Abstract
The human pelvis is a complex anatomical structure that consists of the innominate bones, sacrum and coccyx to form the pelvic ring. Even though considered to be a symmetric entity, asymmetry of the pelvic ring (APR) might occur to alter its anatomy, function, or biomechanics or to impact assessment and treatment of clinical cases. APR and its assessment is complicated by the intricate anatomy of the pelvic ring. There is only limited information and understanding about APR with no established evaluation methods existing. The objective of the present study was to adopt CT‐based 3D statistical modeling and analysis to assess APR within the complex anatomy of the pelvic ring. We were interested to establish a better understanding of APR with knowledge and applications transferred to human anatomy, related research, and development subjects and to clinical settings. A series of 150 routine, clinical, pelvic CT protocols of European and Asian males and females (64 ± 15 (20–90) years old) were post‐processed to compute gender‐ and ancestry‐specific 3D statistical models of the pelvic ring. Evaluations comprised principal component analysis (PCA) that included size, shape, and asymmetry patterns and their variations to be assessed. Four different CT‐based 3D statistical models of the entire pelvic ring were computed according to the gender and ancestry specific groups. PCA mainly displayed size and shape variations. Examination of additional PCA modes permitted six distinct asymmetry patterns to be identified. They were located at the sacrum, iliac crest, pelvic brim, pubic symphysis, inferior pubic ramus, and near to the acetabulum. Accordingly, the pelvic ring demonstrated not to be entirely symmetric. Assessment of its asymmetry proved to be a challenging task. Using CT‐based 3D statistical modeling and PCA, we identified six distinct APRs that were located at different anatomical regions. These regions are more prone to APRs than other sites. Minor asymmetry patterns have to be distinguished from the distinct APRs. Side differences with regard to size, shape, and/or position require to be taken into account. APRs may be due different load mechanisms applied via spine or lower extremity or locally. There is a need for simpler and efficient, yet reliable methods to be routinely transferred to human anatomy, related research, and development subjects and to clinical settings.
Keywords: 3D statistical model, asymmetry, computed tomography, pelvic ring, principal component analysis
Using CT‐based 3D statistical modeling and principal component analysis we were able to identify six distinct regions of the pelvic ring, which can exhibit asymmetries. PC 7 of the European females is an example showing all of the six asymmetries of the pelvic ring located at the sacrum, the iliac crest, the pelvic brim, the acetabulum, the pubic symphysis and the inferior pubic ramus.
1. INTRODUCTION
The human pelvis is composed of the innominate bones, sacrum, and coccyx to form the pelvic ring. It connects the torso with the lower extremities (Putz & Muller‐Gerbl, 1990). It is thought to be a symmetric entity. The innominate bones are paired bones with the ipsilateral side being a mirror image of the contralateral side. In contrast, the sacrum and coccyx are unpaired bones located at the posterior pelvic ring in between the innominate bones. They are composed of fused vertebrae. Each of them is divided by the mid‐sagittal/ symmetry plane into an ipsilateral and contralateral part.
Even though considered to be a symmetric entity, asymmetry of the pelvic ring (APR) might occur to alter its anatomy, function, or biomechanics or to impact treatment of clinical cases. APR may be due to bony anomalies (e.g., hip dysplasia) (Li et al., 2016; Putz & Muller‐Gerbl, 1990; Wells et al., 2017) degenerative or inflammatory processes (Garvey & Hazard, 2014; Stover et al., 2017), fractures or disruptions of the pelvis (Rommens & Hofmann, 2017; Tile et al., 2015). Assessment of APR has been demonstrated to be useful for anatomical studies, analysis of functional behavior, biomechanical considerations, implant development, and other clinical applications (Boulay et al., 2006; DeSilva & Rosenberg, 2017; Osterhoff et al., 2019; Tobolsky et al., 2016). Despite available techniques, little is known about APR and its assessment.
Three‐dimensional (3D) computed tomography (CT)‐based pelvic models as well as 3D statistical modeling and analysis techniques have been used to characterize the complex anatomy of the pelvic ring (Arand et al., 2019). We hypothesize that they could also be used to enhance our knowledge and understanding with regard to APR whilst its intricate anatomy being taken into account.
The objective of the present study was to adopt CT‐based 3D statistical modeling to assess APR within the complex anatomy of the pelvic ring. We were interested to establish a better understanding of APR with knowledge and applications transferred to human anatomy, related research, and development and to clinical settings.
2. MATERIAL AND METHODS
2.1. CT images
A series of 150 pelvic CT scans were included in this study. They consisted of four subgroups as shown in Table 1.
TABLE 1.
Sample demographics of the female and male European and Asian sub‐groups.
| Males | Females | |
|---|---|---|
| European |
51 CT scans (clinical protocol) Mean age: 60.8 years SD ±13 years min.: 25, max.: 85 years |
49 CT scans (clinical protocol) Mean age: 58.6 years SD ±14.7 years min.: 20, max.: 86 years |
| Asian |
30 CT scans (postmortem) Mean age: 68.4 years SD ±16.4 years min.: 26, max.: 89 years |
20 CT scans (postmortem) Mean age: 80.3 years SD ±6.7 years min.: 65, max.: 90 years |
CT data were obtained from the CT database of the AO Research Institute Davos, Davos, Switzerland (Messmer et al., 2007), registered at the “Eidgenössischer Öffentlichkeits‐ und Datenschutzbeauftragter” (EDÖP, Bern, Switzerland). CTs from European females and males corresponded to standard, anonymized, clinical CT protocols. All patients agreed to anonymous research use of their CT data, which have been obtained for clinical reasons. CT scans from Asian females and males were acquired from postmortem specimens. Consent of the local ethics committee was given. Only sacra with five fused sacral vertebrae were included. CT samples with radiographic signs of osseous pathologies other than osteopenia, osteoporosis, or osteoarthritis were excluded.
For Asian CTs, a standard CT scanner (LightSpeed VCT, GE Healthcare, Chalfont St. Giles, UK) was used applying a standard CT protocol with a tube peak voltage of 120 kVp and the GE STANDARD reconstruction kernel. The voxel size was about 0.63 × 0.63 × 0.63 mm in all scans. For the European data Siemens Somatom Definition AS+scanner (Siemens Healthcare GmbH, Erlangen, Germany) was used applying a standard clinical CT protocol with a peak voltage of 140 kVP, the average image resolution was 0.75 × 0.75 × 0.6 mm. Pelvic CT scans were transferred in DICOM (Digital Imaging and Communications in Medicine) format to a standard laptop computer.
2.2. CT post‐processing to generate CT‐based 3D statistical models of the pelvic ring
Pelvic CTs were post‐processed using Amira software (Amira Version 6.7.0; Thermo Fisher Scientific, Waltham, MA, USA). Using the soft‐ and hardware framework, a CT‐based 3D statistical model of the pelvic ring was generated for each of the afore mentioned CT data groups according to methods described by Arand et al. (Arand et al., 2019).
2.3. Model evaluation using principal component analysis (PCA)
The four CT‐based 3D statistical models as generated in previous steps, were assessed via PCA. PCA is a common method for statistical model analysis, described by Bookstein and Zollikofer (Bookstein, 1997; Zollikofer & Leon, 2005), which orders the types of variation of shape and size in decreasing variance (Arand et al, 2019; Heimann & Meinzer, 2009; Heimann et al., 2009; Kamer et al., 2010; Noser et al, 2010). We have been adopting this technique to describe variations in size and shape but also asymmetries of the pelvic ring. For the two European groups principal components (PCs) 1–20, ranging from −3 to +3 SD, were analyzed. Because of the smaller number of Asian CT scans available, PC 1–15, ranging from −2 to +2 SD were analyzed to avoid extrapolation.
Asymmetry patterns of the bony surface were studied and compared to the respective mean model. The identification of asymmetries was done manually, that is, by visual judgment of individual specimen models and PCA models and not by measurements. For each asymmetry pattern identified, a matching 3D CT model was selected. This permitted specific APRs to be demonstrated in given pelvic CT samples.
3. RESULTS
Post‐processing of the 150 grouped, pelvic CT scans resulted in four different 3D statistical models of the pelvic ring with ancestry‐ and gender‐specific models generated (also see Figures 1 and 2). PCA predominantly displayed size and shape variations, as previously described by Arand et al. (Arand et al., 2019).
FIGURE 1.
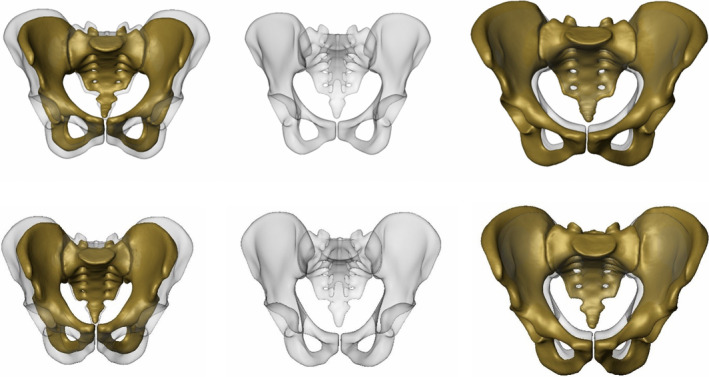
CT‐based 3D statistical models of European females (top row) and males (bottom row) with PC 1 predominantly displaying size variation: Anteroposterior views with mean models (semi‐transparent grey) and with PC 1 models −3 SD (left) and +3 SD in yellow (right)
FIGURE 2.
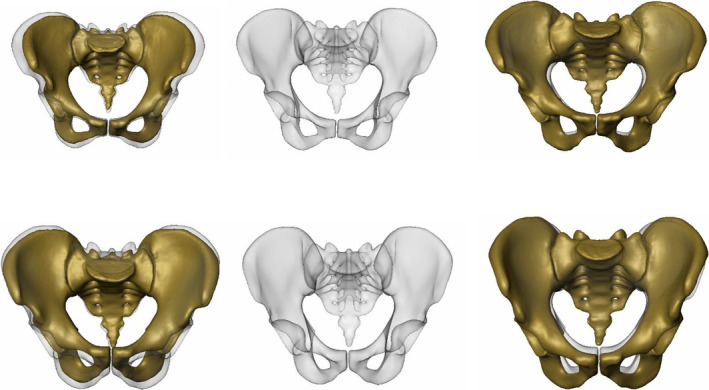
CT‐based 3D statistical model of the Asian females (top row) and males (bottom row) with PC 1 predominantly displaying size variation: Anteroposterior views with mean models in semi‐transparent grey and with PC 1 models −2 SD (left) and +2 SD models in yellow (right)
When extending PCA to comprise PC 1 to PC 20 for the European models (PC 1 to PC 15 for the Asian models), the 3D statistical models also exhibited the following six distinct APRs at different anatomical regions of the pelvic ring:
sacral asymmetry: asymmetric oriented ilio‐sacral joints with/ without oblique and lateralized sacral base.
iliac crest asymmetry: with the ASISs located at different anteroposterior and/ or mediolateral positions.
pelvic brim asymmetry: with asymmetric iliopectineal lines.
acetabular asymmetry: with asymmetric inclination and anteversion (Murray, 1993), craniocaudal, mediolateral, anteroposterior orientation of the acetabula.
pubic symphysis asymmetry: with the pubic symphysis located at different anteroposterior and craniocaudal positions.
inferior pubic ramus asymmetry: with the inferior pubic rami located at different anteroposterior positions.
In Figure 3, the 3D statistical model of the European female model and its PC 7 is displayed as an example for a principal component with major asymmetric changes. PC 7 demonstrated all the six distinct APRs as given by PCA.
FIGURE 3.
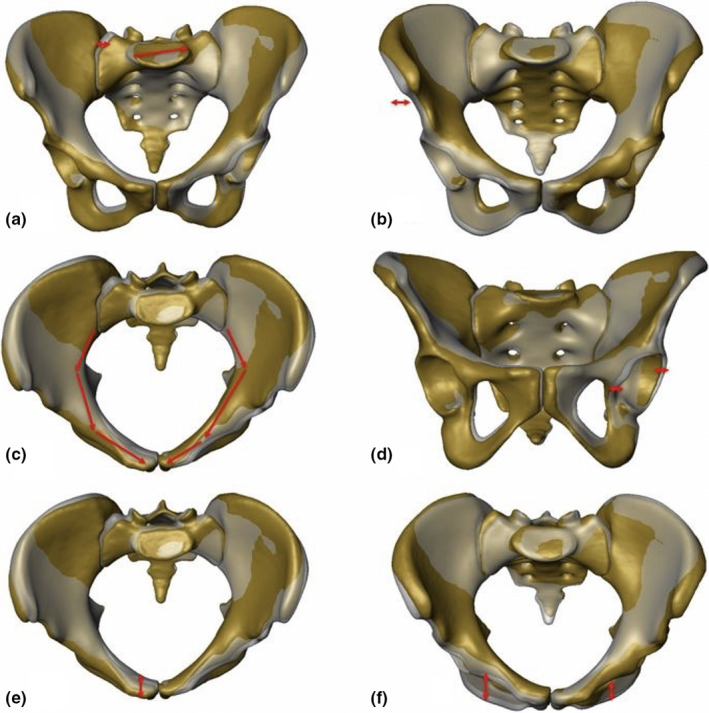
CT‐based 3D statistical model and PCA of the European females with PC 7 to illustrate the six distinct APRs and locations (mean model (semitransparent grey), PC 7 −3 SD and +3 SD models (yellow) and red arrows). (a) PC 7, ‐3 SD: anteroposterior view, red arrows showing asymmetry of the sacrum. (b) PC 7, +3 SD: anteroposterior view, red arrow showing asymmetry of the iliac crest. (c) PC 7, ‐3 SD: inlet view, red arrows showing asymmetry of the pelvic brim. (d) PC 7, ‐3 SD: outlet view, red arrows showing asymmetry of the acetabulum. (e) PC 7, ‐3 SD: inlet view, red arrow showing asymmetry of the pubic symphysis. (f) PC 7, +3 SD: inlet view, red arrows showing asymmetry of the inferior pubic ramus
The afore mentioned six distinct APRs with their locations were unevenly distributed among the gender and ancestry‐specific 3D statistical models and within their PCs, respectively. For example, the European female model displayed first asymmetry patterns in PC 2, whereas for the European male model asymmetry patterns with their locations were observed starting from PC 10. Furthermore, merely the European female model displayed notable pubic symphysis asymmetries in PC 2, PC 7, and PC 8. Interestingly, an asymmetric pubic symphysis was predominantly associated with female gender. PCA only exhibited moderate asymmetries for both the Asian models. Sites other than the ones displaying the distinct APRs only presented minor asymmetries. Remarkably, all 150 pelvic 3D CT models under evaluation demonstrated either distinct APRs, minor ones or combinations thereof.
In Figure 4, all the six distinct APRs with their locations were illustrated in given 3D CT models.
FIGURE 4.
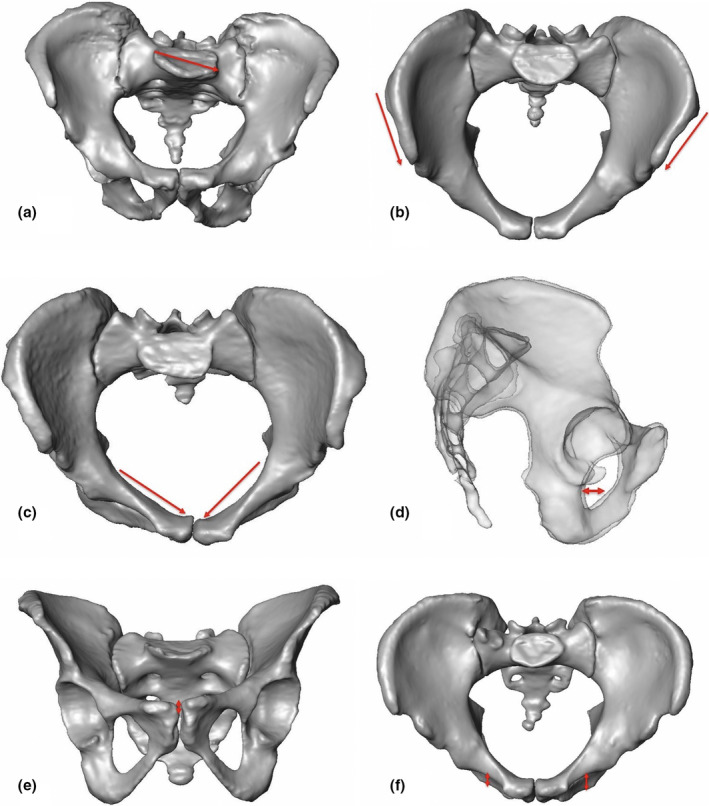
Given 3D CT models to display the six APRs with their locations: (a) 88 years old Asian female with sacral asymmetry: 3D CT model in anteroposterior view with asymmetric oriented sacrum and lateralized and oblique sacral basis (case p457). (b) 3D CT model of a 27 years old Asian male: Inlet view demonstrates iliac crest asymmetry (asymmetric position of left versus right ASIS (case p415)). (c) 3D CT model of a 54 years old European female: Inlet view exhibits asymmetric pelvic brim (case p601). (d) Semi‐transparent lateral view of a 65 years old European female with asymmetric acetabula (case p634). (e) 3D CT model of an 86 years old European female: Outlet view with asymmetric pubic symphysis (case p526). (f) 3D CT model of a 62 years old Asian male: Inlet view with asymmetric inferior pubic ramus (case p424)
4. DISCUSSION
The pelvic ring, composed of the sacrum, coccyx, and innominate bones, is known to be a complex anatomical structure and to demonstrate a variety of anatomical variations. Currently, there is only limited knowledge and understanding about APR and how to assess it. The objective of the present study was to adopt CT‐based 3D statistical modeling and analysis to assess APR. Considering the complex anatomy of the pelvic ring, we expected a better understanding for APR to be established with knowledge and applications obtained transferred to human anatomy, related research, and development subjects and to clinical settings.
Yet there is no established approach to assess the pelvic asymmetry. We studied small differences in side‐to‐side morphology of structures that are essentially symmetrical. Using CT‐based 3D statistical modeling and PCA we identified distinct pelvic asymmetry patterns demonstrated by given pelvic specimens collected (also see Figure 4a–f) and were able to show that asymmetry of the pelvic ring (APR) existed even in subjects displaying no major pathologies such as local fractures or deformities. Quantitative evaluations were not subject of this study.
We have been computing ancestry‐ and gender‐specific CT‐based 3D statistical models of the pelvic ring and performing PCA thereof. Due to the use of clinical and postmortem CT scans, the age differed in the studied groups. Age may have an influence on a bone's shape and size, however, static pelvic parameters like pelvic incidence were shown not to differ with aging (Weinberg et al, 2016). As expected, PCAs exhibited major variation patterns with regard to shape and size as well as to position of the ring‐building bones, as previously described by Arand et al. (Arand et al., 2019). When computing additional PC modes, six distinct asymmetry patterns (i.e., distinct APRs) were observed that were located at different anatomical regions: At the sacral region, it was mainly the sacral base that demonstrated obliquity. Furthermore, asymmetric oriented ilio‐sacral joints were observed. At the iliac crest, asymmetry was primarily due to different anteroposterior or mediolateral orientation when comparing left versus right ASIS. Further asymmetries were located at the pelvic brim, acetabulum, inferior pubic ramus, and pubic symphysis. Interestingly, an asymmetric pubic symphysis was predominantly associated with female gender. This asymmetry pattern may be due to pregnancy or giving birth. Yet, this assumption needs further confirmation as no information about pregnancy/ giving birth was available to conduct this study. Sites other than the ones displaying distinct asymmetry patterns only presented minor asymmetries. Remarkably, all 150 pelvic 3D CT models under evaluation demonstrated either distinct asymmetries, minor ones, or combinations thereof.
Hence, the pelvic ring cannot be considered to be entirely symmetric. Using CT‐based 3D statistical modeling and PCA, we identified and described six distinct APRs that were located at different anatomical regions. These regions demonstrated to be sites that were typically affected by asymmetry patterns as described afore. Furthermore, they need to be distinguished from sites displaying only minor asymmetries or even from ones with a matching symmetry. Therefore, minor asymmetry patterns have to be distinguished from major ones like the distinct APRs as identified by PCA.
Although PCA enabled identification and location of APRs, they still require further evaluations and considerations to be made. For example, asymmetries near to or at the acetabulum may be triggered by side differences with regard to size, shape, and/ or position of the acetabular sockets or solely by side differences of the periacetabular region. In case of matching acetabular sockets, the contralateral one might be taken as a reference to virtually reconstruct the ipsilateral, affected side. However, potential positional side differences need to be taken into account. Technically, this could be performed via mirror imaging the reference to the contralateral side using the symmetry plane. However, as the pelvic ring does not exhibit an entirely symmetric configuration, the definition of the symmetry plane might become a difficult or even unfeasible task. Flipping (i.e., reflecting an object across a given plane) the contralateral reference and aligning it to the ipsilateral, affected side might therefore be a more viable option.
Currently, there is no best practice in performing asymmetry assessment of the pelvic ring. Our technique facilitated analysis and permitted valuable information to be obtained to enhance our understanding about distinct APRs. Furthermore, our approach permitted efficient and comprehensible analyses within gender‐ and ancestry‐specific models generated. It allowed for distinct APRs to be identified without requiring the 150 pelvic CTs as single samples to be taken into account. Furthermore, we think, that use of simpler methods, for example, visual judgment or manual measurements, would have limited our analyses. However, we acknowledge, that CT‐based 3D statistical modeling and PCA are known to be technically and timewise demanding procedures requiring considerable knowledge and expertise. Moreover, they remain difficult to be applied to routine applications such as used in anatomy settings or clinical applications. Simpler and efficient, yet reliable assessment methods need to be available to be commonly used in daily practice. Symmetry analysis of the pelvic ring via CT‐based 3D statistical modeling remains a technically demanding task. Our approach allowed for specific asymmetry patterns and regions to be identified in a complex bone that is essentially symmetrical. Analyses were made irrespective of symmetry plane. We acknowledge that simpler methods may exist. We recommend additional evaluations to be made with pros and cons of different methods, with/without symmetry planes to be thoroughly evaluated and further quantitative evaluations to be performed.
Human demonstrate species‐wide bilateral asymmetry in long bone dimensions (Auerbach & Ruff, 2006). Systematic or a trend to symmetry differences between upper and lower extremity has been termed as “crossed symmetry” (Plochocki, 2004). Auerbach and Ruff re‐examined the topic in a large, geographically, and temporally diverse sample of 780 modern adult humans using morphometric evaluations. Long bones of the upper extremities demonstrated a systematic asymmetric right‐bias, whereas the lower limb displayed element of bias with a slight left‐bias. Furthermore, gender‐related differences were observed.
Different studies on symmetry assessment of the pelvic ring region have been previously performed: Boulay et al. analyzed pelvic asymmetry using 12 anatomical specimens and a reference coordinate system (Boulay et al., 2006). They studied 71 paired morphometric variables and demonstrated 15 ones being significantly asymmetric. These were located at the sacrum, iliac blades and acetabulum and concerned the iliac width and superior lunate surface of the acetabulum. They concluded that, total asymmetry involving the right and the left pelvis seemed to follow a spiral path in the pelvis; that is, in the upper part, the iliac blades to rotate clockwise, and in the lower part, the pubic symphysis to rotate anticlockwise. Yegyan Kumar et al. analyzed pelvic symmetry in 10 pelvic CT scans by comparing the left‐right volumes and found an average difference in left‐right volume was 1.08% (Yegyan Kumar et al., 2016).
Skeletal asymmetries have been described to originate from genetic, biomechanical, and developmental factors (Tobolsky et al., 2016). In this study, os coxae of 128 skeletons were analyzed and a left bias in the directional asymmetry of the pelvis was detected. There were no sex‐ or population‐related differences found in the directional asymmetry. In biomechanics, different investigations were made on the effect of the load distribution of the pelvis (Arkusz et al., 2018; Dalstra & Huiskes, 1995; Shi et al., 2014). However, none of these demonstrated the effect on changes of the pelvic symmetry. In our study, we found no evidence for side‐, gender‐, or ancestry‐related biases. However, we assume an association between behavioral and morphological asymmetry. Hence, APRs may occur as a skeletal adaption to asymmetric mechanical loading, as similarly described for the limb bones (Auerbach & Ruff, 2006; Weatherholt & Warden, 2016): Asymmetries, such as observed at the sacrum and sacral base, could be primarily due to asymmetric mechanical loading transferred via spine. Conversely, asymmetries located at the periacetabular regions could be predominantly caused by asymmetric mechanical loading transferred via the lower extremity. Thirdly, local factors, such as asymmetric muscle traction or uneven forces due to asymmetric orientation of the pubic symphysis (e.g., caused by a fracture, gravidity, or giving birth) could contribute to APRs. Hereafter, the pelvic ring could be understood to be an anatomical site to withstand and adapt to asymmetric mechanical loading distributed via cranial, caudal parts of the human body or transferred via local sources.
In orthopedic pelvic surgery and trauma care, there is a growing interest to use patient‐specific implants (PSIs). A possibility for its manufacturing is “image mirroring” of the intact, contralateral side to be used as an anatomical reference to reconstruct the ipsilateral, affected side. Bilateral pelvic symmetry for PSI manufacturing with regard to acetabular fracture treatment has been evaluated by Osterhoff et al. (Osterhoff et al., 2019). The authors concluded that the pelvis can be considered sufficiently symmetric for using the mirrored contralateral hemipelvis as a template for PSIs in acetabular fracture fixation. We think “mirror imaging” to be a valid approach if above mentioned considerations have been taken into account (see paragraph 3 and 4 of the discussion section). Hence, “mirror imaging” might be a valuable technique for anatomical regions with no or limited asymmetry.
There are several limitations associated with our study: Firstly, we used advanced computational methods that were time consuming and required demanding software tools and skills. Furthermore, no guidelines were provided to analyze the given asymmetric cases. Thirdly, pelvic CTs, as used in our study, do not allow for soft tissue structures (e.g., ligaments, cartilage) to be properly assessed. Lastly, only a limited number of Asian pelvic CT scans have been evaluated.
5. CONCLUSION
The pelvic ring, known to be a complex anatomical structure, has been demonstrated not to be entirely symmetric. Assessment of its asymmetry proved to be a challenging task. Using CT‐based 3D statistical modeling and PCA, we identified six distinct APRs that were located at different anatomical regions. These regions are more prone to APRs than other sites. Minor asymmetry patterns have to be distinguished from the distinct APRs. Side differences with regard to size, shape, and/ or position require to be taken into account.
APRs may be due to different load mechanisms applied via spine or lower extremity or locally. There is a need for simpler and efficient, yet reliable methods to be routinely applied in human anatomy, related research, and development subjects and to clinical settings.
CONFLICTS OF INTEREST
No conflicts of interest have to be declared by the author or coauthors.
AUTHOR CONTRIBUTIONS
Kristin Handrich: corresponding author, study design, data processing, analysis and interpretation, manuscript. Lukas Kamer: initiation of the project, contribution to concept and study design, data acquisition, critical revision of the manuscript. Keith Mayo: initiation of the project, critical revision of the manuscript. Takeshi Sawaguchi: initiation of the project, data acquisition, critical revision of the manuscript. Hansrudi Noser: study design, data processing, critical revision of the manuscript. Charlotte Arand: initiation and concept of the project, data acquisition, critical revision of the manuscript. Daniel Wagner: initiation and concept of the project, critical revision of the manuscript. Pol M. Rommens: initiation of the project, data interpretation, critical revision of the manuscript.
ACKNOWLEDGEMENTS
This study was supported by AOTauma of the AO Foundation, Davos, Switzerland (study number: AR_2018_02). Kristin Handrich received a Research Fellowship grant from the AO Research Institute Davos, Davos, Switzerland. Open access funding enabled and organized by ProjektDEAL.
DATA AVAILABILITY STATEMENT
Author elects not to share data.
REFERENCES
- Arand, C. , Wagner, D. , Richards, R.G. , Noser, H. , Kamer, L. , Sawaguchi, T. et al. (2019) 3D statistical model of the pelvic ring – A CT‐based statistical evaluation of anatomical variation. Journal of Anatomy, 234, 376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkusz, K. , Klekiel, T. , Niezgoda, N. & Bedzinski, R. (2018) The influence of osteoporotic bone structures of the pelvic‐hip complex on stress distribution under impact load. Acta Bioeng Biomech, 20, 29–38. [PubMed] [Google Scholar]
- Auerbach, B.M. & Ruff, C.B. (2006) Limb bone bilateral asymmetry: Variability and commonality among modern humans. Journal of Human Evolution, 50, 203–218. [DOI] [PubMed] [Google Scholar]
- Bookstein, F.L. (1997) Landmark methods for forms without landmarks: Morphometrics of group differences in outline shape. Medical Image Analysis, 1, 225–243. [DOI] [PubMed] [Google Scholar]
- Boulay, C. , Tardieu, C. , Benaim, C. , Hecquet, J. , Marty, C. , Prat‐Pradal, D. et al. (2006) Three‐dimensional study of pelvic asymmetry on anatomical specimens and its clinical perspectives. Journal of Anatomy, 208, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalstra, M. & Huiskes, R. (1995) Load transfer across the pelvic bone. Journal of Biomechanics, 28, 715–724. [DOI] [PubMed] [Google Scholar]
- Desilva, J.M. & Rosenberg, K.R. (2017) Anatomy, development, and function of the human pelvis. Anatomical Record (Hoboken), 300, 628–632. [DOI] [PubMed] [Google Scholar]
- Garvey, J.F. & Hazard, H. (2014) Sports hernia or groin disruption injury? Chronic athletic groin pain: A retrospective study of 100 patients with long‐term follow‐up. Hernia, 18, 815–823. [DOI] [PubMed] [Google Scholar]
- Heimann, T. & Meinzer, H.P. (2009) Statistical shape models for 3D medical image segmentation: A review. Medical Image Analysis, 13(4), 543–563. [DOI] [PubMed] [Google Scholar]
- Heimann, T. , Van Ginneken, B. , Styner, M.A. , Arzhaeva, Y. , Aurich, V. , Bauer, C. et al. (2009) Comparison and evaluation of methods for liver segmentation from CT datasets. IEEE Transactions on Medical Imaging, 28(8), 1251–1265. [DOI] [PubMed] [Google Scholar]
- Kamer, L. , Noser, H. , Schramm, A. & Hammer, B. (2010) Orbital form analysis: problems with design and positioning of precontoured orbital implants: A serial study using post‐processed clinical CT data in unaffected orbits. International Journal of Oral and Maxillofacial Surgery, 39(7), 666–672. [DOI] [PubMed] [Google Scholar]
- Li, Y.M. , Li, J.H. , Li, B. , Wang, J.X. & Chen, Y.S. (2016) The radiological research for pelvis asymmetry of unilateral developmental dysplasia of the hip in adult. Saudi Medical Journal, 37, 1344–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer, P. , Matthews, F. , Jacob, A.L. , Kikinis, R. , Regazzoni, P. & Noser, H. (2007) A CT database for research, development and education: Concept and potential. Journal of Digital Imaging, 20, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, D.W. (1993) The definition and measurement of acetabular orientation. The Journal of Bone and Joint Surgery. British volume, 75‐B(2), 228–232. 10.1302/0301-620X.75B2.8444942 [DOI] [PubMed] [Google Scholar]
- Noser, H. , Hammer, B. & Kamer, L. (2010) A method for assessing 3D shape variations of fuzzy regions and its application on human bony orbits. Journal of Digital Imaging, 23(4), 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhoff, G. , Petersik, A. , Sprengel, K. & Pape, H.C. (2019) Symmetry matching of the medial acetabular surface – A quantitative analysis in view of patient‐specific implants. Journal of Orthopaedic Trauma, 33, e79–e83. [DOI] [PubMed] [Google Scholar]
- Plochocki, J.H. (2004) Bilateral variation in limb articular surface dimensions. American Journal of Human Biology, 16(3), 328–333. [DOI] [PubMed] [Google Scholar]
- Putz, R. & Muller‐Gerbl, M. (1990) Pelvic abnormalities affecting the statics of the vertebral column. Orthopade, 19, 278–282. [PubMed] [Google Scholar]
- Rommens, P.M. & Hofmann, A. (2017) Fragility fractures of the pelvis. Cham, Switzerland: Springer. [Google Scholar]
- Shi, D. , Wang, F. , Wang, D. , Li, X. & Wang, Q. (2014) 3‐D finite element analysis of the influence of synovial condition in sacroiliac joint on the load transmission in human pelvic system. Medical Engineering & Physics, 36, 745–753. [DOI] [PubMed] [Google Scholar]
- Stover, M.D. , Edelstein, A.I. & Matta, J.M. (2017) Chronic anterior pelvic instability: Diagnosis and management. Journal of American Academy of Orthopaedic Surgeons, 25, 509–517. [DOI] [PubMed] [Google Scholar]
- Tile, M. , Helfet, D. , Kellam, J. , & Vrahas, M. ; Aotrauma . (2015) Fractures of the pelvis and acetabulum: Principles and methods of management. Davos, Switzerland: AOTrauma. Distributor: Stuttgart, Germany; New York, NY: Distributed by Georg Thieme Verlag. [Google Scholar]
- Tobolsky, V.A. , Kurki, H.K. & Stock, J.T. (2016) Patterns of directional asymmetry in the pelvis and pelvic canal. American Journal of Human Biology, 28, 804–810. [DOI] [PubMed] [Google Scholar]
- Weatherholt, A.M. & Warden, S.J. (2016) Tibial bone strength is enhanced in the jump leg of collegiate‐level jumping athletes: A within‐subject controlled cross‐sectional study. Calcified Tissue International, 98(2), 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg, D.S. , Morris, W.Z. , Gebhart, J.J. & Liu, R.W. (2016) Pelvic incidence and anatomic investigation of 880 cadaveric specimens. European Spine Journal, 25(11), 3589–3595. [DOI] [PubMed] [Google Scholar]
- Wells, J. , Nepple, J.J. , Crook, K. , Ross, J.R. , Bedi, A. , Schoenecker, P. et al. (2017) Femoral morphology in the dysplastic hip: Three‐dimensional characterizations with CT. Clinical Orthopaedics and Related Research, 475, 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegyan Kumar, A. , El‐Rich, M. , Jaremko, J. & Duke, K. (2016) Symmetry of the pelvis using volume and deviation analysis. 22nd Congress of the European Society of Biomechanics, July 10 ‐ 13, 2016, Lyon, France. [Google Scholar]
- Zollikofer, C.P. & de Leon, M.P. (2005) Virtual reconstruction: A primer in computer‐assisted paleontology and biomedicine. Hoboken, NJ: Wiley‐Interscience. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Author elects not to share data.


