Abstract
Background and Purpose
Mental illness is disproportionately common in people with epilepsy (PWE). This systematic literature review identified original research articles that reported the prevalence of psychiatric comorbidities based upon clinical assessments in a sample of PWE and assessed the clinical features of the populations found in studies included in our review of mental health comorbidity.
Methods
The included articles were written in English and published from 2008 to 2018, and focused on adults aged ≥18 years who had psychiatric diagnoses determined in clinical assessments, such as those found in medical records, clinician psychiatric evaluations, structured diagnostic interviews, and mental health screening questionnaires specific for a psychiatric disorder. The primary outcome was the prevalence of psychiatric comorbidities as a percentage of the total sample of PWE. Additional data included the overall sample size, mean age, epilepsy type, study design, and method of diagnosis. A modified Newcastle Ottawa Scale was used to assess the quality of the studies. All 23 articles that were consistent with the inclusion criteria were related to observational studies.
Results
Mood disorders and anxiety disorders were the most common psychiatric comorbidities, with prevalence rates of 35.0% and 25.6%, respectively. Major depressive disorder was the most common mood disorder, with a prevalence of 24.2%. Post-traumatic stress disorder (PTSD) had the highest reported prevalence among anxiety disorders, at 14.2%, followed by general anxiety disorder at 11.1%. Other comorbidities included psychosis (5.7%), obsessivecompulsive disorder (3.8%), schizophrenia (1.7%), bipolar disorder (6.2%), and substance abuse (7.9%). The pooled prevalence of suicidality, as reported for two studies, was 9.3%. Temporal lobe epilepsy (TLE) was associated with higher levels of psychiatric comorbidity. Two (8.7%) of the 23 studies compared psychiatric comorbidities in TLE with that of extratemporal lobe epilepsy (ETLE), and one of these two studies found that depression was more common in TLE (53.8%) than in ETLE (25%). Regarding seizure types, partial seizures were associated with a higher prevalence of depression vs generalized seizures.
Conclusions
This systematic literature review of recent original research found a relatively high prevalence of mental health comorbidities in PWE. Mood and anxiety disorders are the most common comorbidities, while psychotic spectrum conditions such as schizophrenia and bipolar disorder are much rarer. The prevalence of comorbidity may vary with the epilepsy type and treatment responsiveness. These findings suggest that screening tools for depression and anxiety should be included as part of the training for epilepsy care, while resources for other relatively common conditions such as PTSD and substance abuse disorders should be readily available to neurology specialists who treat PWE.
Keywords: comorbidity, epilepsy, mental health, psychiatric disorder, seizures
INTRODUCTION
Epilepsy is a chronic neurological condition that presents with a variety of seizure types, in which the occurrence of specific seizure episodes is generally unpredictable. People with epilepsy (PWE) face more-serious adverse health outcomes than the general population and are at higher risks of disability, poor quality of life (QOL), stigma, and premature mortality.1,2 It has been estimated that 20–30% of PWE have psychiatric comorbidities.3,4,5,6,7,8,9 The rate of psychotic illness in this population is6,7,8,9,10,11,12 times higher than in the general population.10
Psychiatric conditions can complicate the management of epilepsy and have been associated with reduced health-seeking behavior, poor adherence with antiepileptic medications, increased seizure frequency, and poor outcome after epilepsy surgery.11 The QOL may be affected more by psychiatric comorbidities than by seizure frequency.12 The reported prevalence of psychiatric disorders in PWE has varied widely.13 Previous reports on mental illness comorbidity in PWE may be biased by imprecision in the methods used to characterize diagnoses, such as billing diagnoses or self-report, and relatively few reports have assessed correlations of psychiatric comorbidity in PWE.14 There is a need to not only clarify the prevalence of mental illness in PWE, but also to understand the epilepsy-related factors associated with their occurrence.
This systematic literature review identified original research articles that reported the prevalence of psychiatric comorbidities based upon clinical evaluations in samples of PWE, and examined clinical features of the populations in the studies included in our review of these comorbidities. We expect that the results of this analysis will help to identify areas where more research data are needed and to guide future studies.
METHODS
Data collection
The electronic PubMed, PsycINFO, Ovid, and Cochrane databases were searched for articles on the prevalence of psychiatric comorbidities in PWE. The included articles were restricted to those written in English and published between 2008 and 2018. Search terms were selected in collaboration with an institutional librarian at a US academic medical center.
The publication list compiled in the present literature search comprised 3,138 articles. Basic source information, including titles and abstracts, was extracted from each report. Two authors individually screened the list of 3,138 articles using software available from the Rayyan Qatar Computing Research Institute (Rayyan QCRI: https://rayyan.qcri.org/) with an a priori list of inclusion and exclusion criteria.15 Any disagreements were resolved by a senior clinician researcher.
A publication was included if the reported study focused on adults aged ≥18 years, only included patients with psychiatric diagnoses determined in clinical assessments such as those found in medical health records, clinician psychiatric evaluations, structured diagnostic interviews [e.g., Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID) or Mini International Neuropsychiatric Interview (MINI)], and mental health screening questionnaires specific for psychiatric disorders [e.g., Patient Health Questionnaire (PHQ)]. Studies with the billing diagnosis as the sole basis for psychiatric comorbidity were excluded, as were those that relied exclusively on patient self-reports for the psychiatric diagnosis. Similarly, studies were included if the diagnosis of epilepsy was established by a neurologist, EEG recording, or medical health record, with those utilizing informal diagnoses such as self-reported seizures being excluded. After the screening process and subsequent searching through article references, 23 articles were finally included in this review. The entire publication selection process is summarized in Fig. 1.
Fig. 1. Consort diagram of the systematic literature review process.
Data extraction
The primary outcome analyzed was the prevalence of psychiatric comorbidities as a percentage of the total sample of PWE. If multiple methods of diagnosis were employed to identify the psychiatric illness, the results from the most-validated measure were extracted (e.g., a structured clinical interview was prioritized over a screening questionnaire). The additional data collected included the sample size, mean age, type of epilepsy, study design, and method of diagnosis. Table 1 summarizes the data extracted for the 23 studies.16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38
Table 1. Study characteristics.
| n | Study design | Epilepsy types | Methods of epilepsy diagnosis | Methods of psychiatric diagnosis | |
|---|---|---|---|---|---|
| Jansen et al.16 | 87 | Cross-sectional | Drug-resistant focal epilepsy | Confirmed with EEG, MRI, and PET (met ILAE criteria) | Structured clinical interview with two psychiatrists based on MINI (met DSM-IV criteria) |
| Patel et al.17 | 397,440 | Database/epidemiological | N/A | NIS database (ICD-9) | NIS database (ICD-9) |
| Nogueira et al.18 | 144 | Cross-sectional | MTLE | Medical records (met ILAE criteria) | SCID-I (met DSM-IV criteria) |
| Vujisicet al.19 | 70 | Cross-sectional | N/A | Supported by EEG (met ILAE criteria) | HAM-D and HAM-A |
| Clarke et al.20 | 208 | Database/epidemiological | N/A | Finnish Hospital Discharge Register (according to ICD-8, ICD-9, or ICD-10 depending on time of diagnosis) | Finnish Hospital Discharge Register (according to ICD-8, ICD-9, or ICD-10 depending on time of diagnosis) |
| Akanuma et al.21 | 157 | Cross-section | IGE | Confirmed with EEG (met ILAE criteria) | Medical records (ICD-10) |
| Amruth et al.22 | 80 | Case–control | N/A | Medical records (met ILAE criteria) | MINI 6.0 (met DSM-IV and ICD-10 criteria) |
| Labudda et al.23 | 42 | Matched cohort | Epilepsy with PNES | Medical record (pre-existing diagnosis made by an experienced epileptologist) | MINI for current axis-I psychiatric disorders (met DSM-IV criteria) and SCID for personality disorders (ICD-10) |
| Balibey et al.24 | 41 | Cross-sectional | Focal epilepsy (temporal vs. extratemporal) | Met ILAE criteria | Beck Depression Inventory and Beck Anxiety Inventory |
| Kanner et al.25 | 188 | Cross-sectional | N/A | Current diagnosis of epilepsy requiring treatment with AED | SCID for current and past mood disorders and MINI for the rest (met DSM-IV criteria) |
| Kwon et al.26 | 150 | Cross-sectional | N/A | Met ILAE criteria | Patients were administered MINI (met DSM-IV criteria) |
| Gonçalves and Cendes27 | 25 | Cross-sectional | Refractory TLE | TLE diagnosis with EEG and MRI | Structured psychiatric interview and MINI (ICD-10) |
| Bakken et al.28 | 33,571 | Database/epidemiological | N/A | Norwegian Patient Register (ICD-10) | Norwegian Patient Register (ICD-10) |
| Ertekin et al.29 | 56 | Case–control | Confirmed with MRI and EEG (met ILAE criteria) | SCID (met DSM-IV criteria) | |
| Chandrasekharan et al.30 | 150 | Cross-sectional | N/A | Met ILAE criteria | PHQ-12 administered (uses DSM-IV criteria) |
| Wiglusz et al.31 | 96 | Cross-sectional | N/A | Met ILAE criteria | SCID-I (met DSM-IV criteria) |
| McLaughlin et al.32 | 64 | Cross-sectional | N/A | Confirmation with EEG, MRI, or CT (met ILAE criteria) | Computerized structured diagnostic interview (ICD-10 and DSM-IV criteria) |
| Rashid et al.33 | 217 | Cross-sectional | N/A | Diagnosed and receiving treatment at outpatient clinic | MINI |
| Salinsky et al.34 | 70 | Matched cohort | TBI | 24-hour video EEG recording (met ILAE classification) | SCID-I and SCID-II (met DSM-IV criteria) |
| Gandy et al.35 | 147 | Cross-sectional | N/A | Checked by neurologist and clinical nurse specialist (met ILAE criteria) | MINI (met DSM-IV criteria) |
| Mula et al.36 | 143 | Cross-sectional | N/A | Met ILAE criteria | MINI (met DSM-IV criteria) |
| de Araújo Filho et al.37 | 372 | Matched cohort | TLE-MTS and JME | Confirmed with MRI and EEG (met ILAE criteria) | SCID-I (met DSM-IV criteria) |
| de Oliveira et al.38 | 73 | Cross-sectional | TLE | Confirmed with MRI and EEG (met ILAE criteria) | MINI (met DSM-IV criteria) |
AED: antiepileptic drug, DSM: Diagnostic and Statistical Manual of Mental Disorders, HAM-A: Hamilton Anxiety Rating Scale, HAM-D: Hamilton Depression Rating Scale, ICD: International Classification of Diseases, IGE: idiopathic generalized epilepsy, ILAE: International League Against Epilepsy, JME: juvenile myoclonic epilepsy, MINI: Mini International Neuropsychiatric Interview, MTLE: mesial temporal lobe epilepsy, MTS: mesial temporal sclerosis, NIS: National Inpatient Sample, PHQ: Patient Health Questionnaire, PNES: psychogenic nonepileptic seizures, SCID-I: Structured Clinical Interview for DSM-IV, SCID-II: Structured Clinical Interview for DSM-5, TBI: traumatic brain injury, TLE: temporal lobe epilepsy
The terminology utilized to identify psychiatric disorders varied depending the classification system used by the study [i.e., tenth revision of the International Classification of Diseases (ICD-10) or fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)]. Furthermore, many studies that utilized the same classification system to determine psychiatric diagnosis (e.g., DSM-5) used different labels or categories in the associated report. Thus, in order to simplify the interpretation, categories of diagnoses with smaller samples were grouped into larger, more-inclusive classes of disorders. For example, alcohol dependence, alcohol use, and alcohol abuse were all included under the larger general category of alcohol abuse.
The psychiatric diagnoses for which prevalence was most commonly reported were major depressive disorder (MDD) and general anxiety disorder (GAD), in 17 (73.9%) and 10 (43.5%) of the 23 articles, respectively. Other anxiety disorders and mood disorders were also well represented, while relatively few articles reported on the prevalence of substance-abuse disorders and psychotic disorders.
All of the 23 articles included in this literature review were related to observational studies. Only two (8.7%) utilized a case-control study design to directly compare a sample of PWE with healthy controls. Eighteen (78.3%) articles reported on the prevalence of psychiatric comorbidities within a sample of PWE with no comparison groups. Fifteen (65.2%) were cross-sectional studies and three (13.0%) were database/epidemiological studies. Three (13.0%) articles compared psychiatric comorbidities among different subtypes of epilepsy based on matched-cohort studies. One of those three studies compared the prevalence of psychiatric comorbidities between psychogenic nonepileptic seizures (PNES) patients and PNES with epilepsy.
Only 2 (8.7%) studies compared PWE with a control sample; there were no control samples in the other 23 (91.3%) studies. Thus, the prevalence rates of psychiatric comorbidities reported for all 23 studies were pooled, and then compared with general population findings reported in the National Comorbidity Survey Replication (NCS-R) and the National Survey on Drug Use and Health (NSDUH).39,40
Bibliometric analysis
The 23 articles were all published during an 11-year period (from 2008 to 2019). The publication frequency was highest in 2008, 2014, 2017, 2018, and 2019, when three articles were published each year.
The 23 articles were published in 12 peer-review journals. Nine (39.1%) of the articles were published in Epilepsy & Behavior, followed by three (13.0%) in Epilepsia and two (8.7%) in Seizure.
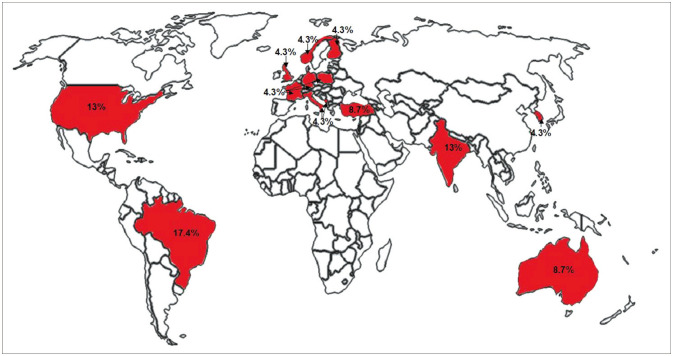
The 23 studies were conducted in 14 different countries. Fig. 2 shows the geographic distribution of the included studies.
Fig. 2. Geographical distribution of the included studies: Brazil (n=4, 17.4%), USA (n=3, 13.0%), India (n=3, 13.0%), Turkey (n=2, 8.7%), Italy (n=1, 4.3%), Australia (n=2, 8.7%), Finland (n=1, 4.3%), South Korea (n=1, 4.3%), Poland (n=1, 4.3%), Norway (n=1, 4.3%), Montenegro (n=1, 4.3%), UK (n=1, 4.3%), France (n=1, 4.3%), and Germany (n=1, 4.3%).
Quality analysis
A modified Newcastle Ottawa Scale (NOS) was used to assess the quality of nonrandomized studies in three categories: 1) the selection of the study group, 2) the comparability of the study group, and 3) the ascertainment of the outcome of interest.41 Two authors independently reviewed the included studies and assigned NOS scores. Any articles that were assigned scores by the two authors that differed by more than 1 point were discussed by them to resolve the conflict.
The NOS scores for each article are listed in Table 2. The maximum score on the modified NOS is 13 points, and the average score across the 23 articles was 9.0 points. The most points were lost for sampling strategy, since many studies only includied samples for a specific epilepsy subtype. Points were also lost for the method of epilepsy diagnosis, since many studies did not verify the diagnosis using EEG.
Table 2. Scores on the modified NOS.
| Sampling strategy | Epilepsy diagnosis | Psychiatric diagnosis | Statistical test | Sample size | Total NOS | |
|---|---|---|---|---|---|---|
| Jansen et al.16 | 1 | 3 | 3 | 1 | 1 | 9 |
| Patel et al.17 | 2 | 1 | 1 | 1 | 1 | 6 |
| Nogueira et al.18 | 1 | 2 | 3 | 1 | 1 | 8 |
| Vujisic et al.19 | 3 | 3 | 2 | 1 | 0 | 9 |
| Clarke et al.20 | 4 | 1 | 1 | 1 | 1 | 8 |
| Akanuma et al.21 | 3 | 3 | 3 | 1 | 1 | 11 |
| Amruth et al.22 | 3 | 2 | 3 | 1 | 1 | 10 |
| Labudda et al.23 | 1 | 1 | 3 | 1 | 1 | 7 |
| Balibey et al.24 | 2 | 2 | 2 | 1 | 1 | 8 |
| Kanner et al.25 | 3 | 1 | 3 | 1 | 1 | 9 |
| Kwon et al.26 | 3 | 2 | 3 | 1 | 0 | 9 |
| Gonçalves and Cendes27 | 1 | 3 | 3 | 1 | 1 | 9 |
| Bakken et al.28 | 4 | 1 | 1 | 1 | 1 | 8 |
| Ertekin et al.29 | 3 | 3 | 3 | 1 | 1 | 11 |
| Chandrasekharan et al.30 | 3 | 2 | 2 | 1 | 1 | 9 |
| Wiglusz et al.31 | 3 | 2 | 3 | 1 | 1 | 10 |
| McLaughlin et al.32 | 1 | 3 | 3 | 1 | 1 | 9 |
| Rashid et al.33 | 3 | 1 | 3 | 1 | 1 | 9 |
| Salinsky et al.34 | 3 | 3 | 3 | 1 | 1 | 11 |
| Gandy et al.35 | 3 | 2 | 3 | 1 | 1 | 10 |
| Mula et al.36 | 3 | 2 | 3 | 1 | 1 | 10 |
| de Araújo Filho et al.37 | 1 | 3 | 3 | 1 | 1 | 9 |
| de Oliveira et al.38 | 1 | 3 | 3 | 1 | 1 | 9 |
| Average | 2.4 | 2.1 | 2.6 | 1.0 | 0.9 | 9.0 |
NOS: Newcastle Ottawa Scale.
RESULTS
The two studies that directly compared control samples (people without epilepsy) with PWE both found that PWE had a larger number of multiple psychiatric conditions.22,29 Both of these studies found that MDD was significantly more prevalent in PWE than controls.22,29 One of the studies found that obsessive-compulsive disorder (OCD) was significantly more prevalent in PWE.29
Table 3 presents the pooled prevalence of psychiatric comorbidities in PWE as reported for the included studies. Mood disorders and anxiety disorders were the most common psychiatric comorbidities, with prevalence rates of 35.0% and 25.6%, respectively. MDD was the most common mood disorder, with a prevalence of 24.2%. Post-traumatic stress disorder (PTSD) was the most common anxiety disorder, with a prevalence of 14.2%, followed by GAD at 11.1%. Other reported comorbidities included psychosis (5.7%), OCD (3.8%), schizophrenia (1.7%), bipolar disorder (6.2%), and substance abuse (7.9%). Although “psychosis” can be used clinically as an umbrella term that includes diagnoses such as schizophrenia and bipolar disorder, it was considered a separate diagnosis in this study, which is consistent with the information in the articles included in this review.
Table 3. Pooled prevalence rates for the included articles.
| Anxiety disorders | Mood disorders | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| GAD | Panic disorder | OCD | PTSD | Total | MDD | Dysthymia | Bipolar disorder | Total | |
| Jansen et al.16 | 10.3 | 12.6 | 2.3 | 3.4 | - | 12.6 | - | - | - |
| Patel et al.17 | - | - | - | - | - | 13.0 | - | - | - |
| Nogueira et al.18 | 0.7 | - | - | - | 12.5 | 9.7 | 2.8 | - | 34.7 |
| Vujisic´ et al.19 | - | - | - | - | 21.42 | 32.85 | - | - | - |
| Clarke et al.20 | - | - | - | - | - | - | - | 1.9 | - |
| Akanuma et al.21 | - | - | - | - | - | - | - | - | 13.4 |
| Amruth et al.22 | - | 1.3 | - | - | - | 26.3 | - | - | - |
| Labudda et al.23 | 7.1 | 7.2 | 2.3 | 16.7 | 40.5 | 4.8 | 7.1 | - | 38.1 |
| Balibey et al.24 | - | - | - | - | 26.8 | 34.14 | - | - | - |
| Kanner et al.25 | 10.6 | 2.1 | 2.7 | - | 14.9 | 16.5 | 2.7 | - | 16.5 |
| Kwon et al.26 | 17.3 | - | - | - | - | 10.0 | - | - | - |
| Gonçalves and Cendes27 | - | - | - | - | - | - | 24 | 12 | 68 |
| Bakken et al.28 | - | - | - | - | - | - | - | 1.5 | - |
| Ertekin et al.29 | 3.6 | - | 7.1 | 1.8 | - | 19.6 | 3.6 | - | - |
| Chandrasekharan et al.30 | - | - | - | - | - | 63.3 | - | - | - |
| Wiglusz et al.31 | 2.1 | 13.5 | - | - | 16.7 | - | - | - | - |
| McLaughlin et al.32 | - | - | - | - | - | 21.9 | 40.6 | - | - |
| Rashid et al.33 | - | - | - | - | - | 41.5 | - | - | - |
| Salinsky et al.34 | - | - | - | 35.0 | 34.9 | 33.0 | - | - | 39.7 |
| Gandy et al.35 | 20.0 | - | 0.7 | - | 30.0 | 24.0 | 7.0 | - | 31.0 |
| Mula et al.36 | - | - | - | - | - | - | - | 11.9 | - |
| de Araújo Filho et al.37 | 14.2 | - | 0.5 | - | 16.1 | 21.2 | 2.4 | 0.5 | 24.2 |
| de Oliveira et al.38 | 21.9 | 9.6 | 11.0 | 42.5 | 21.9 | 2.7 | 9.6 | 49.3 | |
| Average | 11.1 | 7.0 | 3.8 | 14.2 | 25.6 | 10.3 | 6.2 | 35.0 | |
| Substance-abuse disorders | Psychotic disorders | ||||||||
| Alcohol abuse | Drug abuse | Total | Schizophrenia | Psychosis | Total | ||||
| Jansen et al.16 | - | - | - | - | - | 8 | |||
| Patel et al.17 | 8.7 | 7.8 | - | - | 10.4 | - | |||
| Clarke et al.20 | - | - | - | 2.4 | - | 5.2 | |||
| Akanuma et al.21 | - | - | 0.6 | 1.3 | - | - | |||
| Amruth et al.22 | 2.5 | - | - | - | 2.5 | 2.5 | |||
| Labudda et al.23 | - | - | 2.4 | - | - | - | |||
| Bakken et al.28 | 5.74 | 4.32 | - | 1.72 | - | 3.75 | |||
| Ertekin et al.29 | - | - | - | - | - | 1.8 | |||
| Salinsky et al.34 | - | - | 20.6 | - | - | - | |||
| de Araújo Filho et al.37 | 0.5 | - | - | - | - | 9.4 | |||
| de Oliveira et al.38 | - | - | - | 1.4 | 4.1 | 5.5 | |||
| Average | 4.4 | 6.1 | 7.9 | 1.7 | 5.7 | 5.2 | |||
GAD: generalized anxiety disorder, MDD: major depressive disorder, OCD: obsessive-compulsive disorder, PTSD: post-traumatic stress disorder.
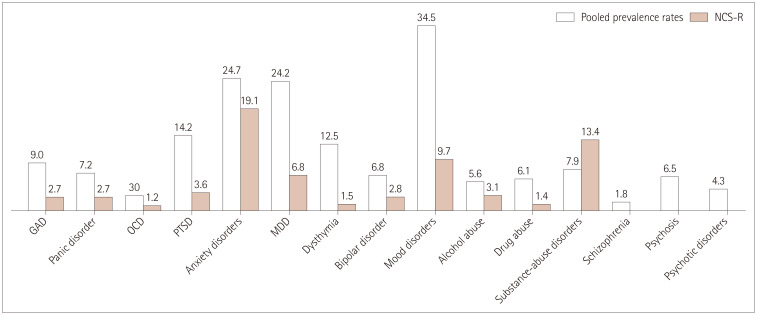
Fig. 3 compares the pooled prevalence rates with those reported for the NCS-R, which was a survey-based study conducted between 2001 and 2003 that used DSM-IV criteria to gather data about the prevalence of mental health conditions in 9,282 randomly selected participants.39 This comparison revealed that PWE had higher rates of anxiety, mood, and psychotic disorders. Though the rates of psychiatric comorbidities were generally higher in PWE than in the general population, this trend was not observed for the combined substance-abuse disorders. However, the separate prevalence rates for drug abuse and alcohol abuse were higher in PWE.
Fig. 3. Comparisons of pooled prevalence rates with those from the NCS-R. GAD: generalized anxiety disorder, MDD: major depressive disorder, NCS-R: National Comorbidity Survey Replication, OCD: obsessive-compulsive disorder, PTSD: post-traumatic stress disorder.
Differences between inpatient and outpatient settings
The reported prevalence of psychiatric comorbidity in PWE can vary depending on the patient setting. For the purpose of this analysis, the patient settings were separated into inpatient and outpatient settings. One (4.3%) article was excluded from this analysis, as it combined patients from the inpatient and outpatient settings,28 while another (4.3%) article was excluded for not reporting whether the subjects were inpatients or outpatients.24 The overall prevalence of psychiatric comorbidity was higher in the inpatient group (n=5, 21.7%) than in the outpatient group (n=16, 69.6%). This difference can be attributed to patients admitted for inpatient care tending to exhibit symptomology that is more severe than for those in outpatient/community settings.
Method of psychiatric diagnosis
The obtained prevalence rate can vary with the method used to diagnose psychiatric conditions due to inherent variations in the diagnostic criteria. The 23 articles included in this analysis were separated into 3 groups based on the method of psychiatric diagnosis. The 16 (69.6%) articles that used clinical interviews such as the MINI and SCID were placed into an interview group, the 4 (17.4%) articles that used information from sources such as medical records and hospital- or population-based registries were placed into a data group, and the 3 (13.0%) articles that utilized questionnaires such as PHQ-9 were placed into a questionnaire group.
The prevalence rates for the interview group were most similar to the overall prevalence rates (Table 3) due to the dominance of articles utilizing clinical interviews. The prevalence of MDD was highest in the questionnaire group (43.4%), followed by 21.8% in the interview group and 13.0% in the data group. Questionnaires such as PHQ-9 are designed to detect symptoms of depression, and hence their use might increase the detection sensitivity and so lead to a higher diagnostic prevalence. In contrast, medical records and databases will only include diagnoses that are observed and documented in the clinical setting. The reported prevalence is dependent on patients choosing to share their symptoms with clinicians, and the prevalence might therefore be underreported in these data sets.
Suicidality
One of the case-control studies found that suicidality was significantly more common in PWE than in healthy and asthmatic controls, and that suicidality increased significantly with the seizure severity.22 The pooled prevalence of suicidality, as reported for two studies, was 9.3%.16,22 This rate is much higher than that of 0.6% in the general US adult population as reported in the 2017 NSDUH by the Substance Abuse and Mental Health Services Administration.40
Multiple psychiatric comorbidities
Nine (39.1%) of the 23 studies had diagnosed multiple psychiatric comorbidities.18,21,23,25,27,28,31,35,37 Six (66.7%) of the nine articles reported on comorbidity of anxiety disorders and mood disorders in PWE, four reported on the prevalence of mixed anxiety disorder and MDD, with a pooled prevalence of 20.5%,18,25,27,35 and one reported the prevalence of mixed GAD and MDD to be 7.3% in patients with temporal lobe epilepsy (TLE).37 One study found that 8.3% of PWE with panic disorder had comorbid MDD,31 while another found that 1.0% of PWE had both substance-abuse and psychotic disorders.28
Psychiatric comorbidities in epilepsy subtypes
TLE was associated with higher levels of psychiatric comorbidity. Two (8.7%) of the 23 studies compared the prevalence of psychiatric comorbidities between TLE and extratemporal lobe epilepsy (ETLE).16,23 Both of these studies found that anxiety was more common in TLE than ETLE, at 37.3% and 14.85%, respectively. One of the two studies found that depression was more common in TLE, at 53.8%, compared with 25% in ETLE.23 Another study compared TLE with idiopathic generalized epilepsy (IGE), and found that the rate of axis-I psychiatric disorders was significantly higher in TLE (75.9%) than in IGE (48.1%).29 One study found that psychotic disorders were significant more common in TLE patients with mesial temporal sclerosis than in those with juvenile myoclonic epilepsy.37
Regarding seizure types, two studies found that depression was more prevalent in partial seizures (37.2%) than generalized seizures (19.2%).19,22 Partial seizures include simple partial seizures, complex partial seizures, and partial seizures with secondary generalization.
Two studies examined differences between left and right focal epilepsy, with both finding no correlation between lateralization and the frequency of psychiatric comorbidities.16,37 One study compared psychiatric comorbidities in limbic and extralimbic epilepsy, but found no difference between these two groups.16
Treatment response
A positive treatment response, such as epilepsy being well controlled through pharmaceutical management, was associated with lower levels of psychiatric comorbidity in PWE. Four (17.4%) of the 23 studies compared the rate of psychiatric comorbidities between pharmacoresistant and treatment-responsive epilepsy. Two of these four articles reported on the prevalence of depression in PWE. MDD was diagnosed in an average of 54.0% of PWE with uncontrolled epilepsy and 12.5% of PWE with controlled epilepsy.19,22 The other two studies investigated the prevalence of comorbid mental disorders, and found that a comorbid mental disorder was present in 46.9% of patients with uncontrolled epilepsy and 26.5% of those with controlled epilepsy.18,21 One article reported on the prevalence of mixed anxiety and mood disorder symptoms: 75% of patients with uncontrolled epilepsy and 25% patients with controlled epilepsy had comorbid anxiety and mood disorders.18
DISCUSSION
This systematic literature review applied rigorous methods to identify original research articles on the determination of psychiatric comorbidity. The findings support the previous literature showing a relatively high prevalence of mental health comorbidity in PWE. Two studies that directly compared samples of PWE with healthy controls found a significantly higher prevalence of psychiatric diagnoses in PWE.22,29 Comparing the pooled review study sample rates with large US nationally representative general population samples39,40 revealed that PWE experience disproportionally higher rates of nearly all psychiatric comorbidities. More data were available on anxiety disorders and mood disorders than other types of psychiatric disorders identified in this literature review. These findings also highlight the need for research into other types of psychiatric comorbidities in PWE, including psychotic and bipolar disorders.
Our review found that mood and anxiety disorders are the most common psychiatric comorbidities in PWE: approximately one in three PWE has a mood disorder and approximately one in four has an anxiety disorder. With respect to specific psychiatric conditions, MDD is seen in nearly one-quarter of PWE and PTSD is seen in more than 14% of them. Psychotic spectrum conditions, such as schizophrenia (1.8% of PWE) and bipolar disorder (6.8% of PWE), are far less common than mood and anxiety conditions.
Substance abuse was present in just under 8% of the PWE included in this review. However, these findings must be interpreted in the context of the relatively small samples in the included studies. There are also potentially confounding issues when interpreting the findings. For example, two large-scale studies of drug abuse and alcohol abuse did not produce information about substance-abuse disorders as a larger category.17,28 Furthermore, two of the three studies of substance-abuse disorders applied a restricted sampling strategy, with one focusing on IGE patients and the other focusing on patients with PNES and epilepsy.21,23 These characteristics may have introduced bias into our pooled prevalence rates.
Some previously systematic reviews have also pooled the prevalence rates of psychosis,42 depression,43,44 and anxiety44 in PWE. It is difficult to perform direct comparisons between such studies due to methodological differences in the analyzed electronic databases, diagnostic terminologies, and statistical analyses. However, the pooled prevalence rates found in the present study were consistent with those reported in other reviews.
With respect to predictors of comorbidity in the context of specific epilepsy types, our review suggests that patients with TLE have higher rates of psychiatric comorbidities than do patients with ETLE.38,45 Similarly, multiple studies have found that the prevalence of psychiatric comorbidities is higher in focal than generalized epilepsy.37 There is less data available for other possible anatomic associations, such as lateralization and limbic/extralimbic epilepsy. The literature contains inconsistent information about whether or not epilepsy lateralization affects psychiatric comorbidities,37,38 and so additional studies with larger samples are needed to provide more detailed anatomical information. Clarifying the relationship between anatomy and pathophysiology will yield prognostic information for guiding clinical care and optimizing epilepsy management.
The present finding that treatment-resistant epilepsy is associated with increased risks of MDD and anxiety disorder is consistent with the previous literature.42 However, this does not explain whether uncontrolled seizures cause psychiatric symptoms or whether there is a shared underlying neurophysiology that causes both intractable epilepsy and psychiatric disorders. Some studies have found that patients with a psychiatric diagnosis are more likely to develop epilepsy, which supports the existence of a common pathology.46,47,48 However, there is need for specific investigations into the relationship between intractable epilepsy and psychiatric conditions.
Finally, the relatively overall high prevalence of psychiatric comorbidity in PWE identified in this review underscores the importance of supporting clinicians who provide epilepsy care to patients with neuropsychiatric symptoms. A recent report from the Task Force on Education of the ILAE Commission on Neuropsychiatry surveyed clinicians from 36 countries about critical areas for skills improvement and their preferred educational format for improving these skills.49 The sampled clinicians prioritized screening for neuropsychiatric conditions and receiving education on managing specific scenarios. The findings of the present review suggest that validated screening tools such as the Neurological Disorders Depression Inventory for Epilepsy50 should be included in the training curriculum for epilepsy care, while information and care referral resources for other relatively common conditions such as PTSD and substance-abuse disorders should be readily available to neurology specialists who treat PWE.
Limitations
As with any literature review, the present analysis was subject to limitations that impact its generalizability and interpretation. This study only assessed articles written in English, and so the geographic distribution may have been biased toward English-speaking countries. Moreover, high variability was present in the type of PWE samples among the 23 studies, with some articles only reporting on TLE and other articles including analyses of large amounts of aggregated data that included all PWE. This was the source of the greatest variability among the studies, as evidenced by the variance in the modified-NOS scores. Furthermore, there were huge differences in the sample size, which ranged from 25 to 397,440 across the 23 studies. Unfortunately, there is no ideal statistical approach for balancing the effects of such a large variation in sample sizes across the included studies, which were performed in different locations, at different times, and using diverse tools for determining diagnoses. This could have resulted in studies that included small samples of specific types of epilepsy or other biasing factors disproportionately influencing the pooled prevalence rates. The pooled prevalence rates were also compared with those for the general population from US representative surveys. Furthermore, this review only included studies reported on between 2008 and 2018. The original database search was completed in mid-2019, and we chose to focus on the most recent decade in order to ensure that the included studies would have the greatest impact on current clinical care. The exclusion of original research published prior to 2008 or after 2018 therefore represents another potential limitation. Another database search was completed early in 2020 in order to identify recent studies that may have been prematurely excluded from our review. Although two articles were identified, they did not describe how epilepsy was diagnosed, and they might not have met our inclusion criteria.51,52 In the future, larger systematic literature reviews that include a wider range of publication years, more diverse samples, and studies with more consistent diagnostic evaluations could provide a better understanding of the prevalence of psychiatric comorbidity in PWE. Finally, this study performed a qualitative analysis, and thus might not have been able to identify gaps in clinical care. However, we hope that our results will be informative to future meta-analyses on this topic.
CONCLUSIONS
This systematic literature review has revealed that the prevalence of mental health comorbidities is relatively high in PWE. Mood and anxiety disorders are the most common comorbidities, while psychotic spectrum conditions such as schizophrenia and bipolar disorder are much rarer. The prevalence of psychiatric diagnoses in PWE may differ with epilepsy type and treatment response. These findings suggest that screening tools for depression and anxiety should be included as part of the training for epilepsy care, while resources for other relatively common conditions such as PTSD and substance-abuse disorders should be readily available to neurology specialists who treat PWE.
Acknowledgements
None
Footnotes
- Conceptualization: Martha Sajatovic.
- Data curation: Elaine Lu, Nataliya Pyatka.
- Formal analysis: Elaine Lu.
- Methodology: Elaine Lu.
- Writing—original draft: Elaine Lu, Nataliya Pyatka, Christopher J Burant.
- Writing—review & editing: all authors.
Conflicts of Interest: Author Sajatovic has the following conflicts of interest to disclose: In the last 3 years she has received research grants from Nuromate, Otsuka, Alkermes, International Society for Bipolar Disorders (ISBD), National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), Patient-Centered Outcomes Research Institute (PCORI); Consultant: Alkermes, Otsuka, Janssen, Myriad, Health Analytics, Frontline Medical Communications; Royalties: Springer Press, Johns Hopkins University Press, Oxford Press, UpToDate; Compensation for preparation of CME activities: American Physician's Institute, MCM Education, CMEology, Potomac Center for Medical Education, Global Medical Education, Creative Educational Concepts, Psychopharmacology Institute. The other authors have no potential conflicts of interest to disclose.
References
- 1.England MJ, Liverman CT, Schultz AM, Strawbridge LM. Epilepsy across the spectrum: promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy Behav. 2012;25:266–276. doi: 10.1016/j.yebeh.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Epilepsy Fast Facts [Internet] Atlanta, GA: Centers for Disease Control and Prevention (CDC); [cited 2020 Mar 23]. Available from: http://www.cdc.gov/epilepsy/basics/fast_facts.htm. [Google Scholar]
- 3.Vuilleumier P, Jallon P. Epilepsy and psychiatric disorders: epidemiological data. Rev Neurol (Paris) 1998;154:305–317. [PubMed] [Google Scholar]
- 4.Barry JJ, Lembke A, Gisbert PA, Gilliam F. Affective disorders in epilepsy. In: Ettinger AB, Kanner AM, editors. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 203–247. [Google Scholar]
- 5.Jobe PC, Dailey JW, Wernicke JF. A noradrenergic and serotonergic hypothesis of the linkage between epilepsy and affective disorders. Crit Rev Neurobiol. 1999;13:317–356. doi: 10.1615/critrevneurobiol.v13.i4.10. [DOI] [PubMed] [Google Scholar]
- 6.Jobe PC. Common pathogenic mechanisms between depression and epilepsy: an experimental perspective. Epilepsy Behav. 2003;4 Suppl 3:S14–S24. doi: 10.1016/j.yebeh.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Tellez-Zenteno JF, Patten SB, Jetté N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007;48:2336–2344. doi: 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- 8.Ettinger A, Reed M, Cramer J Epilepsy Impact Project Group. Depression and comorbidity in community-based patients with epilepsy or asthma. Neurology. 2004;63:1008–1014. doi: 10.1212/01.wnl.0000138430.11829.61. [DOI] [PubMed] [Google Scholar]
- 9.Kobau R, DiIorio CA, Price PH, Thurman DJ, Martin LM, Ridings DL, et al. Prevalence of epilepsy and health status of adults with epilepsy in Georgia and Tennessee: Behavioral Risk Factor Surveillance System, 2002. Epilepsy Behav. 2004;5:358–366. doi: 10.1016/j.yebeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40 Suppl 10:S2–S20. doi: 10.1111/j.1528-1157.1999.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 11.Boylan LS, Flint LA, Labovitz DL, Jackson SC, Starner K, Devinsky O. Depression but not seizure frequency predicts quality of life in treatment-resistant epilepsy. Neurology. 2004;62:258–261. doi: 10.1212/01.wnl.0000103282.62353.85. [DOI] [PubMed] [Google Scholar]
- 12.Rafnsson V, Olafsson E, Hauser WA, Gudmundsson G. Cause-specific mortality in adults with unprovoked seizures. A population-based incidence cohort study. Neuroepidemiology. 2001;20:232–236. doi: 10.1159/000054795. [DOI] [PubMed] [Google Scholar]
- 13.Brooks-Kayal AR, Bath KG, Berg AT, Galanopoulou AS, Holmes GL, Jensen FE, et al. Issues related to symptomatic and disease-modifying treatments affecting cognitive and neuropsychiatric comorbidities of epilepsy. Epilepsia. 2013;54 Suppl 4:44–60. doi: 10.1111/epi.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loughman A, Bowden SC, D'Souza WJ. Self and informant report ratings of psychopathology in genetic generalized epilepsy. Epilepsy Behav. 2017;67:13–19. doi: 10.1016/j.yebeh.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen C, Francomme L, Vignal JP, Jacquot C, Schwan R, Tyvaert L, et al. Interictal psychiatric comorbidities of drug-resistant focal epilepsy: prevalence and influence of the localization of the epilepsy. Epilepsy Behav. 2019;94:288–296. doi: 10.1016/j.yebeh.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 17.Patel RS, Elmaadawi A, Mansuri Z, Kaur M, Shah K, Nasr S. Psychiatric comorbidities and outcomes in epilepsy patients: an insight from a nationwide inpatient analysis in the United States. Cureus. 2017;9:e1686. doi: 10.7759/cureus.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogueira MH, Yasuda CL, Coan AC, Kanner AM, Cendes F. Concurrent mood and anxiety disorders are associated with pharmaco-resistant seizures in patients with MTLE. Epilepsia. 2017;58:1268–1276. doi: 10.1111/epi.13781. [DOI] [PubMed] [Google Scholar]
- 19.Vujisić S, Vodopić S, Radulović L, Injac-Stevović L. Psychiatric comorbidities among patients with epilepsy in Montenegro. Acta Clin Croat. 2014;53:411–416. [PubMed] [Google Scholar]
- 20.Clarke MC, Tanskanen A, Huttunen MO, Clancy M, Cotter DR, Cannon M. Evidence for shared susceptibility to epilepsy and psychosis: a population-based family study. Biol Psychiatry. 2012;71:836–839. doi: 10.1016/j.biopsych.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Akanuma N, Hara E, Adachi N, Hara K, Koutroumanidis M. Psychiatric comorbidity in adult patients with idiopathic generalized epilepsy. Epilepsy Behav. 2008;13:248–251. doi: 10.1016/j.yebeh.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Amruth G, Praveen-Kumar S, Nataraju B, Kasturi P. Study of psychiatric comorbidities in epilepsy by using the Mini International Neuropsychiatric Interview. Epilepsy Behav. 2014;33:94–100. doi: 10.1016/j.yebeh.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Labudda K, Frauenheim M, Illies D, Miller I, Schrecke M, Vietmeier N, et al. Psychiatric disorders and trauma history in patients with pure PNES and patients with PNES and coexisting epilepsy. Epilepsy Behav. 2018;88:41–48. doi: 10.1016/j.yebeh.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Balibey H, Yasar H, Tekeli H, Bayar N. Frequency of anxiety and depression in epileptic patients. Klin Psikofarmakol B. 2015;25:136–140. [Google Scholar]
- 25.Kanner AM, Barry JJ, Gilliam F, Hermann B, Meador KJ. Anxiety disorders, subsyndromic depressive episodes, and major depressive episodes: do they differ on their impact on the quality of life of patients with epilepsy? Epilepsia. 2010;51:1152–1158. doi: 10.1111/j.1528-1167.2010.02582.x. [DOI] [PubMed] [Google Scholar]
- 26.Kwon OY, Park SP. Validity of the Liverpool Adverse Events Profile as a screening tool for detecting comorbid depression or anxiety disorder in people with epilepsy. J Epilepsy Res. 2018;8:74–80. doi: 10.14581/jer.18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonçalves EB, Cendes F. Depression in patients with refractory temporal lobe epilepsy. Arq Neuropsiquiatr. 2011;69:775–777. doi: 10.1590/s0004-282x2011000600010. [DOI] [PubMed] [Google Scholar]
- 28.Bakken IJ, Revdal E, Nesvåg R, Brenner E, Knudsen GP, Surén P, et al. Substance use disorders and psychotic disorders in epilepsy: a population-based registry study. Epilepsy Res. 2014;108:1435–1443. doi: 10.1016/j.eplepsyres.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Ertekin BA, Kulaksizoğlu IB, Ertekin E, Gürses C, Bebek N, Gökyiğit A, et al. A comparative study of obsessive-compulsive disorder and other psychiatric comorbidities in patients with temporal lobe epilepsy and idiopathic generalized epilepsy. Epilepsy Behav. 2009;14:634–639. doi: 10.1016/j.yebeh.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Chandrasekharan SC, Menon V, Wadwekar V, Nair PP. High frequency of depressive symptoms among adults with epilepsy: results from a hospital-based study. J Neurosci Rural Pract. 2017;8(Suppl 1):S13–S19. doi: 10.4103/jnrp.jnrp_21_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiglusz MS, Landowski J, Cubała WJ. Psychometric properties of the Polish version of the Hamilton Anxiety Rating Scale in patients with epilepsy with and without comorbid anxiety disorder. Epilepsy Behav. 2019;94:9–13. doi: 10.1016/j.yebeh.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin DP, Pachana NA, McFarland K. Depression in a community-dwelling sample of older adults with late-onset or lifetime epilepsy. Epilepsy Behav. 2008;12:281–285. doi: 10.1016/j.yebeh.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Rashid H, Katyal J, Tripathi M, Sood M, Gupta YK. Validation of the Indian version of Neurological Disorders Depression Inventory for Epilepsy (NDDI-E) Epilepsy Behav. 2019;95:75–78. doi: 10.1016/j.yebeh.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 34.Salinsky M, Rutecki P, Parko K, Goy E, Storzbach D, O'Neil M, et al. Psychiatric comorbidity and traumatic brain injury attribution in patients with psychogenic nonepileptic or epileptic seizures: a multicenter study of US veterans. Epilepsia. 2018;59:1945–1953. doi: 10.1111/epi.14542. [DOI] [PubMed] [Google Scholar]
- 35.Gandy M, Sharpe L, Perry KN, Miller L, Thayer Z, Boserio J, et al. Anxiety in epilepsy: a neglected disorder. J Psychosom Res. 2015;78:149–155. doi: 10.1016/j.jpsychores.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Mula M, Schmitz B, Jauch R, Cavanna A, Cantello R, Monaco F, et al. On the prevalence of bipolar disorder in epilepsy. Epilepsy Behav. 2008;13:658–661. doi: 10.1016/j.yebeh.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 37.de Araújo Filho GM, Mazetto L, da Silva JM, Caboclo LO, Yacubian EM. Psychiatric comorbidity in patients with two prototypes of focal versus generalized epilepsy syndromes. Seizure. 2011;20:383–386. doi: 10.1016/j.seizure.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 38.de Oliveira GN, Kummer A, Salgado JV, Portela EJ, Sousa-Pereira SR, David AS, et al. Psychiatric disorders in temporal lobe epilepsy: an overview from a tertiary service in Brazil. Seizure. 2010;19:479–484. doi: 10.1016/j.seizure.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Harvard Medical School. National Comorbidity Survey (NSC): NCS-R twelve-month prevalence estimates [Internet] Boston, MA: Harvard Medical School; [cited 2020 Mar 23]. Available from: https://www.hcp.med.harvard.edu/ncs/index.php. [Google Scholar]
- 40.Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health: 2017 methodological summary and definitions [Internet] Rockville, ML: Substance Abuse and Mental Health Services Administration (SAMHSA); [cited 2018 Dec 11]. Available from: https://www.samhsa.gov/data/report/2017-methodological-summary-and-definitions. [Google Scholar]
- 41.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] Ottawa: Ottawa Hospital Research Institute; [cited 2019 Sep]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 42.Clancy MJ, Clarke MC, Connor DJ, Cannon M, Cotter DR. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. 2014;14:75. doi: 10.1186/1471-244X-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiest KM, Dykeman J, Patten SB, Wiebe S, Kaplan GG, Maxwell CJ, et al. Depression in epilepsy: a systematic review and meta-analysis. Neurology. 2013;80:590–599. doi: 10.1212/WNL.0b013e31827b1ae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott AJ, Sharpe L, Hunt C, Gandy M. Anxiety and depressive disorders in people with epilepsy: a meta-analysis. Epilepsia. 2017;58:973–982. doi: 10.1111/epi.13769. [DOI] [PubMed] [Google Scholar]
- 45.Perez MM, Trimble MR, Murray NM, Reider I. Epileptic psychosis: an evaluation of PSE profiles. Br J Psychiatry. 1985;146:155–163. doi: 10.1192/bjp.146.2.155. [DOI] [PubMed] [Google Scholar]
- 46.Hesdorffer DC, Ishihara L, Mynepalli L, Webb DJ, Weil J, Hauser WA. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol. 2012;72:184–191. doi: 10.1002/ana.23601. [DOI] [PubMed] [Google Scholar]
- 47.Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59:35–41. doi: 10.1002/ana.20685. [DOI] [PubMed] [Google Scholar]
- 48.Chung MC, Allen RD, Dennis I. The impact of self-efficacy, alexithymia and multiple traumas on posttraumatic stress disorder and psychiatric co-morbidity following epileptic seizures: a moderated mediation analysis. Psychiatry Res. 2013;210:1033–1041. doi: 10.1016/j.psychres.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 49.Mula M, Cavalheiro E, Guekht A, Kanner AM, Lee HW, Özkara Ç, et al. Educational needs of epileptologists regarding psychiatric comorbidities of the epilepsies: a descriptive quantitative survey. Epileptic Disord. 2017;19:178–185. doi: 10.1684/epd.2017.0915. [DOI] [PubMed] [Google Scholar]
- 50.Gill SJ, Lukmanji S, Fiest KM, Patten SB, Wiebe S, Jetté N. Depression screening tools in persons with epilepsy: a systematic review of validated tools. Epilepsia. 2017;58:695–705. doi: 10.1111/epi.13651. [DOI] [PubMed] [Google Scholar]
- 51.Kim J, Kim Y, Bae JS, Lee JH, Song HK. Concomitant psychiatric symptoms in neurological outpatients. Int J Environ Res Public Health. 2019;16:860. doi: 10.3390/ijerph16050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tareke M, Birehanu M, Amare D, Abate A. Common mental illness among epilepsy patients in Bahir Dar city, Ethiopia: a cross-sectional study. PLoS One. 2020;15:e0227854. doi: 10.1371/journal.pone.0227854. [DOI] [PMC free article] [PubMed] [Google Scholar]