Abstract
While Coronavirus 2019 (COVID-19) typically presents with respiratory tract symptoms, atypical manifestations have been reported. We present a case of a 46-year-old man who presented with fever but no respiratory tract symptoms, and later develops bilateral parotitis. We review the literature for all other reported cases of parotitis and describe common features of these cases. It is important to consider COVID-19 in cases of parotitis, as this impacts patient management and ensures important infection control measures are undertaken.
Keywords: COVID-19, Parotitis, Atypical presentations of COVID-19, Salivary gland, Sialadenitis
As Coronavirus Disease 2019 (COVID-19), caused by the SARS-CoV-2 virus, ravages the globe, infecting more than 60 million people in less than a year [1], atypical presentations of COVID-19 are not unexpected. Typically, the spectrum of COVID-19 ranges from minimally symptomatic disease to severe pneumonia and critical disease. 70 % of patients with COVID-19 are asymptomatic or report symptoms indistinct from a mild “flu-like” illness with fever, malaise, dry cough, and sore-throat. Up to 14 % however develop a clinical/radiological pneumonia which required oxygen supplementation, and 5 % of cases develop critical disease with respiratory and multi-organ failure [2]. Atypical presentations of COVID-19, even in the absence of respiratory symptoms, have been reported, prompting the consideration of whether such patients require evaluation via a nasopharyngeal swab for SARS-CoV-2. Atypical presentations include COVID-19 related myocarditis in both mild [3] and severe [4] presentations of COVID-19, stroke [5], liver-injury and gastrointestinal symptoms [6], ocular manifestations [7] and a variety of neurological manifestations [8]. Atypical manifestations of COVID-19 are important for clinicians to recognize to ensure that such patients receive effective management, including isolation precautions to minimize the transmission of COVID-19.
COVID-19 associated parotitis has been previously described in the literature (Table 1). While the pathophysiology of COVID-19 associated parotitis is unclear, SARS-CoV-2 may have a predilection for the salivary gland through the high expression of ACE2 receptors, which are required for entry into host cells [9], on the epithelial cells of oral mucosa and within salivary glands. In this report, we describe a case of COVID-19 associated parotitis, and review the literature to date on COVID-19 associated parotitis.
Table 1.
Summary of cases of COVID-19 associated parotitis in literature.
| Reference | n, Sex, Age (years) |
Clinical Presentation | COVID-19 testing | Imaging | Management |
|---|---|---|---|---|---|
| Fisher et al [14] | n = 1, F, 21 |
8-day history of fever, cough and dyspnea Unilateral parotitis developed after improvement in respiratory symptoms |
Not reported | CT: Diffuse asymmetric enlargement and swelling; periparotid inflammatory fat stranding; free fluid extending into the left submandibular, submental, and parapharyngeal spaces and along the left sternocleidomastoid and strap muscles | Antibiotics, warm compress sialagogues, hydration |
| Lechien et al [15] | n = 3, F, 23–31 |
Unilateral parotitis occurred at the onset of the disease in 2 patients and over the clinical course of the disease in the other. All 3 had mild upper respiratory tract symptoms and hyposmia. |
PCR + (NP) | MRI: Intraparotid lymphadenitis; multiple unilateral or bilateral intraglandular lymph nodes in the deep and surface layers, in a relatively normal-sized gland. No juxtaglandular fat infiltration or thickening of the fascia. | Analgesia |
| Capaccio et al [20] | n = 1, M 26 |
Unilateral parotitis developed concurrently with fever and myalgia. Patient reported hyposmia and ageusia. | PCR negative (NP, Saliva) IgG +, IgM negative |
US: enlarged and diffuse hypoechoic parotid gland structure, with increased vascularization on color Doppler; no salivary duct enlargement or stones were identified | Not reported |
| Riad et al [21] | n = 6, M n = 9, F 10–73 |
Unilateral parotitis in all. 46.7 % presented with headache, 33.3 % with earache and 20 % with pharyngitis Presence of fever/ other respiratory symptoms not reported. |
PCR + (NP) | Not reported | Not reported |
| Rayan et al [22] | n = 1, M 7 |
Unilateral parotitis, patient did not have respiratory symptoms. | PCR + (NP) | Not reported | Analgesia |
| Chern et al [23] | n = 1, F, 88 | Unilateral pre-auricular swelling and pain Failure to thrive and poor oral intake (fever or respiratory symptoms not reported) |
PCR + (NP) | CT: enlarged right parotid gland with heterogeneous enhancement, surrounding fat stranding and fascial thickening. | Antibiotics, supportive therapy |
| n = 1, M, 64 | Bilateral pre-auricular swelling Fever, diarrhea, hyponatremia |
PCR + (NP) | CT: enlarged parotid glands with areas of heterogeneous enhancement, surrounding fat stranding and fascial thickening; no fluid collection or obstructing sialolith; retropharyngeal edema without rim enhancement as well as enlargement and heterogeneous enhancement of the submandibular glands | Antibiotics, warm compress, sialagogues, hydration |
PCR = polymerase chain reaction.
NP = nasopharyngeal.
Case description
Migrant workers living in large, overcrowded dormitories account for the majority of cases in Singapore. To control the spread of infection within these confined living environments, active case finding through the screening of symptomatic and asymptomatic migrant workers was undertaken. For the first 4 months of the epidemic in Singapore, all COVID-19 cases were isolated in public hospitals [10]. A 46-year-old male migrant worker from northern Thailand was admitted after he was found on screening to be positive for SARS-CoV-2 via a nasopharyngeal swab before admission. On admission the patient was febrile (temperature 38.5⁰C), tachycardic (heart rate 135 beats per minute) and hypertensive (blood pressure 175/114 mmHg) with oxygen saturations of 96 % on room air. The patient reported having intermittent fevers 6 days prior to his admission, had no respiratory symptoms, and did not report any facial swelling. Examination was otherwise unremarkable. Blood tests showed a total white count was 8660 cells/μL with neutrophils of 6300 cells/μL and lymphocytes of 1630 cells/μL. C-reactive protein, ferritin and lactate dehydrogenase were within normal range, but erythrocyte sedimentation rate (ESR) was elevated at 52 mm/hour. A chest radiograph showed no parenchymal lung changes suggestive of a pneumonic process.
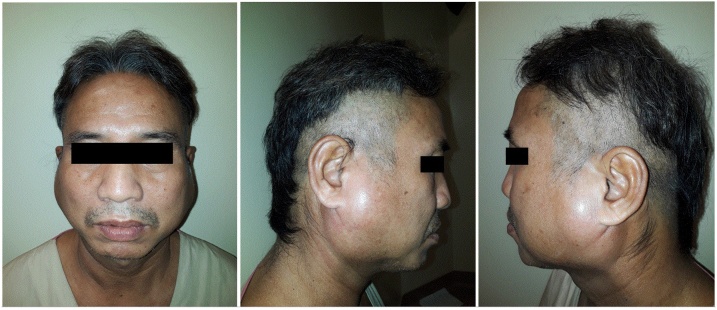
2 days after admission, on 9 days post illness onset (PIO), the patient developed a firm, non-fluctuant, bilateral parotid swelling (Fig. 1) which was mildly tender on palpation. There was no associated cervical lymphadenopathy, and oral and testicular examination was normal. A Computed Tomography scan of his head and neck revealed bilateral acute parotitis with symmetrical enlarged parotid glands with homogenous enhancement and mild surrounding fat stranding, and no obstructive sialolith. There was also mild thickening of platysma muscles on the right with a fluid track along the right sternocleidomastoid muscle and enhancing but not enlarged cervical chain lymph nodes.
Fig. 1.
Front and lateral views of bilateral parotid swelling.
As the patient was suspected to have COVID-19 associated parotitis, saliva and a throat swab were processed for SARS-CoV-2 RNA by reverse transcriptase-polymerase chain reaction (RT-PCR), both of which were negative. The patient also had mumps serology done and a buccal swab sent for Mumps and Paramyxoviruses RNA. His mumps serology was IgM negative, but IgG positive at 36 U/mL; consistent with the patient’s reported history of having received the Measles, Mumps, Rubella (MMR) vaccine in childhood. Mumps and Paramyxoviruses RNA was not detected. An HIV screening test was negative.
At 14 days PIO, the patient underwent 2 nasopharyngeal swabs 24 -hs apart; SARS-CoV-2 RNA was not detected in either. The patient was de-isolated and underwent naso-endoscopy which revealed mildly enlarged bilateral parotid glands with some features of chronic rhinosinusitis. The parotitis improved substantially at 28 days PIO (19 days after the parotitis first evolved), at which point in time he was discharged from hospital. He returned for an outpatient review 3 weeks post-discharge (50 days PIO) where he reported having remained well, and the parotitis had resolved completely.
Discussion
Viral parotitis is most commonly associated with the mumps virus, the only etiologic agent known to cause epidemic parotitis among humans prior to the development and dissemination of an effective mumps vaccine. Other viruses that have been implicated in the minority of cases of infective parotitis include influenza, human parainfluenza viruses, adenoviruses, enteroviruses, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, human herpes viruses 6 and parvovirus B-19 [11].
COVID-19 associated parotitis has been observed during the COVID-19 pandemic with 24 reported cases, including this case (Table 1), in peer-reviewed literature. 14/24 were female, with a median age of 28.5 years. Whilst mumps most commonly affects those aged 5–9 years old [12], COVID-19 associated parotitis has thus far been more commonly described in adults. This may be because since children are typically spared from substantial COVID-19 illness, fewer diagnoses of COVID-19 in children are made. Furthermore, since parotitis is commonly a childhood illness, it may be that such presentations in children are attributed only to the mumps virus without confirmatory serologic or PCR testing [13]. There are other differences from parotitis associated with mumps – most of the reported cases of COVID-19 associated parotitis have unilateral rather than the bilateral parotitis of mumps. Imaging studies in 7 patients showed a diffusely enlarged parotid gland, and at least one other case, like our case, had fluid tracking along muscles of the neck [14]. 3 patients who underwent Magnetic Resonance Imaging (MRI) were found to have intra-parotid lymphadenitis in an otherwise well-preserved gland [15]. The finding of intra-parotid lymphoid tissue inflammation, suggests a different pathogenesis of parotid swelling to the mumps virus which replicates within parotid glandular tissues and can result in oedema and necrosis of acinar and epithelial duct cells [16]. On the other hand, animal models of the highly related SARS-CoV virus showed direct infection of the epithelial cells on the salivary gland through ACE2 receptors [17]. Additionally, the presence of SARS-CoV-2 in saliva [18] and the finding in some studies that up to 56 % of patients with COVID-19 report xerostomia [19], indirectly suggests that the invasion of SARS-CoV-2 into the gland is the most plausible pathogenesis COVID-19 associated parotitis.
Conclusion
Atypical presentations of COVID-19 are to be expected given the magnitude and breadth of the pandemic globally. Thus, we should expect more cases of COVID-19 associated parotitis to be reported. However, since mumps is the virus typically associated with epidemic parotitis, many might misattribute this presentation to mumps, particularly in regions where MMR vaccine coverage is incomplete and circulating mumps virus is endemic. The misdiagnosis of COVID-19 associated parotitis as mumps would result in the erroneous reporting of two diseases, both of which have substantial public health importance. Therefore awareness of this atypical presentation of COVID-19 will potentially reduce the risk of transmission, whilst also improving the public health reporting of this important disease.
Author statement
Zhen Yu Lim: data curation, writing – original draft preparation. Alicia XY Ang: writing – review & editing. Gail B Cross – data curation, writing – original draft preparation, review & editing
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.John Hopkins University of Medicine . Coronavirus Resource Center; 2020. COVID-19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University.https://coronavirus.jhu.edu/map.html [Google Scholar]
- 2.Cascella M., Rajnik M., Cuomo A. Features, evaluation, and treatment of Coronavirus. https://www.ncbi.nlm.nih.gov/books/NBK554776/ [Updated 2020 Oct 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: [PubMed]
- 3.Rajpal S., Tong M.S., Borchers J. Cardiovascular Magnetic Resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2020;11 doi: 10.1001/jamacardio.2020.4916. Published online September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fifi J.T., Mocco J. COVID-19 related stroke in young individuals. Lancet Neurol. 2020;19(9):713–715. doi: 10.1016/S1474-4422(20)30272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao R., Qiu Y., He J.-S., Tan J.-Y., Li X.-H., Li X.-H. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–668. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu P., Duan F., Luo C. Characteristics of ocular findings of patients with Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138(5):575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H., Zhong L., Deng J. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(8) doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngiam J.N., Chew N., Tham S.M. Demographic shift in COVID-19 patients in Singapore from an aged, at-risk population to young, migrant workers with reduced risk of severe disease. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.11.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbadawi L.I., Talley P., Rolfes M.A. Non-mumps viral parotitis during the 2014-2015 Influenza season in the United States. Clin Infect Dis. 2018;67(4):493–501. doi: 10.1093/cid/ciy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galazka A.M., Robertson S.E., Kraigher A. Mumps and mumps vaccine: a global review. Bull World Health Organ. 1999;77(1):3–14. [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical Features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39(May (5)):355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher J., Monette D.L., Patel K.R., Kelley B.P., Kennedy M. COVID-19 associated parotitis: a case report [published online ahead of print, 2020 Jun 27] Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.06.059. S0735-6757(20)30549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lechien J.R., Chetrit A., Chekkoury-Idrissi Y., Distinguin L., Circiu M., Saussez S. Parotitis-like symptoms associated with COVID-19, France, March–April 2020. Emerg Infect Dis. 2020;26(9):2270–2271. doi: 10.3201/eid2609.202059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin S., Eckhaus M., Rennick L.J., Bamford C.G., Duprex W.P. Molecular biology, pathogenesis and pathology of mumps virus. J Pathol. 2015;235(Jan (2)):242–252. doi: 10.1002/path.4445.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C., Wu H., Ding X., Ji H., Jiao P., Song H. Does infection of 2019 novel coronavirus cause acute and/or chronic sialadenitis? Med Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyllie A.L., Fournier J., Casanovas-Massana A., Campbell M., Tokuyama M. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020;383(Sep (13)):1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biadsee A., Kassem F., Dagan O., Masarwa S., Ormianer Z. Olfactory and oral Manifestations of COVID-19: sex-related Symptoms-a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722–728. doi: 10.1177/0194599820934380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capaccio P., Pignataro L., Corbellino M., Popescu-Dutruit S., Torretta S. Acute parotitis: a possible precocious clinical Manifestation of SARS-CoV-2 infection? Otolaryngol Head Neck Surg. 2020;163(Jul (1)):182–183. doi: 10.1177/0194599820926992. [DOI] [PubMed] [Google Scholar]
- 21.Riad A., Kassem I., Badrah M., Klugar M. Acute parotitis as a presentation of COVID-19? Oral Dis. 2020;(July) doi: 10.1111/odi.13571. [DOI] [PubMed] [Google Scholar]
- 22.Rayan M.A., Bader J.A., Feras A.A., Shaikha J.A. COVID-19 associated parotitis in pediatrics. Glob J Ped Neonatol Care. 2020;2(4) doi: 10.33552/GJPNC.2020.02.000545. [DOI] [Google Scholar]
- 23.Chern A., Famuyide A.O., Moonis G., Lalwani A.K. Sialadenitis: a possible early manifestation of COVID‐19. Laryngoscope. 2020;130:2595–2597. doi: 10.1002/lary.29083. [DOI] [PMC free article] [PubMed] [Google Scholar]