Abstract
Annual cervical cancer screening with Papanicolaou (Pap) and HPV (human papillomavirus) testing after stem cell transplant (SCT) is recommended, but the uptake is unknown. We aimed to determine the prevalence and predictors of cervical cancer screening in patients with hematologic malignancies. We searched MarketScan Commercial Claims database for women who underwent allogeneic or autologous SCT. The primary outcome was cervical cancer screening, defined as procedures or abnormal results for HPV and/or Pap testing according administrative codes within 2 years after SCT. A multivariable logistic regression model was fitted with cancer type, SCT year, age, geographic area, insurance plan, comorbidity, and presence of graft-versus-host disease (GVHD).The study included 1484 patients; 1048 patients (70.6%) had autologous and 436 (29.4%) allogeneic SCT. Mean age was 52.5 years. Overall, 660 patients (44.5%) had screening within 2 years after SCT, 214 (49.1%) with allogeneic SCT and 446 (42.6%) with autologous SCT (p=0.02). In the allogeneic SCT group, patients with GVHD had a lower rate of screening than patients without GVHD (42.5% vs. 55.4%, p<0.01), and GVHD was associated with lower odds of screening (OR 0.50; 95% CI 0.32–0.79). In the autologous SCT group, patients with comorbid medical conditions had a lower rate of screening than patients without comorbidity (36.0% vs. 45.7%, p<0.01). In both allogeneic and autologous SCT groups, older patients had lower odds of screening. Cervical cancer screening rates after SCT are low, particularly in patients with GVHD, who are at significant risk of second malignancies. Future work is needed to develop strategies to increase uptake.
Keywords: cervical cancer screening, human papillomavirus, stem cell transplant, second malignancies
Introduction
Human papillomavirus (HPV) is the cause of virtually all cases of cervical cancer.1 Patients who have undergone stem cell transplant (SCT) are at high risk of HPV-related dysplasias, including cervical dysplasia,2 and HPV-related second malignancies,3, 4 including cervical cancer,5, 6 as a result of HPV persistence or reactivation due to immunosuppression.7, 8 The incidence of cervical cancer after SCT has been reported to be 2% to 67%, 13 times as high as the incidence in the general population.5 Long duration of immunosuppressive therapy after SCT and chronic graft-versus-host disease (GVHD) are predictors of cervical cancer in SCT recipients.2, 3, 6, 9
Cervical cancer screening with Papanicolaou (Pap) and HPV co-tests is recommended for immunocompromised patients,10 including patients who have undergone SCT.11 International blood and marrow transplant societies recommend annual cervical cancer testing after SCT.12 Suboptimal rates of cervical cancer screening have been reported through surveys conducted with survivors many years after allogeneic SCT (63% to 66% screened) 13, 14 or autologous SCT (77% screened).14 To date, cervical screening rates and timing after SCT is not clear. In this study, we aimed to determine the prevalence and predictors of cervical cancer screening among patients with hematologic malignancies within 2 years after allogeneic or autologous SCT using a large population-based commercial claims administrative database.
Materials and Methods
We utilized the MarketScan Commercial Claims and Encounters database (Truven Health Analytics), which is a large, nationwide, employment-based database that provides information about health administrative claims and health care expenditure for employees and dependents receiving insurance coverage through private companies. This database includes data from 45 large employers, with claims collected from more than 100 payers,15 and results totaling nearly 230 million unique patients since 1995.16 MarketScan data is currently available through December 2015.
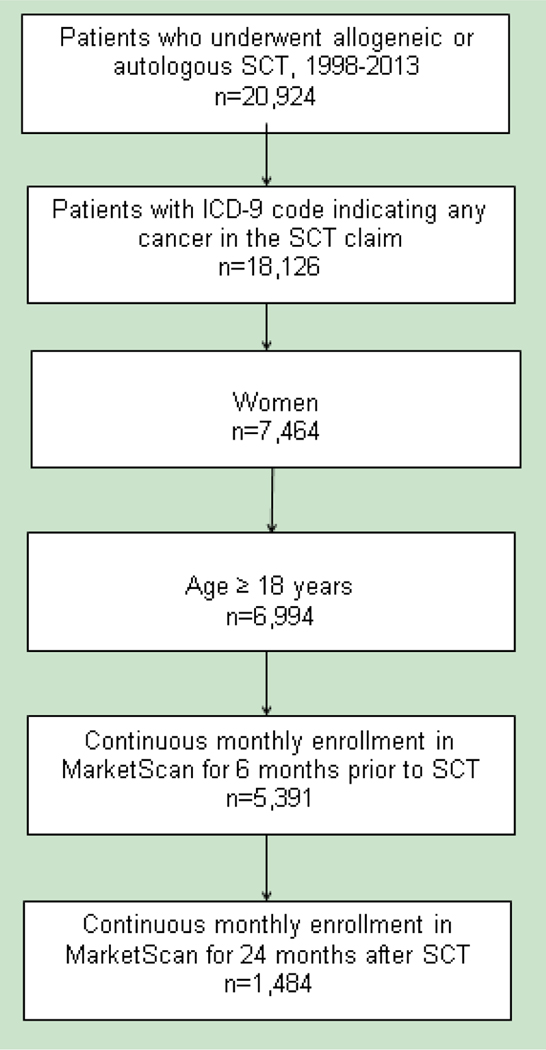
Using International Classification of Diseases 9 (ICD-9) procedure codes and Healthcare Common Procedure Coding System (HCPCS) codes (Supplementary Table), we identified women with malignancy who underwent allogeneic or autologous SCT during the period from January 1998 through January 2013 and had a diagnosis code for any cancer in the SCT claims (Figure 1). We excluded patients who were younger than 18 years. The date of the first SCT claim was used as the index date. Given our intention to study comorbidity during the 6 months before SCT and cervical cancer screening during a period of at least 2 years after SCT, we limited our cohort to individuals with continuous enrollment from 6 months before to 2 years after the index date.
Figure 1. Cohort selection.
ICD-9, International Classification of Diseases 9; SCT, stem cell transplant.
The MarketScan enrollment file provided data on sex, age, geographic area (Northeast, North Central, South, or West), and insurance type (health maintenance organization, preferred provider organization, or other). Comorbid medical conditions, defined using the system of Deyo et al, were identified by review of claims for the 6 months prior to the index date.17 Using ICD-9 codes (Supplementary Table), we determined whether GVHD was present prior to cervical cancer screening. The primary outcome was cervical cancer screening, which was defined as Pap and/or HPV testing or abnormal results for these tests as indicated by ICD-9 or HCPCS codes (Supplementary Table) in any claim during the 2 years after SCT.
Cervical cancer screening during the 2 years after SCT was classified as a binary variable, and unadjusted associations with covariates were tested using the Pearson χ2 test. The screening rate in each 6-month period after SCT was calculated by using the number of patients who had screening during that 6 month interval divided by the total individuals who had not yet received any screening at the beginning of that interval. A multivariable logistic regression model was fitted with cancer type, SCT year, age group, geographic area, insurance type, comorbidity, and presence of GVHD without differentiation between acute and chronic GVHD. Goodness of fit was evaluated using the test of Hosmer and Lemeshow, and results were expressed in terms of odds ratios (ORs) and 95% confidence intervals (CIs). P values less than 0.05 were considered statistically significant; all tests were 2-sided. Statistical analyses were carried out using SAS 9.4 (SAS Institute Inc., Cary, NC). This study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center.
Results
We identified 1484 women with cancer who received allogeneic or autologous SCT between 1998 and 2013 (Figure 1). The mean age of the patients was 52.5 years (standard deviation, 11.8 years). Of the 1484 patients, 608 (41%) had myeloma, 385 (25.9%) had leukemia, and 340 (22.9%) had lymphoma. A total of 1048 patients (70.6%) had autologous SCT, and 436 (29.4%) had allogeneic SCT. A total of 660 patients (44.5%) had cervical cancer screening within 2 years after SCT.
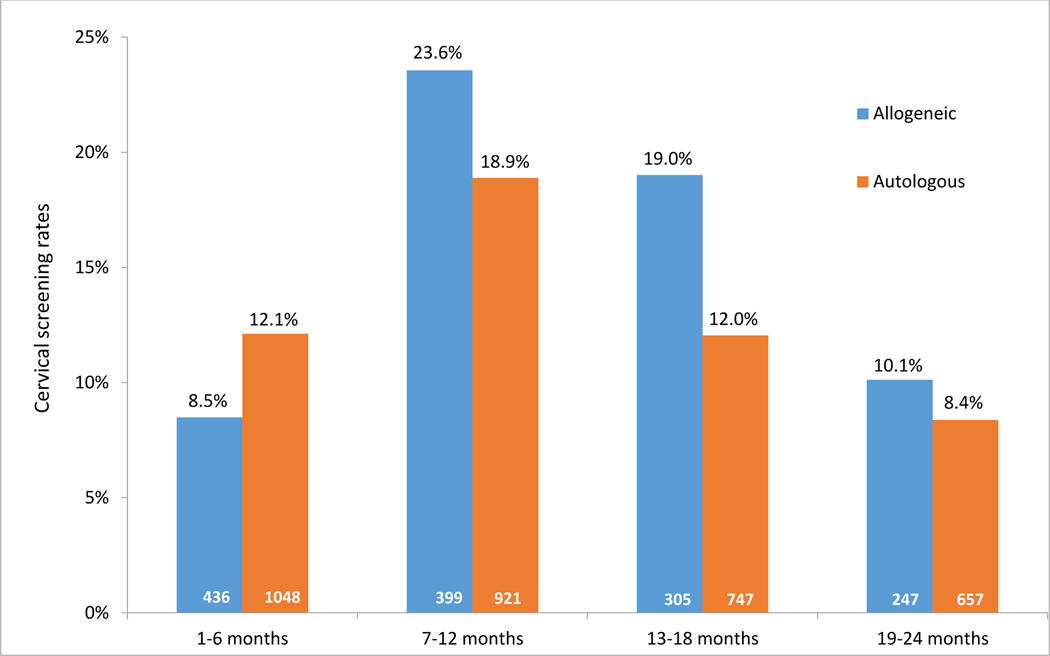
Patient characteristics by SCT type and cervical cancer screening status are summarized in Table 1. The rate of screening within 2 years after SCT was higher among patients who had allogeneic SCT than among those who had autologous SCT (49.1% vs. 42.6%, p=0.02). The incidence of screening was highest during the period from the beginning of month 7 through the end of month 12 after SCT, when the incidence was 23.6% in the allogeneic SCT group and 18.9% in the autologous SCT group (Figure 2). We also explored the rates of cervical cancer screening in the 3rd year after SCT. In a subgroup analysis of allogeneic and autologous SCT patients with 3 years of full insurance coverage after SCT (n=784), we found that the rate of cervical screening rates in the third year was lower than the second year (data not shown).
Table 1:
Characteristics of patients who underwent cervical cancer screening within 2 years after stem cell transplant (SCT)
| Characteristic | Allogeneic SCT (N=436) | Autologous SCT (N=1048) | ||||
|---|---|---|---|---|---|---|
| Total (col %) | Received screening | Total (col %) | Received screening | |||
| N (row %) | p | N (row %) | p | |||
| Total | 436 | 214 (49.1) | 1048 | 446 (42.6) | ||
| Cancer type | ||||||
| Leukemia | 335 (80.0) | 176 (52.5) | 0.05 | 50 (4.8) | 27 (54.0) | <0.01 |
| Lymphoma | 65 (14.9) | 25 (38.5) | 275 (26.2) | 120 (43.6) | ||
| Myeloma | 16 (3.7) | 7 (43.8) | 592 (56.5) | 229 (38.7) | ||
| Other | 20 (4.6) | 6 (30.0) | 131 (12.5) | 70 (53.4) | ||
| Year of SCT | ||||||
| 1998–2001 | 26 (6.0) | 15 (57.7) | 0.83 | 56 (5.3) | 26 (46.4) | 0.19 |
| 2002–2005 | 44 (10.1) | 22 (50.0) | 124 (11.8) | 63 (50.8) | ||
| 2006–2009 | 107 (24.5) | 51 (47.7) | 272 (26.0) | 115 (42.3) | ||
| 2010–2013 | 259 (59.4) | 126 (48.6) | 596 (56.9) | 242 (40.6) | ||
| Age group, yr | ||||||
| <30 | 40 (9.2) | 16 (40.0) | <0.01 | 51 (4.9) | 31 (60.8) | <0.01 |
| 30–40 | 61 (14.0) | 35 (57.4) | 66 (6.3) | 42 (63.6) | ||
| 41–50 | 109 (25.0) | 66 (60.6) | 197 (18.8) | 112 (56.9) | ||
| 51–60 | 154 (35.3) | 74 (48.1) | 431 (41.1) | 190 (44.1) | ||
| 61–65 | 54 (12.4) | 21 (38.9) | 173 (16.5) | 58 (33.5) | ||
| ≥66 | 18 (4.1) | 2 (11.1) | 130 (12.4) | 13 (10.0) | ||
| Region | ||||||
| Northeast | 109 (25.0) | 50 (45.9) | 0.27 | 279 (26.6) | 111 (39.8) | 0.79 |
| North Central | 95 (21.8) | 47 (49.5) | 173 (16.5) | 73 (42.2) | ||
| South | 139 (31.9) | 76 (54.7) | 356 (34.0) | 154 (43.3) | ||
| West | 84 (19.3) | 35 (41.7) | 209 (19.9) | 93 (44.5) | ||
| Unknown | 9 (2.1) | 6 (66.7) | 31 (3.0) | 15 (48.4) | ||
| Insurance type | ||||||
| PPO | 259 (59.4) | 122 (47.1) | 0.59 | 531 (50.7) | 237 (44.6) | 0.15 |
| HMO | 55 (12.6) | 28 (50.9) | 163 (15.6) | 73 (44.8) | ||
| Other | 122 (28.0) | 64 (52.5) | 354 (33.8) | 136 (38.4) | ||
| Comorbiditya | ||||||
| None | 283 (64.9) | 140 (49.5) | 0.83 | 704 (67.2) | 322 (45.7) | <0.01 |
| Yes | 153 (35.1) | 74 (48.4) | 344 (32.8) | 124 (36.0) | ||
| GVHD | ||||||
| No | 224 (51.4) | 124 (55.4) | <0.01 | -- | -- | |
| Yes | 212 (48.6) | 90 (42.5) | -- | -- | ||
GVHD: graft vs. host disease; HMO, health maintenance organization; PPO, preferred provider organization.
Comorbidity based on Deyo comorbidity index [reference 18: Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–619].
Figure 2. Cervical cancer screening rates within 24 months after stem cell transplant, by 6-month periods.
All cervical cancer screening rates between the allogeneic SCT and autologous SCT groups were significantly different (p≤0.05), except at 19–24 months. The screening rate in each 6-month period after SCT was calculated by using the number of persons who had screening during that 6 month interval divided by the total number of persons who had not yet received any screening at the beginning of that interval (in white).
In both the allogeneic and autologous SCT groups, the rate of cervical cancer screening within 2 years after SCT was higher among patients with leukemia than among those with lymphoma and decreased with increasing age. In the allogeneic SCT group, patients with GVHD had a lower rate of cervical cancer screening than those without GVHD (42.5% vs. 55.4%, p<0.01). In the autologous SCT group, patients with at least 1 comorbidity within 6 months before SCT had a lower rate of screening than patients without comorbid conditions (36.0% vs. 45.7%, p<0.01).
The multivariable logistic regression model showed that in the allogeneic SCT group, age over 60 years, a diagnosis of lymphoma, and a diagnosis of GVHD were associated with significantly lower odds of cervical cancer screening, and in the autologous SCT group, age over 50 years was associated with significantly lower odds of cervical cancer screening (Table 2). Because autologous transplant for myeloma is considered palliative, we performed a sensitivity analysis which included only leukemia and lymphoma patients. We found similar results as compared to our complete dataset of patients with hematologic malignancies, except that having a comorbidity was no longer significantly associated with lower odds of cervical cancer screening (Supplemental Tables 2 and 3).
Table 2:
Multivariable logistic regression model for cervical cancer screening within 2 years after stem cell transplant (SCT)
| Variable | Allogeneic SCT (n=436) | Autologous SCT (n=1048) | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
| Cancer type | ||||
| Leukemia | Ref. | Ref. | ||
| Lymphoma | 0.55 (0.31–1.00) | 0.05 | 0.73 (0.39–1.38) | 0.33 |
| Myeloma | 0.78 (0.26–2.34) | 0.66 | 0.72 (0.39–1.35) | 0.31 |
| Other | 0.32 (0.11–0.92) | 0.04 | 0.74 (0.37–1.50) | 0.41 |
| Year of SCT | ||||
| 1998–2001 | Ref. | Ref. | ||
| 2002–2005 | 0.94 (0.33–2.65) | 0.90 | 1.28 (0.65–2.52) | 0.48 |
| 2006–2009 | 1.00 (0.38–2.64) | 0.99 | 1.20 (0.63–2.29) | 0.59 |
| 2010–2013 | 1.28 (0.50–3.27) | 0.60 | 1.24 (0.67–2.31) | 0.50 |
| Age group, yr | ||||
| <30 | 0.42 (0.18–0.99) | 0.05 | 0.86 (0.40–1.85) | 0.70 |
| 30–40 | Ref. | Ref. | ||
| 41–50 | 1.07 (0.54–2.11) | 0.84 | 0.79 (0.44–1.43) | 0.43 |
| 51–60 | 0.64 (0.34–1.22) | 0.18 | 0.48 (0.27–0.85) | 0.01 |
| 61–65 | 0.45 (0.20–1.00) | 0.05 | 0.32 (0.17–0.59) | <0.01 |
| ≥66 | 0.08 (0.02–0.38) | <0.01 | 0.07 (0.03–0.15) | <.001 |
| Region | ||||
| Northeast | Ref. | Ref. | ||
| North Central | 0.88 (0.49–1.58) | 0.67 | 0.87 (0.58–1.32) | 0.52 |
| South | 1.25 (0.71–2.19) | 0.45 | 0.89 (0.60–1.32) | 0.56 |
| West | 0.67 (0.35–1.28) | 0.23 | 0.96 (0.62–1.50) | 0.86 |
| Unknown | 2.08 (0.46–9.44) | 0.34 | 1.29 (0.57–2.96) | 0.54 |
| Insurance type | ||||
| PPO | Ref. | Ref. | ||
| HMO | 1.16 (0.61–2.19) | 0.65 | 0.97 (0.67–1.42) | 0.88 |
| Other | 1.39 (0.87–2.21) | 0.17 | 0.96 (0.71–1.29) | 0.77 |
| Comorbiditya | ||||
| None | Ref. | Ref. | ||
| Yes | 1.11 (0.72–1.71) | 0.64 | 0.77 (0.58–1.02) | 0.06 |
| GVHD | ||||
| No | Ref. | -- | ||
| Yes | 0.50 (0.32–0.79) | <0.01 | ||
GVHD, graft vs. host disease; HMO, health maintenance organization; PPO, preferred provider organization.
Comorbidity based on Deyo comorbidity index [reference 18: Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–619].
Discussion
We found that the prevalence of cervical cancer screening within 2 years after SCT was 44.5% for women with commercial insurance. Among patients who underwent allogeneic SCT, patients with GVHD were less likely to have screening than were those without GVHD. Among patients who underwent autologous SCT, we found a trend among patients with comorbid medical conditions to be less likely to have screening than were those without comorbidities. Younger age was a predictor of cervical cancer screening in both the allogeneic and autologous SCT groups.
For the general population, current guidelines recommend Pap testing every 3 years in women age 21 to 29 years, Pap and HPV co-testing every 5 years in women age 30 to 65 years, and discontinuation of screening at age 65 years if the patient has a normal screening history and no new risk factors.18 However, for immunocompromised SCT patients who at high risk for cervical cancer, international blood and marrow transplant societies recommend annual cervical cancer testing after stem cell transplant.5 Certain cancer patients who have poor expected outcomes may need a personalized approach that takes into account their long-term prognosis and weighs the costs vs. benefits of cervical cancer screening.
In a study surveying allogeneic bone marrow transplant recipients in Australia more than 1 year after transplant, 63.4% of female participants reported ever receiving a Pap test since transplant.13 In another survey, female SCT survivors reported Pap testing rates of 66% and 77% for patients who underwent allogeneic and autologous SCT, respectively, at any time after SCT.14 Our study results showed lower rates of cervical cancer screening, which may reflect methodological differences between survey studies, which have a potential for recall bias, and our study, which was based on actual claims in an administrative database. Importantly, the lower screening rates in our study likely reflect a higher level of illness severity in the first 2 years after SCT, which may have led to lower screening rates.
We report that younger age was a predictor of cervical cancer screening, as reported in a previous study.13 This may reflect that providers may be following the general population recommendations to perform cervical cancer screening more frequently in younger patients (every 3 years in women age 21–29 years vs. every 5 years in women 30–65 years).
We found that in the allogeneic SCT group, patients with GVHD had lower odds of cervical cancer screening than those without GVHD. We were not able to differentiate between acute GVHD and chronic GVHD, which is associated with a higher risk of second malignancies. Chronic GVHD has been shown to be a risk factor for cervical cancer,3, 6 and patients with chronic GVHD need cervical cancer screening. However, patients with chronic GVHD often have multiple medical issues associated with GVHD as well as side effects of immunosuppressive therapy. In addition, patients with GVHD have a higher likelihood of cytopenias, including thrombocytopenia, which could preclude cervical procedures. Future prospective or population-based stem cell transplant registry data should be reviewed to discern the effect of chronic vs. acute GVHD on the risk of cervical dysplasias and secondary malignancies. Beyond GVHD, chronic diseases have previously been determined to be barriers to cervical cancer screening19 and may account for the lower rate of screening among patients with comorbid medical conditions in our autologous SCT group. Myeloma patients, who tend to be older and thus have more comorbidities, likely accounted for this finding, as the association of lower screening rates with comorbidities was no longer seen when myeloma patients were excluded from the analysis.
Our study had several limitations. We limited our population to women who were alive with insurance coverage for at least 24 months after SCT, and as such we did not include patients who died shortly after SCT or who lost their insurance coverage shortly after SCT. Though we used the most recently available data from MarketScan, most data collected occurred before guidelines were published or shortly thereafter. As such, more recent data, especially from SCT-focused survivorship clinics, may demonstrate an improvement in screening rates. This was a retrospective study using an administrative database that did not include the results of the cervical cancer screening. Due to the observational nature of the data, we were not able to ascertain the causality or barriers to screening. However, the MarketScan data come from an insured population including relatively young adults, who are often missing in other administrative databases such as Surveillance, Epidemiology, and End Results-Medicare, and Pap and HPV testing as well as HPV vaccination may be especially valuable in secondary cancer prevention among younger survivors of hematologic malignancies. Our study cohort of patients who were continuously insured for 2 years after SCT was selected to accurately ascertain cervical screening rates in a group where we were confident that we would be able to pick up screening activities independent of insurance status. Our cohort may not be representative of the general transplant population which faces substantial financial burdens after SCT20, 21, and thus our study’s screening rates are likely overestimates making the actual problem potentially even more pronounced among underinsured persons post-SCT.
In conclusion, we found low rates of cervical cancer testing in allogeneic and autologous SCT patients during the recommended time period after SCT. Future work is needed to disseminate and implement international blood and marrow recommendations to perform annual cervical cancer screening after SCT. Dedicated GVHD and survivorships clinics could ensure timely cervical cancer screening.
Supplementary Material
Highlights.
Stem cell transplant (SCT) recipients are at high risk for cervical dysplasia, and cervical cancer screening is recommended. However, screening rates and timing after stem cell transplant (SCT) is not clear.
Using a large commercial claims database, we found that that the screening rates was low, 44.5%, within 2 years after allogeneic or autologous SCT.
Younger age, a diagnosis of leukemia, and not having graft-versus-host disease or comorbid medical conditions were predictors of cervical cancer screening.
Acknowledgments:
This research was supported by the Duncan Family Institute Seed Funding (PI: Hwang), and AIDS Malignancy Consortium at Baylor College of Medicine Dan L. Duncan Cancer Center (grant P30CA125123–04S1, PI: Chiao). This work was also supported in part by resources provided by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (CA016672) and by the Center for Innovations in Quality, Effectiveness and Safety (grant CIN 13–413) at the Michael E. DeBakey Veterans Affairs Medical Center. The authors acknowledge Stephanie Deming of the MD Anderson Department of Scientific Publications for editorial review and Laurissa Gann of the MD Anderson Research Medical Library for assistance with literature searching.
Footnotes
Financial disclosures: The authors report the following potential conflicts of interest: Hwang: Gilead, Merck; Suarez-Almazor: Pfizer, Endo Pharmaceuticals, Bristol-Myers Squibb. All other authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. Human papillomavirus (HPV)-associated cancers. Available at: www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed December 8, 2017.
- 2.Savani BN, Stratton P, Shenoy A, Kozanas E, Goodman S, Barrett AJ. Increased risk of cervical dysplasia in long-term survivors of allogeneic stem cell transplantation—implications for screening and HPV vaccination. Biol. Blood Marrow Transplant. 2008;14:1072–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majhail NS, Brazauskas R, Rizzo JD, et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inamoto Y, Shah N, Savani B, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50:1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey Tirri B, Häusermann P, Bertz H, et al. Clinical guidelines for gynecologic care after hematopoietic SCT. Report from the international consensus project on clinical practice in chronic GVHD. Bone Marrow Transplant. 2015;50:3–9. [DOI] [PubMed] [Google Scholar]
- 7.Maglennon GA, McIntosh PB, Doorbar J. Immunosuppression facilitates the reactivation of latent papillomavirus infections. J. Virol. 2014;88:710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doorbar J. Latent papillomavirus infections and their regulation. Curr. Opin. Virol. 2013;3:416–421. [DOI] [PubMed] [Google Scholar]
- 9.Lee DG. Vaccination of hematopoietic stem cell transplantation recipients: perspective in Korea. Infect Chemother. 2013;45:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Committee on Practice Bulletins-Gynecology. Practice Bulletin No. 168: Cervical Cancer Screening and Prevention. Obstet. Gynecol. 2016;128:e111–130. [DOI] [PubMed] [Google Scholar]
- 11.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2012;18:348–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inamoto Y, Shah NN, Savani BN, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50:1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyer G, Larsen SR, Gilroy N, et al. Adherence to cancer screening guidelines in Australian survivors of allogeneic blood and marrow transplantation (BMT). Cancer Med. 2016;5:1702–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol. Blood Marrow Transplant. 2010;16:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truven Health MarketScan Database Dictionary: Commercial Claims & Encounters, Medicare Supplemental & COB: Truven Health Analytics, Ann Arbor, MI; 2013.
- 16.Truven Health Analytics. Putting Research Data into Your Hands with the MarketScan Databases. 2017; Available at: http://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases. Accessed December 8, 2017.
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 18.Moyer VA. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2012;156:880–891. [DOI] [PubMed] [Google Scholar]
- 19.Crawford A, Benard V, King J, Thomas CC. Understanding barriers to cervical cancer screening in women with access to care, Behavioral Risk Factor Surveillance System, 2014. Prev. Chronic Dis. 2016;13:E154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahn T, Paplham P, Austin-Ketch T, et al. Ascertainment of Unmet Needs and Participation in Health Maintenance and Screening of Adult Hematopoietic Cell Transplantation Survivors Followed in a Formal Survivorship Program. Biol. Blood Marrow Transplant. 2017. [DOI] [PubMed] [Google Scholar]
- 21.Khera N, Chang YH, Hashmi S, et al. Financial burden in recipients of allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2014;20:1375–1381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.