Abstract
Background and Purpose
A major concern with ocular myasthenia gravis (MG) is the potential conversion to generalized MG. This study was conducted to determine if the repetitive nerve stimulation (RNS) test could predict the conversion from ocular to generalized MG.
Methods
The RNS test was conducted in a consistent manner on five muscles in the face and limbs in every patient. Subjects were divided into those who remained as ocular MG (ROMG group) and those who experienced conversion to generalized MG during follow-up (GOMG group).
Results
Conversion to generalized MG occurred in 24 (21.4%) of 112 MG patients with ocular onset. The proportion of patients displaying abnormal decreases in responses in the trapezius, abductor digiti minimi, or flexor carpi ulnaris muscles on the RNS test was higher in the GOMG group (p<0.001, p=0.002, and p<0.001, respectively). The Cox proportional-hazards model revealed that an abnormal result on the RNS test was significantly associated with conversion to generalized MG [hazard ratio (HR)=3.13, 95% confidence interval (CI)=1.18–8.32]. Notably, the HR was higher for abnormal results on the RNS test for the limb muscles, at 5.19 (95% CI=2.09–12.90).
Conclusions
An abnormal result on the RNS test, especially in the limb muscles, is an independent predictor of the conversion from ocular to generalized MG. Applying the RNS test to limb muscles could be useful for predicting the conversion to generalized MG in patients with ocular onset.
Keywords: ocular myasthenia gravis, generalized myasthenia gravis, repetitive nerve stimulation test
INTRODUCTION
Myasthenia gravis (MG) is an autoimmune disorder of the neuromuscular junction characterized by fatigable muscle weakness.1,2 Reportedly 60–85% of MG patients initially present with ocular symptoms such as ptosis or diplopia, and a significant proportion of these patients subsequently progress to generalized MG.2,3,4,5,6 When managing MG patients with ocular onset, one of the main concerns is symptom conversion to a generalized form. It is well known that old age at onset, thymoma, and high acetylcholine receptor (AChR) antibody titer predict the conversion from ocular to generalized MG.7,8,9,10
The repetitive nerve stimulation (RNS) test is a useful and the most commonly applied neurophysiological test for neuromuscular junction disorders including MG. Applying repetitive electrical stimulation to a motor nerve gradually depletes the releasable synaptic vesicles in presynaptic nerve terminals. In patients with MG, this can reduce the endplate potential below the threshold required for the generation of muscle fiber action potentials. Theoretically, this decreased response is correlated with muscle fatigability. However, the role of the RNS test in predicting conversion to generalized MG among patients with ocular MG has been controversial. Some previous studies showed that the RNS test does not predict symptom conversion,11,12,13 whereas others have suggested that the RNS test could predict the conversion to generalized MG. Hong et al.8 reported that an abnormal result on the RNS test is more frequently observed in patients who developed generalized MG than in those who remained as ocular MG. Other studies have also suggested that abnormal results on the RNS test could predict symptom conversion.10,14 However, in those studies the RNS test was not applied to all subjects and the investigated muscles differed between patients. Therefore, there are some limitations in interpreting the effect of the RNS test in predicting conversion to generalized MG based on the current evidence.
This study aimed to determine whether an initial RNS test can predict the conversion to generalized MG in patients with ocular onset. The RNS test was conducted in a consistent manner on five muscles of the limbs and face in every patient.
METHODS
Subject enrollment and data collection
This study had a retrospective cohort design. We retrospectively reviewed the medical records of the patients with MG who visited the Department of Neurology at Severance Hospital, Seoul, South Korea from June 2009 to December 2018. MG was diagnosed based on the presence of typical muscle weakness with fatigue, the results of the RNS and neostigmine challenge tests, and the results of serological tests including AChR antibody and muscle-specific kinase (MuSK) antibody. Subjects were excluded when their symptoms were better explained by another disease. Patients were defined as ocular MG when symptoms were limited to the levator palpebrae, extraocular, and orbicularis oculi (OO) muscles at the time of diagnosis.
Inclusion criteria were 1) diagnosed as ocular MG, 2) positivity in the neostigmine challenge test and/or in the serological test for autoantibodies, including AChR antibody and MuSK antibody, and 3) the RNS test conducted at five muscles [OO, nasalis (NA), abductor digiti minimi (ADM), flexor carpi ulnaris (FCU), and trapezius (TR)] during the diagnostic workup. Patients with a follow-up duration of <6 months or age <15 years at the onset were excluded.
Clinical features of the patients were collected by reviewing the medical records. Clinical information was collected on age, sex, age at symptom onset, age at diagnosis, initial thymus status on chest computed tomography (CT), and history of thymectomy. If the patient's symptoms remained ocular, information was collected on medications for MG administered within 1 year from the diagnosis. Otherwise, information on medication administered before symptom conversion was collected in the patients who experienced symptom conversion to generalized MG during the study period.
The Severance Hospital Institutional Review Board approved this study (approval no.: 4-2020-1712), and the study was conducted in accordance with the Declaration of Helsinki.
RNS testing
We analyzed the results of RNS tests performed during the diagnostic workup for MG. The RNS test was conducted using the following standard protocol in every patient: The baseline compound muscle action potential (CMAP) was measured during supramaximal stimulation, and the postexercise CMAP was obtained after 20 seconds of maximal exercise.15 Five low-frequency (3-Hz) RNSs were applied immediately after and at 1, 3, and 5 minutes after maximal exercise. During each stimulus train, the amplitude of the first CMAP and the lowest amplitude of the remaining CMAPs were calculated. A decrease in CMAP amplitude in any stimuli train of ≥10% was defined as abnormal.16 The RNS test was conducted in a consistent manner on the following five muscles in every patient: the OO, NA, ADM, FCU, and TR muscles. To minimize any immobilization problems, the stimulator was secured with tape during the test. The test was performed on the more-affected side if the ocular symptom distribution was asymmetric. Patients who responded abnormally on the RNS test for any muscle were classified as the RNS-test-positive group, while other patients were classified as the RNS-test-negative group.
Symptom conversion to generalized MG
All of the included subjects only had ocular symptoms including diplopia and/or ptosis at the time of diagnosis. A thorough neurological examination was conducted by two neurologists during the diagnostic workup, which did not detect muscle weakness other than in the levator palpebrae, extraocular, and OO muscles. Symptom conversion to generalized MG was defined as the progression of weakness to an oropharyngeal, neck, limb, or respiratory muscle. The patients whose symptoms remained ocular during follow-up were classified as the remained as ocular MG (ROMG) group, and those who experienced conversion to generalized MG were classified as the generalized MG during follow-up (GOMG) group.
Statistical analysis
Clinical and laboratory data were compared between the ROMG and GOMG groups. Data are reported as number and percentage values for categorical variables, and as mean±SD or median and interquartile-range values for continuous variables depending on conformity with the normality assumption. The Pearson chi-square test or Fisher's exact test was used for categorical variables. Data that conformed to a normal distribution were compared between two groups using t-tests, while other data were compared using the Mann-Whitney U test.
A Kaplan-Meier analysis was conducted to compare the cumulative incidence of conversion to generalized MG between the RNS-test-positive and RNS-test-negative groups, and plots were compared using the log-rank test. The Cox proportional-hazards model was used to estimate the effect of the initial RNS test on symptom conversion to generalized MG. Variables with p<0.05 in the univariate analyses were entered into the multivariate model to identify independent predictors of conversion to generalized MG. The results of the Cox proportional-hazards model are reported as hazard ratios (HRs) with 95% confidence intervals (95% CIs). Loss of follow-up was processed as censored data in the Cox proportional-hazards model. Statistical analyses were performed using SPSS for Windows (version 25, IBM Corp., Armonk, NY, USA). Differences with a p value of <0.05 were regarded as significant.
RESULTS
Patients
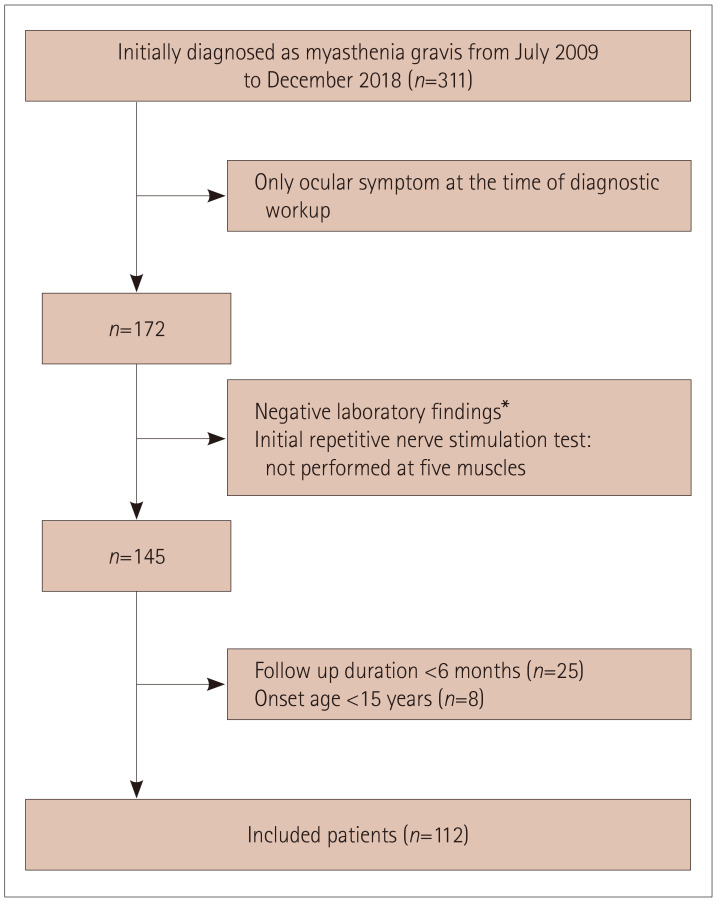
A flow diagram of subject inclusion is displayed in Fig. 1. During the study period, 311 patients were newly diagnosed with MG, and 172 patients had only ocular symptoms at the time of the diagnostic workup. Then, 145 subjects for whom there was sufficient evidence of MG and who had also undergone the RNS test at the five muscles were selected. After excluding patients with a follow-up duration of <6 months or age <15 years at the onset, 112 patients with ocular MG were analyzed in this study. The analyzed patients included 78 (69.6%) with a positive result in the AChR antibody test (>0.5 nmol/L) and no MuSK-antibody-positive MG. The follow-up duration was 44±38 months, and 24 (21.4%) patients experienced conversion to generalized MG during follow-up. Thirteen (54.2%) patients showed limb weakness and seven (29.2%) had bulbar symptoms as the initial clinical feature of generalized MG. Neck weakness and dyspnea were reported for two patients each.
Fig. 1. Patient enrollment. *Positivity in neostigmine challenge test and/or serological test for autoantibodies including acetylcholine receptor antibody and muscle-specific kinase antibody.
Comparison of clinical features between ROMG and GOMG groups
The clinical features of ROMG and GOMG groups are listed in Table 1. The median duration from the diagnosis of MG to the conversion to generalized MG in the GOMG group was 15.9 months (interquartile range 5.2–30.8 months). The median follow-up time for patients in the ROMG group was 38.4 months (interquartile range 19.0–89.0 months). The age at onset was significantly higher in the GOMG group (52.2±12.4 years) than in the ROMG group (45.3±17.0 years, p=0.032). The AChR antibody titer was also higher in the GOMG group (9.1 nmol/L) than in the ROMG group (5.0 nmol/L, p<0.001), as was the proportion of patients who used oral prednisolone (58.0% vs. 33.3%, p=0.039). Among 24 patients who experienced symptom conversion to generalized MG, 6 (25.0%) received only corticosteroids and 2 (8.3%) received both corticosteroids and azathioprine. The median dose for eight patients who received prednisolone before conversion in the GOMG group was 25 mg (range 5–100 mg), and the median dose for 51 patients who received prednisolone within 1 year from the diagnosis in the ROMG group was 20 mg (range 5–100 mg). The dose of prednisolone did not differ significantly between the two groups (p=0.858). The only immunosuppressive agent used was azathioprine. Two patients in the GOMG group and three patients in the ROMG group received azathioprine, at a dose of 100 mg for four patients and 150 mg for one patient.
Table 1. Clinical features of patients in the ROMG and GOMG groups.
| ROMG (n=88) | GOMG (n=24) | p | |
|---|---|---|---|
| Age at onset, years | 45.3±17.0 | 52.2±12.4 | 0.032 |
| Male | 43 (50.0) | 14 (58.3) | 0.469 |
| Duration from onset to MG diagnosis, months | 2.9 [1.4–10.0] | 1.9 [0.7–4.6] | 0.082 |
| Follow-up duration, months | 38.4 [19.0–89.0] | - | |
| Duration from onset to conversion to generalized MG, months | - | 15.9 [5.2–30.8] | |
| AChR-antibody positivity, >0.5 nmol/L | 57 (66.3) | 20 (83.3) | 0.100 |
| Titer of AChR antibody, nmol/L | 5.0±3.7 | 9.1±3.6 | <0.001 |
| Positivity in neostigmine challenge test | 62 (87.3) | 19 (90.5) | 0.725 |
| Thymus on chest CT* | 0.449 | ||
| Normal | 45 (57.0) | 11 (45.8) | |
| Thymic hyperplasia | 20 (25.3) | 6 (25.0) | |
| Thymoma | 14 (17.7) | 7 (29.2) | |
| Thymectomy | 17 (19.3) | 9 (37.5) | 0.061 |
| Thymoma confirmed by pathology | 14 (15.9) | 7 (29.2) | 0.140 |
| Treatment† | |||
| Pyridostigmine | 83 (94.3) | 24 (100) | 0.227 |
| Oral prednisolone | 51 (58.0) | 8 (33.3) | 0.039 |
| Use of immunosuppressive agent | 3 (3.4) | 2 (8.3) | 0.291 |
Data are mean±SD, median [interquartile range], or n (%) values.
*Chest CT was performed in 79 patients in the ROMG group, †Medication administration within 1 year from the diagnosis in the ROMG group or before conversion in the GOMG group.
AChR: acetylcholine receptor, CT: computed tomography, GOMG: symptom conversion to generalized MG, MG: myasthenia gravis, ROMG: remained as ocular MG.
There were no significant differences between the ROMG and GOMG groups in sex, duration from the onset of MG to diagnosis, initial thymus status on chest CT, or proportion of patients who had a thymectomy.
Comparison of the RNS test between ROMG and GOMG groups
The results of the RNS test in the ROMG and GOMG groups are summarized in Table 2. The proportion of patients displaying decreased responses in the OO and NA muscles did not differ between the ROMG and GOMG groups. In contrast, the proportions of patients showing decreased responses in the TR, ADM, and FCU muscles were significantly higher in the GOMG group (37.5%, 16.7%, and 37.5%, respectively) than in the ROMG group (8.0%, 0%, and 8.0%; p<0.001, p=0.002, and p<0.001, respectively). The proportion of subjects showing a decreased response in at least one of the five muscles was also significantly higher in the GOMG group (75.0%) than in the ROMG group (38.6%, p=0.002). When the RNS results for the facial and limb muscles were analyzed separately, the RNS test results in the limb muscles (TR, ADM, and FCU) differed significantly between the ROMG and GOMG groups, whereas those in the facial muscles (OO and NA) did not.
Table 2. Initial RNS test results in the ROMG and GOMG groups.
| ROMG (n=88) | GOMG (n=24) | p | |
|---|---|---|---|
| Abnormal RNS test* | |||
| OO | 20 (22.7) | 9 (37.5) | 0.143 |
| NA | 25 (28.4) | 10 (41.7) | 0.214 |
| TR | 7 (8.0) | 9 (37.5) | <0.001 |
| ADM | 0 (0) | 4 (16.7) | 0.002 |
| FCU | 7 (8.0) | 9 (37.5) | <0.001 |
| At least one of OO, NA | 31 (35.2) | 13 (54.2) | 0.092 |
| At least one of TR, ADM, FCU | 12 (13.6) | 14 (58.3) | <0.001 |
| At least one of ADM, FCU | 7 (8.0) | 11 (45.8) | <0.001 |
| Any muscle | 34 (38.6) | 18 (75.0) | 0.002 |
Data are n (%) values.
*≥10% decrease in compound muscle action potential after low-frequency supramaximal RNS was defined as abnormal.
ADM: abductor digiti minimi, FCU: flexor carpi ulnaris, GOMG: symptom conversion to generalized myasthenia gravis, NA: nasalis, OO: orbicularis oculi, RNS: repetitive nerve stimulation, ROMG: remained as ocular myasthenia gravis, TR: trapezius.
The magnitudes of the decreases are compared in Supplementary Table 1 (in the online-only Data Supplement). Similar to the comparison of the proportion of abnormal responses based on a threshold of 10%, the decreases in ADM, FCU, and TR muscles were significantly larger in the GOMG group than in the ROMG group (p=0.011, p=0.009, and p=0.044, respectively). In contrast, the decreases in the OO and NA muscles did not differ between the ROMG and GOMG groups.
Cumulative incidence and Cox proportional-hazard models for evaluating the predictive value of the RNS test
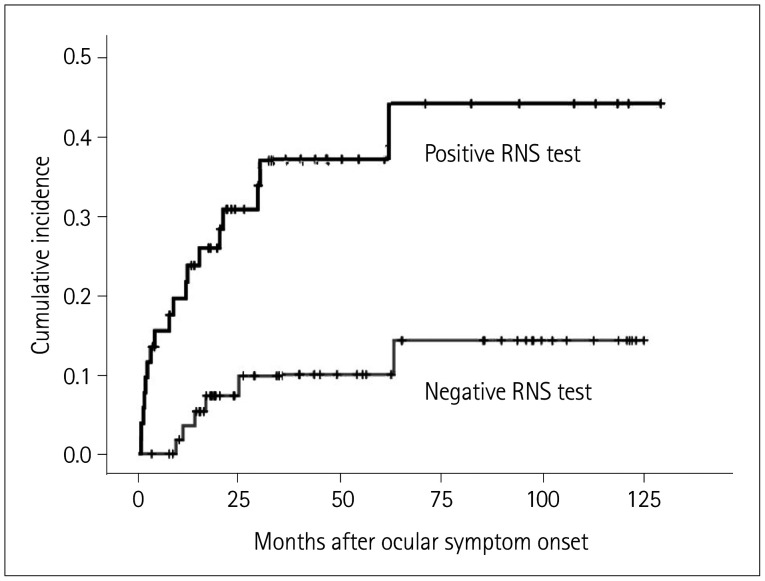
The cumulative incidence of conversion to generalized MG in the RNS-test-positive and RNS-test-negative groups as determined using the Kaplan-Meier method is displayed in Fig. 2. The incidence of symptom conversion to generalized MG was significantly higher in the RNS-test-positive group than in the RNS-test-negative group (log-rank test, p=0.001). The Cox proportional-hazards model was used to assess the effect of the initial RNS test on conversion to generalized MG. Table 3 presents the results of the Cox proportional-hazards model for evaluating the predictive value of the RNS test. In the multivariate analyses, the AChR antibody titer (HR=1.11, 95% CI=1.02–1.22) and an abnormal response on the RNS test for any muscle (HR=3.13, 95% CI=1.18–8.32) were significantly associated with symptom conversion from ocular MG. Additionally, for evaluating the impact of a reduction of the decreased response, these reductions relative to the baseline CMAP were divided into three groups: 10–20%, ≥20%, and ≥30%. The HR in the Cox proportional-hazards models was higher when there was a larger decrease in the response.
Fig. 2. Cumulative incidence of conversion to generalized MG according to the RNS test. The Kaplan-Meier survival curves show the cumulative incidence of conversion to generalized MG from ocular symptom onset according to the RNS test. The cumulative incidence of conversion to generalized MG was higher in the RNS-test-positive group than in the RNS-test-negative group (p=0.001). MG: myasthenia gravis, RNS: repetitive nerve stimulation.
Table 3. Results from Cox proportional-hazards models for variables associated with symptom conversion to generalized myasthenia gravis.
| Univariate p | HR (95% CI) | Multivariate* p | HR (95% CI) | |
|---|---|---|---|---|
| Sex | 0.577 | 1.26 (0.56–2.84) | ||
| Age at onset | 0.091 | 1.02 (0.99–1.05) | ||
| Titer of acetylcholine receptor antibody | <0.001 | 1.17 (1.08–1.28) | 0.023 | 1.11 (1.02–1.22) |
| Thymoma | 0.159 | 1.89 (0.78–4.54) | ||
| Prednisolone use before conversion | 0.036 | 0.40 (0.17–0.94) | 0.110 | |
| Abnormal repetitive nerve stimulation test† | 0.002 | 4.34 (1.72–10.97) | 0.022 | 3.13 (1.18–8.32) |
| Response decrease of 10–20% | 0.351 | 1.50 (0.64–3.52) | ||
| Response decrease ≥20% | 0.001 | 4.15 (1.84–9.36) | ||
| Response decrease ≥30% | <0.001 | 5.62 (2.31–13.67) |
*Variables with p<0.05 in the univariate model were entered into the multivariate model, †Decreased response in at least one of the five muscles.
CI: confidence interval, HR: hazard ratio.
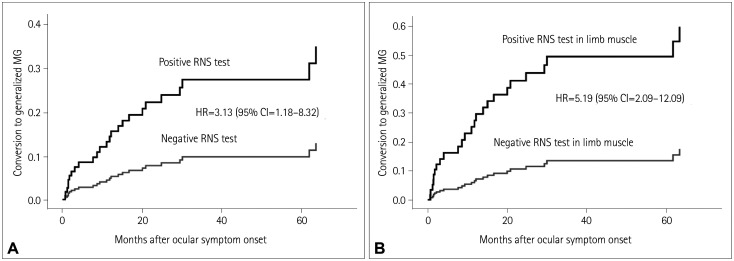
The response decrease on the RNS test was further analyzed by dividing muscles into specific muscle groups. Differences of predictive values according to subgroup RNS test are presented in Table 4. Abnormal findings on the RNS test for the FCU and ADM limb muscles showed a higher HR of 5.19 (95% CI=2.09–12.90). The effects of abnormal responses on the RNS test for any muscle and for limb muscles were compared by constructing cumulative incidence curves using the Cox proportional-hazards model (Fig. 3A and B, respectively).
Table 4. Differences in predictive values according to subgroup RNS test in Cox proportional-hazards models.
| Variable | Abnormal response in OO or NA | Abnormal response in TR, FCU, or ADM | Abnormal response in FCU or ADM | |||
|---|---|---|---|---|---|---|
| p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | |
| Titer of AChR antibody | 0.005 | 1.14 (1.04–1.25) | 0.053 | 0.046 | 1.09 (1.01–1.18) | |
| Prednisolone use | 0.111 | 0.396 | 0.364 | |||
| Subgroup RNS test | 0.364 | <0.001 | 5.08 (2.07–12.52) | <0.001 | 5.19 (2.09–12.90) | |
AChR: acetylcholine receptor, ADM: abductor digiti minimi, CI: confidence interval, FCU: flexor carpi ulnaris, HR: hazard ratio, NA: nasalis, OO: orbicularis oculi, RNS: repetitive nerve stimulation, TR: trapezius.
Fig. 3. Cumulative incidence curves for the Cox proportional-hazards model. A: Cumulative incidence curves in the RNS-test-positive and RNS-test-negative groups. The risk of conversion to generalized MG was 3.13-fold higher in the RNS-test-positive group than in the RNS-test-negative group after adjusting for acetylcholine receptor antibody titer and prednisolone administration (95% CI=1.18–8.32). B: Cumulative incidence curves for positive and negative RNS tests in limb muscles. The HR was higher for abnormal findings on the RNS test for limb muscles, at 5.19 (95% CI=2.09–12.90). CI: confidence interval, HR: hazard ratio, RNS: repetitive nerve stimulation.
DISCUSSION
The present findings demonstrate that the initial RNS test results can predict the conversion to generalized MG among patients with ocular MG. An abnormal decrease in the response on the RNS test was observed more frequently in the GOMG group than in the ROMG group. The MG patients with ocular onset who displayed a decreased response on the RNS test had a 3.13-fold higher risk of conversion to generalized MG compared with those with a normal result on the RNS test after adjusting other variables. Furthermore, abnormal findings on the RNS test for limb muscles showed a higher HR. The decreased response observed in the limb muscles indicates the presence of subclinical weakness that might not be noticed by the patient or detected in a neurological examination.17 The RNS test, especially in limb muscles, could be considered in patients with ocular MG to predict conversion to generalized MG.
When an abnormal electrophysiological response in limb muscles is detected in MG patients with only ocular symptoms, subtype classification of MG could be confusing. MG is currently classified into ocular and generalized forms based on clinical features rather than laboratory findings.1,2,18 Thus, MG patients whose symptoms remain ocular are classified as ocular MG even if an abnormal electrophysiological response in limb muscles is detected. A decreased response on the RNS test suggests dysfunction of neuromuscular transmission in the tested muscle. A decreased response on the RNS test in limb muscles can be assumed to indicate dysfunction of neuromuscular transmission. Therefore, the RNS test can detect this subclinical weakness among MG patients with only ocular symptoms.
Among the 68 patients who exhibited no decreases in the responses of the OO and NA muscles on the RNS test, 7 (10.3%) showed an abnormally decreased response in limb muscles. This discrepancy may be attributed to differences between the tested muscles and the muscles causing ptosis and diplopia. Major ocular symptoms such as ptosis and diplopia are caused by weakness in the levator palpebrae and extraocular muscles, whereas OO and NA—which are actually tested in the RNS test—are facial muscles. Those seven patients had normal facial muscle function or only mild eyelid closure weakness, and their quantitative MG score in tests performed simultaneously with an RNS test for facial muscles was 0 in five patients and 1 in two patients (data not shown). Well-preserved facial muscle function is presumed to the cause of normal responses for the OO and NA in the RNS test. These findings suggest that performing RNS testing in the OO may be insufficient for evaluating neurotransmission in extraocular muscles.
Several previous studies have found that decreased responses were more frequent in patients who experienced conversion to generalized MG than in those who remained ocular.8,10,14 However, several points should be considered when interpreting these data, especially regarding the results of the RNS test. In one study, the results were not statistically significant after adjusting for other variables.8 In addition, the muscles in which RNS tests were performed not selected in a consistent manner, with TR and FCU muscles assessed in only 43% and 28% of the patients, respectively. In another study of positive RNS tests for independently predicting conversion to generalized MG, the RNS test was conducted in only 74% of the patients, and detailed data on the examined muscles were not provided.10 A recent study that showed that a positive RNS test for the facial muscle was an independent predictor only analyzed subjects with ocular MG under immunosuppressive therapy.14 In addition, the RNS test was conducted mainly on the facial nerve, and applied to the ulnar nerve in only 34% of the patients. Since the RNS test was not conducted in every patient in a consistent manner, the possibility of selection bias exists. In the present study, five muscles were examined in a consistent manner in every patient and the RNS test was conducted after 20 seconds of maximal exercise in order to increase the test sensitivity.15,19 By applying this method the present study was able to focus on the usefulness of the initial RNS test in predicting symptom conversion from ocular MG.
In previous studies the proportion of patients who converted from ocular to generalized MG ranged from 10% to 60%.5,8,9,10,12,14 However, the rates of conversion only ranged from 10% to 23% in studies involving Asian patients, which is very similar to the 21.4% found in the present study. Conversion from ocular to generalized MG usually occurs within 2 years.8,20 In the present study, the median duration from onset to the conversion to generalized MG was 16 months. A higher AChR antibody titer was associated with conversion to generalized MG both in the present and previous studies.7,8,21 The proportion of patients who initially took oral prednisolone was higher in the ROMG group than in the GOMG group. Although there were no significant results after adjusting for other variables in the Cox proportional-hazards model, previous studies found a trend of immunosuppressive therapy reducing the rate of conversion of ocular to generalized MG.8,11
In contrast to previous studies,8,10,20 thymic histology was not significantly associated with conversion to generalized MG in the present study. This discrepancy may have been due to the times of thymectomy and prednisolone administration after surgery in this study. Fourteen of 21 subjects who had thymoma had not converted to generalized MG during follow-up. Among them, 12 patients had undergone thymectomy within 3 months of their MG diagnosis, and another 2 patients received surgery before their MG diagnosis. In addition, 6 of these 14 patients were treated with prednisolone before and after thymectomy. In a previous study that showed that the presence of thymoma predicts conversion to generalized MG from ocular MG, the median duration from symptom onset to thymectomy was 12 months.20 Another two studies did not provide information about the time interval between symptom onset and thymectomy.8,10 It is thought that early removal of thymoma and prednisolone administration may have prevented symptom progression. Moreover, we could not find data on anterior mediastinum radiological evaluations or imaging for nine patients in the ROMG group, which could also have impacted the statistical results.
The present study was subject to several limitations. First, this study was conducted at a single tertiary hospital and so there may have been selection bias for more-severe ocular MG. Additionally, our exclusion criterion of a follow-up period of shorter than 6 months could also have caused selection bias, which might have influenced the conversion rate to generalized MG and other associated risk factors. Second, some errors may have been present due to the inherent nature of the RNS test. Immobilization can cause pseudofacilitation and reduce decreases in the responses, especially in the TR muscle, and is the most difficult problem when performing the RNS test.22 However, the RNS test was applied in a consistent manner throughout the study period, and so it is unlikely that this error markedly affected the analyses. Third, since this study had a retrospective design, it is difficult to be certain that the patients initially had only ocular symptoms. The rate of symptom conversion to generalized MG could have been either overestimated or underestimated. It would be interesting to prospectively investigate the conversion of ocular to generalized MG using a combination of clinical symptoms and MG-specific scales. Finally, the sample was too small to identify other risk factors for symptom conversion to generalized MG. Previous studies with large samples revealed that AChR-antibody positivity, presence of thymoma, and corticosteroid use were risk factors for conversion to generalized MG.8,9,10,20 In the present study, the proportion of patients taking prednisolone was significantly higher in the ROMG group, but this difference was not statistically significant after adjusting for other variables in the Cox proportional-hazards model. The proportions of AChR-antibody positivity and presence of thymoma were also higher in the GOMG group than in the ROMG group, but the differences did not reach statistical significance. Considering the above factors, discrepancies from previous studies may have be due to the small sample in this study.
In conclusion, this study has revealed that the RNS test can predict symptom conversion from ocular MG to generalized MG. An abnormal RNS test for the limb muscles had a greater predictive value. Therefore, applying an RNS test to the limb muscles could be beneficial for predicting the conversion of ocular MG to generalized MG. We suggest that the RNS test including limb muscles should be performed even in MG patients with only ocular symptoms.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2020R1C1C1010130).
Footnotes
- Conceptualization: Ki Hoon Kim, Ha Young Shin.
- Data curation: Ki Hoon Kim.
- Formal analysis: all authors.
- Methodology: all authors.
- Resources: all authors.
- Supervision: Seung Woo Kim, Ha Young Shin.
- Visualization: Ki Hoon Kim.
- Writing—original draft: Ki Hoon Kim.
- Writing—review & editing: all authors.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2021.17.2.265.
Comparison of response decreases on the initial RNS test between the ROMG and the GOMG groups
References
- 1.Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375:2570–2581. doi: 10.1056/NEJMra1602678. [DOI] [PubMed] [Google Scholar]
- 2.Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14:1023–1036. doi: 10.1016/S1474-4422(15)00145-3. [DOI] [PubMed] [Google Scholar]
- 3.Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37:141–149. doi: 10.1002/mus.20950. [DOI] [PubMed] [Google Scholar]
- 4.Barton JJ, Fouladvand M. Ocular aspects of myasthenia gravis. Semin Neurol. 2000;20:7–20. doi: 10.1055/s-2000-6829. [DOI] [PubMed] [Google Scholar]
- 5.Bever CT, Jr, Aquino AV, Penn AS, Lovelace RE, Rowland LP. Prognosis of ocular myasthenia. Ann Neurol. 1983;14:516–519. doi: 10.1002/ana.410140504. [DOI] [PubMed] [Google Scholar]
- 6.Oosterhuis HJ. The natural course of myasthenia gravis: a long term follow up study. J Neurol Neurosurg Psychiatry. 1989;52:1121–1127. doi: 10.1136/jnnp.52.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguirre F, Villa AM. Prognosis of ocular myasthenia gravis in an Argentinian population. Eur Neurol. 2018;79:113–117. doi: 10.1159/000487132. [DOI] [PubMed] [Google Scholar]
- 8.Hong YH, Kwon SB, Kim BJ, Kim BJ, Kim SH, Kim JK, et al. Prognosis of ocular myasthenia in Korea: a retrospective multicenter analysis of 202 patients. J Neurol Sci. 2008;273:10–14. doi: 10.1016/j.jns.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Mazzoli M, Ariatti A, Valzania F, Kaleci S, Tondelli M, Nichelli PF, et al. Factors affecting outcome in ocular myasthenia gravis. Int J Neurosci. 2018;128:15–24. doi: 10.1080/00207454.2017.1344237. [DOI] [PubMed] [Google Scholar]
- 10.Teo KY, Tow SL, Haaland B, Gosavi TD, Jing-Liang L, Yew Long LO, et al. Low conversion rate of ocular to generalized myasthenia gravis in Singapore. Muscle Nerve. 2018;57:756–760. doi: 10.1002/mus.25983. [DOI] [PubMed] [Google Scholar]
- 11.Allen JA, Scala S, Jones HR. Ocular myasthenia gravis in a senior population: diagnosis, therapy, and prognosis. Muscle Nerve. 2010;41:379–384. doi: 10.1002/mus.21555. [DOI] [PubMed] [Google Scholar]
- 12.Sommer N, Sigg B, Melms A, Weller M, Schepelmann K, Herzau V, et al. Ocular myasthenia gravis: response to long-term immunosuppressive treatment. J Neurol Neurosurg Psychiatry. 1997;62:156–162. doi: 10.1136/jnnp.62.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberg DH, Rizzo JF, 3rd, Hayes MT, Kneeland MD, Kelly JJ., Jr Ocular myasthenia gravis: predictive value of single-fiber electromyography. Muscle Nerve. 1999;22:1222–1227. doi: 10.1002/(sici)1097-4598(199909)22:9<1222::aid-mus8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Ding J, Zhao S, Ren K, Dang D, Li H, Wu F, et al. Prediction of generalization of ocular myasthenia gravis under immunosuppressive therapy in Northwest China. BMC Neurol. 2020;20:238. doi: 10.1186/s12883-020-01805-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo YL, Dan YF, Leoh TH, Tan YE, Nurjannah S, Ratnagopal P. Effect of exercise on repetitive nerve stimulation studies: new appraisal of an old technique. J Clin Neurophysiol. 2004;21:110–113. doi: 10.1097/00004691-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Preston DC, Shapiro BE. Electromyography and neuromuscular disorders: clinical-electrophysiologic correlations. 3rd ed. New York, NY: Elsevier; 2013. pp. 57–60. [Google Scholar]
- 17.Abraham A, Breiner A, Barnett C, Katzberg HD, Lovblom LE, Rt MN, et al. Electrophysiological testing is correlated with myasthenia gravis severity. Muscle Nerve. 2017;56:445–448. doi: 10.1002/mus.25539. [DOI] [PubMed] [Google Scholar]
- 18.Jaretzki A, 3rd, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, et al. Myasthenia gravis: recommendations for clinical research standards. Ann Thorac Surg. 2000;70:327–334. doi: 10.1016/s0003-4975(00)01595-2. [DOI] [PubMed] [Google Scholar]
- 19.Costa J, Evangelista T, Conceição I, de Carvalho M. Repetitive nerve stimulation in myasthenia gravis--relative sensitivity of different muscles. Clin Neurophysiol. 2004;115:2776–2782. doi: 10.1016/j.clinph.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Li F, Hotter B, Swierzy M, Ismail M, Meisel A, Rückert JC. Generalization after ocular onset in myasthenia gravis: a case series in Germany. J Neurol. 2018;265:2773–2782. doi: 10.1007/s00415-018-9056-8. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Li Y, Feng H, Chen P, Liu W. Clinical characteristics of juvenile myasthenia gravis in Southern China. Front Neurol. 2018;9:77. doi: 10.3389/fneur.2018.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa G, Sonoo M, Hatanaka Y, Kaida K, Kamakura K. A new maneuver for repetitive nerve stimulation testing in the trapezius muscle. Muscle Nerve. 2013;47:668–672. doi: 10.1002/mus.23664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of response decreases on the initial RNS test between the ROMG and the GOMG groups