Abstract
Immune checkpoint inhibitors (ICIs), despite their ability to potentiate antitumor T-cell responses, may cause various immune-related adverse events. Most cases of thrombocytopenia induced by ICIs have revealed a pathophysiologic mechanism of immune thrombocytopenia with increased platelet destruction and preserved megakaryocytes. Acquired amegakaryocytic thrombocytopenic purpura (AATP) is an unusual disorder characterized by thrombocytopenia with markedly diminished bone marrow megakaryocytes in the presence of otherwise normal hematopoiesis. AATP caused by ICIs has not been reported on. Herein, we present the case of a 79-year-old man diagnosed with squamous cell carcinoma of the lung who developed AATP after two courses of durvalumab, a drug targeting programmed death-ligand 1. Two weeks after the second cycle, his platelet count decreased to 2.1 × 104/μL. After the patient underwent platelet transfusion, his platelet count increased to 8.1 × 104/μL the next day but subsequently decreased repeatedly even after the ICI was discontinued. Six weeks after the second cycle, he developed interstitial pneumonia and was administered prednisolone (50 mg/day). However, thrombocytopenia did not improve. Bone marrow biopsy showed scarce megakaryocytes (< 1 megakaryocyte/10 high-power fields) with preservation of myeloid and erythroid series. Myelodysplasia, myelofibrosis, or metastatic lesions were not observed. Cytogenetic analysis showed a normal male karyotype of 46XY. Hence, the patient received eltrombopag, a thrombopoietin receptor agonist, and his platelet count subsequently improved. After recovery, bone marrow aspiration revealed a normal number of megakaryocytes. AATP is rarely the type of thrombocytopenia induced by ICIs and may be successfully treated with thrombopoietin receptor agonists.
Keywords: : acquired amegakaryocytic thrombocytopenia, immune-related thrombocytopenia, immune checkpoint inhibitor, durvalumab, eltrombopag
INTRODUCTION
Immune checkpoint inhibitors (ICIs), despite their ability to potentiate antitumor T-cell responses, may cause various immune-related adverse events (irAEs). Hematologic irAEs (hem-irAEs) occur less frequently at a rate of 0.5% for grade ≥ 2 events (graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events. version 4.03).1,2 Among the hem-irAEs, immune-related thrombocytopenia is the most common type, along with autoimmune hemolytic anemia and neutropenia, during programmed death-1 /programmed death-ligand 1 (PD-L1) treatment.2 Most cases of thrombocytopenia induced by ICIs have revealed an immune mechanism with increased platelet destruction and preserved megakaryocytes.
Acquired amegakaryocytic thrombocytopenic purpura (AATP) is a rare hematological disorder manifesting as thrombocytopenia with a remarkable reduction of megakaryocytes in the bone marrow and preserved myeloid and erythroid series. AATP caused by ICIs has not yet been reported on. Herein, we report the case of a patient with squamous cell carcinoma of the lung who developed AATP after two cycles of durvalumab (an ICI targeting PD-L1) and was successfully treated with eltrombopag.
CASE REPORT
A 78-year-old man with stage IV squamous cell carcinoma of the lung was treated with four cycles of nab-paclitaxel and carboplatin in April 2018. However, in March 2019, tumor enlargement was observed in this patient. Hence, four cycles of nab-paclitaxel and carboplatin were reinitiated, and he also received radiation therapy with 60 Gy/30 fr since July 2019. He was subsequently treated with durvalumab in September 2019 (Day 0). His platelet count was 14.7 × 104/µL, white cell count 4,890/µL with 74.5% neutrophils, and hemoglobin level 9.9 g/dL immediately before the start of durvalumab treatment. Two weeks after the first cycle, his platelet count was 10.9 × 104/µL, and he received a second cycle (Day 14). Two weeks after the second cycle of durvalumab, the platelet count decreased to 2.1 × 104/µL (Day 28). The patient’s white blood cell count was 6,960/µL with 0.5% myelocytes, 68.5% neutrophils, 6.0% lymphocytes, 8.0% monocytes, 15.0% eosinophils, 1.5% basophils, and 0.5% atypical lymphocytes. The hemoglobin level was 10.8 g/dL, and blood film microscopy was negative for schistocytes. The coagulation test results were normal. He had no history of hematologic disease. His regular medication consisted only of silodosin for prostatic hypertrophy, and other than anti-PD-L1 treatment, no other medication was recently prescribed. The third cycle of durvalumab was discontinued. The patient underwent platelet transfusion successfully, considering that his platelet count increased to 8.1 × 104/µL the next day. Despite discontinuing ICI, platelet counts decreased after repeated platelet transfusion (Figure 1).
Fig. 1.
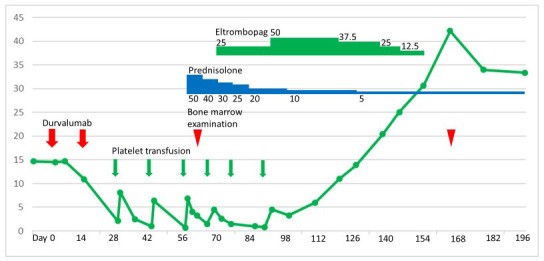
Clinical course illustrating thrombocytopenia
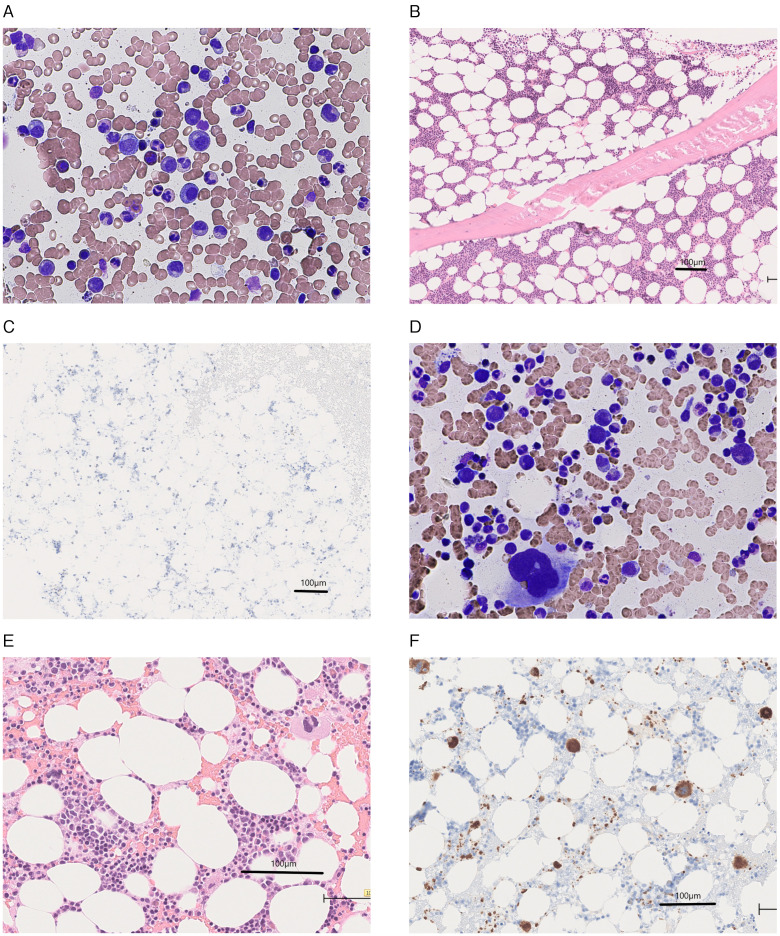
Six weeks after the second cycle of durvalumab (Day 56), he developed interstitial pneumonia, which was considered to be drug-induced, that is, immune-related interstitial pneumonia. He was admitted to our hospital. His platelet count was 0.7 ×104/µL, white cell count 10,850/µL with 81.0% neutrophils, and hemoglobin level 9.6 g/dL. Treatment with prednisolone (50 mg/day) and antibiotics was initiated. Subsequently, although his dyspnea subsided, thrombocytopenia persisted. The coagulation test results were normal. Antibodies against Helicobacter pylori were not detected. The platelet-associated immunoglobulin G level was 243.5 ng/107 (normal range < 30.2 ng/107). The bone marrow smear test showed moderately hypocellular marrow (nucleated cell count [NCC], 12.6 × 104/µL [normal range, 10–25 × 104/µL]). However, megakaryocytes were not observed on the glass slide (megakaryocyte levels ˂ 3.0/µL [normal range, 10–49/µL]), despite the preservation of myeloid, erythroid, and lymphoid series. Myelodysplasia, myelofibrosis, or metastatic lesions were not observed (Figure 2, A). In the bone marrow core biopsy result, moderate hypocellularity of 20%–30% was observed, with significantly rare megakaryocytes (i.e., <1 megakaryocyte/10 high-power fields) (Figure 2, B–C). The thrombocytopenia was considered to be due to AATP caused by ICI. A remarkable decrease in his hemoglobin level was not noticed. Therefore, we considered AATP rather than aplastic anemia. Cytogenetic analysis of the bone marrow showed a normal male karyotype of 46XY. Prednisolone for 13 days failed to improve thrombocytopenia. We initiated eltrombopag treatment on Day 69. In response, the platelet count subsequently improved. Platelet transfusion was no longer required after the last transfusion on Day 89. Eltrombopag was tapered off on Day 155. After recovery (on Day 166), a repeat bone marrow examination revealed a normal number of megakaryocytes (NCC, 16.0 × 104/µL; megakaryocyte level, 28.0/µL) (Figure 2, D–F). The platelet count was in the range of 22.3–48.0 × 104/µL until the patient’s death in July 2020 due to exacerbation of his squamous cell carcinoma of the lung.
Fig. 2.
Bone marrow examination at disease onset (A-C) and after recovery (D-F): (A) May-Giemsa-stained bone marrow smear showing the absence of megakaryocytes and the maintenance of myeloid, erythroid, and lymphoid series without dysplasia or leukemic cells. (B) Hematoxylin- and eosin-stained sections of bone marrow biopsy showing the absence of megakaryocytes without myelofibrosis. (C) Immunohistochemical staining for cluster of differentiation (CD) 61, a platelet glycoprotein IIIa, which is expressed on megakaryocytes and platelets, showing the absence of megakaryocytes. (D) May-Giemsa-stained bone marrow smear showing megakaryocytes. (E) Hematoxylin- and eosin-stained sections of the bone marrow clot showing megakaryocytes. (F) Immunohistochemical staining for CD61 showing megakaryocytes and a myriad of platelets.
DISCUSSION
ICI treatment can lead to irAEs that involve multiple organs. In some cases, irAEs are recognized as being caused by an auto-inflammatory response driven by systemic activation of innate immunity. In other cases, they are more likely to be autoimmune in nature, with the presence of autoantibodies, and yet in other cases, antigen-specific memory T-cell responses indicative of adaptive immunity have been documented.3
To date, most cases of thrombocytopenia induced by ICIs have been shown to be of immune origin with increased platelet destruction and preserved megakaryocytes. These patients’ thrombocytopenia was refractory to platelet transfusions and improved with corticosteroid administration. Of the 15 immune-related thrombocytopenic patients, the megakaryocyte levels were categorized as follows: 6, “elevated”; 1, “normal”; 1, “maintained”; and 7, “present” (Table 1). The platelet counts of the eight patients with information regarding platelet transfusion did not increase (Table 1). The platelet counts of seven out of eight patients treated with steroids alone increased (Table 1). AATP caused by ICIs has not been previously reported, although central immune cytopenias causing hematopoietic stem cell depletion, such as aplastic anemia and pure red cell aplasia, have been reported as irAEs.
Table 1. Characteristics of immune-related thrombocytopenia induced by immune checkpoint inhibitors.
| Author Ref. (year) | Age/ sex | Agents/cycle | platelet count × 104/µL | IPF | Megakaryocyte | Platelet transfusion | Treatment |
|---|---|---|---|---|---|---|---|
| Ahmad et al.4 (2011) | 57/M | Ipili/2 | 0.4 | Not reported | Elevated | No effect | S○ I● |
| Kopecky et al.5 (2015) | 54/M | Ipili/1 | 0.3 | Not reported | & | No effect | S○ |
| Bagley et al.6 (2016) | 34/M | Nivo/8 | 3.3 | Not reported | Not examined | Not used | Romiplostim○ |
| Kanameishi et al.7 (2016) | 77/F | Nivo/2 | 0.2 | Not reported # | Not examined | No effect | S+I+TRA○ |
| Le Roy et al.8 (2016), Burel et al.9 (2017) | 34/M | Pem/1 | 0.01 | Not reported | Elevated | No effect | S+I○ |
| 51/F | Pem/several | 0.9 | Not reported | Normal | Not used | S○ | |
| Karakas et al.10 (2017) | 78/M | Nivo/6 | 0.5 | Not reported | Elevated | No effect | S○ |
| Pfohler et al.11 (2017) | 73/M | Nivo/2,Pem/2 | 1.4 | Not reported | Not examined | Not used | S● |
| Shiuan et al.12 (2017) | 47/F | Nivo+Ipili/ | 0.5> | IPF15.4% | Elevated | No effect | S+I●, R+Romiplostim○ |
| 45/F | Nivo/, Ipili/ | 0.8 | Not reported | Not examined | Not reported | S+I+R○ | |
| Burel et al.9 (2017) | 73/M | PD1+CTLA4/ | 2.0 | Not reported | Elevated | Not reported | Not reported |
| Jotatsu et al.13 (2018) | 62/M | Nivo/2 | 0.16 | IPF9.3% | Elevated | Not used | S○ |
| Hasegawa et al.14 (2019) | 82/F | Nivo/2 | 0.2 | Not reported | Not examined | Not reported | S+I+TRA○ |
| Mori et al.15 (2019) | 77/M | Nivo/1 | 0.2 | Not reported | Maintained | No effect | S○ |
| Delanoy et al.2 (2019) | $ | ||||||
| Mouri et al.16 (2020) | 66/M | Pem/1 | 0.3 | Not reported | Not described | No effect | S○ |
# Antiplatelet antibodies (+)
& The bone marrow was diagnosed with immune thrombocytopenic purpura; however, the status of megakaryocytes was not determined.
$ Bone marrow aspirate analysis in the seven patients with available information about platelet transfusion showed the presence of megakaryocytes.
S, steroids; I, intravenous immunoglobulin; R, rituximab; TRA, thrombopoietin receptor agonist; IPF, immature platelet fraction; Ipili, ipilimumab; Nivo, nivolumab; Pem, pembrolizumab
○, effective; ●, ineffective
An autoimmune mechanism, such as dysregulated humoral immunity, with an antibody directed against “c-Mpl” (thrombopoietin receptor),17,18 or cell-mediated suppression of megakaryopoiesis,19,20 has been considered for the pathogenesis of AATP.21 In AATP caused by anti-c-Mpl antibodies, the response to glucocorticoid, rituximab, or intravenous immunoglobulin was delayed and insufficient.18,22 In contrast, in AATP caused by cell-mediated suppression of megakaryopoiesis, platelet count after glucocorticoid therapy rapidly improved.19,22 Considering that glucocorticoid treatment was not effective in our patient, humoral dysregulation against megakaryocytes might have played a critical role, although the presence of anti-c-Mpl antibodies had not been investigated in this patient. High blood thrombopoietin levels have been reported in patients with AATP.23 In our patient, the blood thrombopoietin level was not measured.
Standard treatment guidelines have not been established for AATP. Several patients have reported successful treatment with cyclosporine, anti-thymocyte globulin, or rituximab, although some of them were unresponsive to glucocorticoid or intravenous immunoglobulin. AATP is associated with central immune cytopenia, causing megakaryocyte depletion. Thrombopoietin receptor agonists, such as eltrombopag, are effective for aplastic anemia derived from central immune cytopenia. Therefore, the patient was administered eltrombopag. The patient’s outcome suggested that AATP induced by ICIs may be successfully treated with eltrombopag. However, it is possible that our patient improved spontaneously with the discontinuation of ICI, considering that the half-life of durvalumab is approximately 18 days.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Calvo R. Hematological side effects of immune checkpoint inhibitors: the example of immune-related thrombocytopenia. Front Pharmacol. 2019; 10: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delanoy N, Michot JM, Comont T, et al. Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: a descriptive observational study. Lancet Haematol. 2019; 6: e48-e57. [DOI] [PubMed] [Google Scholar]
- 3.Young A, Quandt Z, Bluestone JA. The balancing act between cancer immunity and autoimmunity in response to immunotherapy. Cancer Immunol Res. 2018; 6: 1445-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad S, Lewis M, Corrie P, Iddawela M. Ipilimumab-induced thrombocytopenia in a patient with metastatic melanoma. J Oncol Pharm Pract. 2012; 18: 287-292. [DOI] [PubMed] [Google Scholar]
- 5.Kopecký J, Trojanová P, Kubeček O, Kopecký O. Treatment possibilities of ipilimumab-induced thrombocytopenia—case study and literature review. Jpn J Clin Oncol. 2015; 45: 381-384. [DOI] [PubMed] [Google Scholar]
- 6.Bagley SJ, Kosteva JA, Evans TL, Langer CJ. Immune thrombocytopenia exacerbated by nivolumab in a patient with non-small-cell lung cancer. Cancer Treat Commun. 2016; 6: 20-23. [Google Scholar]
- 7.Kanameishi S, Otsuka A, Nonomura Y, et al. Idiopathic thrombocytopenic purpura induced by nivolumab in a metastatic melanoma patient with elevated PD-1 expression on B cells. Ann Oncol. 2016; 27: 546-547. [DOI] [PubMed] [Google Scholar]
- 8.Le Roy A, Kempf E, Ackermann F, et al. Two cases of immune thrombocytopenia associated with pembrolizumab. Eur J Cancer. 2016; 54: 172-174. [DOI] [PubMed] [Google Scholar]
- 9.Le Burel S, Champiat S, Mateus C, et al. Prevalence of immune-related systemic adverse events in patients treated with anti-Programmed cell Death 1/anti-Programmed cell Death-Ligand 1 agents: A single-centre pharmacovigilance database analysis. Eur J Cancer. 2017; 82: 34-44. [DOI] [PubMed] [Google Scholar]
- 10.Karakas Y, Yuce D, Kılıckap S. Immune thrombocytopenia induced by nivolumab in a metastatic non-small cell lung cancer patient. Oncol Res Treat. 2017; 40: 621-622. [DOI] [PubMed] [Google Scholar]
- 11.Pföhler C, Eichler H, Burgard B, et al. A case of immune thrombocytopenia as a rare side effect of an immunotherapy with PD1-blocking agents for metastatic melanoma. Transfus Med Hemother. 2017; 44: 426-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiuan E, Beckermann KE, Ozgun A, et al. Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. J Immunother Cancer. 2017; 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jotatsu T, Oda K, Yamaguchi Y, et al. Immune-mediated thrombocytopenia and hypothyroidism in a lung cancer patient treated with nivolumab. Immunotherapy. 2018; 10: 85-91. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa T, Ozaki Y, Inoue T, et al. Nivolumab-related severe thrombocytopenia in a patient with relapsed lung adenocarcinoma: a case report and review of the literature. J Med Case Reports. 2019; 13: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori H, Sakai C, Iwai M, et al. Immune thrombocytopenia induced by nivolumab in a patient with non-small cell lung cancer. Respir Med Case Rep. 2019; 28: 100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mouri A, Kaira K, Shiono A, Miura Y, Kagamu H. Severe thrombocytopenia associated with pembrolizumab in a patients with non-small cell lung cancer (NSCLC): A case report and literature review. In Vivo. 2020; 34: 877-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwana M, Okazaki Y, Kajihara M, et al. Autoantibody to c-Mpl (thrombopoietin receptor) in systemic lupus erythematosus: relationship to thrombocytopenia with megakaryocytic hypoplasia. Arthritis Rheum. 2002; 46: 2148-2159. [DOI] [PubMed] [Google Scholar]
- 18.Kuwana M, Kaburaki J, Okazaki Y, Miyazaki H, Ikeda Y. Two types of autoantibody-mediated thrombocytopenia in patients with systemic lupus erythematosus. Rheumatology. 2006; 45: 851-854. [DOI] [PubMed] [Google Scholar]
- 19.Gewirtz AM, Sacchetti MK, Bien R, Barry WE. Cell-mediated suppression of megakaryocytopoiesis in acquired amegakaryocytic thrombocytopenic purpura. Blood. 1986; 68: 619-626. [PubMed] [Google Scholar]
- 20.Benedetti F, de Sabata D, Perona G. T suppressor activated lymphocytes (CD8 + /DR +) inhibit megakaryocyte progenitor cell differentiation in a case of acquired amegakaryocytic thrombocytopenic purpura. Stem Cells. 1994; 12: 205-213. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal N, Spahr JE, Werner TL, Newton DL, Rodgers GM. Acquired amegakaryocytic thrombocytopenic purpura. Am J Hematol. 2006; 81: 132-135. [DOI] [PubMed] [Google Scholar]
- 22.Nishino S, Kodaka T, Sawada Y, et al. Marked rebound thrombocytosis in response to glucocorticoids in a patient with acquired amegakaryocytic thrombocytopenia. J Clin Exp Hematop. 2018; 58: 166-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukai HY, Kojima H, Todokoro K, et al. Serum thrombopoietin (TPO) levels in patients with amegakaryocytic thrombocytopenia are much higher than those with immune thrombocytopenic purpura. Thromb Haemost. 1996; 76: 675-678. [PubMed] [Google Scholar]