Abstract
The clinical characteristics of B-cell lymphoma (BCL) were studied through the combined analysis of six clinical trials conducted by the Japan Clinical Oncology Group - Lymphoma Study Group (JCOG-LSG) for aggressive lymphoma in the 1990s, before the introduction of rituximab. Through a central pathological review, 829 patients were diagnosed with BCL according to the World Health Organization classification and treated with doxorubicin-containing combination chemotherapies. Of these patients, 642, 104, 30, and 24 patients were diagnosed with diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL), respectively. The overall survival (OS) of FL and MZL patients was higher than that of patients with DLBCL and MCL. The OS of the MCL patients was higher than that of DLBCL patients in the first 5 years, but MCL had the lowest survival after 5 years. The OS of DLBCL patients was clearly stratified by the international prognostic index and showed data compatible with that of aggressive lymphoma in the pre-rituximab era. These results established the clinical aspects of BCL in a large number of patients treated in prospective studies during the pre-rituximab era in Japan.
Keywords: : aggressive lymphoma, B-cell lymphoma, diffuse large B-cell lymphoma, international prognostic index, clinical trials
INTRODUCTION
Malignant lymphoma is a heterogeneous group of mature lymphoid neoplasms categorized as Hodgkin lymphoma (HL), B-cell lymphoma (BCL), and T/NK-cell lymphoma. BCL and T/NK-cell lymphoma, both of which are categorized as non-Hodgkin lymphomas (NHL), are composed of a number of disease entities.1 Approximately 70% of malignant lymphoma cases are BCL, although the relative incidence of malignant lymphoma subtypes is known to differ according to geographic location. Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of BCL both in Japan and in Western countries, while follicular lymphoma (FL) was found less frequently in Japan (6.7%) than in Western countries in the 1990s (11-33%).2,3 The second most common subtype of BCL in Japan in the 1990s was marginal zone lymphoma (MZL);2 however, the incidence of FL has gradually increased and has become the second most common subtype in Japan,4 which is similar to that observed in Western countries.3
Each lymphoma type has distinct pathological, immunophenotypic, molecular/cytogenetic, and clinical properties, responds differently to treatment, and has a distinct prognosis.5 There are many clinical and biological factors associated with these divergent treatment responses and prognoses, and prognostic models for various types of lymphoma have been proposed. For NHL, the international prognostic index (IPI) has been established as a useful prognostic model and applied to various types of NHL, including DLBCL.6 In Japan, the IPI has been shown to be a useful model for aggressive NHLs, such as DLBCL;7 however, prospective chemotherapy studies of a large number of Japanese patients with DLBCL conducted to determine the IPI’s validity have been limited.
The Japan Clinical Oncology Group - Lymphoma Study Group (JCOG-LSG) has conducted several clinical trials of combination chemotherapies for aggressive lymphoma. In these studies, patients were registered based on institutional pathological diagnoses according to the Working Formulation (WF) classification,8 and a central pathological review was conducted according to the World Health Organization (WHO) classification.9 To determine the clinical characteristics of malignant lymphoma in Japan based on the WHO classification, the JCOG-LSG conducted a combined analysis of these prospective studies, and the results of HL and peripheral T- and NK-cell lymphomas (PT/NKCLs) have been reported previously.10,11 In this study, we report the clinical characteristics of BCL after a combined analysis of the JCOG-LSG clinical trials.
MATERIALS AND METHODS
Details regarding the included studies’ designs, materials, and methods have been reported previously.10,11 The design of this study, JCOG0108A, is outlined below.
Patient selection
A total of 1,141 patients were enrolled in the following six JCOG-LSG multicenter clinical trials of adult, advanced, aggressive lymphomas according to the WF classification8 that were conducted consecutively in the 1990s: JCOG9002,12 JCOG9203,13 JCOG9505,14 JCOG9506,15 JCOG9508,16 and JCOG9809.17 The major eligible subtypes of BCL were as follows: diffuse large cells, diffuse small cleaved cells, diffuse mixed cells, large immunoblastic cells, follicular predominantly large cells, and small non-cleaved cell lymphomas.8 Patients with Mycosis Fungoides (MF), Sézary syndrome, adult T-cell leukemia-lymphoma, and precursor T-lymphoblastic leukemia/lymphoma were excluded from all the studies. Detailed descriptions of the eligibility criteria for these six trials have been previously reported.12-17 All the protocols described above, including the informed consent document, were approved by both the JCOG Protocol Review Committee and the institutional review board of each participating institution. JCOG0108A for NHL is an analysis of these six studies combined, and the first edition of JCOG0108A was written on June 27, 2001 and was approved by the JCOG Protocol Review Committee on October 31, 2001.
Treatment
All patients were enrolled in multicenter prospective studies and were treated with doxorubicin (DXR)-containing second- or third-generation multidrug combination chemotherapies in the JCOG9002 and JCOG9203 studies12,13 or with cyclophosphamide (CPA), DXR, vincristine (VCR), and prednisolone (PSL) CHOP-like regimens in the JCOG9505, JCOG9506, JCOG9508, and JCOG9809 studies.14-17
JCOG9002 was a randomized phase III study evaluating the dose-intensification strategy for doxorubicin and cyclophosphamide in third-generation multi-agent combination chemotherapy, the LSG9 regimen with second-generation combination chemotherapy and the mLSG4 regimen, for patients with aggressive lymphoma.12 JCOG9203 was a phase II study of second-generation combination chemotherapy, the LSG12 regimen, for elderly patients with aggressive lymphoma.13 JCOG9505 was a randomized phase II study of CHOP every 2 weeks (CHOP-14) and dose-escalated CHOP for high-intermediate and high (HI/H)-risk patients with advanced-stage aggressive lymphoma.14 JCOG9506 was a phase II study of upfront, high-dose chemotherapy followed by autologous hematopoietic stem cell transplantation for HI/H-risk patients with aggressive lymphoma.15 JCOG9508 was a phase II study of standard CHOP every 3 weeks (CHOP-21) for low and low-intermediate (L/LI)-risk patients with advanced-stage aggressive lymphoma.16 JCOG9809 was a randomized phase III study comparing CHOP-14 with standard CHOP-21 for patients with advanced-stage aggressive lymphoma in all IPI risk groups.17 All studies included newly-diagnosed patients, and JCOG9002, JCOG9203, and JCOG9809 were clinical trials including patients in all IPI risk groups.
Histopathological and immunohisBlootochemical analyses by central review
An expert panel of six hematopathologists (Kiyoshi Mukai, Shigeo Nakamura, Koichi Ohshima, Masahiro Kikuchi, Yoshihiro Matsuno, and Tadashi Yoshino) and two clinicians (Tomomitsu Hotta and Masanori Shimoyama) reviewed the histopathologic diagnoses of 1,023 of the 1,141 patients who were enrolled in the six studies. A consensus diagnosis was reached following a histological review of each biopsy specimen in accordance with the third edition of the WHO classification system,9 by a panel of hematopathologists and clinicians as described previously.10,11 Briefly, immunohistochemical studies were conducted on paraffin sections using the avidin-biotin-peroxidase complex technique and a panel of monoclonal antibodies. Antibodies that were routinely used for the central review of the pathological diagnosis were for CD20 (L26; Dako, Glostrup, Denmark) and CD3 (PS1; Novocastra, Newcastle upon Tyne, UK). When further immunohistochemical staining was necessary to establish a specific diagnosis, antibodies to the following antigens were also used: cyclin D1 (SP4; Nichirei, Tokyo, Japan) for MCL and CD10 (56C6; Novocastra) and bcl-2 (124; Dako) for FL.
Response criteria
Response to treatment was evaluated according to the WHO criteria.18 Complete response (CR) was defined as the disappearance of all measurable lesions and symptoms of the disease for at least 4 weeks. Partial response (PR) was defined as a reduction of 50% or more in the products of the perpendicular diameters of all measurable lesions added together and the lack of the appearance of new lesions for at least 4 weeks. Uncertain CR (CRu) was defined as maintenance of PR without chemotherapy for more than 3 months after completing treatment. Progressive disease (PD) was defined as an increase of ≥ 25% in the size of any existing lesion or the development of any new lesions. When there were no signs of PD and the response also did not fulfill the PR criteria, it was defined as no change (NC). The CR rate was calculated by dividing the number of patients with CR or CRu by the total number of patients.
Statistical analysis
The OS was calculated from the date of enrollment in each study until the last follow-up or date of death, and OS curves were estimated using the Kaplan–Meier method. The OS curves of B-NHL, PT/NKCL, and major histological types of B-NHL (DLBCL, FL, MCL, and MZL) were analyzed. In patients with DLBCL, OS curves and CR rates according to the IPI risk group were also presented. All statistical analyses were performed in the JCOG Data Center using SAS version 9.1.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Table 1 summarizes the number of patients included in the six clinical trials conducted by JCOG-LSG. A total of 1,141 patients were included in this study, and the number of patients included in JCOG9002, JCOG9203, JCOG9505, JCOG9506, JCOG9508, and JCOG9809 were 447, 45, 70, 43, 213, and 323, respectively. Of the 1,023 patients who received central pathological reviews, 829 were diagnosed with BCL and 136 were diagnosed with PT/NKCL. The numbers of patients with major histological types of BCL according to the WHO classification are summarized in Table 2. DLBCL was the most common type, followed by FL, MCL, and MZL, which included extranodal MZL of mucosa-associated lymphoid tissue (MALT lymphoma) and nodal MZL.
Table 1. Number of patients registered in six JCOG-LSG clinical trials.
| JCOG study | No. of patients | (%) |
|---|---|---|
| JCOG900212) | 447 | 39.2 |
| JCOG920313) | 45 | 3.9 |
| JCOG950514) | 70 | 6.1 |
| JCOG950615) | 43 | 3.8 |
| JCOG950816) | 213 | 18.7 |
| JCOG980917) | 323 | 28.3 |
| Total number | 1141 |
Table 2. Number of patients diagnosed with each B-cell lymphoma subtype.
| No | % | |
|---|---|---|
| B-LbL | 5 | 1 |
| B-CLL/SLL | 5 | 1 |
| MCL | 30 | 4 |
| FL | 104 | 13 |
| Grade 1+2 | (59) | (7) |
| Grade 3 | (45) | (5) |
| MZL | 24 | 3 |
| DLBCL | 642 | 77 |
| PC | 1 | 0 |
| BCL-U | 18 | 2 |
| Total | 829 |
B-LbL, B-lymphoblastic lymphoma; B-CLL/SLL, B-chronic lymphocytic leukemia/small lymphocytic lymphoma; MCL, mantle cell lymphoma; FL, follicular lymphoma; MZL, marginal zone lymphoma; DLBCL, diffuse large B-cell lymphoma; PC, plasmacytoma/myeloma; BCL-U, B-cell lymphoma, unclassified
The major clinical characteristics of each histological type are summarized in Table 3. The patients with DLBCL were predominantly male. Approximately two-thirds of the patients had advanced disease, and most patients had a good Eastern Cooperative Oncology Group (ECOG) performance status (PS). Two-thirds of the patients with DLBCL were categorized into the lower risk groups of the IPI.
Table 3. Clinical characteristics of the four major B-cell lymphoma subtypes, with percentages of each characteristic indicated.
| DLBCL (n=642) % |
FL (n=104) % |
MCL (n=30) % |
MZL (n=24) % |
|
|---|---|---|---|---|
| Male | 60 | 54 | 67 | 50 |
| Age > 60 years | 41 | 22 | 33 | 37 |
| CS 3 or 4 | 61 | 75 | 87 | 63 |
| PS > 1 | 16 | 7 | 7 | 4 |
| LDH > N | 52 | 30 | 27 | 21 |
| No. of extranodal sites > 1 | 20 | 11 | 40 | 33 |
| B symptom, present | 24 | 18 | 30 | 21 |
| IPI (HI/H) | 30 | 12 | 27 | 21 |
CS, clinical stage; PS, performance status; IPI, international prognostic index; N, normal; HI/H, high-intermediate risk and high risk
Of the 104 patients with FL, 54% were male, 78% were aged < 61 years, and three-quarters had advanced disease. More than 80% of the patients did not have B-symptoms, and 85% were in the lower risk groups according to the IPI.
In MCL, two-thirds of the patients were male, one-third of patients were older than 60 years, and around 90% were in an advanced disease stage. Extranodal involvement was observed in 40%, and more than 70% of patients were in the lower IPI risk group.
Twenty-four patients were diagnosed with MZL. Half of them were male, approximately two-thirds had advanced disease, over 90% had a good PS, one-third had more than one extranodal site, and approximately 70% were in the lower risk group according to the IPI.
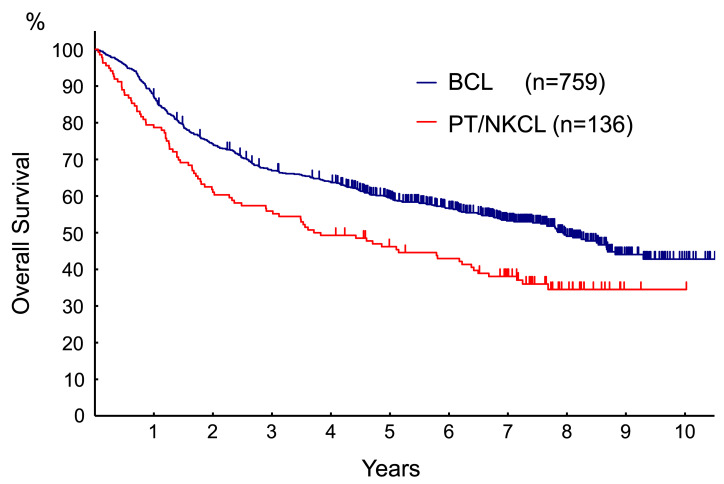
The OS of the 759 patients with BCL consisting of MCL, FL grade 3, MZL, DLBCL, and unclassified BCL was better than that of patients with PT/NKCL (Fig. 1). The five-year OS was 60.4% (95% confidence interval [CI]: 56.7-63.8%) for B-cell lymphoma and 47.2% (95% CI: 38.6-55.4%) for PT/NKCL.
Fig. 1.
The OS of BCL patients including MCL, FL grade 3, MZL, DLBCL, unclassified BCL, and PT/NKCL. The PT/NKCL group included 53 patients with PTCL-NOS, 46 with angioimmunoblastic T-cell lymphoma, 18 with anaplastic large cell lymphoma, 17 with extranodal NK/T-cell lymphoma nasal type, one with subcutaneous panniculitis-like T-cell lymphoma, and one with enteropathy-type T-cell lymphoma as described in a previous report [11]. The OS of BCL patients including MCL, FL grade 3, MZL, DLBCL, unclassified BCL, and PT/NKCL. The PT/NKCL group included 53 patients with PTCL-NOS, 46 with angioimmunoblastic T-cell lymphoma, 18 with anaplastic large cell lymphoma, 17 with extranodal NK/T-cell lymphoma nasal type, one with subcutaneous panniculitis-like T-cell lymphoma, and one with enteropathy-type T-cell lymphoma as described in a previous report [11].
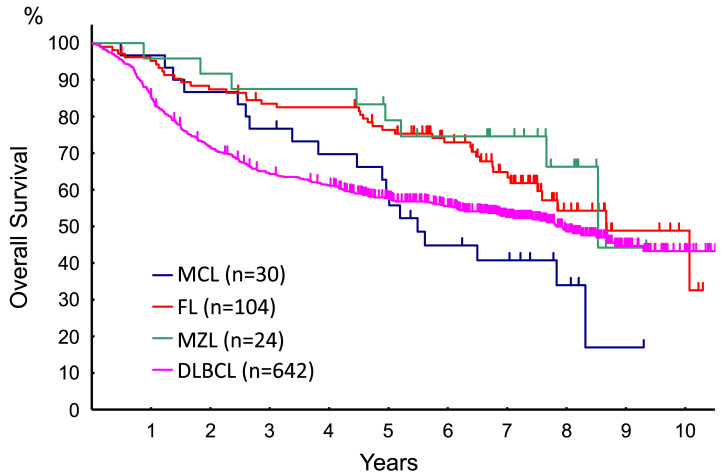
The OS of the BCL subtypes showed different patterns (Fig. 2). The five-year OS of patients with DLBCL, FL, MCL, and MZL were 58.6% (95% CI: 54.6-62.3%), 76.2% (95% CI: 66.6-83.4%), 59.2% (95% CI: 39.5-74.5%), and 82.9% (95% CI: 60.5-93.2%), respectively. DLBCL patients had the poorest OS in the first 5 years, whereas MCL showed the poorest survival after 5 years. The OS of the patients with FL, MCL, and MZL, however, decreased continuously over the observation period.
Fig. 2.
The OS of BCL subtypes. DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma
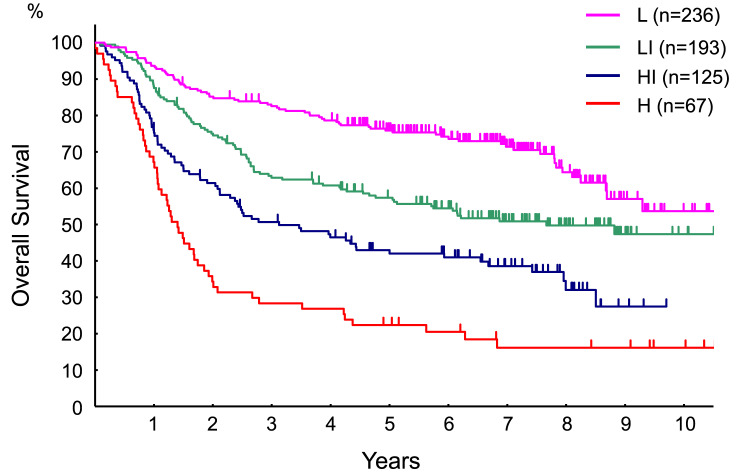
The OS of patients with DLBCL in relation to IPI is shown in Fig. 3. A total of two hundred and thirty-six patients with DLBCL (36.8%) were categorized as having low (L) risk, 193 (30.1%) as low-intermediate (LI) risk, 125 (19.5%) as high-intermediate risk (HI), and 67 (10.4%) as high (H) risk. The five-year OS of L, LI, HI, and H risk DLBCL were 75.7% (95% CI: 69.6-80.7%), 58.0% (95% CI: 50.6-64.7%), 43.5% (95% CI: 34.6-52.0%), and 22.4% (95% CI: 13.3-32.9%), respectively. The CR rates in the L, LI, HI, and H risk DLBCL groups were 80.3%, 72.1%, 57.9%, and 41.8%, respectively.
Fig. 3.
The OS of patients with DLBCL according to the IPI
L, low risk; LI, low-intermediate risk; HI, high-intermediate risk; H, high risk. Significant differences in OS according to IPI were revealed by log-rank test analysis (p < 0.0001).
DISCUSSION
This study revealed the baseline clinical characteristics and treatment outcomes of a large number of patients who were enrolled in six clinical trials for aggressive lymphoma in the pre-rituximab era in Japan. Survival data for BCL in this study showed characteristic patterns. The OS of patients with BCL was higher than that of patients with PT/NKCL during the observation period. For the BCL subtypes, the OS of FL and MZL patients was better than that of DLBCL and MCL patients. The OS for DLBCL patients was the poorest in the first 5 years and then decreased gradually after that, while for FL, MCL, and MZL, the OS decreased continuously throughout the observation period. For MCL patients, the OS curve decreased continuously and was inferior to that of DLBCL after 5 years. These results indicated that the OS of FL and MZL patients had the characteristic patterns of low-grade BCL patients, and the OS of MCL was similar to that of previous reports. Thus, the long-term prognosis of MCL was the worst among the four subtypes of BCL.
The survival of aggressive lymphoma could be stratified using IPI. The five-year OS rates of patients with aggressive lymphoma were 73%, 51%, 43%, and 26% in the L, LI, HI, and H risk groups, respectively.6 Since DLBCL is the most common type of aggressive lymphoma, survival data for aggressive lymphoma should be similar to that for DLBCL. In this study, the 5-year OS of DLBCL according to the IPI was 75.7%, 58.0%, 43.5%, and 22.4% for the L, LI, HI, and H risk groups, respectively. These data were comparable to those for aggressive lymphoma in the pre-rituximab era.6 In this study, a central pathological review conducted according to the WHO classification confirmed the diagnosis, and the data were collected from a large number of patients in prospective studies of aggressive lymphoma. Concerning the survival data for patients with DLBCL according to the IPI in the pre-rituximab era, there have been only two retrospective analysis reports.7,19 Thus, our data should serve as a reference for the survival of patients with DLBCL in prospective clinical trials in the pre-rituximab era.
A previous DLBCL gene expression profiling study identified germinal center B-cell (GCB)-like and activated B-cell (ABC)-like DLBCL subtypes20 diagnosed as GCB and non-GCB subtypes by immunohistochemical analysis.21 These subgroups exhibited distinct characteristics and prognoses, and therefore provided informative data. However, we could not perform immunohistochemical analysis of these subtypes in our study due to the limited number of collected tissue samples.
In general, patients with FL have widespread disease, and most are diagnosed with advanced-stage disease.22 Despite this, patients with FL are usually asymptomatic. The clinical characteristics of FL in this study coincided with those of FL in the literature. Most of the patients had a good PS, an absence of B-symptoms, a low IPI risk, and 75% were diagnosed with advanced stage disease. These results indicate that the clinical characteristics of patients with FL in this study were comparable to the general features of FL.23 According to the WHO, an FL grade 3 classification incorporates 3A and 3B subtypes with characteristic clinical features. Unfortunately, since we did not differentiate FL 3A and 3B in portions of our study we were unable to provide any data for these subtypes.
MCL is predominantly diagnosed in males, most present with advanced stage III or IV disease, and extranodal involvement is a common feature.24,25 In this study, approximately two-thirds of patients with MCL were male, 87% were in stages III or IV, and more than one extranodal disease site was observed in 40% of the patients. The prognosis of MCL patients was poorest among the four types of BCL in this study, and the survival curve showed a constant decrease, with no plateau phase observed. These results were therefore comparable to the general features of MCL.23
MZL includes two types of indolent lymphoma: MALT lymphoma and nodal MZL.9 In general, patients with MALT lymphoma usually present at stage I or II with extranodal disease, and nodal MZL involves peripheral lymph nodes. The advanced and localized stage of MZL showed relatively good survival patterns.26 Since the clinical trials in this combined analysis included patients with advanced-stage disease, the clinical characteristics of patients with MZL in this study were not typical of those with MZL. For example, 63% had advanced stage, 21% had B-symptoms, and 21% had HI/H risk according to the IPI. On the other hand, only 4% had a poor PS, and LDH was elevated in one-fifth of the patients, suggesting that these patients had relatively indolent clinical presentations. The OS of patients with MZL in this study was similar to that of patients with FL. These results indicate that the clinical picture and prognosis were similar to those of MZL in advanced stages.23
In Japan, rituximab was approved for CD20-positive indolent B-cell lymphoma, follicular lymphoma, and mantle cell lymphoma in June 2001, and approved for all types of CD20-positive B-cell lymphoma in June 2003. Consequently, we cannot exclude the possible influence of rituximab treatment on the survival data in our study because some patients might have been be treated with rituximab after relapse, especially after June 2003 when rituximab was approved for all types of CD20-positive B-cell lymphoma. Since we did not collect treatment data after relapse in our study, we could not analyze the possible effect of rituximab treatment on survival.
This study has several limitations. Since all the clinical trials included in this combined analysis were conducted before the introduction of rituximab, data concerning prognosis could not be directly applied to the rituximab era. Second, this study included only patients eligible for clinical trials, and ineligible patients, such as those with poor PS, inadequate organ function, and elderly patients were excluded. Since the current study is a combined analysis of six studies for patients with various age groups and risk groups, and each study incorporated different treatment regimens, the treatment outcomes may be influenced by selection biases. In addition, only a few patients with FL, MZL, and MCL were included because the data were based on clinical trials for aggressive lymphoma. Thus, the proportions of lymphoma subtypes did not reflect a real-world situation, and therefore detailed survival analyses were difficult to conduct due to the small number of patients within the various subtypes.
In conclusion, this study shows that major types of BCL registered in clinical trials for aggressive lymphoma in the pre-rituximab era in Japan displayed characteristic clinical pictures and prognoses. DLBCL, the most common type of BCL, showed survival patterns according to IPI. Survival data were consistent with those of aggressive lymphoma in the pre-rituximab era.
ACKNOWLEDGMENTS
The authors would like to thank the late Masahiro Kikuchi (Fukuoka University), Yoshihiro Matsuno (Hokkaido University), and Tadashi Yoshino (Okayama University) for conducting the pathology review and serving as members of an expert panel and Yoshihiro Matsuno for his assistance in writing the histopathological and immunohistochemical analysis portion of the manuscript. We are grateful to the members of the JCOG Data Center and JCOG Operations Office for their support in manuscript preparation (Drs. Tomoko Kataoka and Keita Sasaki and Mr. Ryunosuke Machida), data management (Ms. Yuko Watanabe), and study management oversight (Dr. Haruhiko Fukuda).
This study was supported by Grants-in-Aid No. 2S-1, 5S-1, 8S-1, 11S-1, 11S-4, 14S-1, 14S-4, 17S-1, and 17S-5 for Cancer Research from the Ministry of Health, Labor and Welfare of Japan.
Footnotes
CONFLICTS OF INTEREST
Dr. Kinoshita reports grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study; Dr. Watanabe reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study, grants from Takara Bio Inc. and United Immunity, Co., Ltd., personal fees from Bristol-Meyers Squibb, personal fees from Astrazeneca Pharmaceutical, and personal fees from Chugai Pharmaceutical outside the submitted work; Dr. Itoh reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study, personal fees from Zenyaku Kogyo Co. Ltd, and personal fees from Fujimoto Pharm. Corp. outside the submitted work; Dr. Yoshimura reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study, personal fees from Chugai Pharma, personal fees from Astra Zeneca, and personal fees from Eli Lilly outside the submitted work; Dr. Tobinai reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study, personal fees from Zenyaku Kogyo, grants and personal fees from Eisai, grants and personal fees from Takeda, grants and personal fees from Mundipharma, personal fees from HUYA Bioscience International, grants and personal fees from Kyowa Kirin, grants and personal fees from Celgene, grants and personal fees from Chugai Pharma, grants and personal fees from Ono Pharmaceutical, personal fees from Yakult, personal fees from Daiichi Sankyo, personal fees from Bristol-Myers Squibb, personal fees from Meiji Seika Kaisha, personal fees from Solasia Pharma, personal fees from Verastem, grants from Janssen, and grants from Abbvie outside the submitted work; Dr. Ogura reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study, personal fees from Eisai, personal fees from Celgene, personal fees from Celltrion, personal fees from Chugai, personal fees from Meiji Seika Pharma, personal fees from Mundi Pharma, personal fees from Verastem, personal fees from Denovo Biopharma, and personal fees from Symbio outside the submitted work; Dr. Yamaguchi reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study, grants from Astellas Pharma, grants from Kyowa Kirin, grants from Ono Pharmaceutical, and grants from Takeda Pharmaceutical outside the submitted work; Dr. Kurosawa reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study; Dr. Imaizumi reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study; Dr. Ota reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study; Dr. Kaba reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study; Dr. Mukai reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study; Dr. Nakamura reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study; Dr. Ohshima reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study; Dr. Hotta reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study; Dr. Tsukasaki reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study; grants and personal fees from Daiich-Sankyo, personal fees from Ono Pharma, grants and personal fees from HUYA, grants and personal fees from Chugai Pharma, grants and personal fees from Celgene, personal fees from Mundy Pharma, personal fees from Kyowa-hakko/Kirin, grants from Eizai, grants from Bayer, and personal fees from Takeda outside the submitted work; Dr. Nagai reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study; grants and personal fees from Janssen Pharmaceutical K. K, grants and personal fees from Celgene Corporation, grants and personal fees from Mundipharma K.K., grants and personal fees from Bayer Yakuhin Ltd., grants from Abbvie G.K., grants and personal fees from Takeda Pharmaceutical Co., Ltd., grants and personal fees from Kyowa Hakko Kirin Co., Ltd., grants and personal fees from Esai Co., Ltd., grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Ono Pharmaceutical Co., Ltd., grants and personal fees from Gilead Sciences Inc., grants and personal fees from Zenyaku Kogyo Co., Ltd., grants from Solasia Pharma K.K., grants from HUYA Bioscience International, grants and personal fees from AstraZeneca plc., grants and personal fees from SymBio Pharmaceuticals Limited, grants from Otsuka Pharmaceutical Co., Ltd., personal fees from Roche Ltd., personal fees from Sanofi K.K., grants from IQVIA serice Japan K.K. outside the submitted work; and Dr. Shimoyama reports receiving grants from the Ministry of Health, Labor and Welfare of Japan while conducting this study.
REFERENCES
- 1.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999; 17: 3835-3849. [DOI] [PubMed] [Google Scholar]
- 2.Lymphoma Study Group of Japanese Pathologists . The World Health Organization classification of malignant lymphomas in Japan: incidence of recently recognized entities. Pathol Int. 2000; 50: 696-702. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin’s lymphomas: distributions of the major subtypes differ by geographic locations. Ann Oncol. 1998; 9: 717-720. [DOI] [PubMed] [Google Scholar]
- 4.Muto R, Miyoshi H, Sato K, et al. Epidemiology and secular trends of malignant lymphoma in Japan: analysis of 9426 cases according to the World Health Organization classification. Cancer Med. 2018; 7: 5843-5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997; 89: 3909-3918. [PubMed] [Google Scholar]
- 6.International Non-Hodgkin’s Lymphoma Prognostic Factors Project . A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993; 329: 987-994. [DOI] [PubMed] [Google Scholar]
- 7.Seki R, Ohshima K, Fujisaki T, et al. Prognostic impact of immunohistochemical biomarkers in diffuse large B-cell lymphoma in the rituximab era. Cancer Sci. 2009; 100: 1842-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National cancer institute sponsored study of classifications of non-hodgkin’s lymphomas. Summary and description of a working formulation for clinical usage. Cancer. 1982; 49: 2112-2135. [DOI] [PubMed] [Google Scholar]
- 9.Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. World Health Organization Classification of Tumors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, IARC Press. 2001. [Google Scholar]
- 10.Itoh K, Kinoshita T, Watanabe T, et al. Prognostic analysis and a new risk model for Hodgkin lymphoma in Japan. Int J Hematol. 2010; 91: 446-455. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe T, Kinoshita T, Itoh K, et al. Pretreatment total serum protein is a significant prognostic factor for the outcome of patients with peripheral T/natural killer-cell lymphomas. Leuk Lymphoma. 2010; 51: 813-821. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita T, Hotta T, Tobinai K, et al. A randomized controlled trial investigating the survival benefit of dose-intensified multidrug combination chemotherapy (LSG9) for intermediate- or high-grade non-Hodgkin’s lymphoma: Japan Clinical Oncology Group Study 9002. Int J Hematol. 2004; 80: 341-350. [DOI] [PubMed] [Google Scholar]
- 13.Mizoroki F, Hirose Y, Sano M, et al. A phase II study of VEPA/FEPP chemotherapy for aggressive lymphoma in elderly patients: Japan Clinical Oncology Group Study JCOG9203. Int J Hematol. 2006; 83: 55-62. [DOI] [PubMed] [Google Scholar]
- 14.Itoh K, Ohtsu T, Fukuda H, et al. Randomized phase II study of biweekly CHOP and dose-escalated CHOP with prophylactic use of lenograstim (glycosylated G-CSF) in aggressive non-Hodgkin’s lymphoma: Japan Clinical Oncology Group Study 9505. Ann Oncol. 2002; 13: 1347-1355. [DOI] [PubMed] [Google Scholar]
- 15.Tobinai K, Hotta T. Clinical trials for malignant lymphoma in Japan. Jpn J Clin Oncol. 2004; 34: 369-378. [DOI] [PubMed] [Google Scholar]
- 16.Kagami Y, Itoh K, Tobinai K, et al. Phase II study of cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP) therapy for newly diagnosed patients with low- and low–intermediate risk, aggressive non-Hodgkin’s lymphoma: final results of the Japan Clinical Oncology Group Study, JCOG9508. Int J Hematol. 2012; 96: 74-83. [DOI] [PubMed] [Google Scholar]
- 17.Ohmachi K, Tobinai K, Kobayashi Y, et al. Phase III trial of CHOP-21 versus CHOP-14 for aggressive non-Hodgkin’s lymphoma: final results of the Japan Clinical Oncology Group Study, JCOG 9809. Ann Oncol. 2011; 22: 1382-1391. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. Geneva, WHO Offset Publication No. 48. 1979.
- 19.Wilder RB, Rodriguez MA, Medeiros LJ, et al. International prognostic index-based outcomes for diffuse large B-cell lymphomas. Cancer. 2002; 94: 3083-3088. [DOI] [PubMed] [Google Scholar]
- 20.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346: 1937-1947. [DOI] [PubMed] [Google Scholar]
- 21.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103: 275-282. [DOI] [PubMed] [Google Scholar]
- 22.Hiddemann W, Cheson BD. How we manage follicular lymphoma. Leukemia. 2014; 28: 1388-1395. [DOI] [PubMed] [Google Scholar]
- 23.Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed, Lyon, IARC. 2017. [Google Scholar]
- 24.Schmidt C, Dreyling M. Therapy of mantle cell lymphoma: current standards and future strategies. Hematol Oncol Clin North Am. 2008; 22: 953-963. [DOI] [PubMed] [Google Scholar]
- 25.Fisher RI, Dahlberg S, Nathwani BN, et al. A clinical analysis of two indolent lymphoma entities: mantle cell lymphoma and marginal zone lymphoma (including the mucosa-associated lymphoid tissue and monocytoid B-cell subcategories): a Southwest Oncology Group study. Blood. 1995; 85: 1075-1082. [PubMed] [Google Scholar]
- 26.Thieblemont C, Berger F, Dumontet C, et al. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood. 2000; 95: 802-806. [PubMed] [Google Scholar]