Abstract
Objectives:
Nonalcoholic fatty liver disease (NAFLD) is common; however, no information is available on how pediatric gastroenterologists in the United States manage NAFLD. Therefore, study objectives were to: (1) understand how pediatric gastroenterologists in the US approach the management of NAFLD (2) identify barriers to care for children with NAFLD.
Methods:
We performed structured one-on-one interviews to ascertain each individual pediatric gastroenterologist’s approach to the management of NAFLD in children. Responses were recorded from open-ended questions regarding screening for comorbidities, recommendations regarding nutrition, physical activity, medications and perceived barriers to care.
Results:
Response rate was 72.0% (486/675). Mean number of patients examined per week was 3 (standard deviation (SD) 3.5). Dietary intervention was recommended by 98.4% of pediatric gastroenterologists. Notably, 18 different dietary recommendations were reported. A majority of physicians provided targets for exercise frequency (72.6%, mean 5.6 days/week, SD 1.6) and duration (69.9%, mean 40.2 minutes/session, SD 16.4). Medications were prescribed by 50.6%. Almost one-half of physicians (47.5%) screened for type 2 diabetes, dyslipidemia, and hypertension. Providers who spent more than 25 minutes at the initial visit were more likely to screen for comorbidities (p=0.003). Barriers to care were reported by 92.8% with 29.0% reporting ≥ 3 barriers.
Conclusions:
The majority of U.S. pediatric gastroenterologists regularly encounter children with NAFLD. Varied recommendations regarding diet and exercise highlight the need for prospective clinical trials. NAFLD requires a multidimensional approach with adequate resources in the home, community, and clinical setting.
Keywords: NASH, clinical practice, obesity
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic pediatric liver disease in the United States, with a prevalence of 9.6% 1. NAFLD can be progressive and serious; approximately 15% of children with NAFLD have advanced fibrosis at diagnosis 2,3. Furthermore, nonalcoholic steatohepatitis (NASH), present in 25-40% of children with NAFLD,1,3 is an advanced form of the disease and the most common indication for liver transplantation in young adults 4. NAFLD is also associated with type 2 diabetes, dyslipidemia, hypertension, and obstructive sleep apnea5-7. The goal for the treatment of NAFLD is reduction of hepatocyte injury and prevention of fibrosis. Research in pediatric NAFLD has increased rapidly over the past two decades8. Based upon this growing body of evidence, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) developed clinical guidelines for the diagnosis and treatment of NAFLD in children9.
Clinical guidelines play an important role in clinical practice; however, the breadth and depth of recommendations are restricted by the quality and volume of available data. For the majority of recommendations in the NAFLD guidelines, the quality of evidence was rated as low or moderate. Consequently, some recommendations lack the specificity needed for standardized implementation. In addition, physicians often encounter barriers as they apply clinical guidelines. It is important to identify these barriers so that solutions may be developed. Currently, standard of care is what a minimally competent physician in the same field would do with the same situation and resources 10. Clinical practice guidelines are recommendations rather than rules. In fact, there are often gaps between evidence-based recommendations and their implementation in clinical practice11,12. Understanding how a condition is managed in real world settings further informs the standard of care, while simultaneously identifying challenges faced when implementing evidence-based recommendations. To date, there are no data available regarding how pediatric gastroenterologists manage NAFLD in the United States. Moreover, barriers to care in children with NAFLD in the United States have not been elucidated.
This study aims were to: (1) understand how pediatric gastroenterologists in the United States approach the management of NAFLD, (2) identify barriers to care, and (3) identify areas needing additional study.
METHODS
Interview design and process
Pediatric gastroenterologists and pediatric gastroenterology fellows conducted interviews from September 1, 2016 through March 31, 2017 regarding individual pediatric gastroenterologists’ approach to the management of NAFLD. Management was considered to be the treatment of NAFLD after the diagnosis itself was confirmed or established based upon local standards. To be included, an interviewee had to be a pediatric gastroenterologist who had completed fellowship training and was in clinical practice in the United States (U.S). Interviewers were trained on a standardized approach to conducting structured interviews using a common template. Participants provided verbal consent prior to the interview. Data were collected on number of years in practice, type of practice, and location of practice (state). Responses were recorded from open-ended questions regarding screening for comorbidities, and recommendations given to patients regarding nutrition, physical activity, and medications. Interviewees were also asked about barriers to care and what they would want addressed by future studies. The study was approved by the IRB at UC San Diego and at participating institutions of co-authors as per institutional guidelines, which followed Helsinki guidelines for medical research.
Data analysis
Sample size calculation determined 323 pediatric gastroenterologists was the minimum number required for a representative sample based upon the population size of pediatric gastroenterologists in the United States (n = 2000), a confidence level of 95% and a margin of error of < 5%. Descriptive statistics (counts, means, standard deviations, percentages) were reported for each outcome of interest. Response rate was determined by the number of respondents divided by the number of people contacted. Mann-Whitney U (2 groups) and Kruskal-Wallis test (>2 groups) rank based methods were used to determine statistically significant differences in continuous variables across groups, and Fisher’s exact test for categorical variables. Significance was set at P < 0.05. All analyses were performed using R version 3.4.013.
RESULTS
Study subjects
We interviewed 486 pediatric gastroenterologists with an overall response rate of 72% (486/675). As shown in Figure 1, 44 U.S. states were represented. The mean clinical practice duration was 12.3 (SD 10.7) years, with 30.2% practicing < 5 years, 21.4% 5-10 years, 16% 10-15 years, and 32.3% for 15+ years. The distribution by practice type was 76% academic, 13% private practice, and 11% community practice. The mean number of patients evaluated for NAFLD per week was 3 (SD 3.5), with 35.7% of respondents evaluating ≤ 1, 38.2% evaluating 1-5, and 26.1% evaluating >5 patients for NAFLD per week. Only eight percent of respondents reported not evaluating patients with NAFLD. Therefore, the final analysis cohort included 445 pediatric gastroenterologists.
FIGURE 1. Participants by State.
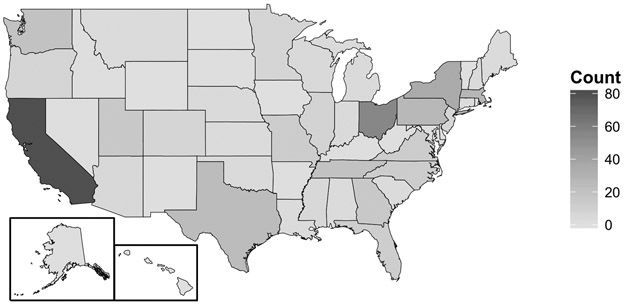
Pictorial histogram depicting number of participants by state.
Clinical Visit Parameters
NASPGHAN guidelines:
It is recommended to follow children with NAFLD on a yearly basis at a minimum to monitor for progression of disease and provide treatment.
When providing lifestyle counseling, more frequent visits are associated with better weight management outcomes in overweight and obese children and therefore may also benefit overweight children with NAFLD/NASH.
Pediatric Gastroenterologists spend an average of 36.9 minutes (SD 12.6, 11.5% < 25, 43.1% 25-39, and 45.4% ≥40 minutes) in an initial visit for NAFLD to explain the diagnosis and provide intervention counseling. Nearly all (97%) pediatric gastroenterologists schedule these patients for follow-up visits with a mean interval of 3.5 months.
Nutrition
NASPGHAN guidelines:
Lifestyle modifications to improve diet and increase physical activity are recommended as the first line treatment for all children with NAFLD.
Avoidance of sugar-sweetened beverages is recommended as a strategy to decrease adiposity.
Almost all pediatric gastroenterologists (98.4%) recommended dietary intervention, with 18 different dietary recommendations reported (Table 1). Changing the consumption of a macronutrient category was recommended by 45.6%, with a low-fat diet recommended by 30.8%, a low-carbohydrate diet by 26.3%, and a high-protein diet by 1.1%. Limiting sweeteners was recommended by 74.6% of respondents with specific advice including: avoiding sugar-sweetened beverages, 63.4%; reducing total sugar intake, 38.0%; and avoiding artificial sweeteners, 9.4%. Moreover, 68.8% of pediatric gastroenterologists counseled on appropriate portion sizes. Additionally, 51.4% of pediatric gastroenterologists made other recommendations regarding eating style, including things such as setting a calorie target, focusing on “clean eating”, and/or choosing healthy snacks.
Table 1.
Recommended Dietary Interventions
| Category | Dietary Focus | Action | Recommend (%) |
|---|---|---|---|
| Macronutrients | 45.6% | ||
| Carbohydrate | Low total intake | 26.3% | |
| Fat | Low total intake | 30.8% | |
| Protein | Increase | 1.1% | |
| Sugar | 74.6% | ||
| Total sugars | Low total intake | 38% | |
| Artificial sweetener | Avoid | 9.4% | |
| Sugar-sweetened beverage | Avoid | 63.4% | |
| Eating Style | 51.2% | ||
| Clean Eating | 5.6% | ||
| Balanced, structure meals | 5.6% | ||
| Calories | Set specific target | 33% | |
| Breakfast | Prioritize | 0.9% | |
| Healthy snacking | 3.4% | ||
| Low glycemic | 12.4% | ||
| Small portion size | 2.0% | ||
| Dining Out | Limit | 4.7% | |
| Other Dietary Components | 10.1% | ||
| Fruits and vegetables | Increase | 5.8% | |
| Water | Increase | 2.0% | |
| Fiber | Increase | 3.4% | |
| Other | |||
| Individualized | 2.2% |
Physical and Sedentary Activity
NASPGHAN guidelines:
Lifestyle modifications to improve diet and increase physical activity are recommended as the first line treatment for all children with NAFLD.
Increasing moderate- to high-intensity physical activity and limiting screen time activities to <2 hours per day is recommended for all children including those with NAFLD.
Exercise intervention was recommended by 97.8% of pediatric gastroenterologists, including 33.9% that made referrals to an exercise program. Specific exercise activities were recommended by 60.0%, most commonly: walking (37.9%), running (16.1%), and biking (14.9%). A specific recommendation was made by 72.6% regarding the target frequency of activity (mean 5.6 days per week). The distribution of recommended exercise frequency is shown in Figure 2. A specific target duration for each exercise encounter was recommended by 69.9%(mean 40.2 +/− 16.4 minutes per session). In addition to exercise, 60.7% (270/445) of pediatric gastroenterologists recommended limiting screen time.
FIGURE 2. Frequency and Duration of Recommended Exercise.
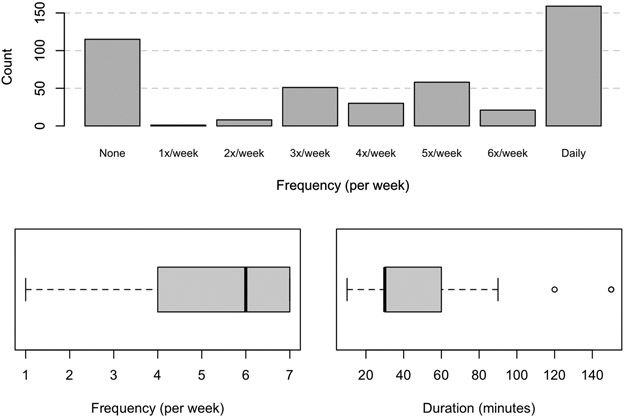
2a. Histogram depicting days per week that pediatric gastroenterologists recommend exercise for patients with NAFLD. 2b.Boxplot showing median and range for days per week that pediatric gastroenterologists recommend exercise for patients with NAFLD. 2c.Boxplot showing median and range for duration of exercise session in minutes that pediatric gastroenterologists recommend for patients with NAFLD.
Medication
NASPGHAN guidelines:
No currently available medications or supplements are recommended to treat NAFLD because none have been proven to benefit the majority of patients with NAFLD.
Medications were used to treat NAFLD by 50.6% of pediatric gastroenterologists. The most commonly used medications were Vitamin E (31.2%), Metformin (9.7%), and Fish Oil (8.3%). In addition, 2.5% of pediatric gastroenterologists reported using medications for weight loss including phentermine, phentermine-topiramate, and liraglutide. Those who evaluated more patients with NAFLD per week were more likely to prescribe medication overall (≤ 1 per week 34.0%; 1-5 per week 57.6%; ≥ 5 per week 62.9%; p < 0.001). However, the use of any particular medication did not differ by the number of patients per week. There was no significant association between years in practice and medication prescribed (p = 0.069).
Referrals for Treatment of Obesity
NASPGHAN guidelines:
Bariatric surgery is not recommended as a specific therapy for NAFLD given lack of outcome data in adolescents. Bariatric surgery may be considered for selected adolescents with BMI >35 kg/m2, who have noncirrhotic NAFLD and other serious comorbidities that are likely to improve with weight loss surgery.
Over half (54.8%) of pediatric gastroenterologists refer patients with NAFLD to a formal weight management clinic. Of those interviewed, 4% reported that they refer patients with NAFLD for bariatric surgery.
Comorbidities
NASPGHAN guidelines:
Children with NAFLD should be screened for dyslipidiemia at diagnosis and periodically as indicated by current lipid guidelines for children.
It is recommended to monitor blood pressure in children with NAFLD.
It is recommended to screen children with NAFLD for diabetes at diagnosis and annually (or sooner if clinical suspicion arises) using either a fasting serum glucose level or a glycosylated hemoglobin).
Providers should remain alert to psychosocial issues and screen children with NAFLD for these when indicated
Most pediatric gastroenterologists, 89% (395/445), reported screening children with NAFLD for one or more comorbidities, most commonly, type 2 diabetes (80%, 356/445), dyslipidemia (72%, 318/445), and hypertension (56%, 248/445), with 47.5% screening for all three of these. Additionally, 42.9% (191/445) reported screening for other conditions, including sleep apnea (29%), bone density (13%), depression (12%), thyroid disease (5%), vitamin D deficiency (3%), and celiac disease (2%). Providers spending >25 minutes at the initial visit were more likely to screen for comorbidities; 74% of those with initial visit length < 25 minutes screen for comorbidities, compared to 92% with initial visit length of 25-40 minutes, and 90% with initial visit length > 40 minutes (p=0.003).
Barriers
Barriers to care were reported by 92.8% of respondents. The 25 barriers reported were categorized using the Social-Ecological Model (Figure 3), which evaluates personal and environmental factors and their effect on behavior14. Individual barriers to care and proposed areas for intervention are detailed in online Table 2. The mean number of barriers reported was 2 (SD 1.2); 29% reported 3 or more barriers. The most common category for barriers was Interpersonal (54.2%), followed by Community Culture (49.0%). The most common specific barriers were access to diet and exercise resources (23.8%) and family motivation and buy-in (22.7%). There were 20.7% of respondents that reported Organizational barriers including inadequate access to a registered dietitian or psychologist. Additionally, 14.8% reported Policy-related barriers such as insurance coverage.
FIGURE 3. Socioeconomic Barriers.
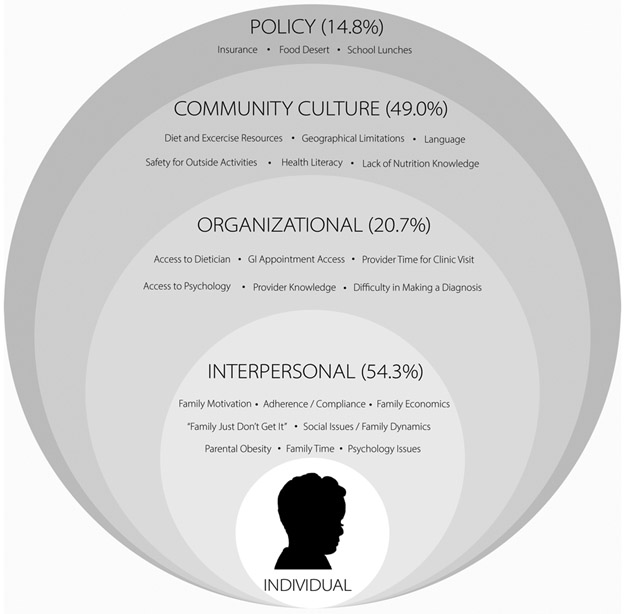
Barriers to the care of patients with NAFLD reported by pediatric gastroenterologists are presented in the context of the Social-Ecological Model. The percentage of pediatric gastroenterologists that reported one or more barriers within each of the model’s four categories is shown.
Table 2.
Individual Barriers in the Management of NAFLD in Children
| Barrier | Number | Percent | Area for Focus |
|---|---|---|---|
| Diet and exercise resources | 106 | 23.8 | Institutional Staffing |
| Family motivation (buy in) | 101 | 22.7 | Patient/provider Education and Communication |
| Adherence /compliance | 89 | 20 | Patient/provider Education and Communication |
| Family economics | 68 | 15.3 | Public Policy |
| Family “just don’t get it” (blaming the patient, the family’s fault) | 55 | 12.4 | Patient/provider Education and Communication |
| Access to RD | 54 | 12.1 | Institutional Staffing |
| Social issues/ family dynamics | 47 | 10.6 | Social Work/Psychology Staffing |
| GI appointment access | 38 | 8.5 | Institutional Staffing |
| Insurance | 37 | 8.3 | Public Policy |
| Geographical limitations | 35 | 7.9 | Telemedicine |
| Language barrier | 35 | 7.9 | Institutional Staffing |
| Provider Time for clinic visit (how long it takes) | 35 | 7.9 | Institutional Staffing |
| Culture | 32 | 7.2 | Patient/provider Education and Communication |
| Access to psychology | 22 | 4.9 | Institutional Staffing |
| Food desert | 16 | 3.6 | Public Policy |
| Safety for outside activities | 15 | 3.4 | Public Policy |
| Health literacy | 14 | 3.1 | Patient Education and Communication |
| Provider knowledge | 11 | 2.5 | Provider Education |
| Psychology issues | 10 | 2.2 | Institutional Staffing |
| Lack of nutrition knowledge | 9 | 2.9 | Institutional Staffing |
| School lunches | 9 | 2.0 | Public Policy |
| Weather | 5 | 1.2 | Public Policy |
Future studies
Many pediatric gastroenterologists wanted studies targeting the treatment of pediatric NAFLD. The most common request (30.1%) was for a medicine to treat pediatric NAFLD. Other common recommendations were studies determining the ideal diet (12.6%) or exercise plan (7.2%) for NAFLD. There was also strong interest in biomarkers for diagnosis (9.4%), determination of disease severity (5.2%), or longitudinal monitoring (4.3%). Additionally, 9.4% suggested studies detailing the natural history of NAFLD in children.
DISCUSSION
We performed direct, systematic, one-on-one interviews with one-in-four pediatric gastroenterologists in clinical practice in the United States. Over 90% of pediatric gastroenterologists surveyed reported caring for children with NAFLD. Treatment of NAFLD most commonly included nutrition and exercise interventions, but we observed wide variability in specific recommendations. Medications were prescribed by approximately one-half of pediatric gastroenterologists. NASPGHAN recommends screening for co-morbidities in children with NAFLD; however, only half of pediatric gastroenterologists performed such screening. Of special note was the substantial range of barriers reported by pediatric gastroenterologists.
Nutrition is the starting point for the management of NAFLD and current guidelines recommended “lifestyle modifications to improve diet” as well as limitation of sugar-sweetened beverage intake9. No specific diet is recommended, reflecting that not all children with NAFLD have the same baseline diet, and that existing literature does not support one specific diet over another8. Consequently, there was no consensus in the recommendations made by pediatric gastroenterologists in clinical practice. Nearly equal numbers recommended a low-fat diet versus a low-carbohydrate diet. These approaches have been shown to have similar short-term effects on hepatic steatosis15. In accordance with the guidelines, the most common dietary recommendation was the avoidance of sugar-sweetened beverages. Added sugars may contribute to both increased adiposity in general, as well as to hepatic de novo lipogenesis17. Dietary approaches have been designed to achieve weight loss or reduce excess total adiposity but have not been adequately studied to determine the effects on liver steatosis, inflammation, or fibrosis. Children with NAFLD may have distinct genetic and/or physiologic mechanisms predisposing them to greater risk of developing the disease16. These are independent of factors driving overweight or obesity alone, and have the potential to influence the efficacy of lifestyle treatments. Optimally, dietary counseling should guide patients on what to eat, not only what to avoid. How this counseling should be distributed between pediatric gastroenterologists and registered dietitians has not been addressed. Many pediatric gastroenterologists noted the lack of access to dietitians and/or the lack of insurance coverage as important barriers.
Exercise is widely believed to improve NAFLD. NASPGHAN guidelines recommend, “increasing moderate to high-intensity physical activity”. Nearly all pediatric gastroenterologists recommend an average of 40 minutes of activity five days per week. Lack of greater specificity on type, frequency, and duration of exercise reflects the current paucity of randomized clinical trials of exercise as a treatment for children with NAFLD. Multiple pediatric trials have used exercise to treat childhood obesity; however, most of the children in these studies did not have NAFLD. In the largest grouping of such studies by Lee et al, only 16% of participants had a liver fat fraction > 5.0%18,19. These studies suggest that liver fat can decrease in the context of exercise, which provides plausibility for the benefit of exercise. However, the effect of exercise in children with NAFLD cannot be extrapolated from these studies. Future studies may address unanswered questions including: Is exercise a therapy by itself or solely an adjuvant therapy, what is the best exercise to treat NAFLD, or how much benefit can be derived from exercise?
There is a dichotomy in the use of medications among pediatric gastroenterologists, with one-half prescribing medications in the treatment of children with NAFLD. Current guidelines do not recommend medications for the treatment of NAFLD in children. It is likely that both physician-driven and patient-driven factors influence the decision to try medication as an intervention. The motivations for medication use were not explored in this study. NAFLD is a heterogeneous disease and individual physicians may have anecdotally observed a benefit of using medications in their patient populations. In contrast, guidelines are based upon aggregate data for the general population of all children with NAFLD and decisions to treat could have been based on the severity of the disease. Moreover, it is challenging to know which children can be fully treated by lifestyle measures. This is underscored by the desire for studies to better characterize the natural history of pediatric NAFLD and to develop predictive biomarkers for the disease. Additionally, medications and lifestyle optimization are not mutually exclusive therapies. However, it may be that the act of prescribing a medication has the unintended consequence of limiting the efforts made by patients to improve their lifestyle20. For the development of effective therapies for children with NAFLD, large, well-designed clinical trials are urgently needed.
NAFLD often occurs in conjunction with extrahepatic manifestations and is a risk factor for comorbidities such as insulin resistance and cardiovascular disease5. NASPGHAN recommends screening for hypertension, dyslipidemia, and type 2 diabetes9. We found that almost half of pediatric gastroenterologists followed these recommendations. Interestingly, the time spent with patients was significantly associated with whether potential comorbidities were addressed. Thus, many providers may not identify comorbidities that have important implications in risk stratification of disease severity due to time constraints. For example, among children with NAFLD, those with high blood pressure are more likely to have worse steatosis6, and those with type 2 diabetes are more likely to have NASH21. Physicians need sufficient time to address comorbidities, as their diagnosis impacts management and have the potential to alter outcomes including morbidity and mortality. Conversely, we found that a number of other comorbidities screened for, such as bone health or mental health, are not formally addressed in the guidelines. Further investigation as to the importance of these comorbidities is needed.
The Social-Ecological Model encompasses five levels illustrating that barriers in the management of NAFLD transcend elements that are in direct control of the patient and the physician22. These barriers include individual, interpersonal and organizational factors; community culture; and policy issues23. One such barrier is limited economic resources, as lifestyle recommendations frequently involve additional costs. For example, fresh fruits and vegetables may be expensive relative to other less healthy food options24. Families may be uncertain how to incorporate cheaper alternatives without specific dietary counseling. Registered dietitians are essential in the care of children with type 2 diabetes; however, for NAFLD the appropriate dietary counseling is often not covered by insurance and/or requires out-of-pocket payments that patients may be unable to afford. Additionally, the cost of organized sports or gym memberships may be prohibitive for many families25. The Organizational level of the model represents patient and family interaction within the clinical care setting. Nearly 20 percent of U.S residents speak a primary language other than English, yet are unable to access medical interpreters26. The use of professional interpreters is associated with improved clinical outcomes27. Patients with language barriers are therefore at increased risk for nonadherence to medical therapy. Pediatric gastroenterologists also noted concerns about access to psychologists for their patients with NAFLD. In a large, multi-center study, over one-third of children with NAFLD were shown to have impaired quality of life7. . Recent data demonstrated that only ten states reimburse for mental health care in the context of the treatment of obesity in children28. Finally, to address those barriers that exist at the policy level, we recommend that pediatric gastroenterologists participate in local, state, and federal advocacy to curb this epidemic of pediatric liver disease.
This study highlights the challenges a clinician faces when trying to adhere to evidence-based recommendations. Major strengths of this study include the high response rate and the large representative sample size. Additionally, the open-ended questions, and one-on-one interview allowed for the fullest range of answers, and provided respondents the opportunity to clarify their answers. One limitation was that the interviews provided self-reported data rather than a direct observation or review of individual medical encounters. Additionally, we did not ascertain the modality used to diagnose NAFLD.
NAFLD is a complex, heterogeneous disease with liver and systemic manifestations. Interviews of pediatric gastroenterologists across the U.S. provided a detailed picture of the current management of pediatric NAFLD. One complicating factor is that there is no universal definition of treatment success. Current treatment is individualized by physician and patient and focuses on optimizing lifestyle parameters. Medication is being used by a substantial proportion of respondents likely reflecting the difficulty of achieving significant and sustained lifestyle changes. This underscores the need to identify effective pharmacologic therapies, particularly for children. Pediatric gastroenterologists identified many barriers to care, some of which are addressable now, and some that will require additional research. Improvements in the management of NAFLD would impact the lives of millions of children and their families as well as the welfare of communities across the U.S.
WHAT IS KNOWN
Clinical guidelines exist for pediatric nonalcoholic fatty liver disease (NAFLD).
No data exist regarding the variability of NAFLD management by pediatric gastroenterologists in the U.S.
WHAT IS NEW
Almost half of pediatric gastroenterologists screen for co-morbidities as recommended by guidelines.
Improvements in nutrition and exercise are the primary interventions recommended but are not standardized as evidenced by the use of 18 different diets.
Medication is used by half of pediatric gastroenterologists underscoring the need to identify effective pharmacologic therapies.
Pediatric gastroenterologists identified many barriers to care, some of which are addressable now.
Acknowledgments
Grant Support: The project described was partially supported by the National Institutes of Health grants UL1TR000100 and UL1TR001442. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
List of abbreviations:
- NAFLD
Nonalcoholic Fatty Liver Disease
- SCALE
Study of Child and Adolescent Liver Epidemiology
- NASH
Nonalcoholic Steatohepatitis
- IRB
Institutional Review Board
- SD
Standard Deviation
- NASPGHAN
North American Society for Pediatric Gastroenterology, Hepatology and Nutrition
- AAP
American Academy of Pediatrics
Footnotes
Disclosures: The authors have nothing to disclose
REFERENCES
- 1.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393. [DOI] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42(3):641–649. [DOI] [PubMed] [Google Scholar]
- 3.Schwimmer JB, Newton KP, Awai HI, et al. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38(10):1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banini BA, Mota M, Behnke M, et al. Nonalcoholic Steatohepatitis (NASH) Has Surpassed Hepatitis C as the Leading Etiology for Listing for Liver Transplant: Implications for NASH in Children and Young Adults. Am J Gastroenterol. 2016;111(S1). [Google Scholar]
- 5.Schwimmer JB, Pardee PE, Lavine JE, et al. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118(3):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwimmer JB, Zepeda A, Newton KP, et al. Longitudinal assessment of high blood pressure in children with nonalcoholic fatty liver disease. PLoS One. 2014;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kistler KD, Molleston J, Unalp A, et al. Symptoms and quality of life in obese children and adolescents with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;31(3):396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeffrey B Schwimmer MD. Clinical Advances in Pediatric Nonalcoholic Fatty Liver Disease. Hepatology. 2016;63(5):1718–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nu. J Pediatr Gastroenterol Nutr. 2017;64(2):319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109–112. [PMC free article] [PubMed] [Google Scholar]
- 11.Cabana MD, Rand CS, Powe NR, et al. Why Don ’ t Physicians Follow A Framework for Improvement. Jama,. 1999;Vol 282:1458–1465. [DOI] [PubMed] [Google Scholar]
- 12.Koot BGP, Nobili V. Screening for non-alcoholic fatty liver disease in children: do guidelines provide enough guidance? Obes Rev. 2017;18(9):1050–1060. [DOI] [PubMed] [Google Scholar]
- 13.R Development Core Team R. R: A language and environment for statistical computing. 2017. [Google Scholar]
- 14.McLeroy K, Bibeau D, Steckler A, et al. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351–377. [DOI] [PubMed] [Google Scholar]
- 15.Ramon-Krauel M, Salsberg SL, Ebbeling CB, et al. A Low-Glycemic-Load versus Low-Fat Diet in the Treatment of Fatty Liver in Obese Children. Child Obes. 2013;9(3):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwimmer JB, Celedon MA, Lavine JE, et al. Heritability of Nonalcoholic Fatty Liver Disease. Gastroenterology. 2009;136(5):1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore JB, Gunn PJ, Fielding BA. The role of dietary sugars and de novo lipogenesis in non-alcoholic fatty liver disease. Nutrients. 2014;6(12):5679–5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, Deldin AR, White D, et al. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: a randomized controlled trial. Am J Physiol Endocrinol Metab. 2013;305(10):E1222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Bacha F, Hannon T, et al. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes. 2012;61(11):2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anthony H, Valinsky L, Inbar Z, et al. Perceptions of hypertension treatment among patients with and without diabetes. BMC Fam Pr. 2012;13(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newton KP, Hou J, Crimmins NA, et al. Prevalence of Prediabetes and Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. 2017;92123(10):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davison KK, Birch LL. Childhood overweight: A contextual model and recommenations for future research. Obes Rev. 2001;2:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotter EW, Hamilton NS, Kelly NR, et al. A Qualitative Examination of Health Barriers and Facilitators Among African American Mothers in a Subsidized Housing Community. Health Promot Pract. 2016;17(5):682–692. [DOI] [PubMed] [Google Scholar]
- 24.Wolfson JA, Bleich SN. Fruit and vegetable consumption and food values: National patterns in the United States by Supplemental Nutrition Assistance Program eligigibility and cooking frequency. Prev Med (Baltim). 2015;76:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill JO, Peters JC. Environmental Contributions to the Obesity Epidemic. Science (80- ). 1998;280:1371–1374. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs EA, Shepard DS, Suaya JA, et al. Overcoming Language Barriers in Health Care: Costs and Benefits of Interpreter Services. Am J Public Health. 2004;94(5):866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karliner LS, Jacobs EA, Chen AH, et al. Do professional interpreters improve clinical care for patients with limited english proficiency? A systematic review of the literature. Health Serv Res. 2007;42(2):727–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JS, Sheer JLO, Lopez N, et al. Coverage of obesity treatment: a state-by-state analysis of Medicaid and state insurance laws. Public Health Rep. 2010;125(4):596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]


