Obesity, which is increasing to epidemic proportions, is strongly associated with increased susceptibility to a variety of diseases. Diabetes and cardiovascular diseases top the list, but many more have also become apparent. Several different types of cancer, such as thyroid, uterine, and liver cancer, are now recognized to have increased incidence or severity in persons with obesity. Although diet has been shown to have direct effects on promoting tumor growth,1 a key link between obesity and each of these diseases is increased chronic inflammation and changes in populations of immune cells. A recent study by Ringel et al.2 identifies the ways in which immunity, tumors, and tumor microenvironments change with obesity (Fig. 1).
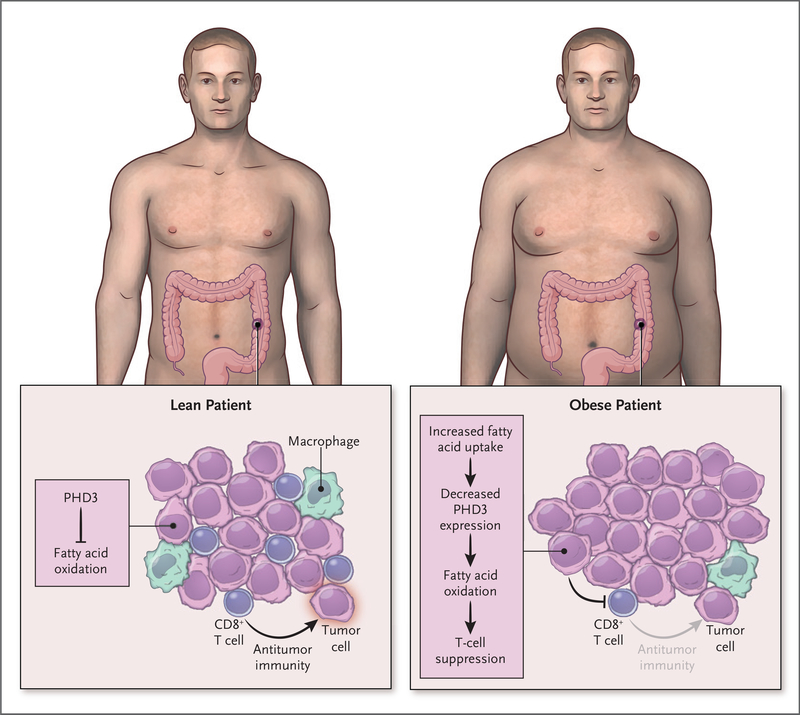
Figure 1. Obesity and the Tumor Immune Microenvironment.
Obesity reshapes the metabolism of cancer cells to impair T-cell inflammatory functions. Obesity remodels the cellular fuel use of tumors to increase cancer-cell fatty acid oxidation while reducing the numbers and spatial positioning of CD8+ T cells to reduce antitumor immunity. A “multi-omic” approach showed that PHD3 is a candidate to mediate these effects because decreased PHD3 expression led to greater lipid metabolism and T-cell suppression.
The observation that obesity can promote inflammation was first reported nearly 30 years ago.3 This finding led to the development of the field of immunometabolism, which seeks to understand how systemic and cellular metabolism influences immunity and inflammation. Obesity and its systemic effects indeed induce changes in T-cell and macrophage populations and promote proinflammatory subsets of these cells. Furthermore, high levels of metabolic hormones that regulate nutrient sufficiency or overabundance, such as insulin and leptin, can promote inflammatory states in T cells and macrophages. Although this effect is particularly apparent in adipose tissues, obesity-induced chronic inflammation and hyperlipidemia act systemically and can exacerbate a wide range of diseases.
The effect of obesity on cancer and the immune cells in the tumor microenvironment has been thought to be pleiotropic. On one level, the Warburg effect of elevated glucose consumption and metabolism was defined in cancer cells, but it is also used by other types of proliferating cells; a similar metabolic program for cancer and immune cells in the tumor microenvironment may lead to nutrient conflicts and competition. Obesity, however, is a setting of nutrient abundance, and the ways in which cancer cells and T cells adapt and interact with altered nutrients remain poorly understood.
Given the prevalence of obesity and the uncertainty of its effect on the tumor microenvironment, Ringel and colleagues2 have investigated the genetic, proteomic, spatial, and metabolomic phenotypes of cancer and immune cells in animal models of obesity-exacerbated cancers. The breadth of their approach, including the generation of a metabolic single-cell atlas of the tumor immune landscape in the context of obesity, is a strength of the study, which yielded several clear findings. The authors found that although cancer cells adopted a more lipid-based metabolism, CD8+ T cells were less abundant, were shifted to less-activated phenotypes, and were spatially excluded from some regions of the tumors. One gene that stood out as selectively down-regulated in tumor cells was PHD3, which encodes prolyl hydroxylase-3. Of interest, experiments with mouse models showed that Phd3 regulates the hypoxic response and represses fatty acid oxidation; rescue of Phd3 expression in cancer cells restored T-cell functions and slowed tumor growth. Consistent with these findings was the observation of low levels of PHD3 in samples of immunologically “cold” tumors (tumors with low levels of immune infiltrate) in five of six different types of cancer.
This study raises several questions. Although T cells showed striking changes in the context of obesity, myeloid cells are most notably increased in the adipose tissue of obese persons and were numerous in the tumors of obese animals. The role of myeloid cells in obesity-induced immune suppression as described by Ringel et al., however, remains unclear. Most important, the ways in which the pathways described here influence the response to therapy and the broader role of PHD3 have yet to be established. Although Ringel et al. have identified a new mechanism by which cancer cells adapt metabolically to suppress T-cell function in obesity, other data support the “obesity paradox,” in which obesity can sensitize tumors to immunotherapy.4 In that scenario, the chronic inflammatory state of obesity is proposed to prime a response to immunotherapy, and hormones, such as leptin, can play proinflammatory roles that are otherwise held in check.
Despite these unknowns, the clinical considerations of these findings are manifold as we gain a deeper understanding of the tumor microenvironment and the increasingly prevalent state of obesity. In broad terms, the “multi-omic” approach2 will provide material to mine and a road map for future studies that will go well beyond these initial findings. More directly, the up-regulation of the PHD3 pathway may provide a new therapeutic target to slow tumor growth in obese patients or, alternatively, to enhance the effects of immunotherapy in nonobese patients. The role of dietary interventions. such as a low-fat, low-carbohydrate diet, has also been widely considered in cancer. The study by Ringel et al. used a high-fat diet to provide new direction and evidence as to how overnutrition may selectively suppress tumor-associated T cells.
The relative role of tumor metabolism and PHD3 to directly suppress T cells within tumors while sensitizing antitumor immunity through a basal state of low-level inflammation and high levels of leptin clearly forms a complex mixture. That said, our growing understanding of the effects of obesity and diet on the tumor microenvironment is yielding new targets and approaches to address the incidence, progression, and treatments of the many cancers exacerbated by the epidemic of obesity.
Footnotes
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
References
- 1.Goncalves MD, Lu C, Tutnauer J, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 2019; 363: 1345–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ringel AE, Drijvers JM, Baker GJ, et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell 2020; 183(7): 1848–1866.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259:8 7–91. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med 2019; 25: 141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]