis and ICU admission. Oman and its region are engulfed with various non-communicable diseases including, obesity, diabetes, hypertension and CKD that contribute significantly to the high mortality among hospitalized patients with COVID-19 infection. Vaccination for COVID-19 should be prioritized based on the risk-groups with significant in-hospital mortality. Appropriately addressing the modifiable risk factors such as heart and liver diseases could reduce morbidity and mortality due to COVID-19.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard – World Health Organization. www.who.int [accessed 08.02.20].
- 2.Khamis F., Al Rashidi B., Al-Zakwani I., Al Wahaibi A.H., Al Awaidy S.T. Epidemiology of COVID-19 infection in Oman: analysis of the first 1304 cases. Oman Med J. 2020;35(3):e145. doi: 10.5001/omj.2020.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khamis F., Al-Zakwani I., Al Naamani H., Al Lawati S., Pandak N., Omar M.B. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J Infect Public Health. 2020;13(7):906–913. doi: 10.1016/j.jiph.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corona virus Disease – Ministry of Health, Oman. www.moh.gov.om>corona [accessed 08.02.21].
- 5.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alqahtani J.S., Oyelade T., Aldhahir A.M., Alghamdi S.M., Almehmadi M., Alqahtani S. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLOS ONE. 2020;15(5) doi: 10.1371/journal.pone.0233147. PMID: 32392262, e0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippi G., Henry B.M. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19) Eur J Intern Med. 2020;75:107–108. doi: 10.1016/j.ejim.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patanavanich R., Glantz S.A. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossato M., Russo L., Mazzocut S., Di Vincenzo A., Fioretto P., Vettor R. Current smoking is not associated with COVID-19. Eur Respir J. 2020;55(6) doi: 10.1183/13993003.01290-2020. 2001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan W.J., Liang W.H., Zhao Y., Liang H., Chen Z., Li Y. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00547-2020. 2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imam Z., Odish F., Gill I., O’Connor D., Armstrong J., Vanood A. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288(4):469–476. doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahim H.F., Sibai A., Khader Y., Hwalla N., Fadhil I., Alsiyabi H. Non-communicable diseases in the Arab world. Lancet. 2014;383(9914):356–367. doi: 10.1016/S0140-6736(13)62383-1. [DOI] [PubMed] [Google Scholar]
- 19.Chahine G., Bitar J., Assouad P., Abi Chaker S. Booz & Co; Illinois: 2013. The $68 billion challenge, quantifying and tackling the burden of chronic diseases in the GCC.http://www.booz.com/me/home/thought_leadership_strategy/reports_and_white_papers_me/display/the-68-billiondollar-challenge?cm_mid=3108890&cm_crmid=95462fe6-3e45-475a-bb64-5f541513f176&cm_medium=email [accessed 07.02.21]. [Google Scholar]
- 20.Alhabib K.F., Sulaiman K., Al-Motarreb A., Almahmeed W., Asaad N., Amin H. Baseline characteristics, management practices, and long-term outcomes of Middle Eastern patients in the Second Gulf Registry of Acute Coronary Events (Gulf RACE-2) Ann Saudi Med. 2012;32(1):9–18. doi: 10.5144/0256-4947.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tailakh A., Evangelista L.S., Mentes J.C., Pike N.A., Phillips L.R., Morisky D.E. Hypertension prevalence, awareness, and control in Arab countries: a systematic review. Nurs Health Sci. 2014;16(1):126–130. doi: 10.1111/nhs.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alhyas L., McKay A., Balasanthiran A., Majeed A. Prevalences of overweight, obesity, hyperglycaemia, hypertension and dyslipidaemia in the Gulf: systematic review. JRSM Short Rep. 2011;2(7):55. doi: 10.1258/shorts.2011.011019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassanien A., Al-Shaikh F., Vamos E., Yadegarfar G., Majeed A. Epidemiology of end-stage renal disease in the countries of the Gulf Cooperation Council: a systematic review. J R Soc Med Sh Rep. 2012;3:38. doi: 10.1258/shorts.2012.011150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization; Geneva: 2020. Transmission of SARS-CoV-2: implications for infection prevention precautions Scientific brief. [Google Scholar]
- 25.Buitrago-Garcia D., Egli-Gany D., Counotte M.J., Hossmann S., Imeri H., Ipekc A.M. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003346. e1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abohamr S.I., Abazid R.M., Aldossari M.A., Amer H.A., Badhawi O.S., Aljunaidi O.M. Clinical characteristics and in-hospital mortality of COVID-19 adult patients in Saudi Arabia. Saudi Med J. 2020;41(11):1217–1226. doi: 10.15537/smj.2020.11.25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almazeedi S., Al-Youha S., Jamal M.H., Al-Haddad M., Al-Muhaini A., Al-Ghimlas F. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24:100448. doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alharthy A., Aletreby W., Faqihi F., Balhamar A., Alaklobi F., Alanezi K. Clinical characteristics and predictors of 28-day mortality in 352 critically ill patients with COVID-19: a retrospective study. J Epidemiol Glob Health. 2020 doi: 10.2991/jegh.k.200928.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayed M., Borahmah A., Yazdani A., Sultan A., Mossad A., Rawdhan H: A. Assessment of clinical characteristics and mortality-associated factors in COVID-19 Critical cases in Kuwait. Med Princ Pract. 2020 doi: 10.1159/000513047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rastad H., Karim H., Ejtahed H.S., Tajbakhsh R., Noorisepher M., Babaei M. Risk and predictors of in-hospital mortality from COVID-19 in patients with diabetes and cardiovascular disease. Diabetol Metab Syndr. 2020;12:57. doi: 10.1186/s13098-020-00565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenthal N., Cao Z., Gundrum J., Sianis J., Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020;3(12):e2029058. doi: 10.1001/jamanetworkopen.2020.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.the Northwell COVID-19 Research Consortium. Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Andrade S.I., Riquena G.V., Rodrigues Goulart B.F., Robinson S.S., Gomes J.C. Antivirals against coronaviruses: candidate drugs for SARS-CoV-2 treatment? Front Microbiol. 2020;11:1818. doi: 10.3389/fmicb.2020.01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–770. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.REMAP-CAP Investigators. Gordon A.C., Mouncey P.R. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021 doi: 10.1056/NEJMoa2100433. https://www.ncbi.nlm.nih.gov/pubmed/33631065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung I.F.N., Lung K.C., Tso E.Y.K., Liu R., Chung T.W.H., Chu M.Y. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smits S.L., de Lang A., van den Brand J.M., Leijten L.M., van Ijcken W.F., Eijkemans M.J.C. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6(2) doi: 10.1371/journal.ppat.1000756. e1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moledina S.M., Maini A.A., Gargan A., Harland W., Jenney H., Phillips G. Clinical characteristics and predictors of mortality in patients with COVID-19 infection outside intensive care. Int J Gen Med. 2020;13:1157–1165. doi: 10.2147/IJGM.S271432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorjee K., Kim H., Bonomo E., Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLOS ONE. 2020;15(12) doi: 10.1371/journal.pone.0243191. e0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castro V.M., McCoy T.H., Perlis R.H. Laboratory findings associated with severe illness and mortality among hospitalized individuals with coronavirus disease 2019 in Eastern Massachusetts. JAMA Netw Open. 2020;3(10) doi: 10.1001/jamanetworkopen.2020.23934. e2023934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi S., Qin M., Shen B., Liu T., Yang F., Gong W. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salvatici M., Barbieri B., Cioffi S.M.G., Morenghi E., Paolo Leone F., Maura F. Association between cardiac troponin I and mortality in patients with COVID-19. Biomarkers. 2020;25(8):634–640. doi: 10.1080/1354750X.2020.1831609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kavsak P.A., Hammarsten O., Worster A., Smith S.W., Apple F.S. Cardiac troponin testing in patients with COVID-19: a strategy for testing and reporting results. Clin Chem. 2021;67(1):107–113. doi: 10.1093/clinchem/hvaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milbrandt E.B., Reade M.C., Lee M., Shook S.L., Angus D.C. Prevalence and significance of coagulation abnormalities in community-acquired pneumonia. Mol Med. 2009;15:438–445. doi: 10.2119/molmed.2009.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodelo J.R., De la Rosa G., Valencia M.L., Ospina S., Arango C.M., Gomez C.I. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med. 2012;30:1991–1999. doi: 10.1016/j.ajem.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 50.Smeeth L., Thomas S.L., Hall A.J., Hubbard R., Farrington P., Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 51.Corrales-Medina V.F., Musher D.M., Wells G.A., Chirinos J.A., Chen L., Fine M.J. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation. 2012;125:773–781. doi: 10.1161/CIRCULATIONAHA.111.040766. [DOI] [PubMed] [Google Scholar]
- 52.Davidson J.A., Warren-Gash C. Cardiovascular complications of acute respiratory infections: current research and future directions. Expert Rev Anti Infect Ther. 2019;17:939–942. doi: 10.1080/14787210.2019.1689817. [DOI] [PubMed] [Google Scholar]
- 53.Gallagher P.E., Ferrario C.M., Tallant E.A. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol. 2008;295(6):H2373–H2379. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mendoza-Torres E., Oyarzún A., Mondaca-Ruff D., Azocar A., Castro P.F., Jalil J.E. ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Ther Adv Cardiovasc Dis. 2015;9:217–237. doi: 10.1177/1753944715597623. [DOI] [PubMed] [Google Scholar]
- 55.Mirzaei R., Goodarzi P., Asadi M., Soltani A., Aljanabi H.A.A., Jeda A.S. Bacterial co-infections with SARS-CoV-2. IUBMB Life. 2020;72(10):2097–2111. doi: 10.1002/iub.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adler, Hugh, Ball R., Fisher M., Mortimer K., Vardhan M.S. Low rate of bacterial co-infection in patients with COVID-19. Lancet Microbe. 2020;1(2):e62. doi: 10.1016/S2666-5247(20)30036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Contou D., Claudinon A., Pajot O., Micaëlo M., Longuet Flandre P., Dubert M. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care. 2020;10(1):119. doi: 10.1186/s13613-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]