Abstract
Background
The pandemic of the new type of corona virus infection 2019 [Covid-19] also affect people with Multiple Sclerosis (pwMS). Currently, the accumulating information on the effects of the infection regarding the demographic and clinical characteristics of the disease, as well as outcomes within different DMTs¸ enable us to have better practices on the management of the Covid-19 infection in pwMS.
Objective
To investigate the incidence of coronavirus disease 2019 (Covid-19) and to reveal the relationship between the demographic-clinical and therapeutic features and the outcome of Covid-19 infection in a multi-center national cohort of pwMS.
Methods
The Turkish Neurological Society-MS Study Group in association with the Italian MuSC-19 Study Group initiated this study. A web-based electronic Case Report Form (eCRF) of Study-MuSC-19 were used to collect the data. The demographic data and MS histories of the patients were obtained from the file tracking forms of the relevant clinics.
Results
309 MS patients with confirmed Covid-19 infection were included in this study. Two hundred nineteen (219) were females (70.9%). The mean age was 36.9, ranging from 18 to 66, 194 of them (62.8%) were under 40. The clinical phenotype was relapsing-remitting in 277 (89.6%) and progressive in 32 (10.4%). Disease duration ranged from 0.2 years to 31.4 years. The median EDSS was 1.5, ranging from 0 to 8.5. The EDSS score was<= 1 in 134 (43%) of the patients. 91.6% of the patients were on a DMT, Fingolimod was the most frequently used drug (22.0%), followed by Interferon (20.1%). The comorbidity rate is 11.7%. We were not able to detect any significant association of DMTs with Covid-19 severity.
Conclusion
The Turkish MS-Covid-19 cohort had confirmed that pwMS are not at risk of having a more severe COVID-19 outcome irrespective of the DMT that they are treated. In addition, due to being a younger population with less comorbidities most had a mild disease further highlight that the only associated risk factors for having a moderate to severe COVID-19 course are similar with the general population such as having comorbid conditions and being older.
Keywords: Multiple Sclerosis, Coronavirus, Disease modifying treatment, Disease severity
1. Introduction
The new type of corona virus infection (coronavirus disease 2019 [Covid-19]) attributable to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first seen in Wuhan, China, in December 2019. By September 30, 2020, as a rapidly spreading infection, the virus infected close to 35 million people and caused nearly one million deaths (WHO Coronavirus Disease, https://covid19.who.int). In Turkey the first official case was reported on March 11, and the first death on 17th March 2020. As of September 30th, when our data entry was stopped the total number of cases were 318663, and the total number of deaths were 8195 in Turkey (https://covid19.saglik.gov.tr, https://covid19.saglik.gov.tr, 2020).
People with Multiple Sclerosis (pwMS) have also been affected in this pandemic. MS is an immune-mediated, neuro-inflammatory and neuro-degenerative disease involving the central nervous system. A considerable number of pwMS are treated with disease modifying therapies (DMT) of which many have immunosuppressive properties. The estimated number of people with MS (pwMS) is 2.8 million worldwide (Browne et al., 2014). This number is around 70,000 in Turkey, of whom approximately 40.000 are treated with a DMT. The impact of Covid-19 infection on pwMS was not clear at the onset of the pandemic. Since then both the increasing clinical experience globally and a limited number of published observational studies enabled us to learn more on the effects of Covid-19 infection in pwMS and their various treatments. Currently, the accumulated information on the effects of the infection regarding the demographic and clinical characteristics of the disease, as well as on the different DMT that pwMS are on, had allowed us to have better estimates on the outcomes and management of the Covid-19 infection in pwMS. However, the unsettled issues are still considerable.
In this study, we have investigated the relationship between the demographic-clinical and therapeutic features of pwMS and the outcome of Covid-19 infection in the Turkish MS cohort. We also compared our outcomes with the largest MS-Covid-19 series published so far, to better understand the Covid-19 impact on MS.
2. Methods
2.1. Data collection
The Turkish Neurological Society-MS Study Group in association with the Italian MuSC-19 Study Group initiated this study. The Turkish Neurological Society MS Study Group is an organization covering all active MS Centers in Turkey. This study was open to any center that volunteered to contribute their data of their MS patients who had Covid-19 infection. Thirty-four centers participated in the study.
We used the web-based electronic Case Report Form (eCRF) of Study-MuSC-19 to collect the data as described in detail previously (Sormani et al., 2021). The demographic data and MS histories of the patients were obtained from the file tracking forms of the relevant clinics. The approvals were obtained from the Republic of Turkey Ministry of Health and Kocaeli University Ethics Committee (E-29549770-903.02.01-4007).
2.2. Study population
The study included pwMS with suspected or confirmed Covid-19 between March 11, 2020 and September 30, 2020. Only patients with a complete follow up to death or recovery were included in this analysis.
The confirmed cases were pwMS, who had at least one positive RT-PCR test for Covid-19. The unconfirmed cases were either pwMS who were not tested or had a negative RT-PCR test but had the clinical symptoms and signs of the infection (cough, fever, shortness of breath, sudden onset of anosmia, ageusia, dysgeusia); positive radiological findings (ground-glass density on chest-CT) and close contact with the infected person and/or had positive serology.
All patients were either seen at the clinical setting or were interviewed by phone with follow-up calls and their data were recorded on the MuSC-19 website.
2.3. Assessed variables and end points
2.3.1. Statistical analysis
Sample size was not calculated since it was a retrospective collection of data and all the patients that met the selection criteria were included and analyzed. Missing baseline values were imputed by applying a multiple imputation method (MICE).
Baseline demographic and clinical characteristics were summarized as count and percentage, mean with range or median with interquartile range.
The association of baseline characteristics and MS therapies to a severe Covid-19 was assessed by univariate and multivariate ordinal logistic models. Severe outcome was defined as a 3-level factors: ICU or death, vs hospitalized, vs others. Age, sex, smoking, BMI, MS type, disease duration, MSSS, presence of comorbidities, previous use of methylprednisolone, and DMT classes were included in the multivariate models as independent variables, using a stepwise selection procedure.
The strength of the associations was expressed with adjusted Odds ratio (OR) and corresponding 95% confidence intervals (CI). All statistical tests were 2-sided and significance level was set at 0.05.
3. Results
3.1. Study population (patients characteristics)
309 patients were included in the study. 291 patients (94.2%) were confirmed Covid-19 on RT-PCR tests. The 18 patients (5.8%) who were negative on nasopharyngeal and nasal swabs, had a clinical, radiological or serologically defined diagnosis of Covid-19, as described above. Data were collected from every region of the country, with the majority being from large cities.
3.2. Demographics and clinical characteristics
The demographic data of the 309 patients included in this study are presented in Table 1 . Two hundred nineteen (219) were females (70.9%). The mean age was 36.9, ranging from 18 to 66, 194 of them (62.8%) were under 40.
Table 1.
Baseline demographic and clinical characteristics (N = 309).
| Age categories | ≤ 40 yrs | 194 (62.8) |
| 41–59 yrs | 110 (35.6) | |
| >60 yrs | 4 (1.3) | |
| Missing data | 1 (0.3) | |
| Age, years | 36.9 (18.0 – 66.0) | |
| Sex | Female | 219 (70.9) |
| Body Mass Index | 24.4 (16.7 – 38.7) | |
| Employment | Any/Not employed | 139 (45.0) |
| Office clerk & workman | 86 (27.8) | |
| Other | 66 (21.4) | |
| Student | 18 (5.8) | |
| Healthcare workers | No | 278 (90.0) |
| Yes | 31 (10.0) | |
| Smoking | Never smoked | 206 (66.7) |
| Current smoker | 50 (16.2) | |
| Former smoker | 46 (14.9) | |
| NA | 7 (2.3) | |
| Presence of at least one comorbidity | 36 (11.7) | |
| Comorbidities | Hypertension | 17 (5.5) |
| Major depressive disorder | 8 (2.6) | |
| Hematological disease | 3 (1.0) | |
| Coronary Heart Disease | 3 (1.0) | |
| Diabetes | 3 (1.0) | |
| HBV infection | 2 (0.6) | |
| Cancer | 2 (0.6) | |
Data are reported as mean (range) and n(%)
The number of healthcare workers (healthcare assistant, nurse and physician) was 31 (10.0%). As about habits, 66.7% out of patients had never smoked, 16.2% were active smokers. Thirty-six (11.7%) had a comorbid disease, hypertension was the most common (17 cases), followed by depression condition (8 cases). of these.
The demographic and clinical features of MS in the study cohort are shown in Table 2 . The clinical phenotype was relapsing-remitting in 277 (89.6%) and progressive in 32 (10.4%). Disease duration ranged from 0.2 years to 31.4 years, with a mean of 6.6 (±5.87) years. The median EDSS was 1.5, ranging from 0 to 8.5. The EDSS score was<= 1 in 134 (43%) of the patients. 91.6% of the patients were on a DMT, Fingolimod was the most frequently used drug (22.0%), followed by Interferon (20.1%). Thirty patients interrupted treatment due to Covid-19 infection.
Table 2.
MS history (N = 309).
| Clinical phenotype | Relapsing remitting MS (RRMS) | 277 (89.6) |
| Secondary progressive MS (SPMS) | 26 (8.4) | |
| Primary progressive MS (PPMS) | 6 (1.9) | |
| MS disease duration, years | 5.0 (2.0 – 10.0) | |
| Last available EDSS | 1.5 (1.0 – 3.0) | |
| Multiple Sclerosis Severity Scores (MSSS) | < 7 | 267 (86.4) |
| ≥ 7 | 42 (13.6) | |
| In treatment | 283 (91.6) | |
| Treatment duration, months | 19.0 (9.0 – 41.0) | |
| Line of treatment | 1st line | 163 (52.8) |
| 2nd line | 120 (38.8) | |
| MS treatment | Fingolimod | 68 (22.0) |
| Interferon | 62 (20.1) | |
| Ocrelizumab | 43 (13.9) | |
| Teriflunomide | 43 (13.9) | |
| Dimethyl fumarate | 30 (9.7) | |
| Glatiramer acetate | 27 (8.7) | |
| None | 26 (8.4) | |
| Natalizumab | 7 (2.4) | |
| Alemtuzumab | 1 (0.3) | |
| Rituximab | 1 (0.3) | |
| Other | 1 (0.3) | |
| Treatment interrupted | 44/283 (15.5) | |
| Reason for interruption | For Covid-19 infection | 30/44 (68.2) |
| Other/Not specified | 6/44 (13.6) | |
| Patient's decision | 5/44 (11.4) | |
| Other* | 3/44 (6.8) | |
| Use of methylprednisolone previous 1 mount | Yes | 30 (9.7) |
Please note that data are reported as median (IQR) and n(%) within the table, whereas in the main manuscript data are reported with minimum and maximum values
Adverse event / side effect, lack of efficacy, pregnancy or pregnancy planning
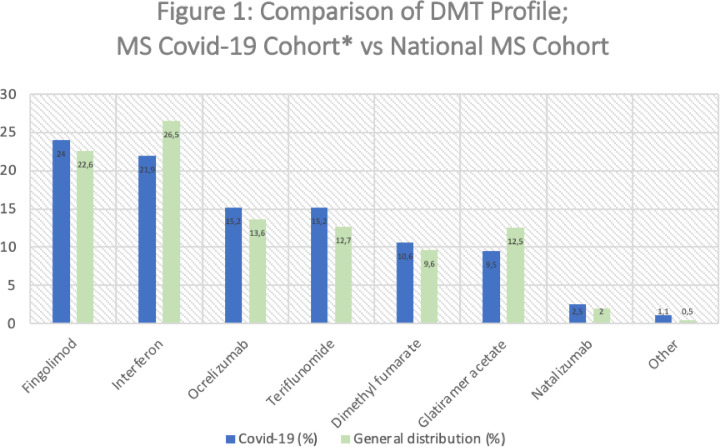
The distribution of DMT use in the Covid-19 MS cohort is compared to the November 2020 MS Turkish Pharma Industry records in Fig. 1 , showing no major differences. Since the number of pwMS not receiving any DMT treatment in the Turkish MS population is unknown, the pwMS were not receiving treatment in the Covid-19 MS cohort were excluded and the comparison was made according to these modified rates.
Fig. 1.
*The differences in the frequencies of DMT use in the Covid-19 MS cohort in Table 2 and Fig. 1 is the result of excluding the untreated Covid-19 MS patients in Fig. 1 for being able to have a direct comparison with the general population, since the number of pwMS not receiving any DMT treatment in the Turkish MS population is unknown.
The signs and symptoms of Covid-19 infection are summarized in Table 3 . The most common symptoms were fatigue, fever and cough and the most common findings were swelling of the tonsils and sore throat. There were 37 (12%) MS patients who were and remained asymptomatic.
Table 3.
Covid signs and symptoms (N = 309).
| Asymptomatic | 37 (12.0) | |
| Symptomatic | 272 (88.0) | |
| Symptoms | Fatigue | 168 (54.4) |
| Fever | 141 (45.6) | |
| Cough | 132 (42.7) | |
| Headache | 106 (34.3) | |
| Sore throat | 90 (29.1) | |
| Bone joint | 84 (27.2) | |
| Loss of smell | 75 (24.3) | |
| Loss of taste | 72 (23.3) | |
| Shortness of breath | 42 (13.6) | |
| Nasal congestion | 23 (7.4) | |
| Sputum production | 18 (5.8) | |
| Chills | 18 (5.8) | |
| Diarrhea | 7 (2.3) | |
| Myalgia | 5 (1.6) | |
| Other | 14 (4.5) | |
| Signs | Throat congestion | 58 (18.8) |
| Tonsils swelling | 21 (6.8) | |
| Rash | 6 (1.9) | |
| Other signs | 5 (1.6) | |
| Lymph nodes enlargement | 4 (1.3) | |
3.3. Risk factors for a severe Covid-19 outcome
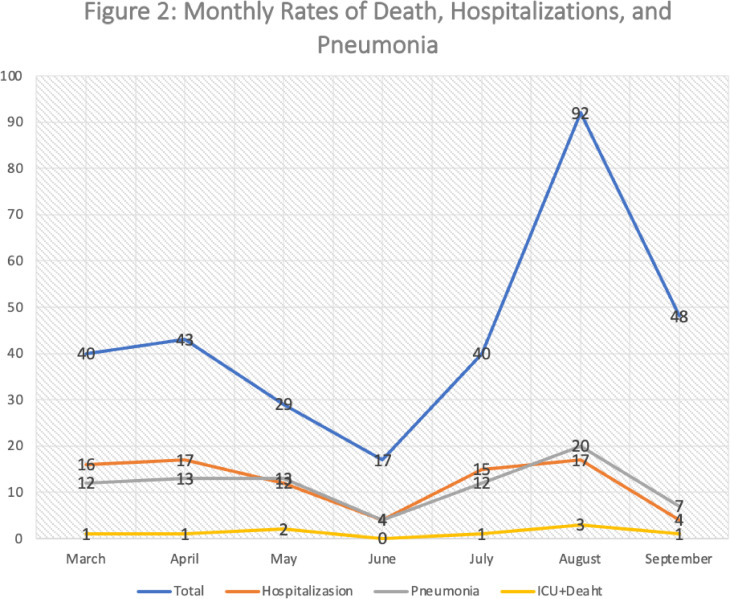
Nine patients (2.9%) were admitted to an ICU, three of them died. Eighty-five (85) patients (27.5%) were only hospitalized (not-ICU), 81 cases of pneumonia were recordedFig. 2. The lower class of Covid-19 severity included 215 patients (69.6%) not even hospitalized.
Fig. 2.
It shows the monthly number of deaths, hospitalizations and pneumonia for the seven month period in which the study was conducted.
Patients’ characteristics are shown in Table 4 .
Table 4.
Patients’ characteristics.
| ICU or death (N = 9) | Hospitalized (N = 85) | Others (N = 215) | ||
|---|---|---|---|---|
| Agemean (range) | 45.3 (19.0 – 66.0) | 38.1 (18.0 - 63.0) | 36.1 (18.0 - 58.0) | |
| Last EDSSmedian (IQR) | 4.5 (3.0 - 6.5) | 2.0 (1.0 - 3.5) | 1.5 (1.0 - 2.0) | |
| MSSS | < 7 | 5 (55.6) | 69 (81.2) | 5 (55.6) |
| ≥ 7 | 4 (44.4) | 16 (18.8) | 4 (44.4) | |
| Clinical phenotype | Relapsing Remitting | 5 (55.6) | 73 (85.9) | 5 (55.6) |
| Progressive | 4 (44.4) | 12 (14.1) | 4 (44.4) | |
Data are reported as n(%), mean (range), or median (IQR)
The rate of hospitalization was quite high in Turkey at the beginning of the pandemic. Because of unfamiliarity with the disease all detected cases were initially hospitalized, without taking into account the severity of the disease. Therefore, people with a positive RT-PCR swab test were hospitalized even if they had mild disease. This approach resulted a hospitalization rate of 40% in the beginning of the pandemic. This rate gradually decreased in the following months.
Older age, progressive MS type, high EDSS and high MSSS were found to be risk factors for severity of Covid-19 at univariate analysis. However, multivariable analysis confirmed only the role of high MSSS (OR = 2.13; 95%IC: 1.07 - 4.23; p = 0.032) and progressive forms of MS (OR = 2.36; 95%IC: 1.09 - 5.13; p = 0.029) as factors with a significant impact on Covid-19 severity. (Table 5 ).
Table 5.
Univariable and multivariable regression models evaluating risk factors for severe Covid-19.
| Univariable Analysis n=309 | Multivariable Analysis n=309 | Stepwise Multivariate Analysis n = 309 | ||||
|---|---|---|---|---|---|---|
| Variable | OR (95% C.I.) | p | OR (95% C.I.) | p | OR (95% C.I.) | OR (95% C.I.) |
| Age | 1.03 (1.01 – 1.06) | 0.016 | 1.02 (0.99 – 1.05) | 0.21 | ||
| Sex (Male vs Female) | 1.30 (0.77 – 2.18) | 0.33 | 1.41 (0.80 – 2.47) | 0.23 | ||
| Smoking (Current/former vs Never) | 0.99 (0.59 – 1.67) | 0.97 | 0.94 (0.54 – 1.65) | 0.83 | ||
| MS type (Progressive vs RR) | 2.93 (1.39 – 6.15) | 0.005 | 1.97 (0.73 – 5.35) | 0.18 | 2.36 (1.09 - 5.13) | 0.029 |
| EDSS | 1.40 (1.21 – 1.62) | <0.001 | / | |||
| Disease duration (years) | 1.01 (0.97 – 1.06) | 0.57 | / | |||
| MSSS (≥ 7 vs <7) | 2.57 (1.33 – 4.97) | 0.005 | 2.17 (1.07 – 4.40) | 0.031 | 2.13 (1.07 - 4.23) | 0.032 |
| BMI | 0.99 (0.94 – 1.04) | 0.58 | 0.99 (0.94 – 1.05) | 0.74 | ||
| Presence of comorbidities | 1.53 (0.74 – 3.19) | 0.26 | 1.32 (0.58 -2.99) | 0.50 | ||
| Methylprednisolone with in 1 month prior to Covid-19 onset | 1.28 (0.59 – 2.78) | 0.53 | 1.26 (0.56 – 2.84) | 0.58 | ||
| Disease modifying therapy | ||||||
| Fingolimod / Natalizumab | Ref. | Ref. | ||||
| Interferon / Glatiramer acetate | 0.57 (0.29 – 1.12) | 0.11 | 0.64 (0.32 - 1.27) | 0.20 | ||
| Teriflunomide / Dymethil fumarate | 0.73 (0.37 – 1.45) | 0.37 | 0.76 (0.38 - 1.52) | 0.44 | ||
| Ocrelizumab/Rituximab | 1.17 (0.54 – 2.53) | 0.69 | 0.64 (0.26 - 1.60) | 0.34 | ||
| None | 1.06 (0.42 – 2.70) | 0.90 | 0.77 (0.28 - 2.11) | 0.61 | ||
OUTCOME = ICU/death vs Only hospitalized vs Others
The relationship between DMTs used by patients and Covid-19 disease severity was investigated. The fingolimod-natalizumab group, which has the highest number of patients, was taken as reference and analyzed accordingly. As a result, no DMT group, including B-cell depleting treatments, was found to be associated with Covid-19 disease severity. The low number of patients in the DMT groups may also have led to this result. (Table 5)
3.4. Death associated with Covid-19
Three patients in whom the infection was confirmed by a positive RT-PCR test for Covid-19 had died (lethality rate=0.9 %).
The first case is a 61-year-old female with hypertension, with secondary progressive MS (SPMS), untreated and with a EDSS = 8. The reported reason for death was pneumonia and a consequent septic shock.
The second case is referred to a 61-year-old male patient with SPMS, EDSS = 8.5, untreated, and presenting hypertension and coronary heart disease as comorbidity.
‘The third case is a 38-year-old female with relapsing remitting MS and an EDSS score of 4, who was treated with fingolimod. She presented with a mild Covid-19 infection; for which she was started antiviral (favipiravir), antibacterial (clarithromycin) and hydroxychloroquine treatments. She had no known previous liver disease. During the one-year period of using fingolimod there was no impairment of liver functions. When Covid-19 symptoms had started her initial liver function tests were within normal limits and deteriorated after she was put on Covid-19 treatments. She was then diagnosed with toxic hepatitis along with multi-organ failure and aspiration pneumonia leading to death. Mortality data are shown in Table 6. ’
Table 6.
Characteristics of deceased patients.
| Sex | Age | Sex | Type of MS | MS disease duration years | MS treatment | Treatment duration months | Last EDSS | MSSS | Comorbidity | Presence of pneumonia | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | 38 | Female | RRMS | 13.0 | Fingolimod | 12.0 | 4.0 | 4.38 | Major depressive disorder | Yes* | Toxic hepatitis, multiple system failure, pneumonia* |
| Male | 61 | Male | SPMS | 28.0 | None | . | 8.5 | 9.45 | Coronary heart disease; Hypertension | Yes | Pneumonia |
| Female | 61 | Female | SPMS | 6.0 | None | . | 8.0 | 9.81 | Hypertension | Yes | Pneumonia, septic shock |
Aspiration pneumonia
At the time of this study submission of our study, there were 5 large MS&Covid-19 published series. We compared our findings to such previous analyses in Table 7 .
Table 7.
MS&Covid-19 relationship.
| Reference | Data of Turkey | Sormani M.P. et al. (4) | Louapre C. et al. (6) | Loonstra FC. et al. (7) | Parrotta E. et al. (8) | Zalbalza A. et al. (9) |
|---|---|---|---|---|---|---|
| Total number of cases | 309 | 844 | 347 | 86 | 76 | 48 |
| Age range | 18-66 | 18-82 | - | 20-71 | 13-71 | |
| Age mean | 36.9 | 45 | 44.6 | 45.5 | 44.9 | 43.9 |
| Comorbidity % | 12.3 | 22.3 | 32 | 26.70 | 46.1 | 35.4 |
| RRMS patient rate % | 89.6 | 80.1 | 79.5 | 80.20 | 72.3 | 72.9 |
| MS disease duration (years) | 7.43 | 10.2 | 13.5 | - | 15.2 | 14.5 |
| EDSS (mean) | 1.5 | 2 | 2 | 3.00 | - | 2 |
| Covid-19 PCR (+) rate % | 94.2 | 33.1 | 55 | 50.00 | 48.7 | 45.8 |
| Mortality % | 0.9 | 1.54 | 3.5 | 4.70 | 7.9 | 4.2 |
| Mortality number | 3 | 13 | 12 | 4 | 6 | 2 |
| pwMS on DMT %* | 91.6 | 82.1 | 81.8 | 86 | 84.2 | 89.6 |
4. Discussion
A positive aspect of our study as compared to those previously published on the same topic is the high RT-PCR positivity rate (Sormani et al., 2021; Louapre et al., 2020; Loonstra et al., 2020; Parrotta et al., 2020; Zabalza et al., 2020). The few patients without a positive PCR test were confirmed with either diagnostic studies and/or later by positive serology for Covid-19. The likely explanation for the high rate of RT-PCR positivity in patients is an extensive screening program as well as repeated testing in suspected cases. An immediate observation from our national MS cohort with Covid-19 is that the majority of the Turkish pwMS had a very mild disease, as shown by the very low rate of ICU admission and death.
When comparing the Turkish MS-Covid-19 cohort with other national or regional studies, noticeably the Turkish cohort is younger, has a lower rate of disability and low rate of co-morbidities. The female/male ratio of the population of our study is 2.44 which is comparable to Turkish pwMS in MsBase's 'TurkMSBase' gender distribution sub-study, in which this ratio was 2.34, in 11596 patients (https://www.msbase.org/sub-studies/(Sub-studies)).
The rate of patients under the age of 40 is approximately two-third in our cohort. Mean age was found to be 36.9, whereas it is approximately 45 in previously published studies [Table 7]. However, this is representative of our national population. According to the 2020 Turkish national data 61.2% of the total population in under the age of 40 (https://www.nufusu.com/turkiye-nufusu-yas-gruplari(Türkiye Nüfusu Yaş, 2020)). In our series, this ratio was 62.8%, consistent with the general national population. The rate of patients under the age of 40 was found to be 33.1% in the study of Sormani et al. (Sormani et al., 2021). It has been shown that one of the main factors for Covid-19 infection severity is age (Möhn et al., 2020), and since our cohort is younger this may be one other explanation for the better outcome observed in our cohort.
Another factor that determines the severity of Covid-19 infection is the presence of comorbidities. In our series, the comorbidity rate is 11.7%. The most important reason for the low rate of comorbidity can also be explained with the younger age of our patients. Since, in similar studies, the rate of comorbidity ranged from 22% to 46%, (Sormani et al., 2021; Louapre et al., 2020; Loonstra et al., 2020; Parrotta et al., 2020; Zabalza et al., 2020) our low rate of comorbidity may be another explanation related to the low mortality seen in Turkish pwMS.
Long disease duration and increased disability are among the major variables associated with the worst outcome in pwMS (Degenhardt et al., 2009). In our cohort, as both disease duration and mean EDSS scores were low these findings may also be associated with low morbidity and mortality observed. The MSSS is a parameter that reveals the relationship of the disease with disability and disease duration in MS (Roxburgh et al., 2005). In our study, the severity of Covid-19 was found to be higher in the patient group with high MSSS score confirming earlier observations (Sormani et al., 2021; Louapre et al., 2020; Loonstra et al., 2020; Parrotta et al., 2020; Zabalza et al., 2020).
From the beginning of the pandemic individuals over the age of 65 were locked down and have been well isolated in Turkey. It's likely that pwMS with advanced age were less infected as well as pwMS with higher disability levels who due to social isolation were less exposed to Covid-19. Since these most vulnerable MS populations were better isolated, they were further protected from infection.
Impact of Covid-19 on MS has been investigated since the early days of the pandemic. Due to difficulties in reaching all patients, alternative methods were implicated. One such multinational project was the use of wearable devices and smartphone technology to explore the impact of Covid-19 among MS patients (Costa et al., 2020). Such studies may provide some information and shed light on this effect, however, the confirmed cases with this specific infection remain low in these populations. So, it may be difficult to reach definite conclusions with this methodology at this stage.
Another fact that it would be of crucial importance is the impact of DMTs in pwMS with Covid-19. We were not able to detect any significant association of DMTs with Covid-19 severity. The question on whether some of the DMT are partly protective and some may be associated with Covid-19 is still controversial (Sormani et al., 2021; Louapre et al., 2020; Loonstra et al., 2020; Parrotta et al., 2020; Zabalza et al., 2020; Baker et al., 2020; Laroni et al., 2020; Zrzavy et al., 2020). Only B-cell depleting treatments were shown to increase the risk for a more severe Covid-19 infection. In general, the infections are likely to be associated with increased morbidity in patients receiving DMT treatments with immunosuppressive properties (Winkelmann et al., 2016). It is known that the frequency of herpes virus infections and opportunistic infections are increased with high efficacy treatments (Luna et al). This risk of infections is associated with lymphopenia, especially suppression of CD8 T cells for viral infections and/or hypogammaglobulinemia (Baker et al., 2020; Vollenhoven et al., 2015; Hauser et al., 2008). It can be speculated that the effect of DMTs on Covid-19 can be either protective or negative by increasing the severity of the infection based on their mode of action. However, in most studies such a negative effect couldn't be established, but the probability of a preventive effect on the multisystem inflammatory syndrome that is associated with Covid-19 severity had been proposed in some other studies in which similar immunosuppressive agents were used in people with various neurologic and systemic autoimmune diseases (Akiyama et al., 2020; Altobrando et al., 2020; Montero-Escribano et al., 2020; Kovvuru et al., 2021).
Two of the patients who died in our series were older patients who weren't on any DMT but had severe disability and comorbidities. The third patient's death was accepted to be due to indirect causes and not directly related to Covid-19. Our experience is mostly consistent with the previously reported large series showing that disease severity and fatality were mostly related to longer disease duration, high disability and older age (Sormani et al., 2021; Louapre et al., 2020; Loonstra et al., 2020; Parrotta et al., 2020; Zabalza et al., 2020). In our national cohort of pwMS none of the DMTs had a significant effect on the outcome of Covid-19 severity. Although a tendency for the B-cell depleting therapies for a more severe disease course was observed, this observation didn't reach statistical significance. Since the distribution of pwMS on DMTs is proportional to the national MS population treated [Fig. 1], it may be suggested that our observation may be representative of our national MS population as well. As our study population was younger with a lower disability level these features may explain the overall better outcome observed in our cohort. Since a number of variables and biases may have influenced the included pwMS in the reported cohorts and the sample size is too small to discriminate the effects of the different DMTs on the risk of severe Covid-19, we believe that the current study does not allow to reach a definite conclusion regarding whether any DMT carries a higher risk independent of other variables. Therefore, we agree with the statements that all approved disease-modifying therapies in pwMS should be continued unaltered and that risk management may be made on individual basis (Zrzavy et al., 2020; Korsukewitz et al., 2020).
5. Limitations
In our cohort, asymptomatic patients were not considered as a separate group, but were included together with the mild disease group. Separate analysis of this selected population may provide more information about the course of the disease, however since their number was limited, they weren't evaluated as a subgroup. Since from the beginning of the pandemic individuals over the age of 65 were locked down and have been well isolated in Turkey, it's likely that pwMS of advanced age were less infected and therefore are under-represented in our cohort. Lack of well documented data for people within the general population with Covid-19 infection didn't allow us to compare the outcomes on national basis. Only 10.2% of the patients in our study population had progressive MS. The most likely explanation for the lower rate of Covid-19 infected MS patients with the progressive forms of disease may be that this population was most carefully following public health guidelines, staying at home and being in less contact with infected people as they know that they are at higher risk and therefore were less likely to be infected. Further limitations were the lack of detailed laboratory and serologic testing for Covid-19-IgG since running such tests was not common practice at the time of our study conduction. Therefore, we cannot reach any conclusion on the immunological profiles of our MS Covid-19 cohort and outcomes.
6. Conclusion
The Turkish MS-Covid-19 study revealed that pwMS are not at a higher risk of Covid-19 severity according to the DMT used, while it confirmed that MS severity, assessed by the EDSS or by a progressive course, was a significant risk factor. In addition, due to the young age and the low proportion of patients with comorbidities, the Turkish MS population had overall a mild Covid-19 course.
Funding
The author (s) have received no financial support for research, authorship, and/or publication of this article.
Credit author statement
Concept - SS, AS, RK, HE, MPS, IS; Design- SS, AS, MPS; Supervision- AS, RK, HE, MPS; Resource - SS, AS, RK, HE; Materials - AS, HE, RK, MPS, IS; Data Collection - All authors; Analysis and / or Interpretation - IS, SS; Literature Search - SS, RK, AS, HE; Writing - SS, RK, HE, AS, MPS, IS; Critical reviews – AS.
Declaration of Competing Interest
S. Sen has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma.
Rana Karabudak has received honoraria for giving educational lectures, consultancy fees for participating advisory boards, and travel grants for attending scientific congresses or symposia from Roche, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey, Abdi İbrahim İlac, Deva and ARIS.
H. Efendi has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey and Abdi İbrahim İlac.
A Siva has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey and Abdi İbrahim İlac.
The rest of authors declare no conflict of interest with the study project.
Footnotes
**The Turkish MS Study Group:
Acikgoz M., Zonguldak Bulent Ecevit University School of Medicine, Zonguldak/Turkey
Agan K., Marmara University School of Medicine, Istanbul/Turkey
Akcali A., Gaziantep University School of Medicine, Gaziantep/Turkey
Akman G., Florence Nightingale Hospital, Istanbul/Turkey
Altintas A., Koc University School of Medicine, Istanbul/Turkey
Arikan Akkoyun F., Kutahya Health Sciences University School of Medicine, Kutahya/Turkey
Beckmann Y., Katip Celebi University School of Medicine, Izmir/Turkey
Bilge N., Ataturk University School of Medicine, Erzurum/Turkey
Boz C., Karadeniz Technical University School of Medicine, Trabzon/Turkey
Bunul D.S., Kocaeli University School of Medicine, Kocaeli/Turkey
Canbaz Kabay S., Kutahya Health Sciences University School of Medicine, Kutahya/Turkey
Cınar B.P., Zonguldak Bulent Ecevit University School of Medicine, Zonguldak/Turkey
Demir C.F., Firat University School of Medicine, Elazıg/Turkey
Ekmekci O., Ege University School of Medicine, Izmir/Turkey
Ethemoglu O., Harran University School of Medicine, Sanliurfa/Turkey
Gokce S.F., Cumhuriyet University School of Medicine, Sivas/Turkey
Guler S., Trakya University School of Medicine, Edirne/Turkey
Gursoy E., Bezmialem University School of Medicine, Istanbul/Turkey
Guzel V., Bezmialem University School of Medicine, Istanbul/Turkey
Kilic A.K., Kartal Dr. Lutfi Kirdar Research and Training Hospital, Neurology Clinic, Istanbul, Turkey
Kocer B., Gazi University School of Medicine, Ankara/Turkey
Kurtuncu M., I stanbul University Istanbul School of Medicine, Istanbul/Turkey
Mirza M., Erciyes University School of Medicine, Kayseri/Turkey
Mungan S., Ankara City Hospital, Neurology Clinic, Ankara/Turkey
Omerhoca S., Bagcılar Research and Training Hospital, Neurology Clinic, Istanbul, Turkey
Saip S., Istanbul University Cerrahpasa School of Medicine, Istanbul/Turkey
Seferoglu M., Bursa Specialty Training and Research Hospital, Neurology Clinic, Bursa/Turkey
Sevim S., Mersin University School of Medicine, Mersin/Turkey
Sivaci A.O., Bursa Specialty Training and Research Hospital, Neurology Clinic, Bursa/Turkey
Tamam Y., Dicle University School of Medicine, Diyarbakir/Turkey
Turkoglu R., Haydarpasa Research and Training Hospital, Neurology Clinic, Istanbul, Turkey
Yuceyar N. Ege University School of Medicine, Izmir/Turkey
References
- Akiyama S, Hamdeh S, Micic D, et al. Prevalence and clinical outcomes of Covid-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218946. Oct 13;annrheumdis-2020-218946. [DOI] [PubMed] [Google Scholar]
- Altobrando AD, Patrizi A, Bardazzi F. Should SARS-CoV-2 influence immunosuppressive therapy for autoimmune blistering diseases? J. Eur. Acad. Dermatol. Venereol. 2020;34(7):e295–e297. doi: 10.1111/jdv.16491. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Amor S, Kang AS, et al. The underpinning biology relating to multiple sclerosis disease modifying treatments during the COVID-19 pandemic. Mult. Scler. Relat. Disord. 2020;43 doi: 10.1016/j.msard.2020.102174. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne P, Chandraratna D, Angood C, et al. Atlas of Multiple Sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014;83(11):1022–1024. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa GD, Leocani L, Montalban X, et al. Real-time assessment of COVID-19 prevalence among multiple sclerosis patients: a multicenter European study. Neurol. Sci. 2020;41(7):1647–1650. doi: 10.1007/s10072-020-04519-x. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt A, Ramagopalan SV, Scalfari A, et al. Clinical prognostic factors in multiple sclerosis: a natural history review. Nat. Rev. Neurol. 2009;5(12):672–682. doi: 10.1038/nrneurol.2009.178. Dec. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008;358(7):676–688. doi: 10.1056/NEJMoa0706383. Feb 14. [DOI] [PubMed] [Google Scholar]
- Korsukewitz C, Reddel SW, Bar-Or A, et al. Neurological immunotherapy in the era of COVID-19 looking for consensus in the literatüre. Nat. Rev. Neurol. 2020;16(9):493–505. doi: 10.1038/s41582-020-0385-8. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovvuru S, Nalleballe K, Onteddu SR, et al. Immunosuppression in chronic autoimmune neurological disorders during the COVID-19 pandemic. J. Neurol. Sci. 2021;420 doi: 10.1016/j.jns.2020.117230. Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroni A, Schiavetti I, Sormani MP, et al. COVID-19 in patients with Multiple Sclerosis undergoing disease-modifying treatments. Mult. Scler. 2020 doi: 10.1177/1352458520971817. Nov 18;1352458520971817. [DOI] [PubMed] [Google Scholar]
- Loonstra FC, Hoitsma E, Kempen ZLV, et al. COVID-19 in multiple sclerosis: the Dutch experience. Mult. Scler. 2020;26(10):1256–1260. doi: 10.1177/1352458520942198. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and Multiple Sclerosis. JAMA Neurol. 2020;77(9):1079–1088. doi: 10.1001/jamaneurol.2020.2581. Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna G, Alping P, Burman J. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. doi: 10.1001/jamaneurol.2019.3365. [DOI] [PMC free article] [PubMed]
- Montero-Escribano P, Matías-Guiu J, Gómez-Iglesias P, et al. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: a case series of 60 patients from Madrid, Spain. Mult. Scler. Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102185. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhn N, Konen FF, Pul R, et al. Experience in Multiple Sclerosis patients with Covid-19 and disease-modifying therapies: a review of 873 published cases. J. Clin. Med. 2020;9(12):4067. doi: 10.3390/jcm9124067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrotta E, DO Kister I, Charvet L, et al. COVID-19 outcomes in MS. Neurol. Neuroimmunol. Neuroinflamm. 2020;7(5):e835. doi: 10.1212/NXI.0000000000000835. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxburgh RHSR, Seaman SR, Masterman T, et al. Multiple Sclerosis severity score using disability and disease duration to rate disease severity. Neurology. 2005;64:1144–1151. doi: 10.1212/01.WNL.0000156155.19270.F8. [DOI] [PubMed] [Google Scholar]
- Sormani MP, Rossi ND, Schiavetti I, et al. Disease modifying therapies and Covid-19 severity in Multiple Sclerosis. Ann. Neurol. 2021 doi: 10.1002/ana.26028. Jan 21. [DOI] [Google Scholar]
- Sub-studies, TurkMSBase, https://www.msbase.org/sub-studies/.
- Türkiye Nüfusu Yaş . Gruplarına Göre Dağılımı 2020. Turkish Statistical Institute; 2020. https://www.nufusu.com/turkiye-nufusu-yas-gruplari [Google Scholar]
- Vollenhoven RFV, Fleischmann RM, Furst DE, et al. Long term safety of rituximab: final report of the rheumatoid arthritis global clinical trial program over 11 years. J. Rheumatol. 2015;42(10):1761–1766. doi: 10.3899/jrheum.150051. Oct. [DOI] [PubMed] [Google Scholar]
- 2020. https://covid19.saglik.gov.tr
- WHO Coronavirus Disease (COVID-19) Dashboard,https://covid19.who.int.
- Winkelmann A, Loebermann M, Reisinger EC. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat. Rev. Neurol. 2016;12(4):217–233. doi: 10.1038/nrneurol.2016.21. Apr. [DOI] [PubMed] [Google Scholar]
- Zabalza A, Cardenas-Robledo S, Tagliani P, et al. COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur. J. Neurol. 2020 doi: 10.1111/ene.14690. Dec 19. [DOI] [PubMed] [Google Scholar]
- Zrzavy T, Wimmer I, Rommer PS, et al. Immunology of COVID-19 and disease-modifying therapies: the good, the bad and the unknown. Eur. J. Neurol. 2020 doi: 10.1111/ene.14578. Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]