Abstract
Background and Purpose
The associations between hearing loss (HL) and the mechanisms underlying cognitive impairment (CI) remain unclear. We evaluated the effects of clinical factors, vascular magnetic resonance imaging (MRI) markers, and CI mechanisms on HL.
Methods
In total, 112 patients with CI (59% demented) and subjective HL prospectively underwent MRI, amyloid positron-emission tomography (PET), hearing evaluations, and neuropsychological tests including a language comprehension test. Patients were categorized into pure-Alzheimer's disease-related CI (ADCI), pure-Lewy-body disease-related CI (LBCI), mixed-ADCI/LBCI, and non-ADCI/LBCI groups based on clinical features and PET biomarkers.
Results
The risk of peripheral HL [defined as a pure-tone average (PTA) threshold >40 dB] was higher in the pure-LBCI group than in the pure-ADCI and mixed-ADCI/LBCI groups, and lower in the presence of ADCI. The non-ADCI/LBCI group had the most-severe vascular MRI markers and showed a higher risk of peripheral HL than did the pure-ADCI and mixed-ADCI/LBCI groups. While the pure-LBCI group had a higher risk of comprehension dysfunction than the pure-ADCI group regardless of the PTA and the score on the Korean version of the Mini Mental State Examination (K-MMSE), those in the pure-LBCI group even with a better K-MMSE score had a risk of comprehension dysfunction comparable to that in the mixed-ADCI/LBCI group due to a worse PTA.
Conclusions
Peripheral HL could be associated with the absence of significant β-amyloid deposition in patients with CI and characteristic of the pure-LBCI and non-ADCI/LBCI groups.
Keywords: hearing loss, Alzheimer's disease, Lewy body disease, Parkinson's disease, vascular dementia, cognitive impairments
INTRODUCTION
Hearing loss (HL) and cognitive dysfunction are prevalent in older adults. Both cross-sectional1,2 and longitudinal3,4,5,6 studies have shown that peripheral HL is associated with cognitive dysfunction and dementia, but this is still controversial7,8,9 due to the underlying mechanism being elusive. Dementia has multiple causes, including Alzheimer's disease (AD), Lewy-body disease (LBD), and vascular cognitive impairment (CI),10 and so the type of dementia must be considered when attempting to elucidate the exact mechanisms underlying the association between HL and dementia.
Previous studies have suggested potential mechanisms for explaining this association. First, older age, microvascular pathology, and vascular risk factors may give a spurious appearance of a connection, since these parameters are all associated with both cognitive dysfunction and HL (the common-cause hypothesis).11 Second, the cognitive overload caused by effortful listening (the cognitive-load hypothesis) or the impoverished sensory input caused by HL (the cascade hypothesis) may accelerate neurodegenerative changes.12
Based on previous studies showing a close relationship between HL and dementia with diverse etiologies,1,2,3,4,5,6 we hypothesized that HL is affected differently depending on the causes of CI and dementia, underpinning the common-cause hypothesis. In this study we assessed the degree of HL using the pure-tone average (PTA) in pure-tone audiometry, and the word recognition score (WRS) in speech audiometry. The relationship between HL and the mechanisms underlying CI was investigated among patients with cognitive dysfunction and subjective HL.
METHODS
Patient recruitment
Between May 2016 and October 2018, consecutive patients complaining of cognitive dysfunction were prospectively enrolled from a university-based memory clinic. Written informed consent was obtained from all participants. All of the included patients underwent hearing evaluations, neuropsychological tests, brain structural magnetic resonance imaging (MRI), and 18F-fluorodeoxyglucose (FDG) positron-emission tomography (PET). 18F-florbetaben (FBB) PET was performed to confirm cerebral β-amyloid deposition in all patients, and 18F-N-(3-fluoropropyl)-2β-carboxymethoxy-3β-(4-iodophenyl) nortropane (FP-CIT) PET was performed to confirm dopamine depletion based on clinical needs. This prospective study was approved by the Institutional Review Board of Yonsei University Medical College (IRB No. 4-2016-0648).
The following exclusion criteria were applied: 1) conductive HL in pure-tone audiometry; 2) HL related to congenital, traumatic, or infective causes; or 3) other causes of cognitive dysfunction including normal-pressure hydrocephalus, traumatic encephalopathy, large-territory cerebral infarction, acute or subacute cerebral infarction or hemorrhage, frontotemporal lobar degeneration, or atypical parkinsonism including progressive supranuclear palsy, multiple-system atrophy, and corticobasal degeneration. This study enrolled 112 patients who underwent a clinical interview, neurological examinations, and laboratory tests including a complete blood count, blood chemistry, vitamin B12, folate, thiamine, syphilis serology, thyroid function, and apolipoprotein E (APOE) genotyping.
Diagnosis and categorization of patients
Because AD and LBD are the two most common degenerative causes of dementia, and vascular disease frequently co-occurs in the elderly,13,14,15 the participants in the present study were categorized into pure-AD-related CI (ADCI), pure-LBD-related CI (LBCI), mixed-ADCI/LBCI, and non-ADCI/LBCI groups. The severity of vascular disease was measured using the modified Fazeka's scale (see MRI scan acquisition and interpretation section). ADCI included AD dementia and mild CI (MCI) due to AD. Clinical AD dementia was diagnosed according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA).16 MCI due to AD was diagnosed based on modified Petersen's criteria.17 LBCI included Parkinson's disease (PD) dementia, MCI due to PD, dementia with Lewy bodies (DLB), and MCI due to DLB. The United Kingdom Parkinson's Disease Society Brain Bank diagnostic criteria for PD were used to diagnose PD,18 and all patients with DLB met the criteria for probable DLB.19
All study participants underwent FDG PET and 18F-FBB PET [Acquisition and interpretation of FBB, FP-CIT, and FDG PET (with CT) scans section], and they were categorized into ADCI and non-ADCI groups based on the 18F-FBB PET results. If participants had a score on the Unified Parkinson's Disease Rating Scale of >16, they were regarded as having significant parkinsonism. Participants with more than two symptoms among cognitive fluctuation, visual hallucination, and parkinsonism were regarded as having DLB. DLB patients and participants with significant parkinsonism and nigrostriatal dopamine depletion on 18F-FP-CIT PET were regarded as having LBCI.
The study participants were categorized into 19 patients with pure ADCI, 38 with pure LBCI, 45 with mixed ADCI/LBCI, and 10 with non-ADCI/LBCI, among whom significant parkinsonism was present in 7, 37, 42, and 9, respectively. Ten DLB patients did not undergo 18F-FP-CIT PET, two of whom did not have significant parkinsonism. All DLB patients had characteristic FDG metabolic increases in the posterior putamen, somatomotor cortex, and vermis.20 All 7 pure-ADCI and 10 non-ADCI/LBCI patients who had significant parkinsonism underwent 18F-FP-CIT PET, and they did not exhibit nigrostriatal dopamine depletion.
Neuropsychological evaluation
A standardized neuropsychological battery [the Seoul Neuropsychological Screening Battery (SNSB)] was performed as we have described previously.21,22 The SNSB includes scorable and nonscorable tests in the attention, memory, visuospatial, language, and frontal/executive domains. General cognitive function was assessed using the Korean version of the Mini Mental State Examination (K-MMSE). Language-related function was dichotomized into normal and abnormal based on the spontaneous speech fluency, comprehension function (assessed by asking five yes/no questions), repetition of five sentences, writing ability, finger-naming test, right-left orientation test, body-part identification test, and buccofacial praxis test. The severity of depressive symptoms was assessed using the Beck Depression Inventory (BDI).
MRI scan acquisition and interpretation
All MRI scans were acquired using a 3-T scanner (Philips Intera, Philips Medical Systems, Best, The Netherlands). We have described the MRI methodology in detail previously.22 Structural abnormalities including territorial cerebral infarction, acute or subacute cerebral infarction or hemorrhage, and normal-pressure hydrocephalus were identified for applying the study exclusion criteria.
The severity of subcortical vascular changes on MRI was measured by applying the modified Fazeka's scale to white-matter hyperintensities (WMHs) and manually counting lacunes and cerebral microbleeds (CMBs). Periventricular WMHs were classified into P1 (cap and band <5 mm), P2 (5 mm≤cap or band <10 mm), and P3 (cap or band ≥10 mm). Deep WMHs were classified as D1 (maximum diameter of deep white-matter lesion <10 mm), D2 (10 mm≤ lesion <25 mm), and D3 (lesion ≥25 mm). Lacunes were defined as small lesions (≥3 mm and ≤15 mm in diameter) with high signals on T2-weighted images, low signals on T1-weighted images, and a perilesional halo on FLAIR images. CMBs were defined as round lesions (<10 mm in diameter) with homogeneous low signal intensities on T2*-weighted gradient recalled-echo images. WMHs, lacunes, and CMBs were rated by a neurologist (B.S.Y.).
Acquisition and interpretation of FBB, FP-CIT, and FDG PET (with CT) scans
FP-CIT PET, FDG PET, and FBB PET scans were acquired using a Discovery 600 device (GE Healthcare, Milwaukee, WI, USA). We have described the acquisition and reconstruction protocols for FP-CIT and FBB PET previously.23 FDG PET scans were acquired for 15 minutes at 60 minutes after intravenously injecting FDG at approximately 4.1 MBq/kg (body weight). Spiral computed tomography scanning for attenuation correction was performed with the following parameters: 0.8-second rotation time, 60 mA, 120 kVp, 3.75-mm section thickness, 0.625-mm collimation, and 9.375-mm table feed per rotation. An expert in nuclear medicine (M.J.Y.) and a neurologist with expertise in dementia (B.S.Y.) performed visual ratings of the brain β-amyloid plaque load (BAPL) score and FP-CIT PET abnormalities. BAPL scores of 2 and 3 were regarded as β-amyloid positive, while BAPL scores of 1 were considered β-amyloid negative.
Hearing evaluation
Audiological evaluations, including pure-tone audiometry and speech audiometry, were performed when patients first visited after their enrollment. The pure-tone air conduction (250–8,000 Hz) and bone conduction (250–8,000 Hz) thresholds were measured using clinical audiometers in a double-walled audio booth. The mean PTA thresholds for air conduction at 500, 1,000, 2,000, and 4,000 Hz (PTA4) were determined. The air-conduction PTA threshold of the better ear was used in this study, since hearing ability is mainly determined by the better ear. PTA-based HL was defined as PTA4 >40 dB HL. Speech audiometry was performed to obtain the WRS, which was measured at the most comfortable hearing level using 50 monosyllabic Korean words that are heard during everyday life. These words were obtained from a validated and standardized resource and were phonetically balanced. WRSbased HL was defined as WRS <70%.
Statistical analyses
All statistical analyses were performed using SPSS software (version 23.0; IBM Corp., Armonk, NY, USA). Student's t-test and analysis of variance were used to compare continuous variables, while chi-square tests were used to compare categorical variables. General linear models were used to identify associations between hearing indices and various factors including age, sex, education level, carrying the E4 variant of the APOE gene (APOE4), BDI score, K-MMSE score, cognitive status (demented vs. nondemented), ADCI diagnosis, LBCI diagnosis, disease group (classified into pure ADCI, pure LBCI, mixed ADCI/LBCI, and non-ADCI/LBCI), and MRI vascular markers including deep WMHs, periventricular WMHs, lacunes, and CMBs.
Logistic regression analyses were performed to identify the associations of the various factors with the risk of HL. To identify significant covariates, univariate analyses were performed after controlling for age, sex, and education level (Supplementary Tables 1 and 2 in the online-only Data Supplement). Variables that showed univariate associations with p<0.05 as covariates were then included in multivariate analyses of the associations of HL with the diagnosis, using the presence of ADCI and LBCI (Model 1) or the disease group (Model 2) as predictors (Tables 2 and 3).
Table 2. Association of clinical factors and diagnosis with the degree of hearing impairment.
| PTA | WRS | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | p | Model 2 | p | Model 1 | p | Model 2 | p | |
| Age | 0.83 [0.23] | <0.001* | 0.83 [0.23] | <0.001* | -0.57 [0.31] | 0.068 | -0.54 [0.31] | 0.083 |
| Sex, male | 5.38 [3.07] | 0.083 | 5.44 [3.11] | 0.083 | -6.03 [4.37] | 0.171 | -6.82 [4.38] | 0.123 |
| Education level | -0.22 [0.32] | 0.493 | -0.21 [0.32] | 0.518 | 0.15 [0.42] | 0.717 | 0.15 [0.42] | 0.713 |
| CMBs | -0.73 [0.32] | 0.023 | -0.79 [0.32] | 0.015* | ||||
| BDI score | -0.43 [0.21] | 0.039 | -0.43 [0.21] | 0.041* | ||||
| K-MMSE score | 0.52 [0.34] | 0.127 | 0.49 [0.34] | 0.152 | ||||
| APOE4 positivity | -2.24 [3.30] | 0.500 | -2.14 [3.31] | 0.519 | ||||
| ADCI diagnosis | -9.92 [3.24] | 0.003* | 11.39 [3.94] | 0.005 | ||||
| LBCI diagnosis | -0.13 [3.28] | 0.968 | -2.89 [4.56] | 0.528 | ||||
| Disease group | ||||||||
| Pure ADCI | -9.03 [4.24] | 0.036* | 12.08 [5.82] | 0.041* | ||||
| Pure LBCI | Reference | Reference | ||||||
| Mixed ADCI/LBCI | -10.76 [3.66] | 0.004 | 14.61 [4.48] | 0.002 | ||||
| Non-ADCI/LBCI | -1.12 [5.18] | 0.829 | 11.16 [7.36] | 0.133 | ||||
Data are beta [SE] values. Data are results of general linear models for hearing indices using age, sex, education level, and factors that showed significant associations with hearing indices in Supplementary Table 1 as covariates. Model 1 used the presence of ADCI and LBCI as predictors. Model 2 used disease group as a predictor. Adjusted R2 values for PTA model 1, PTA model 2, WRS model 1, and WRS model 2 were 0.267, 0.261, 0.199, and 0.208, respectively.
*p<0.05.
ADCI: Alzheimer's disease-related cognitive impairment, APOE4: apolipoprotein E gene E4 variant, BDI: Beck Depression Inventory, CMB: cerebral microbleed, K-MMSE: Korean version of the Mini Mental State Examination, LBCI: Lewy-body disease-related cognitive impairment, PTA: pure-tone average, WRS: word recognition score.
Table 3. Association of clinical factors and diagnosis with the presence of hearing impairment.
| PTA-based HL | WRS-based HL | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | p | Model 2 | p | Model 1 | p | Model 2 | p | |
| Age, years | 1.15 (1.05–1.25) | 0.002* | 1.15 (1.06–1.26) | 0.001* | 1.12 (1.02–1.22) | 0.013 | 1.12 (1.02–1.22) | 0.013* |
| Sex, male | 1.56 (0.57–4.28) | 0.387 | 1.46 (0.53–4.02) | 0.467 | 4.24 (1.20–15.00) | 0.025 | 4.42 (1.23–15.93) | 0.023* |
| Education level, years | 0.94 (0.85–1.04) | 0.244 | 0.94 (0.85–1.04) | 0.246 | 0.93 (0.84–1.02) | 0.135 | 0.93 (0.84–1.03) | 0.146 |
| Periventricular WMHs | 1.55 (0.86–2.82) | 0.147 | 1.55 (0.85–2.85) | 0.154 | ||||
| Smoking | 0.24 (0.06–0.96) | 0.043 | 0.23 (0.06–0.91) | 0.037* | ||||
| ADCI diagnosis | 0.19 (0.07–0.46) | <0.001* | 0.44 (0.18–1.06) | 0.068 | ||||
| LBCI diagnosis | 1.35 (0.48–3.83) | 0.568 | 2.11 (0.66–6.78) | 0.208 | ||||
| Disease group | ||||||||
| Pure ADCI | 0.13 (0.03–0.53) | 0.005* | 0.28 (0.06–1.22) | 0.089 | ||||
| Pure LBCI | Reference | Reference | ||||||
| Mixed ADCI/LBCI | 0.25 (0.09–0.70) | 0.008* | 0.42 (0.16–1.15) | 0.092 | ||||
| Non-ADCI/LBCI | 2.22 (0.34–14.66)† | 0.407 | 0.52 (0.10–2.63) | 0.427 | ||||
Data are OR (95% CI) values. Data are results of logistic regression analyses of the presence of hearing impairment using age, sex, education level, and factors that showed significant associations with HL based on each hearing index in Supplementary Table 2 as covariates. Model 1 used the presence of ADCI and LBCI as predictors. Model 2 used disease group as a predictor. PTA-based HL was defined as PTA4 >40 dB HL and WRS-based HL was defined as WRS <70%. p values in the Hosmer and Lemeshow test for PTA model 1, PTA model 2, WRS model 1, and WRS model 2 were 0.890, 0.279, 0.691, and 0.419, respectively.
*p<0.05, †The OR for PTA-based HL was significantly higher in the non-ADCI/LBCI group than in the pure-ADCI and mixed-ADCI/LBCI groups.
ADCI: Alzheimer's disease-related cognitive impairment, CI: confidence interval, HL: hearing loss, LBCI: Lewy-body disease-related cognitive impairment, OR: odds ratio, PTA: pure-tone average, WMHs: white-matter hyperintensities, WRS: word recognition score.
Comprehension dysfunction was significantly associated with the PTA and WRS (Supplementary Table 1 in the online-only Data Supplement). However, since comprehension dysfunction could be the result of HL rather than the cause, we did not include it as a predictor in the multivariate models. Because peripheral HL can impair comprehension function, logistic regression analyses were performed to evaluate whether the risk of comprehension dysfunction differed according to the disease group. Variables showing significant associations (p<0.05) in univariate logistic regression analyses of comprehension dysfunction after controlling for age, sex, and education level were selected (Supplementary Table 3 in the online-only Data Supplement) for inclusion in multivariate logistic regression analyses. To test whether the risk of comprehension dysfunction was influenced by peripheral HL and general cognition, logistic regression analyses were performed while removing the PTA and both the PTA and K-MMSE score, respectively.
RESULTS
Demographic and clinical characteristics
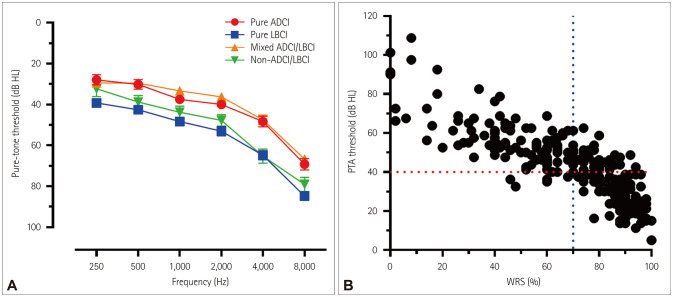
There were no significant intergroup differences in age, education level, BDI score, vascular risk factors including hypertension, diabetes mellitus, dyslipidemia, and smoking, or MRI vascular markers including deep WMHs, periventricular WMHs, and CMBs (Table 1). There were more lacunes in the non-ADCI/LBCI group than in the pure-ADCI, pure-LBCI, and mixed-ADCI/LBCI groups. The proportion of APOE4 carriers was higher in the pure-ADCI and mixed-ADCI/LBCI groups than in the pure-LBCI and non-ADCI/LBCI groups. The proportion of demented patients was higher in the mixed-ADCI/LBCI group than in the pure-ADCI and non-ADCI/LBCI groups. The proportion of demented patients was higher in the pure-LBCI group than in the non-ADCI/LBCI group. The K-MMSE score was lower in the mixed-ADCI/LBCI group than in the pure-ADCI, pure-LBCI, and non-ADCI/LBCI groups. The pure-LBCI group had a higher PTA and lower WRS compared with the pure-ADCI and mixed-ADCI/LBCI groups. The PTA was higher in the non-ADCI/LBCI group than in the pure-ADCI and mixed-ADCI/LBCI groups (Table 1, Fig. 1).
Table 1. Demographic characteristics of the study participants.
| Pure ADCI (n=19) | Pure LBCI (n=38) | Mixed ADCI/LBCI (n=45) | Non-ADCI/LBCI (n=10) | p | |
|---|---|---|---|---|---|
| Age, years | 74.6±7.5 | 78.7±6.5 | 76.8±6.0 | 75.4±4.0 | 0.119 |
| Sex, female | 13 (68.4) | 23 (60.5) | 29 (64.4) | 4 (40.0) | 0.472 |
| Education level, years | 8.2±5.5 | 8.8±5.2 | 9.2±4.9 | 8.0±5.6 | 0.878 |
| Duration of CI, years | 2.3±1.3 | 2.6±1.6 | 2.7±2.2 | 2.4±1.6 | 0.831 |
| Duration of parkinsonism, years | 2.9±2.0 | 2.0±1.8 | 1.9±2.4 | 1.6±1.5 | 0.622 |
| Significant parkinsonism (UPDRS score >16) | 7 | 37 | 42 | 9 | <0.001* |
| Vascular risk factors | |||||
| Hypertension | 13 (68.4) | 24 (63.2) | 27 (60.0) | 7 (70.0) | 0.892 |
| Diabetes mellitus | 3 (15.8) | 15 (39.5) | 11 (24.4) | 2 (20.0) | 0.211 |
| Dyslipidemia | 3 (15.8) | 8 (21.1) | 8 (17.8) | 4 (40.0) | 0.424 |
| Smoking | 5 (26.3) | 8 (21.1) | 10 (22.2) | 3 (30.0) | 0.922 |
| MRI vascular markers | |||||
| Deep WMHs | 1.8±0.7 | 1.8±0.6 | 1.5±0.6 | 2.0±0.6 | 0.157 |
| Periventricular WMHs | 1.8±0.7 | 2.1±0.7 | 1.9±0.8 | 2.4±1.0 | 0.133 |
| Lacunes | 3.3±4.4c | 2.6±4.7e | 1.5±2.1f | 6.9±6.8c,e,f | 0.003 |
| CMBs | 2.2±5.3 | 1.5±3.4 | 0.7±1.5 | 6.3±18.2 | 0.079 |
| Carrier of APOE4 allele | 10 (43.5)a,c | 6 (15.0)a,d | 26 (50.0)d,f | 1 (10.0)c,f | 0.001* |
| Cognitive status | 0.002* | ||||
| Nondemented | 12 (63.2) | 16 (42.1) | 11 (24.4) | 8 (80.0) | |
| Demented | 7 (36.8)b | 22 (57.9)e | 34 (75.6)b,f | 2 (20.0)e,f | |
| Hearing indices | |||||
| PTA, dB HL | 34.3±11.7a | 47.8±17.1a,d | 32.8±15.5d,f | 45.6±8.4f | <0.001* |
| WRS | 79.6±18.0a | 62.8±26.5a,d | 80.3±18.1d | 72.2±19.3 | 0.002* |
| PTA-based HL | 4 (21.1)a,c | 27 (71.1)a,d | 16 (35.6)d,f | 8 (80.0)c,f | <0.001* |
| WRS-based HL | 3 (15.8) | 18 (47.4) | 11 (24.4) | 3 (30.0) | 0.053 |
| BDI score | 11.4±9.1 | 14.3±10.3 | 12.4±8.9 | 12.0±9.0 | 0.687 |
| K-MMSE score | 23.3±4.2b | 23.2±4.0d | 20.4±5.1b,d,f | 24.0±4.5f | 0.013* |
| Comprehension dysfunction | 1 (5.6)a | 15 (41.7)a | 10 (23.8) | 2 (20.0) | 0.034* |
PTA-based HL was defined as PTA4 >40 dB HL and WRS-based HL was defined as WRS <70%. Data are mean±SD or n (%) values. MRI vascular markers refer to the modified Fazeka's scale to WMHs. Group comparisons were performed using chi-square tests or analyses of variance as appropriate.
*p<0.05, aSignificant difference between pure-ADCI and pure-LBCI groups, bSignificant difference between pure-ADCI and mixed-ADCI/LBCI groups, cSignificant difference between pure-ADCI and non-ADCI/LBCI groups, dSignificant difference between pure-LBCI and mixed-ADCI/LBCI groups, eSignificant difference between pure-LBCI and non-ADCI/LBCI groups, fSignificant difference between mixed-ADCI/LBCI and non-ADCI/LBCI groups.
ADCI: Alzheimer's disease-related cognitive impairment, APOE4: apolipoprotein E gene E4 variant, BDI: Beck Depression Inventory, CI: cognitive impairment, CMBs: cerebral microbleeds, HL: hearing loss, K-MMSE: Korean version of the Mini-Mental State Examination, LBCI: Lewy-body disease-related cognitive impairment, MRI: magnetic resonance imaging, PTA: pure-tone average, WMHs: white-matter hyperintensities, WRS: word recognition score.
Fig. 1. Audiological results in patients compared with cognitive dysfunction and subjective hearing impairment. A: Pure-tone audiograms of 112 patients (both ears) are plotted according to the mechanisms underlying cognitive dysfunction. B: The PTA and WRS are plotted. PTA-based HL was defined as PTA4 (average of thresholds at 500, 1,000, 2,000, and 4,000 Hz) >40 dB HL, and WRS-based HL was defined as WRS <70% (red and blue dotted lines, respectively). ADCI: Alzheimer's disease-related cognitive impairment, HL: hearing loss, LBCI: Lewy-body disease-related cognitive impairment, PTA: pure-tone average, WRS: word recognition score.
Univariate associations of clinical and imaging factors with auditory function
Univariate general linear models obtained after controlling for age, sex, and education level showed that a higher PTA was associated with APOE4 negativity, higher K-MMSE score, the presence of comprehension dysfunction, and the absence of ADCI (Supplementary Table 1 in the online-only Data Supplement). A lower WRS was associated with more CMBs, higher BDI score, the presence of comprehension dysfunction, and the absence of ADCI. The pure-LBCI group had a higher PTA and lower WRS compared with the pure-ADCI and mixed-ADCI/LBCI groups.
Univariate logistic regression analyses performed after controlling for age, sex, and education level showed that PTA-based HL (PTA4 >40 dB) was associated with more-severe periventricular WMHs and the absence of ADCI. WRS-based HL (WRS <70%) was associated with the absence of smoking and the absence of ADCI (Supplementary Table 2 in the online-only Data Supplement). The odds ratio (OR) for PTA-based HL was higher for the pure-LBCI group than for the pure-ADCI and mixed-ADCI/LBCI groups. However, the four disease groups had comparable ORs for WRS-based HL.
Multivariate associations of clinical and imaging factors with auditory function
A multivariate general linear model for the PTA showed that a higher PTA was associated with older age and the absence of ADCI, while sex, education level, lacunes, APOE4 positivity, and the presence of LBCI were not associated with the PTA (Table 2). After controlling for age, sex, education level, and APOE4 positivity, the PTA was higher in the pure-LBCI group than in the pure-ADCI and mixed-ADCI/LBCI groups. A multivariate general linear model for the WRS showed that a lower WRS was associated with more CMBs, higher BDI score, and the absence of ADCI, while age, sex, education level, and the presence of LBCI were not associated with the WRS. After controlling for possible confounders, the WRS was lower in the pure-LBCI group than in the pure-ADCI and mixed-ADCI/LBCI groups.
A multivariate logistic regression analysis of PTA-based HL showed that older age and the absence of ADCI were independently associated with a higher OR for PTA-based HL, while sex, education level, periventricular WMHs, and the presence of LBCI were not (Table 3). After controlling for possible confounders, the ORs for PTA-based HL were higher in the pure-LBCI and non-ADCI/LBCI groups than in the pure-ADCI and mixed-ADCI/LBCI groups. A multivariate logistic regression analysis of WRS-based HL showed that older age, male sex, and the absence of smoking were independently associated with a higher OR for WRS-based HL, while education level, the presence of ADCI, and the presence of LBCI were not. After controlling for possible confounders, there was no significant difference in the OR for WRS-based HL between the four disease groups.
Predictors of the presence of comprehension dysfunction
Univariate logistic regression analyses of the presence of comprehension dysfunction after controlling for age, sex, and education level showed that periventricular WMHs, K-MMSE score, PTA, WRS, the presence of LBCI, and disease group were significantly associated with comprehension dysfunction (Supplementary Table 3 in the online-only Data Supplement). Multivariate logistic regression analyses showed that more-severe periventricular WMHs, lower K-MMSE score, and higher PTA were independently associated with higher ORs for comprehension dysfunction (Table 4). The original model obtained after controlling for age, sex, education level, periventricular WMHs, K-MMSE score, and PTA showed that the OR for comprehension dysfunction was higher in the pure-LBCI group than in the pure-ADCI group.
Table 4. Association of clinical factors and diagnosis with the presence of comprehension dysfunction.
| Original model | p | Model without PTA | p | Model without PTA and K-MMSE score | p | |
|---|---|---|---|---|---|---|
| Age | 0.97 (0.85–1.09) | 0.587 | 1.04 (0.94–1.15) | 0.468 | 1.08 (0.99–1.19) | 0.096 |
| Sex, male | 1.46 (0.36–5.94) | 0.598 | 2.06 (0.57–7.40) | 0.269 | 2.53 (0.77–8.37) | 0.127 |
| Education level | 0.85 (0.72–0.99) | 0.042* | 0.87 (0.75–1.00) | 0.057 | 0.79 (0.69–0.91) | 0.001* |
| Periventricular WMHs | 2.90 (1.18–7.14) | 0.020* | 2.42 (1.06–5.55) | 0.037* | 2.49 (1.15–5.40) | 0.021* |
| K-MMSE score | 0.73 (0.59–0.89) | 0.002* | 0.77 (0.65–0.92) | 0.004* | ||
| PTA | 1.07 (1.02–1.12) | 0.006* | ||||
| Disease group | ||||||
| Pure ADCI | 0.04 (0.003–0.490) | 0.013* | 0.04 (0.004–0.480) | 0.010* | 0.06 (0.01–0.60) | 0.016* |
| Pure LBCI | Reference | Reference | Reference | |||
| Mixed ADCI/LBCI | 0.36 (0.09–1.55) | 0.171 | 0.23 (0.06–0.90) | 0.034* | 0.50 (0.17–1.51) | 0.223 |
| Non-ADCI/LBCI | 0.10 (0.01–1.29) | 0.078 | 0.15 (0.01–1.56) | 0.112 | 0.14 (0.02–1.07) | 0.059 |
Data are OR (95% CI) values. Results are based on logistic regression analyses of the presence of comprehension dysfunction using age, sex, and education level as covariates. Predictors included disease group, PTA, and K-MMSE score as appropriate. p values in the Hosmer and Lemeshow test for the original model, model without PTA, and model without PTA and K-MMSE score were 0.524, 0.246, and 0.342, respectively.
*p<0.05.
ADCI: Alzheimer's disease-related cognitive impairment, LBCI: Lewy-body disease-related cognitive impairment, OR: odds ratio, PTA: pure-tone average.
A sensitivity analysis in which the PTA was removed from the original model showed that the OR for comprehension dysfunction was higher in the pure-LBCI group than in the pure-ADCI and mixed-ADCI/LBCI groups. This sensitivity result indicated that given the same level of general cognition, patients with pure LBCI had worse comprehension function by virtue of a worse PTA compared with patients with mixed ADCI/LBCI. Another sensitivity analysis in which both the PTA and K-MMSE score were removed from the original model showed that the pure-LBCI group had an OR that was comparable with that of the mixed-ADCI/LBCI group, as did the original model. This sensitivity analysis indicated that more-severe general cognitive dysfunction in the mixed-ADCI/LBCI group led to a level of comprehension dysfunction that was similar to that in the pure-LBCI group with a worse PTA.
DISCUSSION
This study evaluated the associations between HL and the mechanisms underlying CI in CI patients who were carefully phenotyped and diagnosed with the support of imaging biomarkers. Our major findings are as follows: First, the presence of ADCI was associated with a lower risk of PTA-based HL in patients with CI or dementia. Second, the pure-LBCI group had a worse PTA and a higher risk of PTA-based HL than did the pure-ADCI and mixed-ADCI/LBCI groups. Third, the risk of comprehension dysfunction was higher in the pure-LBCI group than in the pure-ADCI group, independent of the PTA and K-MMSE score. In addition, the pure-LBCI group even had a higher risk of comprehension dysfunction than did the mixed-ADCI/LBCI group given the same level of cognition, which was attributable to worse peripheral hearing.
Taken together, these findings indicate that ADCI is characterized by relative sparing of peripheral hearing function and comprehension function, whereas pure LBCI is associated with an increased risk of peripheral HL (based on the PTA) and comprehension dysfunction. Mixed ADCI/LBCI shows profound cognitive dysfunction, which is further associated with comprehension dysfunction without an increased risk of peripheral HL.
The absence of ADCI was associated with a higher PTA, lower WRS, and higher risks of PTA- and WRS-based HL among the enrolled patients with CI. Considering the high sensitivity and specificity of FBB PET in detecting histopathology-confirmed β-amyloid plaques, the absence of ADCI in the patients with CI suggests the presence of other major causes of CI such as LBD and vascular disease.21,24,25,26,27 However, the presence of LBCI by itself was not correlated with the severity and presence of HL. This could be because our study did not include control subjects with normal cognition and hearing function, which could have led to underestimation of the relationship between the cause of CI and HL. However, the pure-LBCI group had a significantly higher OR for HL based on the PTA than did the pure-ADCI and mixed-ADCI/LBCI groups. The non-ADCI/LBCI group—which had the most-severe vascular MRI markers (Table 1)—also had a higher OR for HL based on the PTA than did the pure-ADCI and mixed-ADCI/LBCI groups (Table 3). Therefore, our findings suggest that LBCI without β-amyloid deposition and severe small-vessel disease as detected in brain MRI are associated with peripheral HL, but not with the presence of β-amyloid deposition on amyloid PET. This point of view is consistent with a recent meta-analysis not finding a significant association between age-related HL and cognitive dysfunction in AD.28 However, since we did not recruit pathologically confirmed patients and we did not evaluate the dose-dependent relationship between β-amyloid deposition and HL, future studies are warranted to validate this interpretation.
Our results regarding the association between peripheral HL (based on the PTA) and pure LBCI are consistent with previous studies showing that patients with PD have peripheral HL.29,30 A previous study suggested that α-synuclein located in the cochlea and stria vascularis31 is related to and plays a role in susceptibility to noise-induced peripheral HL.29 Another study found that cochlear function was restored after dopamine treatment, and suggested that the depletion of dopamine in the olivocochlear system could expose primary auditory neurons to excessive glutamate release from inner hair cells that results in peripheral HL.30 Future studies of the correlation between the severities of nigrostriatal dopamine depletion and HL are warranted to determine if there is further support for this hypothesis.
Both the PTA and WRS were better in the mixed-ADCI/LBCI group than in the pure-LBCI group. This suggests that the presence of LBCI does not directly indicate an increased risk of peripheral HL, and that there is a heterogeneity of LBCI in terms of HL according to the presence of ADCI. The differential diagnosis of DLB and PD dementia is based on an arbitrary distinction regarding the timing of cognitive symptoms relative to motor symptoms.32 Considering that significant cerebral β-amyloid deposition is more likely in patients with DLB than in those with PD dementia,33,34,35 and the heterogeneity in terms of HL in patients with LBCI is strongly correlated with the presence of concomitant β-amyloid deposition, the phenotypical heterogeneity in LBD could be attributable to these β-amyloid deposits. Future clinicopathological correlation studies and longitudinal follow-up studies are needed, but it is plausible that significant β-amyloid deposition leads to more-rapid cognitive decline that prevents the appearance of HL in LBCI patients.
Meanwhile, it is noteworthy that the risk of PTA-based HL differed significantly between the study disease groups, whereas that of WRS-based HL did not. Pure-tone audiometry measures the auditory threshold when detecting pure tones, while the word recognition test evaluates speech perception and repetition ability. Because pure-tone audiometry is solely dependent on the function of cochlea and the neurons connected directly to the primary auditory cortex, it closely reflects the functioning of the peripheral auditory system and neural connectivity related to the primary auditory cortex, with rare exceptions such as in auditory agnosia.36 In contrast, the WRS is affected in a complex manner by the brain functions of the auditory association cortex that involve language comprehension and expression.37 Therefore, PTA-based HL reflects relatively peripheral HL, whereas WRS-based HL reflects more-varied and multifactorial causes. Although the subjects with ADCI experienced subjective hearing difficulties in our study, they exhibited superior auditory function in both pure-tone audiometry and the word recognition tests. This suggests that the subjective hearing difficulty in the ADCI group could not be explained by impaired auditory function, and instead was attributable to cognitive dysfunction in learning and memory.
Our analyses of comprehension dysfunction showed that auditory dysfunction was reflected in a higher PTA, cognitive dysfunction was reflected in lower K-MMSE score, and more-severe periventricular WMHs independently contributed to comprehension dysfunction. The risk of comprehension dysfunction was higher in the pure-LBCI group than in the pure-ADCI group, independent of the PTA and K-MMSE score. Given the same level of cognitive function (i.e., K-MMSE score), the risk of comprehension was higher in the pure-LBCI group than in the mixed-ADCI/LBCI group. These results suggest that the mixed-ADCI/LBCI group had better auditory function but worse general cognitive function, which led to comprehension dysfunction comparable to that in the pure-LBCI group. Considering our previous finding that patients with mixed ADCI/LBCI have additional cortical thinning in the bilateral temporoparietal junction and parietal cortices compared with patients with pure ADCI or pure LBCI,21 more-severe neurodegeneration involving the higher order auditory association cortex could explain the comprehension dysfunction found in the present mixed-ADCI/LBCI group.
This study was subject to several limitations. First, because the study had a cross-sectional design, the causal relationship between HL and CI is not yet clear. Second, the absence of a control group meant that our analyses could have underestimated the strength of the relationship between CI and HL. Third, although FDG PET and FBB PET were performed in all participants, about 10% of the LBCI patients did not undergo FP-CIT PET. However, all of these patients satisfied the clinical criteria for DLB and had supportive findings on FDG PET. Fourth, although we excluded patients who were diagnosed as frontotemporal lobar degeneration and atypical parkinsonism including progressive supranuclear palsy, multiple-system atrophy, and corticobasal degeneration, it is possible that other pathologies such as limbic-predominant age-related TDP-43 encephalopathy or argyrophilic grain disease were included. Fifth, considering the low diagnostic sensitivity of FP-CIT imaging, there was a possibility of underdetection of subclinical LBD,38 since some of the patients classified into pure ADCI or non-ADCI/LBCI could have had subclinical LBD. Sixth, the smallness of the sample in the non-ADCI/LBCI group could have resulted in underestimation of differences in hearing impairment. Lastly, all patients were recruited from a university-based memory clinic, which might limit the generalizability of our results. Notwithstanding the above limitations, this is the first study to have investigated the relationship between CI and HL by considering the mechanisms underlying CI with imaging-biomarker support.
In summary, peripheral HL could be associated the absence of significant β-amyloid deposition in patients with CI. Peripheral HL occurred most frequently in the pure-LBCI group, whereas severe general cognitive dysfunction predominated in the mixed-ADCI/LBCI group, and both could lead to comprehension dysfunction. Our results support the common-cause hypothesis, because the mechanisms underlying CI affected the degree of HL given the same degree of cognitive dysfunction. If the cognitive-load hypothesis or cascade hypothesis were valid, the degree of HL would not have differed with the mechanisms underlying CI.
Acknowledgements
This research was supported by a Yonsei University College of Medicine grant funded by the Dongwha Industry (6-2016-0128 to J.J.), a faculty research grant from Yonsei University College of Medicine (6-2018-0052 to S.Y.B.), and the research program through the National Research Foundation of Korea (NRF) funded by the Korea government (2017M3A9E8029721 to J.J.). These funding bodies played no role in the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit the article for publication.
Footnotes
- Conceptualization: Jinsei Jung, Byung Seok Ye, Mijin Yun.
- Data curation: Jinsei Jung, Seong Hoon Bae, Ji Hyuk Han, Sang Hyun Kwak, Gi-Sung Nam.
- Formal analysis: Jinsei Jung, Seong Hoon Bae, Phil Hyu Lee, Mijin Yun, Young Ho Sohn.
- Funding acquisition: Jinsei Jung, Seong Hoon Bae.
- Investigation: Jinsei Jung, Byung Seok Ye, Mijin Yun.
- Methodology: Jinsei Jung, Seong Hoon Bae, Byung Seok Ye.
- Project administration: Jinsei Jung, Byung Seok Ye.
- Resources: Jinsei Jung, Byung Seok Ye, Mijin Yun.
- Supervision: Jinsei Jung, Byung Seok Ye, Mijin Yun.
- Validation: Byung Seok Ye.
- Visualization: Jinsei Jung, Seong Hoon Bae, Byung Seok Ye.
- Writing—original draft: Jinsei Jung, Seong Hoon Bae, Byung Seok Ye.
- Writing—review & editing: Jinsei Jung, Seong Hoon Bae, Byung Seok Ye.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
SUPPLEMENTARY MATERIALS
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2021.17.2.290.
Univariate association of each factor with the degree of hearing impairment
Univariate association of each factor with the presence of hearing loss
Univariate association of each factor with the presence of comprehension dysfunction
References
- 1.Uhlmann RF, Larson EB, Rees TS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261:1916–1919. [PubMed] [Google Scholar]
- 2.Harrison Bush AL, Lister JJ, Lin FR, Betz J, Edwards JD. Peripheral hearing and cognition: evidence from the staying keen in later life (SKILL) study. Ear Hear. 2015;36:395–407. doi: 10.1097/AUD.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin FR, Yaffe K, Xia J, Xue QL, Harris TB, Purchase-Helzner E, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallacher J, Ilubaera V, Ben-Shlomo Y, Bayer A, Fish M, Babisch W, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79:1583–1590. doi: 10.1212/WNL.0b013e31826e263d. [DOI] [PubMed] [Google Scholar]
- 5.Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68:214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurgel RK, Ward PD, Schwartz S, Norton MC, Foster NL, Tschanz JT. Relationship of hearing loss and dementia: a prospective, population-based study. Otol Neurotol. 2014;35:775–781. doi: 10.1097/MAO.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gennis V, Garry PJ, Haaland KY, Yeo RA, Goodwin JS. Hearing and cognition in the elderly. New findings and a review of the literature. Arch Intern Med. 1991;151:2259–2264. [PubMed] [Google Scholar]
- 8.Li Y, Healy EW, Drane JW, Zhang J. Comorbidity between and risk factors for severe hearing and memory impairment in older Americans. Prev Med. 2006;43:416–421. doi: 10.1016/j.ypmed.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Bucks RS, Dunlop PD, Taljaard DS, Brennan-Jones CG, Hunter M, Wesnes K, et al. Hearing loss and cognition in the Busselton Baby Boomer cohort: an epidemiological study. Laryngoscope. 2016;126:2367–2375. doi: 10.1002/lary.25896. [DOI] [PubMed] [Google Scholar]
- 10.Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther. 2014;6:82. doi: 10.1186/s13195-014-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 12.Uchida Y, Sugiura S, Nishita Y, Saji N, Sone M, Ueda H. Age-related hearing loss and cognitive decline - the potential mechanisms linking the two. Auris Nasus Larynx. 2019;46:1–9. doi: 10.1016/j.anl.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Perry EK, Marshall E, Perry RH, Irving D, Smith CJ, Blessed G, et al. Cholinergic and dopaminergic activities in senile dementia of Lewy body type. Alzheimer Dis Assoc Disord. 1990;4:87–95. [PubMed] [Google Scholar]
- 14.Lennox G, Lowe J, Landon M, Byrne EJ, Mayer RJ, Godwin-Austen RB. Diffuse Lewy body disease: correlative neuropathology using anti-ubiquitin immunocytochemistry. J Neurol Neurosurg Psychiatry. 1989;52:1236–1247. doi: 10.1136/jnnp.52.11.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heidebrink JL. Is dementia with Lewy bodies the second most common cause of dementia? J Geriatr Psychiatry Neurol. 2002;15:182–187. doi: 10.1177/089198870201500402. [DOI] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Ye BS, Kim HJ, Kim YJ, Jung NY, Lee JS, Lee J, et al. Longitudinal outcomes of amyloid positive versus negative amnestic mild cognitive impairments: a three-year longitudinal study. Sci Rep. 2018;8:5557. doi: 10.1038/s41598-018-23676-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanc F, Colloby SJ, Cretin B, de Sousa PL, Demuynck C, O'Brien JT, et al. Grey matter atrophy in prodromal stage of dementia with Lewy bodies and Alzheimer's disease. Alzheimers Res Ther. 2016;8:31. doi: 10.1186/s13195-016-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye BS, Lee S, Yoo H, Chung SJ, Lee YH, Choi Y, et al. Distinguishing between dementia with Lewy bodies and Alzheimer's disease using metabolic patterns. Neurobiol Aging. 2020;87:11–17. doi: 10.1016/j.neurobiolaging.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Kang SW, Jeon S, Yoo HS, Chung SJ, Lee PH, Sohn YH, et al. Effects of Lewy body disease and Alzheimer disease on brain atrophy and cognitive dysfunction. Neurology. 2019;92:e2015–e2026. doi: 10.1212/WNL.0000000000007373. [DOI] [PubMed] [Google Scholar]
- 22.Yoo HS, Jeon S, Chung SJ, Yun M, Lee PH, Sohn YH, et al. Olfactory dysfunction in Alzheimer's disease- and Lewy body-related cognitive impairment. Alzheimers Dement. 2018;14:1243–1252. doi: 10.1016/j.jalz.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Lee YG, Jeon S, Yoo HS, Chung SJ, Lee SK, Lee PH, et al. Amyloid-β-related and unrelated cortical thinning in dementia with Lewy bodies. Neurobiol Aging. 2018;72:32–39. doi: 10.1016/j.neurobiolaging.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Leverenz JB. Advisory council July 2015 Meeting Presentation: Lewy Body dementias [Internet] Washington, D.C.: U.S. Department of Health and Human Services; 2015. Jul 27, [cited 2019 Apr 22]. Available from: https://aspe.hhs.gov/advisory-council-july-2015-meeting-presentation-lewy-body-dementias. [Google Scholar]
- 25.Ye BS, Seo SW, Kim GH, Noh Y, Cho H, Yoon CW, et al. Amyloid burden, cerebrovascular disease, brain atrophy, and cognition in cognitively impaired patients. Alzheimers Dement. 2015;11:494–503. doi: 10.1016/j.jalz.2014.04.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 27.Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease--lessons from pathology. BMC Med. 2014;12:206. doi: 10.1186/s12916-014-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018;144:115–126. doi: 10.1001/jamaoto.2017.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vitale C, Marcelli V, Allocca R, Santangelo G, Riccardi P, Erro R, et al. Hearing impairment in Parkinson's disease: expanding the nonmotor phenotype. Mov Disord. 2012;27:1530–1535. doi: 10.1002/mds.25149. [DOI] [PubMed] [Google Scholar]
- 30.Pisani V, Sisto R, Moleti A, Di Mauro R, Pisani A, Brusa L, et al. An investigation of hearing impairment in de-novo Parkinson's disease patients: a preliminary study. Parkinsonism Relat Disord. 2015;21:987–991. doi: 10.1016/j.parkreldis.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Akil O, Weber CM, Park SN, Ninkina N, Buchman V, Lustig LR. Localization of synucleins in the mammalian cochlea. J Assoc Res Otolaryngol. 2008;9:452–463. doi: 10.1007/s10162-008-0134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jellinger KA, Korczyn AD. Are dementia with Lewy bodies and Parkinson's disease dementia the same disease? BMC Med. 2018;16:34. doi: 10.1186/s12916-018-1016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71:903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrou M, Dwamena BA, Foerster BR, MacEachern MP, Bohnen NI, Müller ML, et al. Amyloid deposition in Parkinson's disease and cognitive impairment: a systematic review. Mov Disord. 2015;30:928–935. doi: 10.1002/mds.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomperts SN, Locascio JJ, Marquie M, Santarlasci AL, Rentz DM, Maye J, et al. Brain amyloid and cognition in Lewy body diseases. Mov Disord. 2012;27:965–973. doi: 10.1002/mds.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffiths TD. Central auditory pathologies. Br Med Bull. 2002;63:107–120. doi: 10.1093/bmb/63.1.107. [DOI] [PubMed] [Google Scholar]
- 37.Trumpp NM, Kliese D, Hoenig K, Haarmeier T, Kiefer M. Losing the sound of concepts: damage to auditory association cortex impairs the processing of sound-related concepts. Cortex. 2013;49:474–486. doi: 10.1016/j.cortex.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 38.McKeith I, O'Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6:305–313. doi: 10.1016/S1474-4422(07)70057-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Univariate association of each factor with the degree of hearing impairment
Univariate association of each factor with the presence of hearing loss
Univariate association of each factor with the presence of comprehension dysfunction