Abstract
Objectives:
Early-phase pediatric nonalcoholic fatty liver disease (NAFLD) clinical trials are designed with non-invasive parameters to assess potential efficacy. Increasingly, these parameters include MRI-derived proton density fat fraction (PDFF) and MR elastography (MRE)-derived shear stiffness as biomarkers of hepatic steatosis and fibrosis, respectively. Understanding fluctuations in these measures is essential for calculating trial sample sizes, interpreting results, and planning clinical drug trials in children with NAFLD. Lack of such data in children comprises a critical knowledge gap. Therefore, the primary aim of this study was to assess whole-liver MRI-PDFF change in adolescents with nonalcoholic steatohepatitis (NASH) over 12 weeks.
Methods:
Adolescents 12-19 years with biopsy-proven NASH undergoing standard-of-care treatment were enrolled. Baseline and week-12 assessments of anthropometrics, transaminases, MRI-PDFF, and MRE-stiffness were obtained.
Results:
Fifteen adolescents were included (mean age 15.7 [SD 2.9] years). Hepatic MRI-PDFF was stable over 12 weeks (mean absolute change −0.8%, p = 0.24). Correlation between baseline and week-12 values of MRI-PDFF was high (ICC = 0.97, 95% CI 0.90 – 0.99). MRE-stiffness was stable (mean percentage change 2.7%, p = 0.44); correlation between baseline and week-12 values was moderate (ICC = 0.47; 95% CI: 0, 0.79). Changes in weight, BMI, and aminotransferases were not statistically significant.
Conclusions:
In adolescents with NASH, fluctuations in hepatic MRI-PDFF and MRE-stiffness over 12 weeks of standard-of-care were small. These data on the natural fluctuations in quantitative imaging biomarkers can serve as a reference for interventional trials in pediatric NASH and inform the interpretation and planning of clinical trials.
Keywords: Nonalcoholic steatohepatitis, NAFLD, MRE, MRI PDFF, steatosis
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in adolescents1 and the leading reason for liver transplantation in young adults.2 The prevalence of NAFLD has risen rapidly among children in the United States.3 NAFLD encompasses a broad spectrum of liver disease severity ranging from isolated steatosis, to nonalcoholic steatohepatitis (NASH) with advanced fibrosis and cirrhosis.4 Children with NASH are at higher risk of serious comorbidities such as type 2 diabetes, hypertension, left ventricular strain, dyslipidemia, and obstructive sleep apnea, and they have a lower quality of life compared to children without NASH.5-10 Despite this large disease burden, there is no proven, safe, and effective medication to treat pediatric NAFLD.
To better enable the planning and execution of clinical trials and develop effective therapies for NAFLD in children, improvements in methodologies evaluating outcome measures of pediatric NAFLD are needed. Currently, the definitive diagnosis and characterization of NAFLD relies on liver histology; however, the invasive nature, potential for sampling error, and cost of liver histology make it impractical as a repeated measure over a short period of time, which is required for early phase clinical trials. Quantitative imaging biomarkers such as magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF) and MR elastography (MRE)-derived shear stiffness (MRE-stiffness) are noninvasive techniques to measure hepatic steatosis and stiffness, respectively, and may be better suited for the task of disease stratification and monitoring in NAFLD.11-14 Additionally, MRI is well suited for the pediatric population in that it does not use ionizing radiation. In a study of 174 children, MRI-PDFF was strongly correlated with hepatic steatosis evaluated by liver histology,15 and in a recent study of 90 children with biopsy-proven NAFLD, MRE was significantly correlated with fibrosis stage.13
Given the promising performance of MR quantitative biomarkers to assess pediatric NAFLD, six out of ten active early-phase pediatric NAFLD clinical trials are utilizing these imaging biomarkers as a primary or secondary outcome measure.16 Yet no pediatric data exist to delineate the natural course of either MRI-PDFF or MRE-stiffness within the timeline of a therapeutic trial. Understanding normal, short-term natural fluctuations in these measures is essential to design and interpret clinical trials. Such fluctuations could affect power analyses to determine clinical trial sample size, and the ability to detect drug and placebo effects. The current lack of data regarding natural fluctuations in NAFLD-related MR imaging biomarkers over the timeframe of pediatric NAFLD clinical trials comprises a critical knowledge gap.
Therefore, the STEATOSIS (Short Term Evaluation in Adolescents, Transitoriness or Stability in Steatosis) observational study was performed with a primary aim to assess change in whole-liver MRI-PDFF in adolescents with NASH over a 12-week time course, such as is commonly used in early-phase clinical trials. Secondary aims were to assess changes over this time course in hepatic MRE-stiffness, as well as in per-segment MRI-PDFF, and to investigate changes in other relevant parameters such as anthropometrics and serum liver transaminases.
METHODS
Participants and Study Design
This was a prospective, longitudinal, observational study of adolescents with NASH over 12-weeks. Participants were enrolled at UC San Diego Altman Clinical and Translational Research Institute. Participants provided written informed assent and their parents provided written informed consent before the screening visit was initiated. Ethics approval was obtained from the Institutional Review Board of the University of California, San Diego.
Adolescents aged 12-19 years with an established clinico-pathological diagnosis of pediatric NASH who were willing to undergo all research procedures and continue standard-of-care treatment for NAFLD17 were eligible for enrollment in this study. No additional intervention or treatment was initiated, but participants were encouraged to continue measures as per their treating gastroenterologist. This study did not provide additional counseling outside of the recommendations of their treating gastroenterologist. The participants were aware that labs and MRI exams would be conducted at two time points with a 12-week interval. Additionally, no medications were started or stopped in the 3 months prior to participation or during participation in this study. To be included, participants had to have a biopsy-proven diagnosis of pediatric NASH and evidence of ongoing disease including ALT ≥ 45 U/L and MRI-PDFF ≥ 10%. Exclusion criteria were: pregnancy; nursing; inability to undergo an MR exam; or non-compensated liver disease. Simultaneous participation in a clinical trial was not allowed.
Clinical Evaluation and Follow-up Evaluation
Assessments were conducted at baseline and at week 12. Each visit consisted of a review of medical history; vital signs (blood pressure, pulse rate, respiratory rate and temperature); anthropometrics (height, weight, waist and hip measurements); and fasting blood collection (complete blood count with white cell differential, comprehensive metabolic panel, alanine aminotransferase [ALT], aspartate aminotransferase [AST], gamma glutamyl transferase [GGT], coagulation tests, and lipid panel). Anthropometrics were performed twice and averages reported.
MR Imaging
All participants underwent MR imaging at baseline and at week 12. Prior to each MR exam, participants were instructed to fast for four hours, and breath-hold instructions were reviewed with the MR Technologist. All scans were performed on a 3T GE scanner (GE DISCOVERY MR750 3.0T; GE Medical Systems, Waukesha, WI) with a torso phase-array surface coil centered over the liver, and a dielectric pad placed between the abdominal wall and the surface coil. Safety readings were performed for each MR exam.
MRI-PDFF
Key MRI-PDFF scanning parameters were: 2D axial spoiled-gradient-echo end-expiration breath-hold acquisitions, TR > 100 ms, six TE values evenly spaced from 1.15 to 6.9 ms, flip angle 10 degrees, number frequency-encoding steps between 140 and 192, number phase-encoding steps between 128 and 140, no filters, no saturation, slice thickness 6 to 10 mm (contiguous), and rectangular field-of-view to accommodate body habitus.15,18
MRI-PDFF values (expressed as a percent) were derived from parametric maps computed pixel-by-pixel from magnitude source images on an Osirix platform (Osirix Foundation, Geneva, Switzerland) using a custom MatLab™ (The MathWorks, Natick, MA, USA) nonlinear, least-squares fitting algorithm. MRI-PDFF values derived from parametric maps have been shown to be nearly equivalent to values calculated directly from regions of interest (ROIs) placed on magnitude MRI-PDFF source images.19 To obtain a whole-liver MRI-PDFF value for each MR exam, a data analyst placed a 1-cm diameter ROI in each of the nine liver Couinaud segments on 5th-echo source images taking care to avoid large blood vessels and bile ducts, liver edges, any lesions, the gallbladder, and artifact. Those ROIs were transferred to the PDFF parametric maps derived from the source images, mean MRI-PDFF values from each of those ROIs was recorded, and a mean value for each MR exam was calculated as the simple mean of those nine values. For each participant, ROI placements for the week-12 exam were co-localized by data analysts to the ROI locations on the baseline exam. MRI-PDFF segmental values were considered unreliable if there was marked artifact in that segment. MRI-PDFF values over 40% derived from parametric maps were checked against values calculated using magnitude (ie Lipoquant) fitting directly from the regions of interest placed on the source images, and in cases of discordancy the values calculated directly from source images were used.
MRE-stiffness
A passive acoustic driver providing continuous vibrations at 60 Hz was placed over the anterior body wall, over the right lobe of the liver at the widest part of the liver, and 2D axial gradient-echo (GRE) MRE was performed with the following scanning parameters: images acquired at four contiguous slice locations; repetition time 50 ms; echo time 20.2 ms; flip angle 30 degrees; matrix 256 x 64; field of view 36 x 36 cm to 48 x 48 cm depending on body habitus; one-signal average; slice thickness 10 mm; interslice gap 0 mm; receiver bandwidth ± 31.25 kHz; array spatial sensitivity encoding technique with parallel imaging acceleration factor 2; motion sensitization along the z-direction; and four phase offsets. Thus, four magnitude and four phase images were acquired at each of four contiguous slice levels, for a total of 32 images per MRE exam.
MRE images were transferred offline for manual analysis by experienced analysts. A parametric shear-stiffness parametric map (calibrated in units of kPa) was computed pixel-by-pixel from wave images for each slice using a previously described two-dimensional direct multimodal inversion algorithm within a custom software package (MRE Quant, Mayo Clinic; Rochester, MN).20 Based on automatically-calculated goodness-of-fit values from regression analysis, computer-derived confidence maps were generated depicting pixels with confidence values > 0.95 (e.g., in pixels for which wave propagation was considered to be adequate). Analysts then manually drew ROIs over the liver on elastogram images for each of the four acquired slice levels, referring to the magnitude images to avoid large blood vessels and bile ducts, edges of the liver, any lesions, the gallbladder, and artifact. Hepatic stiffness was calculated for each MRE scan as the average liver stiffness value for the ROIs drawn on images for the four acquired slice locations, weighted by the areas of those ROIs. Experience in prior studies showing that a minimum of 700 pixels over four acquired slices provides a stable estimate of weighted mean liver stiffness,21 thus MRE-stiffness values were considered to be unreliable if the total ROI was < 700 pixels. Although MR elastograms include the entire liver, only portions of the elastograms contain reliable data, and collecting reliable data from each Couinaud segment is usually not possible. For this reason, MRE does not lend itself to segmental analysis.
Data Analysis
The primary outcome was change in whole-liver hepatic MRI-PDFF from baseline to week 12. Secondary outcomes were change in hepatic MRE-stiffness, per-segment hepatic MRI-PDFF, ALT, AST, GGT, and anthropometrics (height, weight, BMI and waist circumference). A paired t-test was used to assess change in each measure from baseline to week 12. To assess the extent of variability and signal stability between time points, the intraclass correlation coefficient, limits of agreement, and Bland-Altman plots were reported for MRI-PDFF and MRE-stiffness.22
RESULTS
Study Population
A total of 23 adolescents were screened, six of whom were ineligible. Reasons for ineligibility included ALT level < 45 U/L (n = 4), hepatic MRI-PDFF < 10% (n = 1), and liver biopsy showing NAFLD but not NASH (n = 1). Of the 17 adolescents who were enrolled, two did not complete the second required MR scan. Among the 15 participants who completed both the baseline and week-12 scans, all scan results were considered reliable and were included in the analysis. For these 15 participants, the mean NAFLD Activity Score was 4.5 (SD 0.9) and 53% (8/15) had fibrosis. No participant had cirrhosis.
Demographics and Laboratory Values
Mean participant age was 15.7 (SD 2.91) years, with a mean BMI of 34.6 (SD 4.55) kg/m2 and a mean change in BMI over 12 weeks of +0.14 kg/m2 (p = 0.56, SD 0.91). Mean participant weight was 95.2 kg (SD 16.24), and mean change in weight over 12 weeks was +0.97 kg (p = 0.45, SD 2.0). Mean ALT at baseline was 114 U/L (SD 77), and mean change in ALT over 12 weeks was −18 U/L (SD 49). Mean AST was 53 U/L (p = 0.19, SD 26) at baseline, and mean change in AST over 12 weeks was −8 U/L (p = 0.23, SD 26). Mean GGT was 58 U/L (SD 35) at baseline, and mean change in GGT over 12 weeks was −4 U/L (p = 0.45, SD 18). Demographics and laboratory values are summarized in Table 1.
Table 1.
Subject Characteristics and Mean Laboratory Values
| Baseline | Week 12 | p-value | |
|---|---|---|---|
| Sex, N (%) | |||
| Female | 2 (13.3%) | ||
| Male | 13 (86.7%) | ||
| Mean age (SD), yrs | 15.7 (2.2) | ||
| Mean weight (SD), kg | 95.2 (16.2) | 96.2 (18.2) | 0.09 |
| Mean height (SD), cm | 165.5 (8.3) | 166.1 (8.4) | 0.02 |
| Mean BMI (SD), kg/m2 | 34.7 (4.6) | 34.8 (5.1) | 0.56 |
| ALT (SD), U/L | 114 (77) | 96 (67) | 0.19 |
| AST (SD), U/L | 55 (29) | 47 (19) | 0.23 |
| GGT (SD), U/L | 58 (35) | 54 (39) | 0.45 |
| Glucose (SD), mg/dL | 93 (23) | 95 (19) | 0.59 |
| HbA1c (SD), % | 5.5 (0.7) | 5.6 (0.6) | 0.63 |
| Insulin (SD), mU/mL | 34 (11) | 35 (12) | 0.72 |
| Triglycerides (SD), mg/dL | 156 (99) | 165 (100) | 0.69 |
| Cholesterol (SD), mg/dL | 167 (31) | 163 (32) | 0.53 |
| LDL (SD), mg/dL | 103 (25) | 93 (26) | 0.12 |
| HDL (SD), mg/dL | 36 (7) | 38 (9) | 0.26 |
SD = standard deviation; BMI = body mass index; ALT = alanine aminotransferase; AST = aspartate aminotransferase; GGT = gamma glutamyl transferase; Hb = hemoglobin; LDL = low density lipoptrotein; HDL = high density lipoprotein
Change in MRI-PDFF
Whole-liver and per-segment changes in MRI-PDFF are summarized in Table 2.
Table 2.
Mean (SD, range) of PDFF measures and MRE at baseline and 12-week follow-up, as well as their difference, intraclass coefficient (ICC), and limits of agreement
| Baseline | 12-week Follow-up |
Difference | p-value | % change | p-value | ICC (95% CI) |
Limits of Agreement |
|
|---|---|---|---|---|---|---|---|---|
| MRI-PDFF (%) | ||||||||
| Average | 20.6 (10.0, 6.1-41.2) | 19.8 (9.7, 6.2-37.7) | −0.8 (2.6, −5.0-2.5) | 0.24 | −3.8 (12.4) | 0.25 | 0.97 (0.90, 0.99) | (−5.8, 4.2) |
| Segment 1 | 19.3 (9.5, 2.5-35.1) | 19.0 (10.0, 3.4-35.1) | −0.3 (3.5, −8.2-5.4) | 0.74 | −0.4 (19.3) | 0.93 | 0.94 (0.83, 0.98) | (−7.2, 6.6) |
| Segment 2 | 18.1 (10.4, 2.9-41.7) | 17.4 (9.8, 5.3-38.3) | −0.7 (2.1, −4.0-3.5) | 0.22 | −1.7 (14.3) | 0.65 | 0.98 (0.93, 0.99) | (−4.9, 3.5) |
| Segment 3 | 19.1 (10.1, 4.9-40.1) | 18.8 (10.4, 4.2-36.2) | −0.3 (2.7, −4.6-4.4) | 0.69 | −2.7 (15.2) | 0.51 | 0.97 (0.91, 0.99) | (−5.6, 5.0) |
| Segment 4a | 19.3 (10.0, 5.8-41.1) | 18.8 (8.9, 7.7-31.5) | −0.5 (2.9, −6.6-3.4) | 0.53 | 0.7 (18.9) | 0.89 | 0.96 (0.88, 0.99) | (−6.1, 5.2) |
| Segment 4b | 19.9 (9.8, 6.5-38.3) | 19.6 (10.0, −5.0-37.9) | −0.3 (2.3, −3.6-3.3) | 0.65 | −2.2 (14.1) | 0.56 | 0.97 (0.93, 0.99) | (−4.8, 4.3) |
| Segment 5 | 22.4 (10.3, 7.2, 42.3) | 21.1 (9.7, 7.4-38.1) | −1.3 (3.0, −7.7-2.8) | 0.12 | −4.7 (13.5) | 0.20 | 0.95 (0.87, 0.98) | (−7.0, 4.5) |
| Segment 6 | 22.1 (10.5, 6.9-43.5) | 20.8 (10.2, 6.7-39.0) | −1.2 (3.2, −7.4-2.3) | 0.15 | −5.9 (14.6) | 0.14 | 0.95 (0.86, 0.98) | (−7.4, 5.0) |
| Segment 7 | 23.0 (10.1, 8.8-43.6) | 21.2 (9.9, 7.0-40.4) | −1.4 (3.1, −7.3-2.9) | 0.12 | −6.8 (13.0) | 0.07 | 0.95 (0.85, 0.98) | (−7.4, 4.6) |
| Segment 8 | 22.7 (10.7, 7.7-44.7) | 21.1 (10.0, 7.5-39.5) | −1.6 (3.5, −10.4-3.3) | 0.10 | −6.8 (13.4) | 0.07 | 0.93 (0.82, 0.98) | (−8.6, 5.3) |
| MRE-shear stiffness (kPa) | ||||||||
| Average | 2.29 (0.27, 1.9-2.9) | 2.34 (0.34, 1.8-3.0) | 0.05 (0.32, −0.5-0.6) | 0.55 | 2.72 (13.17) | 0.44 | 0.48 (0, 0.79) | (−0.6, 0.7) |
PDFF = proton density fat fraction; MRE = magnetic resonance elastography; CI = confidence interval
Whole-liver change
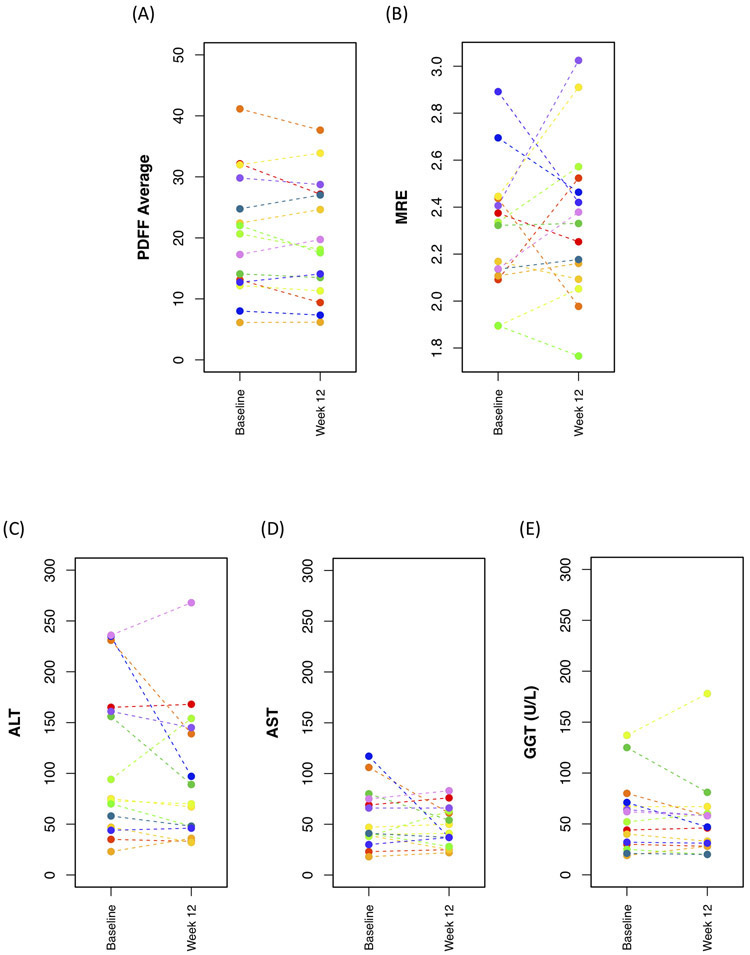
Mean whole-liver hepatic MRI-PDFF at baseline was 20.6% (SD 10%). Mean absolute change in MRI-PDFF from baseline to week 12 was −0.8% PDFF (SD 2.6%; p = 0.24), and mean relative change in MRI-PDFF over that period was −3.8% (p = 0.25). The range of absolute change in MRI-PDFF from baseline to week 12 was +2.3% to −5.0%. The correlation between baseline and week-12 MRI-PDFF was high (ICC = 0.97; 95% CI: 0.90, 0.99). Bland-Altman plots are presented in Figure 2.
Figure 2.
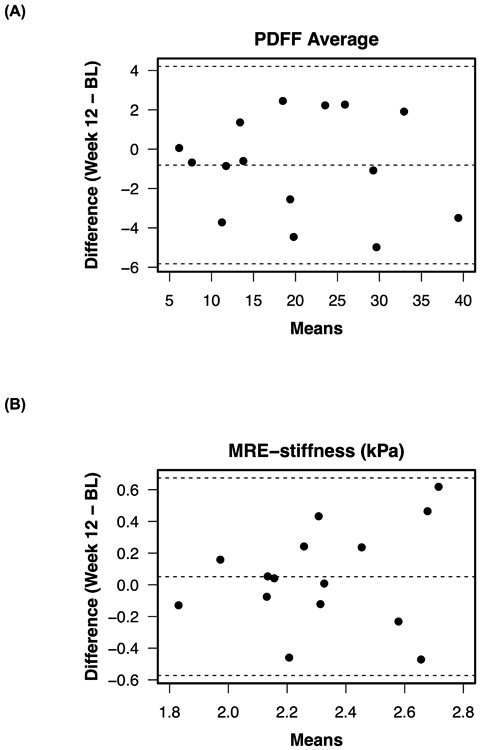
Bland Altman plots for (A) MRE PDFF and (B) MRE-stiffness. BL = Baseline
Per-segment changes
The absolute changes in mean per-segment MRI-PDFF was not significant for any segment (p > 0.05, for each segment individually). The largest mean per-segment relative MRI-PDFF change was for segment 8 (−6.2% PDFF); segment 4a had the smallest mean per-segment absolute change over 12 weeks (+0.7% PDFF).
Change in MRE-stiffness
MRE-stiffness did not change over the 12-week time course of this study (p = 0.54); mean values at baseline and at week 12 were 2.29 kPa and 2.34 kPa, respectively, and relative mean change from baseline to week 12 was +2.7% (SD 13.1%). Correlation between baseline and week-12 MRE-stiffness was moderate (ICC = 0.47; 95% CI: 0, 0.79) (Table 2, Figures 1 and 2).
Figure 1.
Mean change in MRI-estimated and laboratory values at baseline compared to week 12. (A) MRI PDFF (B) MRE-stiffness (C) ALT (D) AST (E) GGT. Each color represents a unique child.
DISCUSSION
We performed a prospective, longitudinal, observational study to determine the change in MRI-PDFF and MRE-stiffness over a 12-week period of standard-of-care treatment in adolescents aged 12-19 years with pediatric NASH. All MR exams were well tolerated. We observed small changes from baseline to week 12 in MRI-PDFF and MRE-stiffness, but those changes were not statistically significant.
The primary outcome of this study was change in hepatic MRI-PDFF, a high precision and accurate quantitative imaging biomarker of hepatic steatosis. Several studies have measured the precision of MRI-PDFF within the same day, but those studies did not assess the natural fluctuation within a timeframe relevant to a clinical trial. In a study of 29 adults with obesity, the inter-examination repeatability of MRI-PDFF between three measurements performed over a two-hour period demonstrated intraclass correlation coefficient of 0.999 and range ≤ 0.45%.23 The authors concluded that an absolute change in PDFF greater than 1.6% is likely to represent true biological change rather than measurement error. Similarly, a study of 36 post-menopausal women undergoing MRI-PDFF twice within the same day reported an agreement of PDFF within ±1.9%.24 The sizes of these normal fluctuations in MRI-PDFF are much smaller than changes that have been reported to represent clinically relevant change in adult clinical trials,12 in whom a greater than 25% decrease in MRI-PDFF from baseline has been associated with improvement in the NAFLD activity score.25 The present study, with a mean absolute change in MRI-PDFF over 12 weeks of standard-of-care treatment of −0.8%, is consistent with these prior studies of MRI-PDFF precision, demonstrating minimal fluctuation.
In the current study, the change in MRE-stiffness was small, 2.7%. There are minimal data available to make a direct comparison. A recent meta-analysis evaluating twelve studies and 274 patients reported that the overall repeatability coefficient of hepatic shear stiffness scores ranged from 12% to 37%.26 Mean sample size in repeatability studies was 23 participants, and studies varied in terms of subject population (healthy vs diseased). Time between imaging studies ranged from several minutes to 6 weeks. Overall mean repeatability coefficient was 22% (95% CI: 16%, 28%). In adults with NAFLD, it is thought that MRE-stiffness measurements are affected mainly by fibrosis and to a lesser extent by inflammation with steatosis having little if any impact. The relative contributions of fibrosis, inflammation, and steatosis on MRE-stiffness in children with NAFLD is unknown and merits investigation. Additionally, there is further work needed to know how much change in MRE-stiffness is required to represent a true change in liver inflammation and/or fibrosis. In our study, the correlation between baseline and week-12 MRE was moderate (ICC = 0.48), suggesting that MRE-stiffness estimates are noisy. In studies evaluating MRE as an outcome measure, multiple acquisitions at each time point may be required to improve the precision of those estimations.
In this observational study, we characterized natural fluctuations of MRI-PDFF and MRE-stiffness in adolescents with NASH and ongoing standard-of-care over a 12-week time course, which is a typical time course for many drug development clinical trials. Because contemporary clinical trials are increasingly utilizing these quantitative imaging biomarkers as outcomes, these data are necessary for designing and interpreting clinical trials in pediatric NASH, including the determination of sample size. In this study, the mean absolute change in MRI-PDFF was −0.8%, and the mean relative change was −3.8%. In 95 percent of children, the change in MRI-PDFF would be between a 5.8% reduction and a 4.2% increase; thus in a clinical trial setting changes within these limits would be reasonably expected in a monitored group and may be considered to reflect natural fluctuations in these measures. For an intervention group as part of a clinical trial, a change greater than this range would be needed to reflect clinically relevant change and not just natural fluctuation. Additionally, in studies that lack a control group, these data could serve as a comparator.
A major strength of this study was that it was conducted in a population of well-characterized adolescents with a clinicopathologic diagnosis of pediatric NASH. Only participants with NASH were included because among children with NAFLD, those with NASH are at greatest risk for adverse liver outcomes and serious comorbidities. Thus, children with NASH are the target population for pharmacologic trials and ultimately treatment with medication. Including only adolescents was also intentional, as there are considerable differences in adolescents compared to younger children with respect to the type of inflammation that is predominant. Among children with NAFLD, those with zone 3 centered NASH are predominantly 13 or older and those with zone 1 centered NASH are often pre-pubertal.4 Another strength was that participants had ongoing standard-of-care management, which reflects the natural course of the disease, whereas data from a clinical trial may be influenced from participation in an intervention, even in the placebo group as patients with NASH receiving placebo can have improvement in histology, ALT, and MRI biomarkers.27 The sample size was consistent with the sample size for early stage clinical trials in children with biopsy-proven disease.28,29
This was the first study to assess longitudinal change in hepatic steatosis and fibrosis, as measured by MRI-PDFF and MRE-stiffness, respectively, in adolescents with NASH to determine the natural course of these measurements over a typical therapeutic clinical trial time course. After 12 weeks of standard-of-care treatment, mean whole-liver MRI-PDFF was stable with an absolute percentage decrease of less than one percent. MRE-stiffness demonstrated greater variability, with a moderate correlation at baseline and week 12. There were no significant changes in anthropometrics, including BMI, or laboratory values, including ALT, over the 12-week time course of this study. These data on the natural fluctuation in quantitative imaging biomarkers can serve as a reference for interventional trials in pediatric NASH and inform the interpretation of clinical trial results for hepatic MRI-PDFF and hepatic MRE-stiffness.
What is Known:
Non-invasive biomarkers are needed for clinical trials in pediatric NAFLD
Advanced MRI techniques can measure liver fat and liver stiffness accurately in children, however how these measures vary with time is unknown
What is New:
In adolescents with nonalcoholic steatohepatitis (NASH), we demonstrated that MRI-derived proton density fat fraction (PDFF) and MR elastography (MRE)-derived shear stiffness are stable over a 12-week time course
These noninvasive tools can be useful to determine whether a change in hepatic steatosis or stiffness is due to natural fluctuations or a specific intervention such as a novel therapy in a clinical trial
Acknowledgments
Financial Support: The project described was partially supported by grants from Galmed Pharmaceuticals LTD and UC San Diego Altman Clinical and Translational Research Institute from the National Institutes of Health Grant UL1TR001442. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest:
Goyal, Sawh, Ugalde-Nicalo, Angeles, Proudfoot, and Newton: no conflicts of interest
Middleton: consults for Bracco, Kowa, Median, Merge Healthcare, Novo Nordisk, Quantitative Insights; is a stockholder with General Electric and Pfizer, and has grant funding from Gilead and Guerbet.
Sirlin: grant funding from Bayer, GE, Philips and Siemens; consults for AMRA, Boehringer and Guerbet; is on the speaker’s bureau for Resoundant and has lab service agreements with Gilead, ICON, Intercept, Shire and Synageva.
Schwimmer: grant funding from Galmed and Intercept.
REFERENCES
- 1.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393. [DOI] [PubMed] [Google Scholar]
- 2.Doycheva I, Issa D, Watt KD, Lopez R, Rifai G, Alkhouri N. Nonalcoholic Steatohepatitis is the Most Rapidly Increasing Indication for Liver Transplantation in Young Adults in the United States. J Clin Gastroenterol. 2018;52(4):339–346. [DOI] [PubMed] [Google Scholar]
- 3.Welsh JA, Rn MPH, Karpen S, Msph MBV. Increasing Prevalence of Nonalcoholic Fatty Liver Disease Among United States Adolescents, 1988-1994 to 2007-2010. 2013:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42(3):641–649. [DOI] [PubMed] [Google Scholar]
- 5.KP N, Hou J, NA C, et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 2016;170(10):e161971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwimmer B, Zepeda A, Newton P, et al. Longitudinal assessment of high blood pressure in children with nonalcoholic fatty liver disease. PLoS One. 2014;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh GK, Vitola BE, Holland MR, et al. Alterations in Ventricular Structure and Function in Obese Adolescents with Nonalcoholic Fatty Liver Disease. J Pediatr. 2013;162(6):1160–1168.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harlow KE, Africa JA, Wells A, et al. Clinically Actionable Hypercholesterolemia and Hypertriglyceridemia in Children with Nonalcoholic Fatty Liver Disease. J Pediatr. 2018;198:76–83.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundaram SS, Sokol RJ, Capocelli KE, et al. Obstructive sleep apnea and hypoxemia are associated with advanced liver histology in pediatric nonalcoholic fatty liver disease. J Pediatr. 2014;164(4):699–706.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kistler KD, Molleston J, Unalp A, Abrams SH, Behling C, Schwimmer JB. Symptoms and quality of life in obese children and adolescents with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;31(3):396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoo T, Serai SD, Pirastehm A, et al. Linearity, Bias, and Precision of Hepatic Proton Density Fat Fraction Measurements by Using MR Imaging: A Meta-Analysis. Radiology. 2018;286(2):486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Middleton MS, Van Natta ML, Heba ER, et al. Diagnostic accuracy of magnetic resonance imaging hepatic proton density fat fraction in pediatric nonalcoholic fatty liver disease. Hepatology. 2018;67(3):858–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwimmer JB, Behling C, Angeles JE, et al. Magnetic resonance elastography measured shear stiffness as a biomarker of fibrosis in pediatric nonalcoholic fatty liver disease. Hepatology. 2017;66(5):1474–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zand KA, Shah A, Heba E, et al. Accuracy of multiecho magnitude-based MRI (M-MRI) for estimation of hepatic proton density fat fraction (PDFF) in children. J Magn Reson Imaging. 2015;42(5):1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwimmer JB, Middleton MS, Behling C, et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology. 2015;61(6):1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US National Library of Medicine. Clinicaltrials.gov. https://clinicaltrials.gov/ct2/results?term=fatty+liver&cond=NAFLD&Search=Apply&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&age=0&gndr=&type=Intr&rslt=. Accessed September 13, 2018.
- 17.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nu. J Pediatr Gastroenterol Nutr. 2017;64(2):319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokoo T, Shiehmorteza M, Hamilton G, et al. Estimation of Hepatic Proton-Density Fat Fraction by Using MR Imaging at 3.0 T. Radiology. 2011;258(3):749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton G, Yokoo T, Bydder M, et al. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed. 2011;24(7):784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkatesh SK, Ehman RL. Magnetic resonance elastography of abdomen. Abdom Imaging. 2015;40(4):745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayakumar S, Middleton MS, Lawitz EJ, et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase II trial of selonsertib. J Hepatol. 2019. January;70(1):133–141. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan DC, Obuchowski NA, Kessler LG, et al. ; RSNA-QIBA Metrology Working Group. Metrology Standards for Quantitative Imaging Biomarkers. Radiology. 2015. December;277(3):813–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negrete LM, Middleton MS, Clark L, et al. Inter-examination precision of magnitude-based MRI for estimation of segmental hepatic proton density fat fraction in obese subjects. J Magn Reson Imaging. 2014;39(5):1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West J, Romu T, Thorell S, et al. Precision of MRI-based body composition measurements of postmenopausal women. PLoS One. 2018;13(2):e0192495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loomba R et al. Reductions in hepatic proton density fat fraction (PDFF) predict histologic improvement in a multi-center clinical trial of subjects with nonalcoholic steatohepatitis (NASH), AASLD meeting abstract. Hepatology. 2017;66(S1):1146A. [Google Scholar]
- 26.Serai SD, Obuchowski NA, Venkatesh SK, et al. Repeatability of MR Elastography of Liver: A Meta-Analysis. Radiology. 2017;285(1):92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han MAT, Altayar O, Hamdeh S, et al. Rates of and Factors Associated With Placebo Response in Trials of Pharmacotherapies for Nonalcoholic Steatohepatitis: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2019. March;17(4):616–629.e26. [DOI] [PubMed] [Google Scholar]
- 28.Vos MB, Jin R, Welsh JA, et al. Losartan Improves Hepatic Inflammation in Children with Nonalcoholic Fatty Liver Disease. Gastroenterology. 2016;150(4):S1036. [Google Scholar]
- 29.Dohil R, Schmeltzer S, Cabrera BL, et al. Enteric-coated cysteamine for the treatment of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;33(9):1036–1044. [DOI] [PubMed] [Google Scholar]