Abstract
Background and Purpose
Brainstem gliomas (BSGs) in adults are rare brain tumors with dismal outcomes. The aim of this study was to determine the clinical and genetic features in a series of BSGs and their association with the prognosis.
Methods
Fifty patients who underwent a stereotactic biopsy between January 2016 and April 2018 at a single institution were collected. Data on clinicopathological characteristics were analyzed and factors associated with patient survival were identified using a Cox regression model.
Results
The median age at diagnosis was 55.5 years, and 62% of the patients were male. Glioblastoma (44%) accounted for the largest proportion of BSGs, and oligodendroglioma (2 of 50) was rarely encountered. The IDH mutation (6 of 44) occurred infrequently in astrocytomas, and IDH-mutant tumors harbored both ATRX loss and MGMT promoter methylation at a relatively low level. Wild-type IDH astrocytomas were identified as having high rates of 1p/19q codeletion (5 of 38) and loss of heterozygosity 1p (8 of 38) or 19q (8 of 38) only. In diffuse midline glioma H3K27M mutant, MGMT promoter methylation occurred in three of four cases. Patients were offered radiotherapy and/or concurrent/adjuvant temozolomide chemotherapy, and their median survival time was 13 months. Multivariate analysis revealed that a low tumor grade, absence of tumor enhancement, duration of symptoms ≥3 months, Karnofsky performance status ≥70, and ATRX loss conferred a survival advantage.
Conclusions
Adult BSGs showed different molecular genetic characteristics, but also resembled supratentorial gliomas in their clinical features associated with oncological outcomes.
Keywords: brain stem neoplasms, glioma, molecular epidemiology, prognosis
INTRODUCTION
Brainstem gliomas (BSGs) are relatively rare tumors of the central nervous system that are more common in children,1 accounting for less than 2% of adult gliomas.2,3 Imaging findings show that adult BSGs are most frequently identified in the pons (60–63%) and also occur in the medulla oblongata (25%) and the midbrain (12–15%), with a combination of these brain structures being involved in most cases.3 Based on radiographic characteristics, adult BSGs have been subdivided into diffuse intrinsic, focal, and exophytically growing tumors, among which infiltrative gliomas represent the most common pathological subtype.4,5 Due to the challenging location, the scope of surgical treatment for these infiltrative tumors is limited to stereotactic biopsy. Treatment efforts have focused on investigating adjuvant therapies, such as radiotherapy and chemotherapy.6 Although the prognoses have been improving, adult patients with high-grade BSG still face a dismal course, with the median survival time reported to range from 5.7 months to 16 months.6,7 Moreover, few studies have focused on the biological characteristics, which has hampered the development of therapeutic strategies and improvements in the survival of patients.
The above-described situation indicates that further investigations of intrinsic traits in adult BSGs are urgently needed. The main purposes of this study were to determine the clinical and pathological features of adult BSGs and identify prognostic factors influencing overall survival (OS), with the aim of improving the management of these patients.
METHODS
Patients and specimens
Patients were collected at the Sixth Medical Center of Chinese PLA General Hospital between January 2016 and April 2018. These patients visited our center by themselves or were referred from other hospitals in Beijing, China. Due to the diffusely infiltrative nature of the neoplasm, the participants were not candidates for tumor resection, but all underwent MRI-guided or CT-guided stereotactic biopsy. Sufficient tumor specimens were obtained for pathological diagnose, while part of each tissue sample was sent out fresh for the DNA detection of a gene panel (Supplementary Table 1 in the online-only Data Supplement) as dictated by the institutional pathology protocol. Additionally, the status of MGMT promoter methylation was detected by bisulfite sequencing.
Subjects were included in our study when they met the following criteria: 1) radiological imaging showing that the center of the tumor bulk intrinsically resided in the brainstem, and 2) age of ≥18 years at the time of the initial diagnosis. Patients were not eligible if they presented with a history of other tumors or received chemotherapy or had radiotherapy prior to the biopsy. Brainstem diffuse astrocytic and oligodendroglial tumors were diagnosed by two experienced pathologists independently according to the 2016 World Health Organization (WHO) Classification of Tumors of the Central Nervous System.
Other clinical data were obtained from the institutional medical database, including: 1) age at the time of diagnosis, 2) sex, 3) main symptoms and their duration, 4) Karnofsky performance status (KPS), 5) location of tumor epicenter, 6) MRI findings (tumor size and tumor enhancement), 7) postoperative treatment, and 8) length of follow-up.
The study protocol was approved by the Tissue Committee and Research Ethics Board of Chinese PLA General Hospital (NGH-01581-C00712). All patients or their legal guardians signed the written informed-consent form about the acquisition and use of human tissue in this study, which compiled with the National Regulations of Clinical Samples in China.
Statistical analysis
The clinical data are described statistically as percentages, means, and medians. Complete follow-up information was recorded. OS was calculated in months from the initial date of diagnosis to the time of death, regardless of cause. Survival was estimated using the Kaplan-Meier method, and variables related to survival were analyzed using log-rank test. Factors for which p<0.2 in the univariate analysis were included in the analysis performed using the multivariate Cox stepwise regression model.
All analyses were conducted using the SPSS software program (versions 18.0, SPSS, Chicago, IL, USA). A p value of <0.05 was considered statistically significant.
RESULTS
Male sex and grade IV tumors predominate in adult BSGs
Fifty patients with diffusely infiltrative BSG were eligible for inclusion in the analysis (Table 1). According to the WHO classification, 52% of the tumors were grade IV [22 glioblastoma (GBM) and 4 diffuse midline glioma (DMLG) H3K27M-mutant (H3K27M-mut)], 24% (n=12) were grade III anaplastic astrocytoma (AA), 22% (n=11) were grade II diffuse astrocytoma (n=9) or oligodendroglioma (n=2), and only 1 case was diagnosed as grade I pilocytic astrocytoma. The age at the time of diagnosis was 53.6±16.9 years (mean±SD; range from 24–93 years), and 62% of the patients were male (n=31). Most of the BSGs were centered in the pons (80%, n=40), 7 tumors were in the midbrain, and the remaining 3 cases mainly involved the medulla oblongata. Moreover, at least two brainstem structures were invaded in 40% of the patients.
Table 1. Clinical features of 50 brainstem gliomas in adult.
| Grade | Type (number) | Sex | Age (years) | Duration of symptoms (months) | KPS | Tumor epicenter | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ≥55 | <55 | ≥3 | <3 | ≥70 | <70 | Midbrain | Pons | Medulla | ||
| WHO IV | GBM (22) | 14 (63.6) | 8 (36.4) | 16 (72.7) | 6 (27.3) | 8 (36.4) | 14 (63.6) | 12 (54.5) | 10 (45.5) | 2 (9.1) | 18 (81.8) | 2 (9.1) |
| DMLG (4) | 3 (75.0) | 1 (25.0) | 3 (75.0) | 1 (25.0) | 2 (50.0) | 2 (50.0) | 2 (50.0) | 2 (50.0) | 2 (50.0) | 2 (50.0) | 0 (0) | |
| WHO III | AA (12) | 7 (58.3) | 5 (41.7) | 8 (66.7) | 4 (33.3) | 9 (75.0) | 3 (25.0) | 7 (58.3) | 5 (41.7) | 1 (8.3) | 10 (83.4) | 1 (8.3) |
| WHO II | DA (9) | 5 (55.6) | 4 (44.4) | 2 (22.2) | 7 (77.8) | 6 (66.7) | 3 (33.3) | 9 (100.0) | 0 (0) | 1 (11.1) | 8 (88.9) | 0 (0) |
| O (2) | 1 (50.0) | 1 (50.0) | 0 (0) | 2 (100.0) | 2 (100.0) | 0 (0) | 1 (50.0) | 1 (50.0) | 0 (0) | 2 (100.0) | 0 (0) | |
| WHO I | PA (1) | 1 (100.0) | 0 (0) | 0 (0) | 1 (100.0) | 1 (100.0) | 0 (0) | 1 (100.0) | 0 (0) | 1 (100.0) | 0 (0) | 0 (0) |
| Total, % | 62 | 38 | 58 | 42 | 56 | 44 | 64 | 36 | 14 | 80 | 6 | |
| Grade | Type (number) | Tumor enhancement | Diameter (cm) | Radiotherapy | Chemotherapy | Status | Median OS (months) | |||||
| Yes | No | ≥2 | <2 | Yes | No | Yes | No | Dead | Alive | |||
| WHO IV | GBM (22) | 14 (63.6) | 8 (36.4) | 10 (45.5) | 12 (54.5) | 22 (100.0) | 0 (0) | 22 (100.0) | 0 (0) | 21 (98.0) | 1 (2.0) | 9.5 |
| DMLG (4) | 2 (50.0) | 2 (50.0) | 2 (50.0) | 2 (50.0) | 4 (100.0) | 0 (0) | 4 (100.0) | 0 (0) | 4 (100.0) | 0 (0) | 7.5 | |
| WHO III | AA (12) | 6 (50.0) | 6 (50.0) | 3 (25.0) | 9 (75.0) | 12 (100.0) | 0 (0) | 12 (100.0) | 0 (0) | 10 (83.3) | 2 (16.7) | 17.5 |
| WHO II | DA (9) | 4 (45.4) | 5 (55.6) | 5 (55.6) | 4 (45.4) | 9 (100.0) | 0 (0) | 5 (55.6) | 4 (45.4) | 7 (77.8) | 2 (22.2) | 32.0 |
| O (2) | 0 (0) | 2 (100.0) | 2 (100.0) | 0 (0) | 2 (100.0) | 0 (0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | 37.0 | |
| WHO I | PA (1) | 0 (0) | 1 (100.0) | 0 (0) | 1 (100.0) | 1 (100.0) | 0 (0) | 0 (0) | 1 (100.0) | 0 (0) | 1 (100.0) | 38.0 |
| Total, % | 52 | 48 | 44 | 56 | 100 | 0 | 88 | 12 | 86 | 14 | ||
Data are presented as n (%).
AA: anaplastic astrocytoma, DA: diffuse astrocytoma, DMLG: diffuse midline glioma, GBM: glioblastoma, KPS: Karnofsky performance status, O: oligodendroglioma, OS: overall survival, PA: pilocytic astrocytoma, WHO: World Health Organization.
The medical histories indicated that the median duration of symptoms was 3 months (range=0.5–25 months). The presenting signs and symptoms mainly included cranial nerve dysfunction and long-tract signs. Headaches and other manifestations caused by obstructive hydrocephalus were also observed. The median KPS at diagnosis was 80 (range=40–100), and 32 participants had a KPS of >70. MRI showed contrast-enhanced masses in 52% of the BSGs (n=26), and the maximum diameter of the tumors ranged from 9.9 mm to 29.3 mm, with a median of 19.4 mm.
Wild-type IDH astrocytomas account for the vast majority of adult BSGs and present with a high rate of loss of heterozygosity of 1p and/or 19q
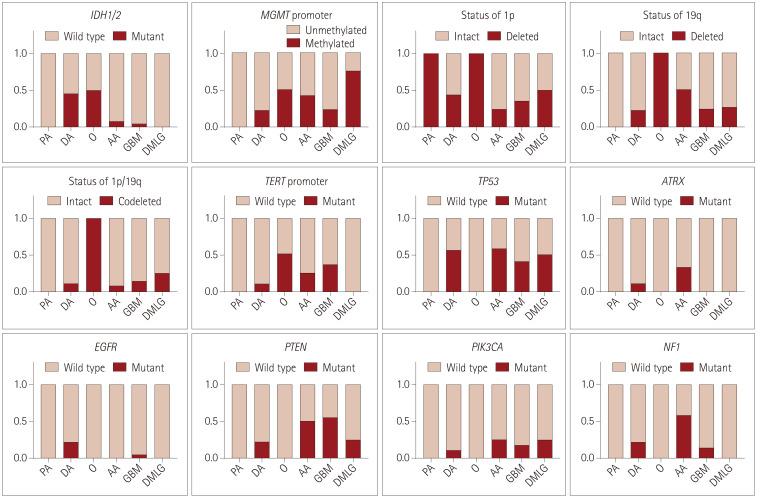
Gene panel sequencing revealed that IDH1/2 mutation occurred in 6 of 44 astrocytic gliomas and 1 of 2 oligodendroglial tumors, but in no DMLG H3K27M-mut (Fig. 1).
Fig. 1. Common genetic alterations detected in BSGs. The proportion of each genetic alteration is shown for different BSG subtypes. AA: anaplastic astrocytoma, BSG: brainstem glioma, DA: diffuse astrocytoma, DMLG: diffuse midline glioma, GBM: glioblastoma, O: oligodendroglioma, PA: pilocytic astrocytoma.
In the IDH mutant (IDH-mut) astrocytomas, TP53 mutation occurred in five cases and only two tumors harbored both ATRX loss and MGMT promoter methylation. Among this subgroup, an AA with an R172S mutation in IDH2 showed TP53 mutation, ATRX loss, MGMT promoter methylation and PIK3CA mutation, while three of the other five IDH1-R132H mutated astrocytomas had a loss of heterozygosity (LOH) of 1p only (Supplementary Table 2 in the online-only Data Supplement).
In the wild-type IDH astrocytic subgroup, 18 of 38 tumors carried a TP53 mutation, and 12 cases harbored a TERT promoter C228T/C250T mutation, but only 3 were identified with ATRX loss. ATRX loss and TERT promoter mutation were mutually exclusive. MGMT promoter methylated tumors accounted for 26.3% (10 of 38) of this subgroup. More than half of the patients (21 of 38) in this subgroup harbored LOH of 1p or 19q, and codeletion of 1p/19q was identified in 5 of these cases. PTEN and NF1 mutations were found in 10 and 12 wild-type IDH astrocytomas, respectively. EGFR, RB1, and PIK3CA mutations were identified in three, three and seven tumors, respectively. Either MSH2 or MSH6 mutation was identified in four cases, all of whom also carried a TP53 mutation. EGFR amplification occurred in seven astrocytic gliomas with a median amplification time of 7.1, and three of these tumors also exhibited CDK4 amplification. Amplifications of CDK4, PDGFRA, and MET were presented in five, three, and two cases, respectively.
In the subgroup of oligodendrogliomas, the wild-type IDH and 1p/19q codeleted tumor did not display any common genetic alterations, while another IDH-mut and 1p/19q codeleted one harbored TERT promoter mutation and MGMT promoter methylation.
In the subgroup of DMLG H3K27M-mut, no mutations of IDH1/2, ATRX, or TERT promoter were found. MGMT promoter methylation was present in three of four cases, of which one harbored TP53 mutation, PTEN mutation, and PDGFRA amplification with a change of 10.6-fold, and the other two carried LOH of 1p and codeletion of 1p/19q. The MGMT promoter unmethylated tumor showed of a TP53 mutation.
No mutations in BRAF V600E, CDK4, CDK6, CDKN2A, CDKN2B, MYCN, or PMS2 and no gene rearrangement in BRAF, FGFR3, RELA, or YAP1 were found in any of the tumors in our cohort. Common genetic alterations in each pathological subtype are listed in Table 2, and the details of the genetic findings are also given in Supplementary Table 2 (in the online-only Data Supplement).
Table 2. Common genetic alterations in adult brainstem gliomas and supratentorial gliomas.
| Pathological types | MGMT promoter methylation | TERT promoter mutation | ATRX loss | TP53 mutation | PTEN mutation | Codeletion of 1p and 19q | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Ref.10 | Cohort | Ref.10 | Cohort | Ref.10 | Cohort | Ref.11 | Cohort | Ref.11 | Cohort | Ref.11 | |
| GBM, IDH-wt | 24 (5/21) | ~40 | 38 (8/21) | >75 | 0 (0/21) | 3 | 38 (8/21) | 20 | 57 (12/21) | 24 | 14 (3/21) | 0 |
| GBM, IDH-mut | 0 (0/1) | ~90 | 0 (0/1) | 12 | 0 (0/1) | 78 | 100 (1/1) | 83 | 0 (0/1) | 0 | 0 (0/1) | 50 |
| Astrocytoma (grade II and III), IDH-wt | 31 (5/16) | ~55 | 25 (4/16) | >60 | 19 (3/16) | 12 | 50 (8/16) | 20 | 50 (8/16) | 23 | 13 (2/16) | 11 |
| Astrocytoma (grade II and III), IDH-mut | 40 (2/5) | ~85 | 0 (0/5) | 5 | 40 (2/5) | 63 | 80 (4/5) | 69 | 0 (0/5) | 0 | 0 (0/5) | 5 |
| Oligodendroglioma (grade II and III) | 50 (1/2) | ~65-100 | 50 (1/2) | 94 | 0 (0/2) | 3 | 0 (0/2) | 13 | 0 (0/2) | 0 | ||
| DMLG | 75 (3/4) | 0 (0/4) | 0 (0/4) | 50 (2/4) | 25 (1/4) | 25 (1/4) | ||||||
Data are presented as % (n).
GBM: glioblastoma, DMLG: diffuse midline glioma, mut: mutant, Ref: reference, wt: wild type.
Adult patients with BSGs suffer undesirable outcomes
All the patients underwent a stereotactic biopsy, and those patients who developed symptomatic hydrocephalus received a ventriculo-peritoneal shunt at the diagnosis or during follow-up. When the diagnosis was made, all BSG patients were offered 30 cycles of radiotherapy with a total dose of 54–60 Gy, and patients with wild-type IDH1/2 grade II tumor or high-grade glioma (HGG) were also suggested to receive temozolomide chemotherapy (Stupp protocol). The 50 patients were followed up for 0.5 months to 42 months (median=13.3 months), and 43 patients had died at the final follow-up. The median OS of all adult BSGs was 13 months.
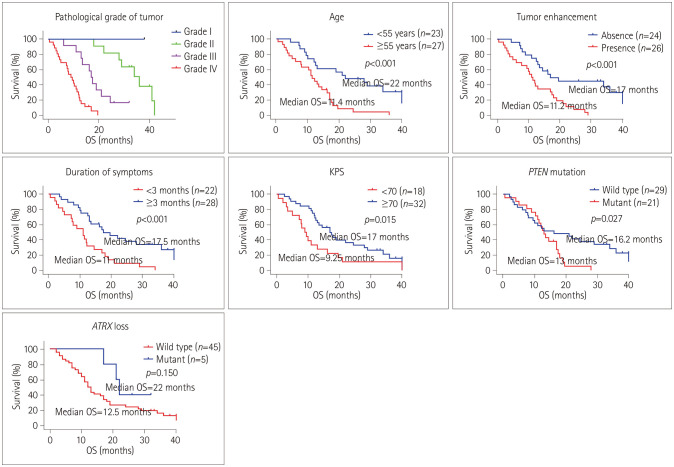
The OS differed significantly between with the pathological grade, being shorter in grade IV (median=9 months) than in grade III (median=17.5 months) and grade II (median=33 months) (Fig. 2). Kaplan-Meier log rank testing also showed that age ≥55 years, KPS <70, HGG, presence of tumor enhancement, duration of symptoms <3 months, temozolomide chemotherapy and PTEN mutation were associated with a worse prognosis (Fig. 2). However, sex, tumor location, tumor diameter, MGMT promoter methylation, IDH1/2 mutation, LOH of 1p/19q, TERT promoter mutation, TP53 mutation, EGFR amplification, NF1 mutation, and PIK3CA mutation had no significant impact on survival in the adult BSG patients. After adjusting for the confounding effects of each variable in the multivariate analysis, low tumor grade, absence of tumor enhancement, duration of symptoms ≥3 months, and KPS ≥70 conferred a survival advantage for patients (Table 3). Although not significant in the univariate analysis, ATRX loss was also identified as an independent factor predicting a favorable prognosis (Table 3).
Fig. 2. Clinicopathological factors associated with OS of brainstem glioma patients. Kaplan-Meier curves were used to analyze the relationships of age, KPS, tumor grade, tumor enhancement, duration of symptoms, PTEN mutation, and ATRX loss with the OS of all patients. OS: overall survival, KPS: Karnofsky performance status.
Table 3. Results of univariate and multivariate analyses of factors associated with OS of 50 patients with brainstem gliomas.
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| p | HR | 95% CI | Median OS (months) | p | |
| Age (≥55 years vs. <55 years) | 0.001 | 11.4 vs. 22.0 | 0.197 | ||
| Sex (female vs. male) | 0.578 | 14.5 vs. 13.0 | |||
| Grade (HGG vs. LGG) | <0.001 | 15.109 | 3.604–63.335 | 11.0 vs. 36.0 | <0.001 |
| KPS (≥70 vs. <70) | 0.015 | 0.317 | 0.147–0.686 | 17.0 vs. 9.0 | 0.004 |
| Course of disease (≥3 months vs. <3 months) | 0.001 | 0.401 | 0.201–0.802 | 17.5 vs. 11.0 | 0.010 |
| Diameter (≥2 cm vs. <2 cm) | 0.729 | 12.0 vs. 14.5 | |||
| Location (pons vs. midbrain vs. medulla) | 0.989 | 13.5 vs. 13.0 vs. 12.2 | |||
| Tumor enhancement (yes vs. no) | 0.001 | 2.892 | 1.355–6.173 | 11.0 vs. 17.0 | 0.006 |
| Chemotherapy (yes vs. no) | 0.012 | 12.2 vs. 34.0 | 0.424 | ||
| IDH (mutant vs. wild type) | 0.107 | 29.0 vs. 12.5 | 0.328 | ||
| MGMT promoter (methylated vs. unmethylated) | 0.508 | 16.2 vs. 13.0 | |||
| 1p/19q (LOH vs. intact) | 0.460 | 11.4 vs. 13.0 | |||
| TERT promoter (mutant vs. wild type) | 0.656 | 11.4 vs. 13.5 | |||
| ATRX (mutant vs. wild type) | 0.150 | 0.107 | 0.027–0.418 | 22.0 vs. 12.5 | 0.001 |
| TP53 (mutant vs. wild type) | 0.977 | 13.0 vs. 16.2 | |||
| PTEN (mutant vs. wild type) | 0.029 | 13.0 vs. 16.2 | 0.722 | ||
| NF1 (mutant vs. wild type) | 0.619 | 17.0 vs. 12.2 | |||
| PIK3CA (mutant vs. wild type) | 0.891 | 12.5 vs. 13.5 | |||
| EGFR amplification (yes vs. no) | 0.309 | 11.0 vs. 13.5 | |||
CI: confidence interval, HGG: high-grade glioma, HR: hazard ratio, KPS: Karnofsky performance status, LGG: low-grade glioma, LOH: loss of heterozygosity, OS: overall survival.
The findings of the subgroup analyses of the Cox model are presented in Supplementary Tables 3, 4, and 5 (in the online-only Data Supplement). These tables indicate that grade IV and tumor enhancement were independent risk predictors in patients with brainstem HGG, KPS<70, high tumor grade, duration of symptoms <3 months, tumor enhancement, and ATRX loss were independent risk predictors in brainstem astrocytoma patients; and absence of tumor enhancement was an independent factor predicting a favorable outcome in brainstem GBM patients.
DISCUSSION
This study retrospectively analyzed the general features and genetic characteristics of 50 adult BSGs at a single institution and identified prognostic factors that may be helpful in improving the management of these patients.
The anatomic characteristics of brainstem tumors mean that controversy remains about whether to perform a stereotactic biopsy procedure due to the presumed risk for histological diagnosis of lesions located in this area.8 A meta-analysis of 735 patients found that a stereotactic biopsy was valuable in diagnosing these patients, with a 96% success rate and a very low rate of procedure-related complications (0.6%).9 This was confirmed in the present study, since 50 adult BSG patients who underwent a stereotactic biopsy were accurately diagnosed, despite a glioma having been considered an intratumoral heterogeneous tumor, and the only complication was a minor hemorrhage in 1 patient. Moreover, the use of a stereotactic biopsy also allowed successful tissue sampling for molecular characterization, which primarily was incorporated into the definition of brain tumors according to the 2016 WHO classification. We can also briefly report on the distribution and genetic features of reclassified diffuse gliomas of the brainstem in adult patients.
Based on previous studies and the present findings, both supratentorial and brainstem GBMs constitute the majority of adult gliomas located in their respective areas.10 Consistent with the findings for supratentorial GBMs, IDH mutations (1 of 22, 4.5%) were rarely found in adult brainstem GBMs.11 Moreover, IDH mutations were infrequently seen in our brainstem WHO grade-II and -III diffuse gliomas (6 of 23, 26.1%), in contrast to rates of up to 70% in supratentorial WHO grade-II and -III astrocytic and oligodendroglial tumors.11 Several recent studies have suggested that non-IDH1-R132H mutations (IDH1-R132C/G and IDH2-R172S/G), which constitute only 5% of IDH mutations in adult supratentorial gliomas, occur more frequently in adult BSGs.12,13 In the present study, an R172S mutation in IDH2 was detected in one AA (WHO grade III). All of the IDH mutations were present in patients younger than 50 years, showing that our findings are consistent with previous reports of IDH mutations mainly occurring in young adults, and being rare in patients older than 55 years.14
Regarding astrocytomas, there is robust evidence that IDH mutations usually occur together with a loss-of-function mutation in TP53 and ATRX genes.15,16 We also found a high prevalence of TP53 mutation in the IDH-mut astrocytic group (5 of 6, 83.3%) resembling their supratentorial counterparts; however, a lower proportion of ATRX loss was detected in the IDH-mut astrocytomas (2 of 6, 33.3%) compared with that in cerebral astrocytomas (96%),13 indicating genetic differences between these two entities.17 Moreover, patients with ATRX loss had a longer OS in our group analyzed using the Cox model (Table 3 and Supplementary Table 4 in the online-only Data Supplement). Similar findings were obtained in cerebral gliomas, with ATRX loss identified as a subgroup of IDH-mut astrocytic tumors with a better prognosis and associated with a high survival rate in the GBM group.18,19 Given that all ATRX losses occurred in astrocytomas at grades II and III and were weakly related to IDH mutation in our cohort, the results suggest that ATRX alteration refines the classification of adult brainstem astrocytomas; this needs to be investigated further in a larger sample.
IDH mutations and codeletion of chromosomes 1p and 19q are associated with the oligodendroglial histological type, which is enriched for wild-type TP53 and TERT promoter mutation.17 However, this oligodendroglial entity is rarely encountered in the brainstem of either pediatric or adult patients,20,21 with only one eligible case found in our cohort. We might be able to report the second case of a brainstem oligodendroglioma with 1p/19q codeletion but wild-type IDH1/2,21 which showed different genetic alterations from the IDH-mut one and warranted further investigations. It is particularly interesting that 1p/19q codeletion was also found not to correspond to a diagnosis of oligodendroglioma. A retrospective study of 359 fibrillary astrocytomas found that 11 tumors (3.1%) had 1p/19q codeletion and only 1 of these cases demonstrated IDH1 mutation.22 In addition, 19 and 35 of the 359 cases harbored losses only on chromosomes 1p and 19q, respectively.22 Though 1p/19q codeletion is evidently rarely detected in adult BSGs, brainstem astrocytic gliomas with wild-type IDH in our study frequently had 1p/19q codeletion (5 of 38, 11.4%), which mainly occurred in GBM (3 of 21, 14%), and increased trends of LOH of 1p only (13 of 38, 25%) and LOH of 19q only (8 of 38, 18.2%) in the wild-type IDH subgroup and LOH of 1p only (3 of 6, 50%) in the IDH-mut subgroup were also found. Genomic instability might account for complete 1p/19q codeletion in GBM; moreover, our findings revealing oligodendroglioma components in a few GBM cases according to the 2007 morphological criteria might explain the high rate of codeleted 1p/19q. Besides, TP53 mutation appearing at a high rate (3 of 5, 60%) in the 1p/19q codeleted astrocytic tumors may help to avoid misclassification of such tumors as oligodendroglioma.23
More recently, landmark genomic studies have shown that diffuse intrinsic pontine glioma is driven by somatic mutations in histone H3, either H3.1 (HIST1H3B/C) or H3.3 variants (H3F3A), which defines distinct subgroup entities with different biological and clinical phenotypes and prognose.24,25 These histone H3 mutations rarely occur in supratentorial gliomas, but they have been detected in a much higher proportion of adult diffuse intrinsic BSGs, such as in 53.6% (15 of 28) of samples harboring H3F3A K27M mutations.12,26 Moreover, the presence of H3K27M mutation seemed to be strongly correlated with wild-type IDH and was potentially associated with a short survival time.26 We then identified four BSGs (accounting for only 8% of our cohort) as DMLG H3K27M-mut, and H3K27M and IDH mutations were mutually exclusive in the subgroup that showed a poor prognosis similar to GBM patients (median OS=7.5 months vs. 9.5 months). This subgroup could harbor mutations in TP53, PTEN, PIK3CA, PPM1D, and methylated MGMT promoter (Supplementary Table 2 in the online-only Data Supplement), as well as PDGFRA amplification that tended to confer a proneural phenotype and a prometastatic gene expression pattern in tumors with PDGFRA activation.24
Another important biological marker is MGMT promoter methylation, which has been reported in 30–80% of supratentorial gliomas, but it has not been adequately evaluated in adult BSGs.27,28 Negativity for MGMT expression was identified in 35.3% of 34 adult high-grade BSGs.27 Similarly, our study found MGMT promoter methylation in 25% of low-grade BSGs and approximately 34% of high-grade ones, indicating a low effective rate of response to alkylating agents such as temozolomide.29 Moreover, only two out of six IDH-mut astrocytomas presented with MGMT promoter methylation in our study, while about 96% of supratentorial IDH-mut astrocytomas displayed MGMT promoter methylation.13 Moreover, the rate of MGMT methylation was as high as 75% (three of four) in DMLG H3K27M-mut, which contrasts to previous reports of H3K27M-mut gliomas rarely (<5%) having a methylated MGMT promoter.30 This discrepancy is probably due to the smallness of our sample and the average proportion of methylated CpG sites in MGMT promoter being less than 20% in the four DMLG H3K27M-mut (3.25%, 11%, 18%, and 18%, with a cutoff value of 10%), in contrast with this proportion being up to 80–100% in other subtypes of gliomas. PI3K signaling is a critical regulator of glioma progression, and it is activated by mutations in the PI3KCA or PIK3R1 subunit and loss of tumor suppressor PTEN that is present in 15–40% of GBMs.31 In our cohort, the incidence of PTEN mutations was high in the GBM group (12 of 22, 54.5%) and the loss of PTEN was mutually exclusive with IDH mutations, pointing to the most aggressive type of glioma and the worst prognosis of the patients. The PTEN mutation was associated with a shorter OS of adult patients with BSG compared with wild-type PTEN (Fig. 2).
Furthermore, univariate analysis identified that several clinical factors were associated with a prolonged OS, including being younger than 55 years, low tumor grade, absence of tumor enhancement, duration of symptoms ≥3 months and KPS ≥70, and the latter four factors were seen to be favorable independent predictors of survival. Age <55 years was excluded from the multivariate analysis since high-grade tumors tended to occur in the older patients of our cohort. Previous studies of clinical prognostic factors of adult BSG patients have obtained consistent findings.32,33 Likewise, these factors have been demonstrated to be prognostically significant in predicting survival in supratentorial gliomas,34 indicating that the clinical course could be at least partly shared by gliomas located in different areas.
In contrast with therapeutic regimens containing surgery and postoperative radiotherapy being applied to most supratentorial gliomas, there is still no consensus on the effective treatments for adult BSGs. The role of resection (excluding a stereotactic biopsy that is mainly applied for diagnostic purposes) in the treatment of diffuse BSGs is controversial, due to the significant morbidity and mortality associated with operations in the highly eloquent region. With the application of electrophysiological monitoring, navigation, and neuroimaging, the microsurgical resection of such lesions via a brainstem safe entry zone may become feasible.
A recent study of 502 adult brainstem HGGs from the Surveillance, Epidemiology, and End Results Program (SEER) database concluded that for surgically accessible tumors, partial resection and gross total resection are associated with three- and fourfold increases in OS, respectively, relative to biopsy only.7 However, the value of resection in improving the survival of adult BSG patients needs further verification. Radiotherapy with concurrent or adjuvant chemotherapy is currently the most commonly treatment.
Another analysis based on the National Cancer Database collected 422 adult patients with high-grade BSG, and found that median OS was longer for radio-chemotherapy (14.2 months) than radiation alone (5.7 months) and no postoperative treatment (1.8 months).6 However, we found that the median OS was shorter in patients receiving temozolomide than that in patients without chemotherapy (12.2 months vs. 34 months), which was mainly due to all high-grade and wild-type IDH low-grade gliomas being in the chemotherapy group. Despite receiving these treatments, the conditions of the patients deteriorated, and so novel therapies such as targeted therapy and immunotherapy are required to prolong their survival in the future.
Some limitations of this study need to be considered. Firstly, this was a retrospective study and so its findings are not as reliable as those of a randomized controlled trial. Secondly, the total number of participants is small and the pathological types were highly heterogeneous, with both of these features reducing the power of the statistical analyses, especially for the multivariate models. Thirdly, adjuvant treatments such as targeted therapy and immunotherapy were not applied to these patients, and so their effects could not be evaluated in this study.
It was conclusively found that adult BSGs have different molecular genetic characteristics (Table 2), but also that some of their clinical features associated with the oncological prognosis partly resemble those of supratentorial gliomas.34 Further studies including large populations are needed to explore more comprehensive profiles of adult BSGs and incorporate specific markers when stratifying patients and developing therapeutic regimens, in order to improve the OS of this population.
Acknowledgements
This study was supported by the Specialized Research Program of Capital Health Development (Grant No. 2020-4-5116).
Footnotes
- Conceptualization: Jianning Zhang, Chunhui Zhou.
- Data curation: Fan Yang, Hao Zhao.
- Formal analysis: Fan Yang, Chao Dong.
- Funding acquisition: Jianning Zhang.
- Investigation: Luokai Huangfu, Hao Zhao.
- Methodology: Chunhui Zhou.
- Supervision: Shuwei Wang.
- Validation: Luokai Huangfu.
- Writing—original draft: Chunhui Zhou.
- Writing—review & editing: Hao Zhao, Jianning Zhang, Shuwei Wang.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2021.17.2.220.
Gene panel for detecting adult brainstem gliomas at our institution
Common genetic alterations and overall survival of 50 adult brainstem glioma patients
Results of univariate and multivariate analyses of factors associated with OS of 38 patients with brainstem high-grade glioma
Results of univariate and multivariate analyses of factors associated with OS of 44 patients with brainstem astrocytoma
Results of univariate and multivariate analyses of factors associated with OS of 22 patients with brainstem glioblastoma
References
- 1.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19:v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalid SI, Kelly R, Adogwa O, Carlton A, Tam E, Naqvi S, et al. Pediatric Brainstem gliomas: a retrospective study of 180 patients from the SEER database. Pediatr Neurosurg. 2019;54:151–164. doi: 10.1159/000497440. [DOI] [PubMed] [Google Scholar]
- 3.Eisele SC, Reardon DA. Adult brainstem gliomas. Cancer. 2016;122:2799–2809. doi: 10.1002/cncr.29920. [DOI] [PubMed] [Google Scholar]
- 4.Guillamo JS, Monjour A, Taillandier L, Devaux B, Varlet P, Haie-Meder C, et al. Brainstem gliomas in adults: prognostic factors and classification. Brain. 2001;124:2528–2539. doi: 10.1093/brain/124.12.2528. [DOI] [PubMed] [Google Scholar]
- 5.Landolfi JC, Thaler HT, DeAngelis LM. Adult brainstem gliomas. Neurology. 1998;51:1136–1139. doi: 10.1212/wnl.51.4.1136. [DOI] [PubMed] [Google Scholar]
- 6.Kerezoudis P, Goyal A, Lu VM, Alvi MA, Bydon M, Kizilbash SH, et al. The role of radiation and chemotherapy in adult patients with high-grade brainstem gliomas: results from the National Cancer Database. J Neurooncol. 2020;146:303–310. doi: 10.1007/s11060-019-03374-x. [DOI] [PubMed] [Google Scholar]
- 7.Doyle J, Khalafallah AM, Yang W, Sun Y, Bettegowda C, Mukherjee D. Association between extent of resection on survival in adult brainstem high-grade glioma patients. J Neurooncol. 2019;145:479–486. doi: 10.1007/s11060-019-03313-w. [DOI] [PubMed] [Google Scholar]
- 8.Rachinger W, Grau S, Holtmannspötter M, Herms J, Tonn JC, Kreth FW. Serial stereotactic biopsy of brainstem lesions in adults improves diagnostic accuracy compared with MRI only. J Neurol Neurosurg Psychiatry. 2009;80:1134–1139. doi: 10.1136/jnnp.2009.174250. [DOI] [PubMed] [Google Scholar]
- 9.Hamisch C, Kickingereder P, Fischer M, Simon T, Ruge MI. Update on the diagnostic value and safety of stereotactic biopsy for pediatric brainstem tumors: a systematic review and meta-analysis of 735 cases. J Neurosurg Pediatr. 2017;20:261–268. doi: 10.3171/2017.2.PEDS1665. [DOI] [PubMed] [Google Scholar]
- 10.Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019;15:405–417. doi: 10.1038/s41582-019-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reyes-Botero G, Giry M, Mokhtari K, Labussière M, Idbaih A, Delattre JY, et al. Molecular analysis of diffuse intrinsic brainstem gliomas in adults. J Neurooncol. 2014;116:405–411. doi: 10.1007/s11060-013-1312-2. [DOI] [PubMed] [Google Scholar]
- 13.Banan R, Stichel D, Bleck A, Hong B, Lehmann U, Suwala A, et al. Infratentorial IDH-mutant astrocytoma is a distinct subtype. Acta Neuropathol. 2020;140:569–581. doi: 10.1007/s00401-020-02194-y. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Voronovich Z, Clark K, Hands I, Mannas J, Walsh M, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16:1478–1483. doi: 10.1093/neuonc/nou097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelsche C, et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015;129:133–146. doi: 10.1007/s00401-014-1370-3. [DOI] [PubMed] [Google Scholar]
- 16.Karsy M, Guan J, Cohen AL, Jensen RL, Colman H. New molecular considerations for glioma: IDH, ATRX, BRAF, TERT, H3 K27M. Curr Neurol Neurosci Rep. 2017;17:19. doi: 10.1007/s11910-017-0722-5. [DOI] [PubMed] [Google Scholar]
- 17.Ohba S, Kuwahara K, Yamada S, Abe M, Hirose Y. Correlation between IDH, ATRX, and TERT promoter mutations in glioma. Brain Tumor Pathol. 2020;37:33–40. doi: 10.1007/s10014-020-00360-4. [DOI] [PubMed] [Google Scholar]
- 18.Wiestler B, Capper D, Holland-Letz T, Korshunov A, von Deimling A, Pfister SM, et al. ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol. 2013;126:443–451. doi: 10.1007/s00401-013-1156-z. [DOI] [PubMed] [Google Scholar]
- 19.Xie Y, Tan Y, Yang C, Zhang X, Xu C, Qiao X, et al. Omics-based integrated analysis identified ATRX as a biomarker associated with glioma diagnosis and prognosis. Cancer Biol Med. 2019;16:784–796. doi: 10.20892/j.issn.2095-3941.2019.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuoka K, Yanagisawa T, Watanabe Y, Suzuki T, Shirahata M, Adachi J, et al. Brainstem oligodendroglial tumors in children: two case reports and review of literatures. Childs Nerv Syst. 2015;31:449–455. doi: 10.1007/s00381-014-2563-8. [DOI] [PubMed] [Google Scholar]
- 21.Hodges SD, Malafronte P, Gilhooly J, Skinner W, Carter C, Theeler BJ. Rare brainstem oligodendroglioma in an adult patient: presentation, molecular characteristics and treatment response. J Neurol Sci. 2015;355:209–210. doi: 10.1016/j.jns.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Andrews C, Prayson RA. 1p/19q co-deleted fibrillary astrocytomas: not everything that is co-deleted is an oligodendroglioma. Ann Diagn Pathol. 2020;46:151519. doi: 10.1016/j.anndiagpath.2020.151519. [DOI] [PubMed] [Google Scholar]
- 23.Xiong J, Liu Y, Wang Y, Ke RH, Mao Y, Ye ZR. Chromosome 1p/19q status combined with expression of p53 protein improves the diagnostic and prognostic evaluation of oligodendrogliomas. Chin Med J (Engl) 2010;123:3566–3573. [PubMed] [Google Scholar]
- 24.Castel D, Philippe C, Calmon R, Le Dret L, Truffaux N, Boddaert N, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015;130:815–827. doi: 10.1007/s00401-015-1478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duchatel RJ, Jackson ER, Alvaro F, Nixon B, Hondermarck H, Dun MD. Signal transduction in diffuse intrinsic pontine glioma. Proteomics. 2019;19:e1800479. doi: 10.1002/pmic.201800479. [DOI] [PubMed] [Google Scholar]
- 26.Feng J, Hao S, Pan C, Wang Y, Wu Z, Zhang J, et al. The H3.3 K27M mutation results in a poorer prognosis in brainstem gliomas than thalamic gliomas in adults. Hum Pathol. 2015;46:1626–1632. doi: 10.1016/j.humpath.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Babu R, Kranz PG, Agarwal V, McLendon RE, Thomas S, Friedman AH, et al. Malignant brainstem gliomas in adults: clinicopathological characteristics and prognostic factors. J Neurooncol. 2014;119:177–185. doi: 10.1007/s11060-014-1471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boots-Sprenger SH, Sijben A, Rijntjes J, Tops BB, Idema AJ, Rivera AL, et al. Significance of complete 1p/19q co-deletion, IDH1 mutation and MGMT promoter methylation in gliomas: use with caution. Mod Pathol. 2013;26:922–929. doi: 10.1038/modpathol.2012.166. [DOI] [PubMed] [Google Scholar]
- 29.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 30.Meyronet D, Esteban-Mader M, Bonnet C, Joly MO, Uro-Coste E, Amiel-Benouaich A, et al. Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol. 2017;19:1127–1134. doi: 10.1093/neuonc/now274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filbin MG, Suvà ML. Gliomas genomics and epigenomics: arriving at the start and knowing it for the first time. Annu Rev Pathol. 2016;11:497–521. doi: 10.1146/annurev-pathol-012615-044208. [DOI] [PubMed] [Google Scholar]
- 32.Hu J, Western S, Kesari S. Brainstem glioma in adults. Front Oncol. 2016;6:180. doi: 10.3389/fonc.2016.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reithmeier T, Kuzeawu A, Hentschel B, Loeffler M, Trippel M, Nikkhah G. Retrospective analysis of 104 histologically proven adult brainstem gliomas: clinical symptoms, therapeutic approaches and prognostic factors. BMC Cancer. 2014;14:115. doi: 10.1186/1471-2407-14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392:432–446. doi: 10.1016/S0140-6736(18)30990-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene panel for detecting adult brainstem gliomas at our institution
Common genetic alterations and overall survival of 50 adult brainstem glioma patients
Results of univariate and multivariate analyses of factors associated with OS of 38 patients with brainstem high-grade glioma
Results of univariate and multivariate analyses of factors associated with OS of 44 patients with brainstem astrocytoma
Results of univariate and multivariate analyses of factors associated with OS of 22 patients with brainstem glioblastoma