Abstract
Background and Purpose
Guillain-Barré syndrome (GBS) is rare, but its symptoms are severe and they occasionally lead to long-term disability. Country-specific epidemiological evidence is useful for detecting potential problems at the population level. This study investigated the epidemiological and economic characteristics of GBS in South Korea.
Methods
The Korean National Health Insurance Service claims data from 2010 to 2016 were used to identify incident cases as newly hospitalized patients with a primary diagnosis of GBS (the 10th revision of the International Classification Disease code of G61.0). New cases were defined as patients not having claim records for GBS within one year prior to the hospital admission for GBS.
Results
The incidence rate increased by 45.6% between 2010 and 2016, from 1.28 to 1.82 per 100,000 population. All age groups other than <20 years showed increasing trends. The incidence rate was highest in those aged 65 years to 74 years. Approximately 72% of the incident GBS cases had antecedent infection within 42 days before GBS was diagnosed. Children younger than 10 years constituted the highest proportion of antecedent infections (93.7%). The average length of stay per GBS hospitalization was 33.5 days. Patients had an average of 7.48 outpatient visits for GBS treatment per year. The economic burden from a societal perspective of treating GBS during the first year was USD 16,428.
Conclusions
The increasing incidence trend and substantial economic burden of GBS strongly advocate the development of effective strategies for preventing and managing GBS.
Keywords: economic cost, epidemiology, Guillain-Barré syndrome, incidence, South Korea
INTRODUCTION
Guillain-Barré syndrome (GBS) is a disorder of the peripheral nervous system. Its acute onset progresses over a few days to weeks, and its progression lasts for 4 weeks.1,2 The most recognizable symptoms of GBS are ascending and relatively symmetric weakness or paralysis in limbs, with autonomic dysfunction and absent tendon reflexes. Symptoms can disappear following treatment with either immunoglobulin (IVIg) or plasma exchange,3 however, long-term disability is a possibility.4 Although the underlying etiology of GBS has not been clearly identified, the risk may increase after an abnormal immune response caused by preceding infection.5,6 Campylobacter jejuni, gastrointestinal tract infections, upper respiratory infections, and influenza vaccinations are known causes of GBS, yet these associations remain controversial, as suggested in earlier studies.7,8,9,10
The global incidence of GBS quantified as cases per 100,000 person-years ranges from 0.81 (Canada) to 1.89 (Italy),11 making it a ‘rare condition’ according to the World Health Organization. However, the serious symptoms and complications of GBS can cause long-term disability, and so the clinical and economic burden to patients cannot be neglected. The reported mortality rate has varied widely, from 1% to 18%,12 and is mostly associated with respiratory or cardiac complications following GBS. GBS can occur in any age group, but its incidence increases with age.13 South Korea has recently become an aged society, and is expected to become a superaged society by 2026.14 Despite concerns about geriatric disorders, including GBS, increasing in Korea, few epidemiological studies have been conducted at the population level.15
Most studies of the epidemiological or economic burden of GBS have been conducted in Western countries,11,16 with there being only a few Asian studies.17,18,19 The generic differences between Western and Asian populations20 and variations in the predominant risk factors for GBS across populations indicate the need to acquire country-specific epidemiological evidence. Moreover, variations in the practice patterns of clinicians and insurance reimbursement systems across countries mean that local studies are needed to examine the economic burden of GBS.
We therefore conducted a study to investigate the epidemiological characteristics and economic burden of GBS in South Korea using nationwide population-based administrative claims data. Specifically, the population-adjusted annual incidence rate was estimated in order to examine the magnitude of the disease burden in Korean society. We analyzed selected patient and provider characteristics available from claims data, the extent of health-care resources used, and the costs associated with treating GBS. We also examined the types of infections commonly preceding GBS with the aim of determining strategies for preventing GBS or promptly detecting its onset. We expected that our results would provide new knowledge regarding GBS and contribute to a better understanding of GBS in the Asian population.
METHODS
Data source and study population
We used Korean National Health Insurance Service (NHIS) claims data from 2010 to 2016. South Korea has a universal health insurance system that comprises the National Health Insurance (NHI) and Medical Aid (MA) programs. The NHI program is a wage-based, contributory program that covers about 97% of the population, while the MA program is a government-subsidized public assistance program for the most poverty-stricken of the population.21 NHIS claims data include information on the covered medical services provided to the beneficiaries enrolled in both the NHI and MA programs. The NHIS claims data provide information on the diagnoses and medical procedures for each claim, such as surgery, diagnostic tests, and prescribed medicines. The data also include details such as the age and sex of the selected patients, and characteristics of the health-care providers such as the type and location of health-care institutions and the specialty type of clinicians.
The study subjects were incident cases of GBS, defined as patients who were newly hospitalized with a primary diagnosis of GBS [code of G61.0 in the 10th revision of the International Classification Disease (ICD-10)]. This was based on earlier evidence that most GBS patients require hospitalization.22,23 To confirm that the GBS cases were new, the study subjects were required to not have claim records with diagnosis codes of GBS, or atypical forms of GBS, within one year prior to the first day of the present hospital admission with GBS.24 Atypical forms of GBS are categorized as ICD-10 codes of G61.8 (other inflammatory polyneuropathies), G61.9 (inflammatory neuropathy, unspecified), G62.8 (other specified polyneuropathies), and G62.9 (polyneuropathy, unspecified).
This study was approved by the Institutional Review Board of Gangnam Severance Hospital (approval number: 3-2018-0115). The need to obtain written informed consent from the participants was waived by the board.
Incidence rate and preceding infection
For each year, the population-adjusted incidence rate of GBS per 100,000 population was calculated by dividing the total number of patients with incident GBS by the standard population size of that year, then multiplying this by 100,000. We examined the selected patient and provider characteristics available from claims data for the pooled cases from 2010 to 2016. The extent of health-care resources used to treat GBS was also examined.
It has been reported that preceding infection is associated with the occurrence of GBS.2,3 The common infections observed prior to the manifestation of GBS are Campylobacter jejuni, Haemophilus influenzae, hepatitis, cytomegalovirus, Epstein-Barr virus, Mycoplasma pneumonia, and influenza A virus.5,6,7,8,22,25,26 If a patient had a claim record with a diagnosis of infection within 42 days prior to the onset of GBS, we considered the patient as having a preceding infection. The time window of 42 days27 and the type of infections included in the analysis6 were chosen based on a review of the literature.
Economic burden
The economic burden of GBS during the year after its onset was estimated from a societal perspective. Insurance-covered medical costs were estimated by summing the costs from claim records for diagnoses of GBS. We estimated the medical costs for non-insurance-covered services provided to treat GBS using the ratio of insurance-covered to non-insurancecovered costs (i.e., 95.1:4.9) among patients with rare diseases. This ratio was obtained from a 2017 survey of medical expenses for frequently provided medical services published by the NHIS.28 The average two-way transportation costs for visiting health-care institutions derived from the Korean National Health and Nutrition Examination Survey of 200629 were multiplied by the average number of hospital admissions and outpatient visits for a diagnosis of GBS per patient during one year. The costs of lost productivity due to hospitalization for GBS were determined by multiplying the average number of inpatient days for each 10-year age group by the average daily wage for the corresponding age group as obtained from the Korean Statistical Information Service. The weighted costs of lost productivity were then calculated using the proportion of patients in each 10-year age group as a weight. For the cost of lost productivity due to outpatient visits for GBS, the average number of outpatient visits for each 10-year age group was multiplied by one-third of the average daily wage for the corresponding age group. The cost of lost productivity due to premature death was not considered because GBS does not have a high mortality rate.30 All costs were converted into equivalent 2016 values using the consumer price index and the NHIS price index.
Statistical analysis
The incidence rate was estimated based on the mid-year population, and its corresponding 95% confidence intervals were estimated under the assumption of a Poisson distribution. For each age group (i.e., ≤9, 10–19, 20–49, 50–64, and ≥65 years), autoregression was carried out to test for significant secular trends in the incidence rate. All statistical analyses were performed with SAS software (version 9.4, SAS Institute, Cary, NC, USA).
RESULTS
The total number of patients with newly diagnosed GBS in South Korea increased from 648 in 2010 to 941 in 2016, corresponding to a 45.2% increase (Table 1). To account for the population size varying between years, we calculated the population-adjusted incidence rate as the number of patients per 100,000 population. On average, there were 1.48 cases of GBS per 100,000 population annually over the study period. The population-adjusted incidence rate increased by 45.6% between 2010 and 2016, from 1.28 to 1.82 per 100,000, which is very similar to the increase of 45.2% in the total number of patients with GBS. This implies that the increase in GBS incidence might not be attributable to the change in the size of the population. The incidence rate was 44% higher in males than females throughout the study period.
Table 1. Incidence of GBS in Korea.
| Year | Total no. of GBS patients | Incidence rate (patients per 100,000 population) | Male-to-female rate ratio | ||
|---|---|---|---|---|---|
| Total | Males | Females | |||
| 2010 | 648 | 1.28 (1.19–1.39) | 1.47 (1.33–1.63) | 1.09 (0.97–1.23) | 1.35 (1.25–1.46) |
| 2011 | 630 | 1.24 (1.15–1.34) | 1.47 (1.33–1.62) | 1.41 (1.27–1.56) | 1.04 (0.97–1.12) |
| 2012 | 640 | 1.26 (1.16–1.36) | 1.56 (1.41–1.72) | 0.95 (0.84–1.08) | 1.64 (1.51–1.78) |
| 2013 | 741 | 1.45 (1.35–1.56) | 1.70 (1.55–1.87) | 1.20 (1.07–1.34) | 1.42 (1.32–1.53) |
| 2014 | 838 | 1.63 (1.53–1.75) | 1.93 (1.77–2.11) | 1.34 (1.20–1.49) | 1.44 (1.34–1.55) |
| 2015 | 849 | 1.65 (1.54–1.76) | 1.92 (1.76–2.09) | 1.38 (1.24–1.53) | 1.39 (1.30–1.49) |
| 2016 | 941 | 1.82 (1.71–1.94) | 2.14 (1.97–2.32) | 1.50 (1.36–1.66) | 1.42 (1.33–1.52) |
| p for trend | <0.001 | 0.001 | <0.001 | 0.141 | - |
| Increase from 2010 to 2016, % | 45.2 | 38.5 | 40.0 | 36.3 | - |
Parentheses contain 95% confidence intervals.
GBS: Guillain-Barré syndrome.
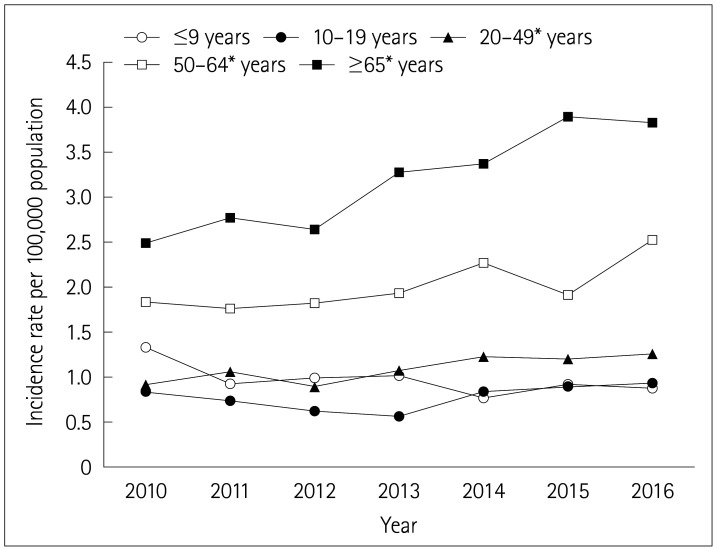
To determine whether the increasing trend of the incidence rate was consistent across different age groups, we examined the secular trends of the population-adjusted age-specific incidence rates of GBS (Fig. 1). All age groups other than <20 years showed significantly increasing trends in the incidence of GBS (p for trend <0.05). Throughout the 7-year study period, the population-adjusted incidence rate of GBS was lowest in teens, followed by those younger than 10 years. The highest incidence rate and the most-rapid increase were observed in those aged ≥65 years.
Fig. 1. Secular trends of the population-adjusted age-specific incidence rates of Guillain-Barré syndrome. *p<0.05.
The proportion of patients with GBS enrolled in the MA program was 5.0% (Table 2). This is higher than the proportion of the enrollees of the MA program among the general population (about 3%), implying that patients with GBS are poorer. The distribution of patients with GBS by income level also supports this finding, with a higher proportion of patients belonging to the lower half of the income-level group (61.6%) than to the upper half of the income-level group (38.4%).
Table 2. Socioeconomic and health-care utilization characteristics of patients with newly diagnosed GBS during 2010 to 2016.
| Characteristic | Value |
|---|---|
| Age, years | 48.6±26.5 |
| Males | 47.2±28.8 |
| Females | 50.7±25.6 |
| Sex | |
| Male | 3,120 (59.0) |
| Female | 2,167 (41.0) |
| Type of national health insurance program | |
| National Health Insurance | 5,021 (95.0) |
| Medical Aid | 266 (5.0) |
| Income level | |
| Top quartile* | 894 (18.2) |
| Second quartile | 993 (20.2) |
| Third quartile | 1,302 (26.5) |
| Bottom quartile | 1,729 (35.1) |
| Type of health-care institution diagnosing GBS | |
| Tertiary-care hospital | 5,020 (94.9) |
| General hospital | 162 (3.1) |
| Clinic | 46 (0.9) |
| Annual number of hospital admissions for GBS per GBS patient | 1.36±0.79 |
| Length of stay per admission for GBS, days | 33.50±34.44 |
Data are mean±SD or n (%) values.
*Highest income.
GBS: Guillain-Barré syndrome.
Selected health-care-utilization characteristics of patients with GBS were analyzed (Table 2). About 95% of the patients with GBS were diagnosed at tertiary-care hospitals. Patients with GBS had an average of 1.36 hospital admissions for GBS per year, and on average stayed in the hospital for more than a month (37.65 days) when they were hospitalized for GBS. About 72% of the incident GBS cases had a history of antecedent infection within 42 days before GBS was diagnosed, which suggests that infection is causally associated with GBS (Table 3). Among the different age groups, children younger than 10 years comprised the highest proportion of patients experiencing antecedent infection (93.7%), while subjects aged 20–49 years comprised the lowest proportion (65.1%). Among those younger than 65 years, the most common type of infection was respiratory infection (66.1–94.6%), followed by gastrointestinal infection (17.0–42.2%) and Haemophilus influenzae (9.8–31.1%). Similarly, respiratory infection occurred most frequently in those aged ≥65 years (61.5%), followed by influenza (19.3%) and gastrointestinal infection (14.3%). It appears that patients experiencing antecedent infection suffered from multiple infections. Each patient experienced an average of 1.12 antecedent infections, and this rate was higher in younger patients, being almost two sfor children younger than 10 years old.
Table 3. Antecedent infections of patients with GBS during 2010 to 2016.
| Infection | ICD-10 codes | Age range, years | |||||
| Total | ≤9 | 10–19 | 20–49 | 50–64 | ≥65 | ||
| Total no. of GBS patients | 5,287 (100) | 316 (100) | 333 (100) | 1,743 (100) | 1,485 (100) | 1,410 (100) | |
| Antecedent infection | |||||||
| No | 1,486 (28.1) | 20 (6.3) | 85 (25.5) | 609 (34.9) | 431 (29.0) | 341 (24.2) | |
| Yes | 3,801 (71.9) | 296 (93.7) | 248 (74.5) | 1,134 (65.1) | 1,054 (71.0) | 1,069 (75.8) | |
| Anatomic infection* | |||||||
| Gastrointestinal | A08, A09 | 825 (21.7) | 125 (42.2) | 67 (27.0) | 301 (26.5) | 179 (17.0) | 153 (14.3) |
| Respiratory | J00–J06, J20–J22, J40–J42, H65–H67, J440 | 2,585 (68.0) | 280 (94.6) | 195 (78.6) | 750 (66.1) | 703 (66.7) | 657 (61.5) |
| Pathogenic infection* | |||||||
| Hemophilus influenzae | A41.3, A49.2, J20.1, G00, J09–J18 | 591 (15.5) | 92 (31.1) | 55 (22.2) | 111 (9.8) | 127 (12.1) | 206 (19.3) |
| Campylobacter jejuni | A04.5 | 4 (0.1) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.1) | 2 (0.2) |
| Varicella zoster virus | B01 | 109 (2.9) | 5 (1.7) | 10 (4.0) | 29 (2.6) | 31 (2.9) | 34 (3.2) |
| Rickettsia tsutsugamushi | A75.3 | 25 (0.7) | 0 (0.0) | 0 (0.0) | 3 (0.3) | 6 (0.6) | 16 (1.5) |
| Mycoplasma pneumonia | A49.3, B96, J29 | 26 (0.7) | 8 (2.7) | 7 (2.8) | 5 (0.4) | 2 (0.2) | 4 (0.4) |
| Cytomegalovirus | B25 | 9 (0.2) | 1 (0.3) | 2 (0.8) | 2 (0.2) | 2 (0.2) | 2 (0.2) |
| Herpes virus | B00 | 93 (2.4) | 16 (5.4) | 10 (4.0) | 27 (2.4) | 23 (2.2) | 17 (1.6) |
| Epstein-Barr virus | B27 | 18 (0.5) | 6 (2.0) | 4 (1.6) | 3 (0.3) | 4 (0.4) | 1 (0.1) |
| Parainfluenza virus | J20.4, J21.81 | 2 (0.1) | 2 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total no. of infections | 4,287 | 535 | 351 | 1231 | 1,078 | 1092 | |
| Average no. of infections per patient with infection† | 1.13 | 1.81 | 1.42 | 1.09 | 1.02 | 1.02 | |
Data are n (%) values.
*The sum of the proportions for each type of infection can exceed 100% because some patients had multiple infections, †p for trend <0.05.
GBS: Guillain-Barré syndrome, ICD-10: the 10th revision of the International Classification Disease.
We estimated the costs of treating GBS during one year following the incident case (Table 4). The total cost was USD 16,428 per patient, with about 96% of this cost (USD 15,791) associated with hospitalization. Medical costs and the costs of lost productivity were the dominant cost components for both inpatient and outpatient services.
Table 4. Average costs of treating Guillain-Barré syndrome per patient during the year after onset from a societal perspective.
| Type of cost | Cost, USD |
|---|---|
| Hospital admission | |
| Direct medical costs | |
| Insurance-covered services | 9,327 |
| Non-covered services | 481 |
| Direct non-medical costs | |
| Transportation costs | 14 |
| Indirect costs | |
| Cost of lost productivity | 5,970 |
| Total hospital admission costs | 15,791 |
| Outpatient visit | |
| Direct medical costs | |
| Insurance-covered services | 343 |
| Non-covered services | 18 |
| Direct non-medical costs | |
| Transportation costs | 66 |
| Indirect costs | |
| Cost of lost productivity | 210 |
| Total outpatient visit costs | 637 |
| Total | 16,428 |
All costs are in 2016 dollars.
DISCUSSION
This study has revealed epidemiological aspects of GBS in South Korea using nationwide population-based data from 2010 to 2016. On average there were 1.48 GBS patients per 100,000 population annually, which is higher than the rate found in an earlier study (1.24 GBS cases per 100,000 person-years) conducted from 2011 to 2015 in Korea.31 The GBS cases in that previous study were identified from administrative data in the NHIS database for patients registered for co-payment assistance for rare and incurable diseases. That database only includes patients who met the diagnostic criteria on the basis of comprehensive medical tests, which would have resulted in very conservative estimates of incidence rates.
The incidence of GBS varies across countries. A median incidence rate of 1.11 GBS cases per 100,000 person-years was reported for a systematic review of 16 studies performed in 7 Western countries,11 which is lower than the incidence rate in Korea. Taiwan showed a higher incidence rate (1.65 per 100,000 person-years) than Korea,17 whereas those in the Harbin and Jiangsu provinces of China were 0.66 per 100,000 person-years18 and 0.59 per 100,000 person-years,32 respectively, and Japan also showed a lower incidence rate (1.14 per 100,000 person-years) than Korea.19 Our results indicate that Korea is one of the countries with a relatively high GBS incidence rates. These differences in the incidence rates across countries might be attributable to differences in insurance coverage for diagnostic tests, accessibility to medical services, and the epidemiological characteristics of the populations. Koreans generally have easier accessibility to medical services and higher medical standards relative to other countries. Nerve conduction studies (NCSs) and diagnostic tests using cerebrospinal fluid (CSF) and intravenous IVIg—which are major diagnostic tests and treatment for GBS, respectively—are covered by the NHIS. Furthermore, the differences in the clinical features of GBS,20 predominant risk factors, and demographic characteristics in Korea could contribute to its higher incidence rate.
Over the 7-year study period, the incidence rate and number of GBS patients gradually increased by 39% and 45%, respectively, which are comparable with the results of earlier cohort studies: nearly 40% in a 15-year study in Taiwan,17 and 10% in a 10-year study in the USA.33 The increasing incidence of GBS could be due to an increase in its actual occurrence or to other causes, such as an improved detection rate resulting from advances in the diagnostic tests. GBS is mainly diagnosed based on the clinical presentation of patients and the findings of diagnostic tests using CSF or an NCS. To the best of our knowledge, no advanced diagnostic methods have been developed recently for detecting GBS. Thus, the most-plausible explanation is an improved awareness of GBS, partly influenced by the results of a recent study for the relationship between preceding infections or vaccinations and GBS incidence.17
With respect to the sex-specific incidence, GBS occurred 45% more in males than in females in Korea. This result is consistent with previous international studies finding male-to-female rate ratios of 1.41–1.52.13,33,34,35,36 This contrasts with most autoimmune diseases having a preponderance of females, and the reason for the difference with GBS has not yet been identified.11
We found that 72% of GBS patients had antecedent infections, which is similar to or slightly lower than previous findings.16,37 We found that the proportion was highest (94%) in those younger than 10 years, 89% of whom showed evidence of respiratory infection prior to GBS. It is known that recognizing GBS in this age group can be delayed due to nonspecific clinical symptoms and the difficulty of clinical examinations.38 Therefore, careful monitoring after respiratory infections are diagnosed in young children can facilitate the prevention and prompt detection of GBS.
Since the average income level of GBS patients was lower than that of the general population in Korea, the treatment costs of GBS should be a considerable burden for patients. This study found that the average cost of treating GBS during the year after the onset was USD 16,428. One previous study in the USA estimated that the economic burden per patient during the year after the onset was USD 74,010 (in 2016 dollars).39 That cost was obtained from claims data and panel surveys, and comprised medical costs, lost-productivity costs, and premature-death costs. A study from France estimated the economic burden during the first year for different severity levels of GBS. The cost was even higher in France than it was in the USA, ranging from USD 83,707 for mild GBS to US$ 123,780 for severe GBS (in 2016 dollars).40 The total cost only comprised medical costs and the costs of lost productivity, and data were obtained from clinical trials. The economic burden of GBS in Korea is only 13–22% of those in USA and France, which could be explained by difference in the cost components included in each study, as well as the levels of medical charges and practice patterns in each country.
To the best of our knowledge, this is one of the few epidemiological studies to have analyzed the incidence and economic burden of GBS based on a representative national health insurance claims database. The NHIS database covers 97% of Korean population, and hence its utilization enabled more-reliable and accurate analyses compared with using other data sources that typical cover only a few hospitals. In addition, this study has revealed an important economic profile of GBS treatment in Korea, which may allow real-world evidence to be integrated into local policy-making.
Several methodological limitations arise from studies that use retrospective claims data. Since GBS is a clinical diagnosis, determining the presence of GBS using claims data may be less specific and overestimate the true incidence.41 Electrophysiological tests or CSF examinations are used to clinically diagnose GBS, with specific cases being ascertained using Briton collaboration criteria or the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) criteria. In our study we employed a conservative approach in order to minimize misclassification errors in identifying incident cases of GBS, by using the optimal case definition suggested by a previous study that GBS be defined only as hospitalized cases, with a primary diagnosis of GBS made by a neurologist.38 We defined incident GBS as a hospitalized case with a primary diagnosis of GBS, and no claim records having diagnosis codes of GBS or atypical forms of GBS within the year prior to the first day of the present hospital admission for GBS. Whether a neurologist assessed the patient could not be determined from claims data in our study. However, 94.9% of the GBS patients visited tertiary-care hospitals, most of which employ neurologists. Thus, the incidence rate identified by our study is considered to be close to the true incidence.
Another limitation of the present study was that it did not examine the incidence rates of different subtypes. Since the predominant forms of preceding infection vary among GBS subtypes, identifying subtypes in accordance with predominant forms of infections will further improve GBS prognoses.
Lastly, by applying the conservative case definition of GBS, this study only included classic forms of GBS. Miller Fisher syndrome and other clinical variants that are becoming more common in Asia (overlap syndrome and plus-or-minus syndrome) were not included. Future studies should further examine these subtypes and variants.
In conclusion, this nationwide epidemiological study found that the annual incidence rate of GBS in Korea from 2010 to 2016 was 1.48 patients per 100,000 population. Antecedent infection (mainly respiratory infection) was present in 72% of GBS patients, and the number of infections per patient decreased with age. The economic burden from a societal perspective of treating each GBS patient during the first year was USD 16,428.
Acknowledgements
This work was supported in part by a grant from Korea Centers for Disease Control & Prevention (grant number 2018-E2402-00). The funding source had no involvement in the study design; the collection, analysis and interpretation of data; the writing of the report; or the decision to submit the article for publication.
Footnotes
- Methodology: Ah-Young Kim, Hankil Lee, Hye-Young Kang.
- Project administration: Young-Mock Lee.
- Writing—original draft: Ah-Young Kim.
- Writing—review & editing: Ah-Young Kim, Hye-Young Kang, Young-Mock Lee.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Ho TW, Li CY, Cornblath DR, Gao CY, Asbury AK, Griffin JW, et al. Patterns of recovery in the Guillain-Barre syndromes. Neurology. 1997;48:695–700. doi: 10.1212/wnl.48.3.695. [DOI] [PubMed] [Google Scholar]
- 2.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 3.Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 4.Bersano A, Carpo M, Allaria S, Franciotta D, Citterio A, Nobile-Orazio E. Long term disability and social status change after Guillain-Barré syndrome. J Neurol. 2006;253:214–218. doi: 10.1007/s00415-005-0958-x. [DOI] [PubMed] [Google Scholar]
- 5.Hadden RD, Karch H, Hartung HP, Zielasek J, Weissbrich B, Schubert J, et al. Preceding infections, immune factors, and outcome in Guillain-Barré syndrome. Neurology. 2001;56:758–765. doi: 10.1212/wnl.56.6.758. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs BC, Rothbarth PH, van der Meché FG, Herbrink P, Schmitz PI, de Klerk MA, et al. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. 1998;51:1110–1115. doi: 10.1212/wnl.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 7.González-Suárez I, Sanz-Gallego I, Rodríguez de, Arpa J. Guillain-Barré syndrome: natural history and prognostic factors: a retrospective review of 106 cases. BMC Neurol. 2013;13:95. doi: 10.1186/1471-2377-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson BR, Zegarra JA, López-Gatell H, Sejvar J, Arzate F, Waterman S, et al. Binational outbreak of Guillain-Barré syndrome associated with Campylobacter jejuni infection, Mexico and USA, 2011. Epidemiol Infect. 2014;142:1089–1099. doi: 10.1017/S0950268813001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Souayah N, Michas-Martin PA, Nasar A, Krivitskaya N, Yacoub HA, Khan H, et al. Guillain-Barré syndrome after Gardasil vaccination: data from vaccine adverse event reporting system 2006–2009. Vaccine. 2011;29:886–889. doi: 10.1016/j.vaccine.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Tam CC, O'Brien SJ, Petersen I, Islam A, Hayward A, Rodrigues LC. Guillain-Barré syndrome and preceding infection with campylobacter, influenza and Epstein-Barr virus in the general practice research database. PLoS One. 2007;2:e344. doi: 10.1371/journal.pone.0000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36:123–133. doi: 10.1159/000324710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng KK, Howard RS, Fish DR, Hirsch NP, Wiles CM, Murray NM, et al. Management and outcome of severe Guillain-Barré syndrome. QJM. 1995;88:243–250. [PubMed] [Google Scholar]
- 13.Benedetti MD, Pugliatti M, D'Alessandro R, Beghi E, Chiò A, Logroscino G, et al. A multicentric prospective incidence study of Guillain-Barré syndrome in Italy. The ITANG Study. Neuroepidemiology. 2015;45:90–99. doi: 10.1159/000438752. [DOI] [PubMed] [Google Scholar]
- 14.Statistics Korea. Population Projections for Korea: population trends and projections of the world and Korea [Internet] Daejeon: Statistics Korea; [cited 2020 Feb 27]. Available from: http://kostat.go.kr/portal/eng/pressReleases/8/8/index.board?bmode=read&aSeq=347597&pag- [Google Scholar]
- 15.Kim JK, Bae JS, Kim DS, Kusunoki S, Kim JE, Kim JS, et al. Prevalence of anti-ganglioside antibodies and their clinical correlates with Guillain-Barré syndrome in Korea: a nationwide multicenter study. J Clin Neurol. 2014;10:94–100. doi: 10.3988/jcn.2014.10.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32:150–163. doi: 10.1159/000184748. [DOI] [PubMed] [Google Scholar]
- 17.Huang WC, Lu CL, Chen SC. A 15-year nationwide epidemiological analysis of Guillain-Barré syndrome in Taiwan. Neuroepidemiology. 2015;44:249–254. doi: 10.1159/000430917. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Q, Wang DS, Jiang GX, Han H, Zhang Y, Wang WZ, et al. Distinct pattern of age-specific incidence of Guillain-Barré syndrome in Harbin, China. J Neurol. 2002;249:25–32. doi: 10.1007/pl00007844. [DOI] [PubMed] [Google Scholar]
- 19.Kusumi M, Nakashima K, Nakayama H, Takahashi K. Epidemiology of inflammatory neurological and inflammatory neuromuscular diseases in Tottori Prefecture, Japan. Psychiatry Clin Neurosci. 1995;49:169–174. doi: 10.1111/j.1440-1819.1995.tb02223.x. [DOI] [PubMed] [Google Scholar]
- 20.Bae JS, Yuki N, Kuwabara S, Kim JK, Vucic S, Lin CS, et al. Guillain-Barré syndrome in Asia. J Neurol Neurosurg Psychiatry. 2014;85:907–913. doi: 10.1136/jnnp-2013-306212. [DOI] [PubMed] [Google Scholar]
- 21.Cho H, Choi J, Kim YS, Son SJ, Lee KS, Hwang HJ, et al. Prevalence and predictors of potentially inappropriate prescribing of central nervous system and psychotropic drugs among elderly patients: a national population study in Korea. Arch Gerontol Geriatr. 2018;74:1–8. doi: 10.1016/j.archger.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Chiò A, Cocito D, Leone M, Giordana MT, Mora G, Mutani R, et al. Guillain-Barré syndrome: a prospective, population-based incidence and outcome survey. Neurology. 2003;60:1146–1150. doi: 10.1212/01.wnl.0000055091.96905.d0. [DOI] [PubMed] [Google Scholar]
- 23.Van Koningsveld R, Van Doorn PA, Schmitz PI, Ang CW, Van der Meché FG. Mild forms of Guillain-Barré syndrome in an epidemiologic survey in The Netherlands. Neurology. 2000;54:620–625. doi: 10.1212/wnl.54.3.620. [DOI] [PubMed] [Google Scholar]
- 24.Kwong JC, Vasa PP, Campitelli MA, Hawken S, Wilson K, Rosella LC, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis. 2013;13:769–776. doi: 10.1016/S1473-3099(13)70104-X. [DOI] [PubMed] [Google Scholar]
- 25.Kang JH, Sheu JJ, Lin HC. Increased risk of Guillain-Barré syndrome following recent herpes zoster: a population-based study across Taiwan. Clin Infect Dis. 2010;51:525–530. doi: 10.1086/655136. [DOI] [PubMed] [Google Scholar]
- 26.Mori M, Kuwabara S, Miyake M, Noda M, Kuroki H, Kanno H, et al. Haemophilus influenzae infection and Guillain-Barré syndrome. Brain. 2000;123(Pt 10):2171–2178. doi: 10.1093/brain/123.10.2171. [DOI] [PubMed] [Google Scholar]
- 27.Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012;366:2294–2304. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- 28.National Health Insurance Service. The 2017 survey of medical expenses for medical services frequently provided. Wonju: National Health Insurance Service; 2018. [Google Scholar]
- 29.Korea Centers for Disease Control and Prevention. The Third Korea National Health and Nutrition Examination Survey (KNHANES III), 2005 [Internet] Cheongju: Korea Centers for Disease Control and Prevention; [cited 2020 Feb 26]. Available from: https://knhanes.cdc.go.kr/knhanes/sub03/sub03_02_05.do. [Google Scholar]
- 30.Alshekhlee A, Hussain Z, Sultan B, Katirji B. Guillain-Barré syndrome: incidence and mortality rates in US hospitals. Neurology. 2008;70:1608–1613. doi: 10.1212/01.wnl.0000310983.38724.d4. [DOI] [PubMed] [Google Scholar]
- 31.Lim SS, Lee W, Kim YK, Kim J, Park JH, Park BR, et al. The cumulative incidence and trends of rare diseases in South Korea: a nation-wide study of the administrative data from the National Health Insurance Service database from 2011–2015. Orphanet J Rare Dis. 2019;14:49. doi: 10.1186/s13023-019-1032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Ma F, Zhang J, Chu X, Xu Y. Population incidence of Guillain-Barré syndrome in parts of China: three large populations in Jiangsu province, 2008–2010. Eur J Neurol. 2014;21:124–129. doi: 10.1111/ene.12265. [DOI] [PubMed] [Google Scholar]
- 33.Shui IM, Rett MD, Weintraub E, Marcy M, Amato AA, Sheikh SI, et al. Guillain-Barré syndrome incidence in a large United States cohort (2000–2009) Neuroepidemiology. 2012;39:109–115. doi: 10.1159/000339248. [DOI] [PubMed] [Google Scholar]
- 34.Hense S, Schink T, Kreisel SH, Marcelon L, Simondon F, Tahden M, et al. Estimation of background incidence rates of Guillain-Barré syndrome in Germany - a retrospective cohort study with electronic healthcare data. Neuroepidemiology. 2014;43:244–252. doi: 10.1159/000369344. [DOI] [PubMed] [Google Scholar]
- 35.Rivera-Lillo G, Torres-Castro R, Burgos PI, Varas-Díaz G, Vera-Uribe R, Puppo H, et al. Incidence of Guillain-Barré syndrome in Chile: a population-based study. J Peripher Nerv Syst. 2016;21:339–344. doi: 10.1111/jns.12182. [DOI] [PubMed] [Google Scholar]
- 36.Delannoy A, Rudant J, Chaignot C, Bolgert F, Mikaeloff Y, Weill A. Guillain-Barré syndrome in France: a nationwide epidemiological analysis based on hospital discharge data (2008–2013) J Peripher Nerv Syst. 2017;22:51–58. doi: 10.1111/jns.12202. [DOI] [PubMed] [Google Scholar]
- 37.Winer JB. Guillain-Barré syndrome. BMJ. 2008;337:a671. doi: 10.1136/bmj.a671. [DOI] [PubMed] [Google Scholar]
- 38.Roodbol J, de Wit MC, Walgaard C, de Hoog M, Catsman-Berrevoets CE, Jacobs BC. Recognizing Guillain-Barre syndrome in preschool children. Neurology. 2011;76:807–810. doi: 10.1212/WNL.0b013e31820e7b62. [DOI] [PubMed] [Google Scholar]
- 39.Frenzen PD. Economic cost of Guillain-Barré syndrome in the United States. Neurology. 2008;71:21–27. doi: 10.1212/01.wnl.0000316393.54258.d1. [DOI] [PubMed] [Google Scholar]
- 40.Espérou H, Jars-Guincestre MC, Bolgert F, Raphaël JC, Durand-Zaleski I. Cost analysis of plasma-exchange therapy for the treatment of Guillain-Barré syndrome. French Cooperative Group on plasma exchange in Guillain-Barré syndrome. Intensive Care Med. 2000;26:1094–1100. doi: 10.1007/s001340051323. [DOI] [PubMed] [Google Scholar]
- 41.Funch D, Holick C, Velentgas P, Clifford R, Wahl PM, McMahill-Walraven C, et al. Algorithms for identification of Guillain-Barré syndrome among adolescents in claims databases. Vaccine. 2013;31:2075–2079. doi: 10.1016/j.vaccine.2013.02.009. [DOI] [PubMed] [Google Scholar]