Abstract
Background
To date, more than 105,805,951 cases of COVID-19 have been diagnosed including 2,312,278 deaths. Many patients have cardiovascular risk-factors and/or co-morbidities and a lot of them developed de novo heart conditions during the active or the post-infectious phase of the infection. A number of studies tried to demonstrate an association between poor prognostic outcomes and cardiovascular comorbidities and related damages, but the quality of current evidence is still weak.
Patients and methods
The aim of this single-center report is to describe the prevalence of cardiac injuries among our COVID-19 patients, to explore their association with survival outcomes and to demonstrate the medical care provided in our real-world setting. Our study included 610 COVID-19 patients admitted to the intensive care unit of our university hospital of whom13.77% (n = 84) presented cardiovascular injuries and which we included in this case series.
Results
The average age of our patients was 65 years (27–90). 60 were men (71.42%) while 24 were women (28.55%). Their average BMI was 29.7 kg/m2. Among them, 50 had a pulmonary embolism (59.52%), 12 patients had a myocardial infarction (14.28%), 10 presented pericarditis (11.9%) and 3 developed myocarditis (3.57%). There were 6 cases of ischemia (7.14%), 2 cases of stroke (2.38%), and 1 case of decompensated heart failure (1.19%). Among our patients, 46.42% had diabetes, 32.14% had a high blood pressure, 13.09% had a chronic renal failure and 14.28% had a history of ischemic heart disease. 14 patients (16.66%) had an elevated troponin with higher levels than 1000 ng/mL. The D-dimer value was high in almost all patients (80.95%). Lung damage from COVID-19 was extensive in 27.38%, severe in 32.14%, and critical in 40.47% of enrolled cases. CT chest angiography, ECG, and cardiac ultrasound were performed to the paraclinical confirmatory exploration of cardiac damages of these patients. Medical care was based on isolation, azithromycin, vitamin C, zinc, vitamin D, salicylic acid, dexamethasone followed with methylprednisolone, and anticoagulation for all hospitalized patients. Tocilizumab was indicated for 17 patients with hyperferritinemia (20.23% of patients). The initial respiratory care of our patients required oxygen therapy using nasal cannula (7.14%) high concentration masks (33.33%), high flow nasal cannula treatment (11.9%), non-invasive ventilation (NIV) (5.95%), and mechanical ventilation (41.66%). Thrombolysis was performed in three subjects with myocardial infarction and 2 underwent angioplasty with placement of an active stent at the proximal interventricular anterior artery, which all were successful. Three massive pulmonary embolisms died despite adequate treatment. Colchicine and salicylic acid were administered for pericarditis cases. Thromboprophylaxis was indicated for all patients and was reinforced if a venous thrombotic episode was confirmed. Patients with limb ischemia underwent surgical treatment. Among the 84 patients included in our cohort, 34 (40.47%) died in intensive care unit and 50 (59.52%) had a favorable evolution.
Conclusion
Cardiovascular involvement during COVID-19 should not be neglected and are associated with severe outcomes.
Keywords: COVID-19, Case series, Cardiovascular injury, Intensive care unit, Morocco
Highlights
-
•
Several studies have demonstrated an association between poor prognostic outcomes and cardiovascular comorbidities in COVID-19.
-
•
The cardiovascular events in our case series confirmed these findings.
-
•
Cardiovascular involvement during COVID-19 should not be neglected as it is associated with severe outcomes.
1. Introduction
Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2), or more commonly known as COVID-19 infection, has created a socio-economical and medical global serious crisis. Indeed, its high infectious rate and contagiousness in the latent phase have made this pandemic uncontrollable despite the urgent lockdown worldwide. The first case was described on December 8th, 2019 in Hubei, China. In less than 3 months, the virus has spread to nearly 177 countries, areas, and territories around the world [1]. The World Health Organization (WHO) considered the infection a global pandemic on March 11th, 2020 [2]. Up to date, more than 105,805,951cases of COVID-19 have been identified with 2,312,278 deaths (as of February 08, 2021) [3]. The clinical manifestations of COVID-19 are mainly pulmonary, but cardiovascular complications have also been reported and are associated with a poor prognosis [4]. Cardiovascular damages associated with SARS-CoV-2 infection include acute coronary syndromes, arrhythmias, heart failure, myocarditis, venous thromboembolic complications [5], ischemic lesions [6], and pericarditis [7]. The consequences of COVID-19 on the cardiovascular system are represented by the worsening of the infection and by the onset of de novo heart problems [8]. SARS-CoV-2, the viral agent responsible for the COVID-19 infection is a single-stranded RNA virus that invades body cells through the ACE-2 receptor [9]. The entry of the pathogen into human cells is facilitated by the interaction of a viral tip protein with this receptor. ACE-2 is a membrane-bound mono-carboxy-peptidase, expressed mainly on the surface of lung, kidney, intestinal and heart cells. Thus, a more severe form of the infection in patients with cardiovascular disease could be due to increased secretion of ACE-2 in these patients as compared to healthy individuals [10]. Patients with comorbidities like heart conditions are therefore not only at high risk of becoming infected but also at risk of a more severe and fatal infection [11,12]. In this paper, we describe the cardiovascular manifestations of 84 patients with COVID-19 infection among 610 cases admitted to the intensive care unit of the University Hospital of Oujda, Morocco. We also investigate the incidence of cardiovascular injuries and illustrate the risk factors of these complications and related mortality. To the best of our knowledge, this is the first real-world study in our setting to report this association I this particular population of COVID-19 patients. The findings of this study were reported according to the PROCESS guidelines [13].
2. Patients and Methods
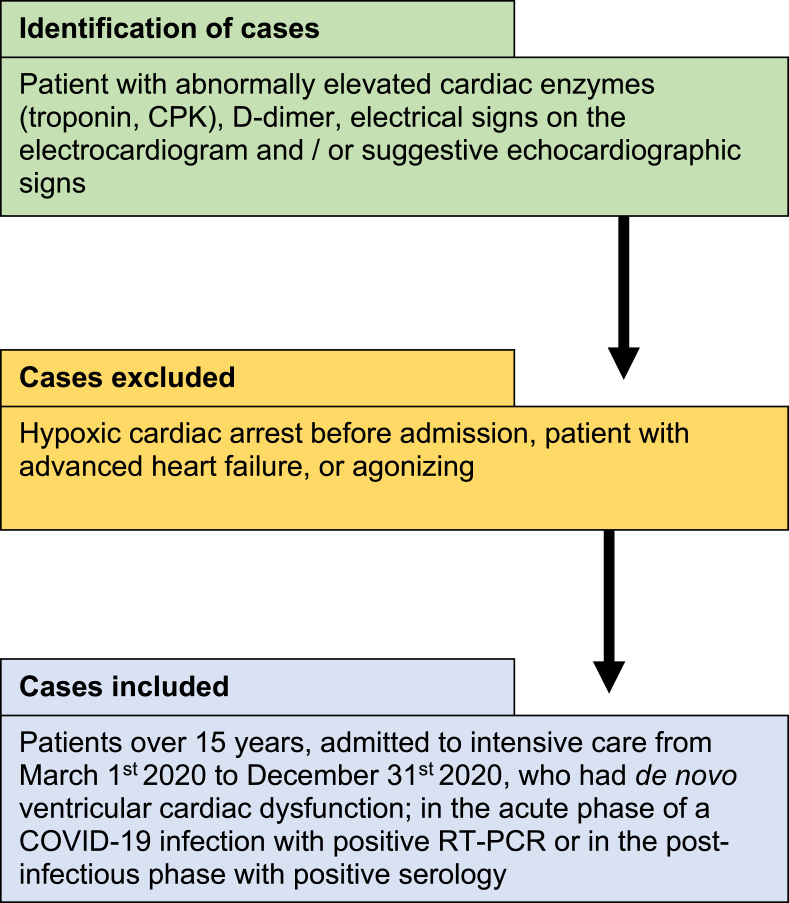
This was a single-center retrospective study conducted in the intensive care department of the University Hospital of Oujda, Morocco. Patient's diagnosis was based on clinical manifestation of COVID-19, associated with a typical chest imaging and confirmed with a positive COVID-19 serology and/or RT-PCR. Among the 610 confirmed patients with COVID-19 pneumonia admitted to our department, 84 were included in our study from March 1st to December 31st, 2020. The inclusion and exclusion criteria are shown in Fig. 1. Epidemiological, clinical, paraclinical, therapeutic and follow-up data were retrospectively collected from patient's medical records.
Fig. 1.
Flowchart of patient's selection.
Descriptive statistics using IBM SPSS Statistics 26 were used for data analysis. Categorical variables were presented as percentages and quantitative data as means (±standard deviation). Access to patients' data was authorized by the Mohammed VI University Hospital. Given the retrospective design of this study, the requirement of patient's consents was waived. Anonymity of data was respected as per national and international guidelines. Our study was registered in ResearchRegistry under the number: 6573.
3. Results
3.1. Epidemiological characteristics
Among the 610 patients diagnosed positive for COVID-19 admitted to intensive care, 84 (13.77%) were included in this study. 72 (85.71%) patients had a positive RT-PCR, while the rest had a positive serology. Of the 84 patients with COVID-19 with cardiovascular disease, 60 (71.42%) were men while 24 (28.55%) were women. The majority of them were elderly, with an average age of 65 years (27–90). The average BMI of our cohort was 29.7 kg/m2. The details of the epidemiological characteristics of our patients are shown in Table 1.
Table 1.
Patients’ clinicopatholgic characteristics of our case series.
| Patients' parameters |
N |
% |
|---|---|---|
| Gender | ||
| Male | 60 | 71.42% |
| Female | 24 | 28.55% |
| Age | ||
| Mean age | 65.11 (±10.85) | |
| ≤35 | 1 | 1.19% |
| 35–65 | 45 | 53.57% |
| ≥65 | 38 | 45.23.% |
| BMI (Kg/m2) | ||
| ≤25 | 6 | 7.14% |
| 25–29.9 | 32 | 3.8% |
| 30–35 | 46 | 54.76% |
| Medical history | ||
| Smoking | 9 | 10.71% |
| Diabetes | 39 | 46.42% |
| High blood pressure | 27 | 32.14% |
| Chronic renal failure | 11 | 13.09% |
| Ischemic heart disease | 12 | 14.28% |
| Asthma/allergic rhinitis | 4 | 4.76% |
| ACFA/dyslipidemia/gout | 5 | 5.59% |
| Breast tumor | 1 | 1.19% |
| Acute adrenal insufficiency | 1 | 1.19% |
| Symptoms | ||
| Dyspnea | 70 | 83.33% |
| Fever | 68 | 80.95% |
| Asthenia | 68 | 80.95% |
| Cough | 35 | 41.66% |
| Myalgia | 31 | 36.9% |
| Chest pain | 29 | 34.52% |
| Anosmia | 18 | 21.42% |
| Ageusia | 13 | 15.47% |
| TTE aspects | ||
| Normal TTE | 25 | 29.76% |
| Dilated right ventriculi | 28 | 33.33% |
| Paradoxical septum | 2 | 2.38% |
| Intra-right ventriculi thrombus | 2 | 2.38% |
| McConnell's sign | 1 | 1.19% |
| Hypokinesia | 8 | 9.52% |
| Akinesia | 6 | 7.14% |
| Pericardial effusion: | 10 | 11.9% |
| -low abundance | 7 | 8.33% |
| -moderate abundance | 3 | 3.57% |
| Respiratory care (oxygen therapy) | ||
| Nasal cannula | 6 | 7.14% |
| High concentration masks | 28 | 33.33% |
| High flow nasal cannula | 10 | 11.9% |
| Noninvasive ventilation | 5 | 5.95% |
| Mechanical ventilation | 35 | 41.66% |
3.2. Cardiovascular injuries
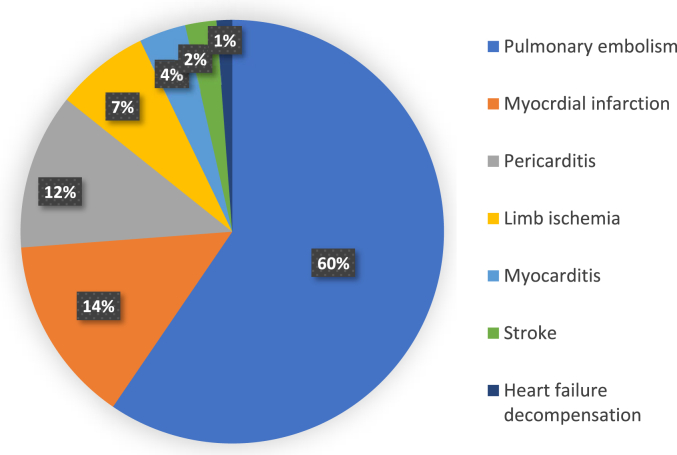
Among these patients, 50 had a pulmonary embolism (59.52%); three of them were massive. Twelve patients had a myocardial infarction (14.28%), ten presented pericarditis (11.9%) and three developed myocarditis (3.57%). In addition, six cases presented an ischemia (7.14%) including two patients with Leriche syndrome (associated with segmental pulmonary embolism in one case), three patients with ischemia of the lower limb, and only 1 case of ischemia of the upper limb. Besides, there were 2 cases of ischemic stroke (2.38%) and 1 case of decompensated heart failure (1.19%). The distribution of cardiovascular injuries is shown in Fig. 2.
Fig. 2.
Frequency of cardiovascular events in our case series.
3.3. Medical history and symptoms
In our study, 9 patients had a history of active smoking. 46.42% had diabetes, 32.14% had a high blood pressure, 13.09% had a chronic renal failure and 14.28% had a history of ischemic heart disease; of them, 3 patients had active stent, 1 with a coronary artery bypass graft surgery, and the rest with medical treatment only. Table 1 summarizes the detailed description of medical history of our case series. The most common symptoms were dyspnea, fever, asthenia, cough, chest pain and diarrhea. Headache, myalgia, anosmia and ageusia were also common signs in our patients (Table 1).
3.4. Clinical, electrocardiographic (ECG) and transthoracic echographic (TTE) signs
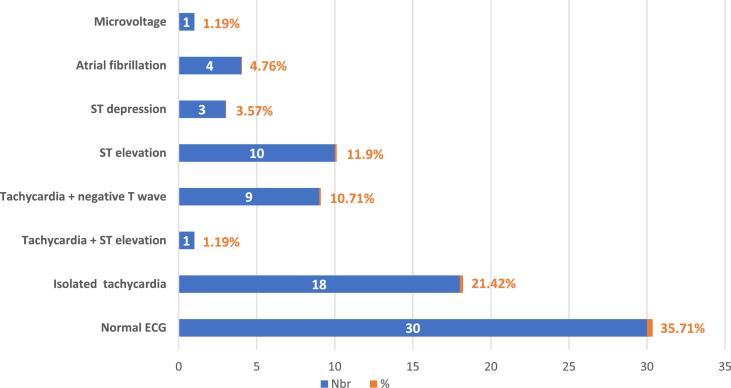
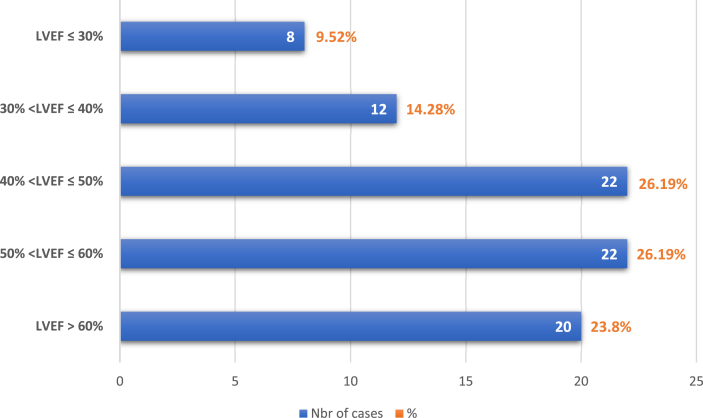
Among our patients, 22.61% of them presented clinical signs of left heart failure (crackling in pulmonary auscultation), 14.28% of right heart failure and only 2 patients (2.38%) had pericardial friction on cardiac auscultation. In our study, all the patients had an ECG; it was normal in 30 cases and showed multiple modifications in 34 cases (Fig. 3). Echocardiography was performed for all patients, except 4 of them due to rapid heart rates or lack of echogenicity. The values of left ventricular ejection fraction (LVEF) are shown in Fig. 4. The modifications found in the TTE were made of dilated right ventricle (RV), paradoxical septal motion, visualization of an intra-RV thrombus, McConnell's sign, hypokinesia, akinesia and pericardial effusion. TTE was normal for 25 patients (29.76%). More details are shown in Table 1.
Fig. 3.
Frequency of electrocardiographic modifications in our included cases.
Fig. 4.
Values of left ventricular ejection fraction (LVEF) of our case series.
3.5. The biological findings
In this series, 14 patients (16.66%) had elevated troponin levels (>1000 ng/mL). These levels ranged from 6400 to 27643 for patients with myocardial infarction (MI) and reached 47369 in one case of myocarditis. The D-dimer value was high in almost all patients (80.95%), but even higher in those with pulmonary embolism (ranging from 580 to 8700 μg/L).
Regarding the blood count, 83.33% of patients presented a hyperleukocytosis greater than 10,000e/mm3, 78.29% had a lymphopenia. Reactive C protein (RCP) values varied between 100 and 200 in 90.47% of patients and were greater than 200 in 7.14% of cases. Ferritin and fibrinogen levels were also high due to the inflammatory syndrome associated with SARS-CoV-2 infection. In this series, the serum ferritin dosage was designed to identify patients who are candidates for a tocilizumab therapy.
3.6. Radiological diagnosis
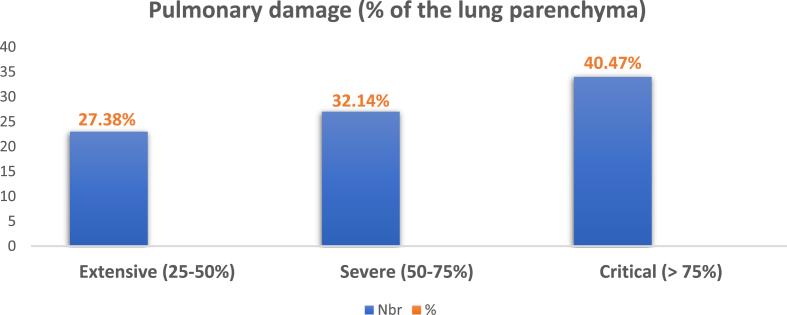
All patients underwent chest CT-scan without contrast injection at their admission. Lung damages from COVID-19 were extensive, severe or critical. No patient had minimal pulmonary involvement in our series (Fig. 5). CT angiography was performed if pulmonary embolism was suspected, or worsening of the respiratory symptoms occurred, except in 3 cases in which the patients were unstable, and for whom TTE alone contributed to the diagnosis of massive pulmonary embolism. In our series, we had 50 cases of pulmonary embolism (59.52%), of which 12 were bilateral (14.28%) and 38 segmental lobar (45.23%). 6 patients with suspicion of ischemic limb underwent CT angiography: 2 had Leriche syndrome, 1 had an occlusion of the right humeral artery and 3 had an occlusion of the right popliteal artery. For the patients in our series with consciousness disorder, brain magnetic resonance imaging (MRI) confirmed the diagnosis of ischemic stroke of the brainstem and both cerebellar hemispheres in 2 cases.
Fig. 5.
Pulmonary damages of our case series.
3.7. Medical care
All the patients were treated in isolation, and received the basic treatment using azithromycin 500 mg the 1st day then 250 mg for 4 days, vitamin C (4 g per day), zinc (90 mg/day) and vitamin D (25,000 IU/week for 4–6 weeks). Dexamethasone (6 mg/day) was prescribed in 68 cases (80.95%), followed by methylprednisolone relay (32 mg/day) with progressive regression. Salicylic acid was given at a dose of 160 mg/day. The prescription of anticoagulation at a curative dose was predominant (90.47%). Its indication took into account the BMI, the fibrinogen and D-dimer levels and the patient's thromboembolic history.
In our series, the initial respiratory care of the patients required oxygen therapy using nasal cannula, high concentration masks, high flow nasal cannula treatment, NIV, and mechanical ventilation (Table 1). 17 patients with hyperferritinemia received an injection of tocilizumab dosed at 400 mg (20.23% of patients). Thrombolysis was performed in three cases of myocardial infarction and 2 patients underwent angioplasty with placement of an active stent at the proximal VIA artery. Two of the 3 massive pulmonary embolisms underwent thrombolysis, the third died at the time of diagnosis. Among the cases of pericarditis, only 4 were initiated with colchicine-based treatment at a dose of 0.5 mg/day for 3–6 weeks, associated with aspirin at an anti-inflammatory dose (1gx3/day for 4 weeks).
3.8. Evolution
Among the 84 patients included in the study, 34 (40.47%) died in intensive care unit and 50 (59.52%) had a favorable evolution and were discharged. In the group of pulmonary embolisms, the clinical outcomes of 30 patients improved with curative anticoagulation, among them only 3 intubated cases, could be extubated, with good evolution and were discharged under an oxygen extractor. Unfortunately, the other 20 patients died including the 3 massive pulmonary embolisms who succumbed to the cardiogenic shock that followed, despite the treatment.
Regarding MI, 7 patients died. Five patients underwent thrombolytic therapy and angioplasty; one of them survived from a cardiac arrest in the operating room after a successful angioplasty. All of them were discharged. Two of the ten patients with pericarditis died from acute respiratory distress syndrome where the lung damage was >75%. One single case of myocarditis did not survive, and it was a 66-year-old man with a history of coronary artery bypass graft surgery who suffered from a cardiogenic shock. This patient presented a blood pressure of 73/45 mmHg at the admission and had troponin at 47,369 ng/mL and in whom TTE found 25% LVEF.
The other two patients who suffered from the brain stroke did not survive. For those who suffered from the vascular damage, the case with Leriche syndrome was able to recover from his pulmonary embolism, he benefited from aorto-di-femoral bypass surgery resulting in revascularization of the limbs. The other case died after the surgery. The two patients with lower limb ischemia and the one with upper limb ischemia underwent a Fogarty catheter thrombectomy with successful revascularization. On the other hand, a case of ischemia of the right lower limb with plantar and toes necrosis, which was amputated, died a few hours later.
4. Discussion
Although the clinical features of SARS-CoV-2 infection are rather respiratory, cardiac lesions have nevertheless been reported by several teams in the field [14]. Up to today, the exact etiology of heart damage in patients with COVID-19 remains under investigation, however the following supposed mechanisms have been suggested. A potential mechanism would be SARS-CoV-2 using ACE-2 as a receptor to enter target cells, it causes direct damage to the heart [15,16] and subsequently inhibits protective signaling pathways in cardiac myocytes [17,18].
In previous studies, the presence of SARS-CoV-1 viral RNA was detected in 35% of hearts in autopsied subjects during the SARS epidemic in Toronto [19]. As molecular structures show great similarities between the receptor domains of SARS-CoV-1 and SARS-CoV-2, this finding could be extended to SARS-CoV-2 [20]. All these data further suggest that SARS-CoV-2 may have a role of direct invasion and damage to the myocardium [21]. In addition, there is an indirect mechanism suggesting the possibility that viral infections can trigger the activation of the host's antiviral response to immune mediation by a severe systemic inflammatory response (cytokine storm) [22]. This includes activation of macrophages, natural killer cells (NK), T lymphocytes (TL), neutrophilia, hyperleukocytosis and hyperglobulinemia [23], high levels of interleukin, and also C-reactive protein; which has been recognized as one of the most common causes of heart damage [24].
The systemic inflammatory response after infection can cause overexpression of macrophages residing in the tissues resulting in reduced coronary blood flow, decreased oxygen supply and destabilization of the coronary artery [11,24]. Le last suggested mechanism is the imbalance between the myocardial oxygen needs and supplies. Indeed, the severe hypoxia that exists during acute lung injury due to SARS-Cov-2 may lead to myocardial ischemia [6,25].
The first retrospective study, involving 41 patients with COVID-19, detected heart damage in 5 patients (12%) [27]. Since then, several retrospective studies have reported an increase in the prevalence of heart damage in patients with COVID-19, with a rate ranging from 5% to 28% [12,[28], [29], [30]]and they constitute 59% of the relative causes of death to SARS-CoV-2 [31]. In our case series, 13.77% of the COVID-19 patients admitted in the intensive care unit had cardiovascular heart damages, and 40.47% of them died, which is in line with our findings. Additionally, current data suggests that patients with previous or underlying cardiovascular disease are likely to have heart damage during their COVID-19 infection [1,32].
Patients with coronary artery disease are believed to be at potentially high risk of coronary plaque rupture secondary to viral infection induced by systemic inflammation [33], which was the case of 2 patients in our study. It was shown that 26% of the patients required intensive cardiology care. Among these, 7.2% patients presented acute coronary syndrome [12]. In our study, myocardial infarction was present in 14.28% of the patients. COVID-19 patients have also presented severe forms of myocarditis with acute impairment of LV function. The diagnosis of myocarditis is not easy in this context. The most typical ECG aspect of myocarditis is usually the appearance of non-specific T wave changes as might be seen in ACS or left ventricular hypertrophy. In myocarditis, very high troponin levels (>10,000 ng/L), signify a sometimes-fulminant cardiac involvement. Regarding the prognosis, a few deaths of COVID-19 patients linked to this myocarditis are reported, but this incidence seems low. Fortunately, it's also the case in our study with only one myocarditis death.
Significant differences between the initial troponinemia levels were observed between the cured and the deceased myocarditis patients [34]. Normalizations of hemodynamic parameters, oxygenation and cardiac function have been reported during the administration of treatments modulating inflammation such as methylprednisolone or immunoglobulins [35], as was the case for us. Coagulation abnormalities and arrhythmias are also common in patients with COVID-19, although the mechanism is still poorly understood [36,37]. Rhythm disturbances have been described in a cohort of 138 patients admitted with COVID-19. In fact, 16.4% of the patients had palpitations with rhythm abnormalities on the ECG [38]. This high prevalence could be explained by metabolic disturbances, hypoxia, neurohormonal stress and inflammation in the context of ARDS. In our case series, 4.76% of the patients had arrhythmia.
ACS, fulminant myocarditis, rapid uncontrolled arrhythmia such as atrial fibrillation or ventricular tachycardia may be accompanied by cardiogenic shock. In the infectious context, the origin of the shock is not always clearly defined. ECG, cardiac ultrasound and invasive hemodynamic monitoring are important elements to aid in the diagnosis.
The prognosis of cardiogenic shock remains in the majority of cases pejorative as our case series found. Currently, the hypothesis of a thrombotic increased risk of COVID-19 is based on the description of cases or series of cases. Klok et al. showed, in a cohort of 184 Dutch patients hospitalized in intensive care and receiving anticoagulant treatment at least at a prophylactic dose, the occurrence of a venous thromboembolic episode in 27% of patients and of arterial thrombosis in 3.7% of between them [39]. Based on the PROTECHT clinical trial and a prospective Canadian cohort [40], 7–10% of ICU patients develop thromboembolic disease despite pharmacologic thromboprophylaxis [41,42]. In our series, 59.52% of the patients developed a pulmonary embolism. This difference may be due to the severity of the hospitalized patient in our study.
COVID-19 infection constitutes an inflammatory and infectious condition which would lead to an increased risk of thrombosis: deep vein thrombosis (DVT) of the lower limbs and pulmonary embolism but also microvascular and arterial thrombosis as seen in six of our patients with limb ischemia. In this scenario, respiratory deterioration associated with other clinical signs of venous thrombosis should raise suspicion of pulmonary embolism [26], which was the case of one patient in our series. The age greater than or equal to 75 years, the existence of a history of venous thromboembolism, obesity, active cancer, cardiac or respiratory insufficiency are also risk factors for DVT that add to the inherent risk of viral infection. In our series, obesity was a major risk factor of thrombo-embolic lesions. Inflammation is often marked in COVID-19 infection, especially in severe forms, leading to sometimes considerable elevations of D-dimers (>10,000 μg/L), fibrinogen (>8 g/L) and CRP (>100 mg/L), which is in line with our findings.
According to another report that enrolled 1099 patients, hypertension was present in almost 15%, diabetes in around 7%, coronary heart disease in only 2.5% [43]. However, our data indicate a higher rate with 46.42% of diabetes, 32.14% of hypertension and 14.28% ischemic heart disease. This difference might be due to the large sample used for that report (severe and non-severe patients) meanwhile our study had just critical COVID-19 patients. Thus, they had more comorbidities. As already observed for similar viral respiratory infections, all of these factors have a poor prognosis and predict the onset of new cardiac damage, or the patient's progression to shock, multisystem failure, or even lethal ventricular arrhythmia [44], as seen in most of our patient which death was caused by theses complications.
To the best of our knowledge, this is the first PROCESS-compliant case series on this topic to be conducted in Morocco. Our research has some limitations. The retrospective design and the small sample size may limit the current conclusions. Future research with prospective enrollment and longer follow up after discharge from intensive care units is needed. Notably, it is recommended to consider all patients with COVID-19 and cardiovascular involvement and/or morbidities as subjects with a high probability of developing severe disease. Therefore, these patients should adequately be managed earlier in the process of their treatment and followed up with reduced time periods to avoid any worsening of their clinical presentation.
5. Conclusion
COVID-19 infection is a disease in which pulmonary symptoms are prominent. However, it can manifest with extra-pulmonary involvement, especially cardiovascular injuries which can sometimes be fatal, such as the acute coronary syndrome, myocarditis, pericarditis, pulmonary embolism and ischemic stroke. The presence of underlying cardiovascular diseases and/or factors represents an increased risk of complications and mortality. Awareness of these various conditions is essential for the rapid and adequate management of patients at risk. The ECG and the TTE are the preferred exams for the diagnosis of damages, without forgetting a careful interpretation of the biological results particularly CRP and troponin levels. Chest CT angiography should be performed whenever worsening respiratory symptoms occur or pulmonary embolism is suspected.
Ethical approval
This is a retrospective case series that does not require a formal ethical committee approval. Data were anonymously registered in our database. Access to data was approved by the head of the department.
Sources of funding
This research was not funded
Author contribution
Dr. Abdelilah El Rhalete and Dr. Inas Rhazi: are principal investigators that collected and analyzed data, wrote the manuscript and prepared the final draft for the submission. Dr. Amine Bensaid and Dr. Ikram Zaid: participated in patients’ management. Prof. Nabila Ismaili and Prof. Nouha Elouafi: collaborated in the project. Prof. Brahim Housni and Prof. Houssam Bkiyer: supervised the research project and approved the final draft for publication All authors approved the final version of the manuscript.
Trial registry number
researchregistry6573.
Consent
This is a retrospective study.
Guarantor
Prof. Brahim Housni.
Dr Abdelilah El Rhalete and Dr Inas Rhazi.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
We would like to thank the medical and nursing teams of Mohammed VI University Hospital for their significant involvement in the management of the patients included in our study. Particular thanks to the director of Mohammed VI University Hospital Prof. Abdelkarim Daoudi for his successful management of this outbreak.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102309.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J. Am. Med. Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.The world health organization coronavirus disease 2019 (COVID-19) situation report Title. https://covid19.who.int [PubMed]
- 3.https://www.worldometers.info/coronavirus/
- 4.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus–infected pneumonia in Wuhan, China. J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan A., Hamilton J.P., Alqahtani S.A., Woreta T A. A narrative review of coronavirus disease 2019 (COVID-19): clinical, epidemiological characteristics, and systemic manifestations. Intern. Emerg. Med. 2021:1–16. doi: 10.1007/s11739-020-02616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Putko R.M., Bedrin M.D., Clark D.M., Piscoya A.S., Dunn J.C., Nesti L.J. SARS-CoV-2 and limb ischemia: a systematic review. J. Clin. Orthop. Trauma. 2020 Nov 29 doi: 10.1016/j.jcot.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra A.K., Lal A., Sahu K.K., Kranis M., Sargent J. Quantifying and reporting cardiac findings in imaging of COVID-19 patients. Monaldi Arch. Chest Dis. 2020 Nov 9;(4):90. doi: 10.4081/monaldi.2020.1394. [DOI] [PubMed] [Google Scholar]
- 8.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metabol. Syndr. 2020;14(3):247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int. J. Infect. Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://www.louvainmedical.be/fr/article/aspects-cardiologiques-de-linfection-par-le-covid-19
- 11.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J. Am. Med. Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agha R.A., Sohrabi C., Mathew G., Franchi T., Kerwan A., O'Neill N., PROCESS Group The PROCESS 2020 guideline: updating consensus preferred reporting OfCasESeries in surgery (PROCESS) guidelines. Int. J. Surg. 2020 Dec;84:231–235. doi: 10.1016/j.ijsu.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 Jul 1;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan W., Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int. J. Cardiol. 2020 Jun 15;309:70–77. doi: 10.1016/j.ijcard.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., Jain S.S., Burkhoff D., Kumaraiah D., Rabbani L., Schwartz A., Uriel N. COVID-19 and cardiovascular disease. Circulation. 2020 May 19;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 17.Alifano M., Alifano P., Forgez P., Iannelli A. Renin-angiotensin system at the heart of COVID-19 pandemic. Biochimie. 2020 Jul;174:30–33. doi: 10.1016/j.biochi.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L., O'Kane A.M., Peng H., Bi Y., Motriuk-Smith D., Ren J. SARS-CoV-2 and cardiovascular complications: from molecular mechanisms to pharmaceutical management. Biochem. Pharmacol. 2020 Aug;178:114114. doi: 10.1016/j.bcp.2020.114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oudit G.Y., Kassiri Z., Jiang C. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 Feb 22;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang C., Jin Z. An acute respiratory infection runs into the most common noncommunicable epidemic-COVID-19 and cardiovascular diseases. JAMA Cardiol. 2020 Jul 1;5(7):743–744. doi: 10.1001/jamacardio.2020.0934. [DOI] [PubMed] [Google Scholar]
- 22.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017 Jul;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fung G., Luo H., Qiu Y., Yang D., McManus B. Myocarditis. Circ. Res. 2016 Feb 5;118(3):496–514. doi: 10.1161/CIRCRESAHA.115.306573. [DOI] [PubMed] [Google Scholar]
- 24.Bonow R.O., Fonarow G.C., O'Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020 Jul1;5(7):751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 25.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei J.-F., Huang F.-Y., Xiong T.-Y., Liu Q., Chen H., Wang H. British Cardiac Society; 2020. Acute Myocardial Injury Is Common in Patients with Covid-19 and Impairs Their Prognosis. Heart. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020 May;109(5):531–538. doi: 10.1007/s00392-020-01626-9. Epub 2020 Mar 11. PMID: 32161990; PMCID: PMC7087935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong T.-Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X., Yu Y., Xu J., Shu H., Xia J. An Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hongde Hu, Fenglian Ma, XinWei, Yuan Fang. Coronavirus Fulminant Myocarditis Treated with Glucocorticoid and Human Immunoglobulin. [DOI] [PMC free article] [PubMed]
- 36.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:E438–E440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kochav S.M., Coromilas E., Nalbandian A., Ranard L.S., Gupta A., Chung M.K. Cardiac arrhythmias in COVID-19 infection. Circ. Arrhyth. Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008719. e008719-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu K., Fang Y.-Y., Deng Y., Liu W., Wang M.-F., Ma J.-P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klok F.A. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020:49–3848. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook D., Crowther M., Meade M. Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit. Care Med. 2005;33 doi: 10.1097/01.ccm.0000171207.95319.b2. [DOI] [PubMed] [Google Scholar]
- 41.The A. New Zealand Intensive Care Society Clinical Trials G, et al. Dalteparin versus unfractionated heparin in critically ill patients. N. Engl. J. Med. 2011;364 doi: 10.1056/NEJMoa1014475. [DOI] [PubMed] [Google Scholar]
- 42.J Helms C Tacquard F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan W.J., Ni Z.Y., Hu Y. China medical treatment expert group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.