Abstract
Background and Purpose
The cerebral cortex has been the focus of investigations of the pathogenesis of migraine for a long time. Transcranial magnetic stimulation (TMS) is a safe and effective technique for evaluating cortex excitability. Previous studies of the duration of the cortical silent period (CSP)—a measure of intracortical inhibition—in migraine patients have yielded conflicting results. We aimed to characterize cortical excitability by applying TMS to female migraineurs during the preovulatory phase of the menstrual cycle, in order to eliminate the effects of variations in sex hormones.
Methods
We enrolled 70 female subjects: 20 migraine with aura (MA) patients, 20 migraine without aura (MO) patients, and 30 healthy controls. We measured the CSP, resting motor threshold (rMT), and motor evoked potential (MEP) induced by TMS to evaluate cortical excitability during the preovulatory phase of the menstrual cycle.
Results
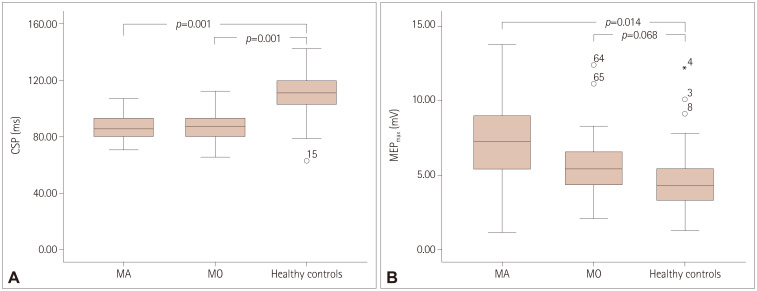
The CSP was shorter in MA patients (88.93±3.82 ms, mean±SEM) and MO patients (86.98±2.72 ms) than in the control group (109.06±2.85 ms) (both p=0.001), and did not differ significantly between the MA and MO groups (p=0.925). The rMT did not differ significantly among the groups (p=0.088). MEPmax was higher in MA patients than in healthy controls (p=0.014), while that in MO patients did not differ from those in MA patients and healthy controls (p=0.079 and p=0.068).
Conclusions
We detected a shorter CSP in both MA and MO patients. This finding may indicate the presence of motor cortex hyperexcitability, which is probably due to reduced GABAergic neuronal inhibition in migraine.
Keywords: migraine, cortical excitability, transcranial magnetic stimulation, cortical silent period, menstrual cycle
INTRODUCTION
Migraine is a complex neurovascular disease that is more common in females and is characterized by usually episodic throbbing headache and certain neurological symptoms such as photophobia, phonophobia, vasomotor symptoms, and motor, emotional, and visual sensory disturbances. Migraine has clinically two main forms: migraine with aura (MA) and migraine without aura (MO).1,2,3,4 Neural and vascular mechanisms have been considered to be responsible for the pathogenesis of migraine. Many clinical and basic-science studies have explored the pathophysiology of migraine using neurophysiological and neuroimaging tests including transcranial Doppler ultrasonography, functional magnetic resonance imaging, and positron-emission tomography, but it remains to be clearly elucidated.4,5,6,7
The cerebral cortex has been the focus of investigations of the pathogenesis of migraine for a long time. Hyperexcitability of the motor and visual cortices during the interictal period has been suggested to play an important role in the pathogenesis of migraineurs,8,9,10 while there have also been some contrasting claims of hypoexcitability in the cortex.11,12 Transcranial magnetic stimulation (TMS) is a safe noninvasive tool for evaluating brain functions in some neurological and psychiatric disorders. TMS has been applied to detect changes in the excitability of the visual and motor cortices in vivo.10,13,14,15 The resting motor threshold (rMT), motor evoked potential (MEP), duration of the cortical silent period (CSP), and short-interval intracortical inhibition (SICI) have been used to evaluate motor cortex excitability in migraine.16,17,18,19
The CSP duration is a measure of intracortical inhibition that reflects the activation of gamma-aminobutyric acid (GABA) interneurons.12,18,19 Only a few studies have evaluated the CSP in patients with migraine, with some finding a shorter CSP in migraine,10,18,19,20 and the others finding no difference.17,21,22 These discrepancies may be due to methodological differences such as in the selection of patients, sample size, and the use of different kinds of measuring device.10,19
Some studies have described a possible relationship between migraine attacks and hormonal variations in female patients. Variations in sex hormones may alter the cortical excitability in females. While estrogen increases neural excitability by activating the glutaminergic system, progesterone leads to central neuronal inhibition by activating the GABAergic system.10,23,24,25 Only one study of CSP in migraineurs took the phases of the menstrual cycle into account, but it did not apply TMS in any specific phase of the menstrual cycle.10
Thus, to eliminate the effect of variations in sex hormones during the menstrual cycle on cortical excitability, we aimed to determine the CSP duration, rMT, and MEP induced by TMS in female subjects that included MA patients, MO patients, and healthy controls during the preovulatory phase of the menstrual cycle.
METHODS
Patients
This study was included 70 female subjects: 20 patients with MA (aged 29.20±1.16 years, mean±SEM), 20 patients with MO (aged 26.10±1.19 years), and 30 healthy controls (aged 26.26±0.80 years). All participants were examined by an expert neurologist, who diagnosed migraine according to the criteria of the International Headache Society. None of the patients had menstrual migraine or received prophylactic migraine treatment, and all of them had a regular menstrual cycle, with all tests performed in preovulatory phase (between days 5 and 10 of the menstrual cycles). All electrophysiological testing was done during a migraine-attack-free period, which was defined as at least 3 days after the last migraine attack and 3 days before the subsequent migraine attack. All subjects were either called by telephone or participated in a face-to-face interview after the procedure to determine whether they had developed any headache attack. If a headache had occurred within 3 days after procedure, all tests were repeated under similar conditions.
Subjects with neuropsychiatric disease, depression, cranial spine surgery, hypertension, metabolic, endocrine or infectious disease, malignancy, pregnancy, lactating, or implants that may be affected by magnetic fields were excluded from the study. None of the subjects had taken any medication during the previous month that could alter cortical excitability, such as sedatives, hypnotics, anticonvulsants, antidepressants, antihistamines, anticholinergics, or contraceptives.
Informed consent was obtained from all subjects, and the study was approved by the local ethical committee (Faculty of Medicine Ethics Committee, Cumhuriyet University, approval number: 26-10-2000-08).
Electrophysiological measurements
Electrophysiological measurements were made with the subject seated in a chair as comfortably as possible in a dimmed and silent room. The motor cortex was stimulated using a 90-mm circular coil with input from a Magstim 200 stimulator (The Magstim Company Ltd; Wales, UK) that produces a maximum transient magnetic field strength of 2 tesla. Electromyography (EMG) activity was recorded using an appropriate device (Neuropack 8 MEB-4200, Nihon Kohden, Tokyo, Japan).
EMG recordings were made from the right first dorsal interosseous (FDI) muscle using Ag-AgCl surface electrodes. The cathode (active electrode) was placed over the muscle belly and the anode (reference electrode) was placed about 3 cm distally. The recording parameters were as follows: bandpass filter from 2 Hz to 3 kHz and sweep speed of 5 ms/div for the MEP measurements, and bandpass filter from 10 Hz to 5 kHz and sweep speed of 30 ms/div for the CSP measurements.
The coil was placed over the vertex with its center at the Cz point. Orienting the coil with its A-side coil upwards resulted in a clockwise current stimulating the left primary motor cortex. The coil was oriented in the anterior-posterior direction with its handle in the posterior direction, and the coil was rotated in order to determine its optimum position. The motor activation threshold was defined as the lowest stimulation intensity required to produce a peak-to-peak MEP amplitude of ≥50 μV in at least five of ten stimulations. The rMT was detected at rest. The process was well tolerated by all participants.
The mean MEP amplitude obtained during three consecutive stimuli at the maximum stimulator output was accepted as MEPmax.
A special setup was used to calculate 20% of the maximum voluntary isometric contraction. The cuff of a fixed sphygmomanometer was squeezed at maximum force using abduction movement of the right index finger to measure (in mm Hg) the maximum voluntary contraction, and then the subject was asked to perform the same movement at 20% of this value. The CSP was recorded during FDI muscle contraction (approximately 20% of the maximum voluntary muscle contraction) while applying a stimulation intensity of 110% of the rMT. The CSP duration was measured as the time that elapsed between the stimulus artifact and the resumption of voluntary EMG activity. The shortest CSP obtained from five consecutive stimuli was used in the subsequent evaluations.
Statistical analyses
All statistical analyses were performed using SPSS (version 17.0, SPSS Inc., Chicago, IL, USA). The data for the TMS parameters of the CSP, rMT, and MEPmax did not conform to a normal distribution, and so parametric tests could not be applied to evaluate those data. The Kruskal-Wallis test was used to compare the data of the three study groups. When a significant p value was determined for any parameter in the Kruskal-Wallis test, the Mann-Whitney U test was used for pairwise comparisons. Categorical variables were compared using the chi-square test. Pearson's correlation analysis was applied for detecting correlations between the TMS parameters of the CSP, rMT, and MEPmax and clinical parameters such as the frequency of migraine attacks, duration of illness, and age. A probability value of p<0.05 was considered statistically significant.
RESULTS
The age distribution did not differ significantly between the three groups, and the duration and frequency of migraine attacks did not differ between the MA and MO groups (Table 1). There were also no correlations between the TMS parameters of the rMT, MEPmax, and CSP duration and clinical factors related to migraine such as the attack frequency and disease duration (Table 2).
Table 1. Demographic data and TMS parameters in the three study groups.
| MA (n=20) | MO (n=20) | Healthy controls (n=30) | p | |
|---|---|---|---|---|
| Age (years) | 29.20±1.16 | 26.10±1.19 | 26.26±0.80 | 0.067 |
| Migraine duration (years) | 9.90±1.06 | 6.95±0.94 | 0.644 | |
| Monthly attack frequency | 2.70±0.23 | 2.50±0.27 | 0.463 | |
| rMT (%) | 39.15±1.15 | 39.40±1.36 | 42.30±1.01 | 0.088 |
| MEPmax (mV) | 7.10±0.78 | 5.81±0.55 | 4.92±0.45 | 0.016* |
| CSP (ms) | 88.93±3.82 | 86.98±2.72 | 109.06±2.85 | 0.001*† |
Data are mean±SEM values.
*Significant difference between MA patients and healthy controls, †Significant difference between MO patients and healthy controls.
CSP: cortical silent period, MA: migraine with aura, MEPmax: maximum amplitude of motor evoked potential, MO: migraine without aura, rMT: resting motor threshold, TMS: transcranial magnetic stimulation.
Table 2. Correlations between TMS parameters and clinical factors of migraine.
| Attack frequency | Disease duration | Age | |
|---|---|---|---|
| CSP duration | r=0.247, p=0.294 | r=–0.373, p=0.105 | r=–0.040, p=0.867 |
| MEPmax | r=0.162, p=0.495 | r=–0.086, p=0.718 | r=–0.110, p=0.645 |
| rMT | r=–0.209, p=0.377 | r=0.054, p=0.820 | r=–0.313, p=0.179 |
CSP: cortical silent period, MEPmax: maximum amplitude of motor evoked potential, rMT: resting motor threshold, TMS: transcranial magnetic stimulation.
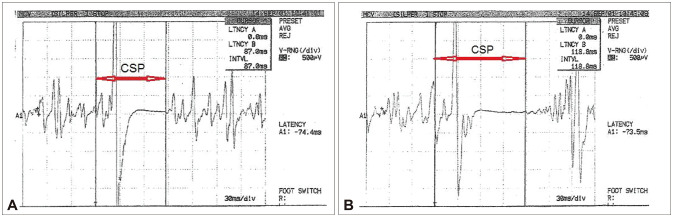
The CSP was shorter in the MA group (88.93±3.82 ms) and MO group (86.98±2.72 ms) than in the control group (109.06±2.85 ms, both p=0.001) (Fig. 1), with no significant difference between the MA and MO groups (p=0.925) (Table 1) (Fig. 2A). MEPmax was significantly higher in MA patients than healthy controls (p=0.014), and did not differ between MO patients and either MA patients (p=0.079) or healthy controls (p=0.068) (Fig. 2B). There were no significant intergroup differences in rMT (Table 1).
Fig. 1. CSP measurements of patients. A: CSP in a case of MA. B: CSP in a healthy control case. CSP: cortical silent period, MA: migraine with aura.
Fig. 2. CSP and MEPmax values in groups. A: CSP duration in the three groups. B: MEPmax in the three groups. CSP: cortical silent period, MA: migraine with aura, MEPmax: maximum amplitude of motor evoked potential, MO: migraine without aura.
DISCUSSION
We found that the CSP was shorter in the MA and MO females than in the healthy controls, while MEPmax was higher in the MA females than in the healthy controls.
Motor cortex excitability in migraine has previously been investigated using rMTs, MEPs, and CSP durations, but the results have demonstrated both hyperexcitability and hypoexcitability of the cortex.10,17,19,20,21,26,27 Van der Kamp et al.28,29 claimed that migraine was associated with motor cortex hyperexcitability, since they found high MEPmax amplitudes in their own studies, which were not found in other studies.10,20,22 The present study found that MEPmax was elevated in MA patients but not in MO patients.
The rMT is related to membrane excitability regulated by voltage-gated sodium channels,19,30 and during the interictal period has been found to be low in some studies10 but high in others.17,31 Like in the present study, Neverdahl et al.19 and Boros et al.23 found similar rMT values in migraine and control patients.
The CSP has been described as an interruption of voluntary motor activity following the application of a magnetic stimulus, and it appears as a silent period on the EMG record. The CSP duration is a measure of motor cortical postsynaptic inhibition associated with the activation of GABAergic interneurons,20,30,32,33 which are inhibitory interneurons in the motor cortex. These interneurons may be selectively damaged by transient hypoxia during migraine attacks due to them being very sensitive to ischemia. Their functions might be selectively suppressed during recurrent cortical spreading depression. Therefore, some authors have claimed that the impairment or primary loss of GABAergic neurons could be responsible for the pathogenesis of migraine, with the damaged inhibitory mechanisms possibly being responsible for the motor cortex hyperexcitability seen in some migraineurs. A short CSP may reflect a reduced activation ability of cortical inhibitory interneurons secondary to the main problems in the motor cortex.20,21,22,34,35 However, contradictory results regarding the CSP duration have been detected, with some studies in migraineurs showing short CSPs10,18,19,20,36 but other studies finding normal CSPs,17,21,22 while the CSP has been found to be longer in patients with chronic migraine than in both MA patients and controls.9 In our study, the shorter CSP found in patients with MA and MO supports the hypothesis of hyperexcitability in the motor cortex during the interictal period due to the diminished activation of GABAergic inhibitory neurons.
The above-mentioned contradictory results may also arise from technical differences such as timing of the procedure, type of coil, and pulse characteristics, as well as factors associated with the patient population such as the sample size, sex, and type of migraine.10,19 Previous evaluations of the CSP in migraine have included both male and female patients, and variations in the sex ratio between study populations might have been responsible for the contradictory results. Hence, we enrolled only female patients in the present study in order to avoid potentially confounding effects of sex.
Variations in female sex hormones are known to affect cortical excitability.25,37 These hormones can be present at different levels depending on the administration of hormonal therapy, pregnancy, and stages of the female reproductive life cycle including premenarche, menarche, premenopause, and menopause. Also, different phases of the menstrual cycle can alter hormone levels so as to affect migraine attacks; for example, estradiol may stimulate neuronal excitability while progesterone metabolites may enhance GABAergic neuronal inhibition.23,25,37,38 Enrolling patients in different menstrual phases can therefore be a reason for the observed inconsistencies in results.23 In our study, all subjects had a regular menstrual cycle and all tests were performed in the preovulatory phase. Boros et al.23 studied the effects of the different phases of the menstrual cycle on cortex excitability by measuring the rMT in migraineurs. They found that the measured rMT was not affected by the phase of the menstrual cycle or the usage of oral contraceptives. However, because that study did not measure certain parameters such as the CSP duration, MEPmax, and SICI,23 we cannot conclude that those tests would not be affected by differences in menstrual phases. Khedr et al.10 examined motor and visual cortical excitability in migraine patients while taking into account the menstrual phase in order to avoid any confounding effects on cortex excitability. Consistent with the results of our study, those authors reported that the CSP was significantly shorter in MA and MO patients. While they were careful not to make measurements during the premenstrual and menstrual phases, they might not have completely excluded the confounding effects of hormonal changes since they did not conduct their tests during a specific phase of the menstrual cycle.10
Mechanisms originating from both cortical and spinal inhibition can affect the CSP duration. The mechanisms responsible for modulation of the CSP have not been clearly demonstrated.13,32 To exclude the effect of spinal inhibition, Curra et al.20 measured the CSP duration originating from cortical inhibition in facial muscle. Those authors found that a short CSP duration was independent of the spinal mechanisms in migraine patients, and so attributed this finding to reduced cortical inhibition in migraine.20
Another factor modulating the CSP duration is the degree of contraction of the muscle used during the measurement. The contraction intensities used in previous studies have ranged from 10% to 100%, and larger muscle contraction has been associated with a shorter CSP. While a large contraction intensity of 40–60% may provide more-accurate CSP measurements, this is difficult to maintain during measurements, which might result in inaccurate CSP evaluations. Therefore, smaller contractions may provide optimal measurements of CSP,32,39,40 and so 20% of the maximum voluntary contraction was used in the present study.
Many studies of patients with migraine have not found significant correlations between the CSP duration and clinical parameters such as the frequency and duration of migraine attacks.17,19,20,22 We also did not find any significant relationships between the CSP duration and migraine parameters such as the attack frequency and disease duration.
The strengths of the present study were as follows: Firstly, all the subjects were female, thereby avoiding any confounding effect of sex. Secondly, all measurements were performed in a specific phase of the menstrual cycle in females with a normal menstrual cycle, thereby avoiding the confounding effect of hormonal changes. Thirdly, none of the patients were taking antimigraine prophylactics or any drugs that would affect measurements of TMS parameters, such as oral contraceptives, hormonal therapy, or drugs that act on the CNS. Fourthly, this study included more migraine cases than previous studies evaluating motor cortex excitability based on CSP measurements.
One limitation of this study is that the parameters were not measured at different stimulus levels, with a second test not performed to show inhibition of GABAergic neurons such as SICI. Such measurements might have allowed more general conclusions to be drawn about cortical excitability in migraine. However, we demonstrated that the CSP was shorter in both MA and MO patients, which may indicate the presence of motor cortex hyperexcitability due to reduced GABAergic neuronal inhibition in migraine.
Acknowledgements
None
Footnotes
- Conceptualization: all authors.
- Investigation: all authors.
- Writing—original draft: all authors.
- Writing—review & editing: all authors.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA. Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol. 2019;18:795–804. doi: 10.1016/S1474-4422(19)30185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain. 2013;154:S44–S53. doi: 10.1016/j.pain.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppola G, Di Lorenzo C, Parisi V, Lisicki M, Serrao M, Pierelli F. Clinical neurophysiology of migraine with aura. J Headache Pain. 2019;20:42. doi: 10.1186/s10194-019-0997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97:553–622. doi: 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day BK, Dodick DW, Schwedt TJ. Neuroimaging in migraine-recent advances and perspectives for the future. US Neurology. 2010;6:82–86. [Google Scholar]
- 6.Clarke BM, Upton AR, Kamath MV, Al-Harbi T, Castellanos CM. Transcranial magnetic stimulation for migraine: clinical effects. J Headache Pain. 2006;7:341–346. doi: 10.1007/s10194-006-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohm PE, Stancampiano FF, Rozen TD. Migraine headache: updates and future developments. Mayo Clin Proc. 2018;93:1648–1653. doi: 10.1016/j.mayocp.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Brighina F, Palermo A, Fierro B. Cortical inhibition and habituation to evoked potentials: relevance for pathophysiology of migraine. J Headache Pain. 2009;10:77–84. doi: 10.1007/s10194-008-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozturk V, Cakmur R, Donmez B, Yener GG, Kursad F, Idiman F. Comparison of cortical excitability in chronic migraine (transformed migraine) and migraine without aura. A transcranial magnetic stimulation study. J Neurol. 2002;249:1268–1271. doi: 10.1007/s00415-002-0834-x. [DOI] [PubMed] [Google Scholar]
- 10.Khedr EM, Ahmed MA, Mohamed KA. Motor and visual cortical excitability in migraineurs patients with or without aura: transcranial magnetic stimulation. Neurophysiol Clin. 2006;36:13–18. doi: 10.1016/j.neucli.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Áfra J. Cortical excitability in migraine. J Headache Pain. 2000;1:73–81. [Google Scholar]
- 12.Maertens de, Pepin JL, Schoenen J, Delwaide PJ. Percutaneous magnetic stimulation of the motor cortex in migraine. Electroencephalogr Clin Neurophysiol. 1992;85:110–115. doi: 10.1016/0168-5597(92)90076-n. [DOI] [PubMed] [Google Scholar]
- 13.Coppola G, Pierelli F, Schoenen J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia. 2007;27:1427–1439. doi: 10.1111/j.1468-2982.2007.01500.x. [DOI] [PubMed] [Google Scholar]
- 14.Fumal A, Bohotin V, Vandenheede M, Schoenen J. Transcranial magnetic stimulation in migraine: a review of facts and controversies. Acta Neurol Belg. 2003;103:144–154. [PubMed] [Google Scholar]
- 15.Brigo F, Storti M, Nardone R, Fiaschi A, Bongiovanni LG, Tezzon F, et al. Transcranial magnetic stimulation of visual cortex in migraine patients: a systematic review with meta-analysis. J Headache Pain. 2012;13:339–349. doi: 10.1007/s10194-012-0445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Afra J, Mascia A, Gérard P, Maertens de Noordhout A, Schoenen J. Interictal cortical excitability in migraine: a study using transcranial magnetic stimulation of motor and visual cortices. Ann Neurol. 1998;44:209–215. doi: 10.1002/ana.410440211. [DOI] [PubMed] [Google Scholar]
- 18.Aurora SK, al-Sayeed F, Welch KM. The cortical silent period is shortened in migraine with aura. Cephalalgia. 1999;19:708–712. doi: 10.1046/j.1468-2982.1999.019008708.x. [DOI] [PubMed] [Google Scholar]
- 19.Neverdahl JP, Omland PM, Uglem M, Engstrøm M, Sand T. Reduced motor cortical inhibition in migraine: a blinded transcranial magnetic stimulation study. Clin Neurophysiol. 2017;128:2411–2418. doi: 10.1016/j.clinph.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Curra A, Pierelli F, Coppola G, Barbanti P, Buzzi MG, Galeotti F, et al. Shortened cortical silent period in facial muscles of patients with migraine. Pain. 2007;132:124–131. doi: 10.1016/j.pain.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Werhahn KJ, Wiseman K, Herzog J, Förderreuther S, Dichgans M, Straube A. Motor cortex excitability in patients with migraine with aura and hemiplegic migraine. Cephalalgia. 2000;20:45–50. doi: 10.1046/j.1468-2982.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- 22.Gunaydin S, Soysal A, Atay T, Arpaci B. Motor and occipital cortex excitability in migraine patients. Can J Neurol Sci. 2006;33:63–67. doi: 10.1017/s0317167100004716. [DOI] [PubMed] [Google Scholar]
- 23.Boros K, Poreisz C, Paulus W, Antal A. Does the menstrual cycle influence the motor and phosphene thresholds in migraine? Eur J Neurol. 2009;16:367–374. doi: 10.1111/j.1468-1331.2008.02500.x. [DOI] [PubMed] [Google Scholar]
- 24.Sacco S, Ricci S, Degan D, Carolei A. Migraine in women: the role of hormones and their impact on vascular diseases. J Headache Pain. 2012;13:177–189. doi: 10.1007/s10194-012-0424-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DA, et al. Menstrual cycle effects on cortical excitability. Neurology. 1999;53:2069–2072. doi: 10.1212/wnl.53.9.2069. [DOI] [PubMed] [Google Scholar]
- 26.Cosentino G, Di Marco S, Ferlisi S, Valentino F, Capitano WM, Fierro B, et al. Intracortical facilitation within the migraine motor cortex depends on the stimulation intensity. A paired-pulse TMS study. J Headache Pain. 2018;19:65. doi: 10.1186/s10194-018-0897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coppola G, Schoenen J. Cortical excitability in chronic migraine. Curr Pain Headache Rep. 2012;16:93–100. doi: 10.1007/s11916-011-0231-1. [DOI] [PubMed] [Google Scholar]
- 28.van der Kamp W, Maassen VanDenBrink A, Ferrari MD, van Dijk JG. Interictal cortical hyperexcitability in migraine patients demonstrated with transcranial magnetic stimulation. J Neurol Sci. 1996;139:106–110. doi: 10.1016/s0022-510x(96)00044-5. [DOI] [PubMed] [Google Scholar]
- 29.van der Kamp W, MaassenVanDenBrink A, Ferrari MD, van Dijk JG. Interictal cortical excitability to magnetic stimulation in familial hemiplegic migraine. Neurology. 1997;48:1462–1464. doi: 10.1212/wnl.48.5.1462. [DOI] [PubMed] [Google Scholar]
- 30.Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, et al. TMS and drugs revisited 2014. Clin Neurophysiol. 2015;126:1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 31.Bettucci D, Cantello R, Gianelli M, Naldi P, Mutani R. Menstrual migraine without aura: cortical excitability to magnetic stimulation. Headache. 1992;32:345–347. doi: 10.1111/j.1526-4610.1992.hed3207345.x. [DOI] [PubMed] [Google Scholar]
- 32.Škarabot J, Mesquita RNO, Brownstein CG, Ansdell P. Myths and methodologies: how loud is the story told by the transcranial magnetic stimulation-evoked silent period? Exp Physiol. 2019;104:635–642. doi: 10.1113/EP087557. [DOI] [PubMed] [Google Scholar]
- 33.Poston B, Kukke SN, Paine RW, Francis S, Hallett M. Cortical silent period duration and its implications for surround inhibition of a hand muscle. Eur J Neurosci. 2012;36:2964–2971. doi: 10.1111/j.1460-9568.2012.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krüger H, Luhmann HJ, Heinemann U. Repetitive spreading depression causes selective suppression of GABAergic function. Neuroreport. 1996;7:2733–2736. doi: 10.1097/00001756-199611040-00065. [DOI] [PubMed] [Google Scholar]
- 35.Sloper JJ, Johnson P, Powell TP. Selective degeneration of interneurons in the motor cortex of infant monkeys following controlled hypoxia: a possible cause of epilepsy. Brain Res. 1980;198:204–209. doi: 10.1016/0006-8993(80)90356-x. [DOI] [PubMed] [Google Scholar]
- 36.Maier J, Sebastian I, Weisbrod M, Freitag CM, Resch F, Bender S. Cortical inhibition at rest and under a focused attention challenge in adults with migraine with and without aura. Cephalalgia. 2011;31:914–924. doi: 10.1177/0333102411408627. [DOI] [PubMed] [Google Scholar]
- 37.Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Effects of ovarian hormones on human cortical excitability. Ann Neurol. 2002;51:599–603. doi: 10.1002/ana.10180. [DOI] [PubMed] [Google Scholar]
- 38.Silberstein SD. Sex hormones and headache. Rev Neurol (Paris) 2000;156 Suppl 4:4S30–4S41. [PubMed] [Google Scholar]
- 39.Matsugi A. Changes in the cortical silent period during force control. Somatosens Mot Res. 2019;36:8–13. doi: 10.1080/08990220.2018.1563536. [DOI] [PubMed] [Google Scholar]
- 40.Säisänen L, Pirinen E, Teitti S, Könönen M, Julkunen P, Määttä S, et al. Factors influencing cortical silent period: optimized stimulus location, intensity and muscle contraction. J Neurosci Methods. 2008;169:231–238. doi: 10.1016/j.jneumeth.2007.12.005. [DOI] [PubMed] [Google Scholar]