Abstract
This study investigates the hypothesis that there is, during childhood, a disproportionate age‐related expansion of the origin of temporalis muscle compared to the growth of the underlying skull. Lateral projections of 50 randomly selected 3D reformatted computerized tomographic (CT) scans (yielding 100 temporalis muscles) of children aged >0.6 to 15 years scanned for conditions that did not affect the shape of their head or face were windowed to provide the optimum delineation of temporalis muscle against the underlying bone. Vertical and anterior–posterior measurements of the muscle made independently by two observers were compared with those of the skull along the same planes. The development of the anterior temporal crest was also assessed. The intraclass correlation coefficient for differences in the measurements made by each observer ranged from good to excellent. The muscle and skull measurements were used to produce a ratio of muscle‐to‐skull lengths in both vertical and horizontal planes. Analysis of these ratios showed a statistically significant increase in the vertical reach of temporalis with age (Pearson correlation coefficient (R) =0.7826; p < 0.05) compared to the growth of the skull along the planes chosen for the study—but less so for its horizontal reach (R = 0.5073. p < .001). There were no significant differences between right/left or male/female measurements. There was also a substantial level of agreement between both observers in their assessment of the development of the temporal crest. The mean age of children in whom a fully formed temporal crest could be identified (10.6 years) was significantly greater (p < 0.001) than that of the 38 remaining subjects (6.0 years). These results confirm that there is, in response to increased masticatory/dietary demands during childhood, a disproportionate increase in the vertical and (to a lesser extent) horizontal reach of temporalis muscle over its origin from the temporal, frontal, sphenoid, and parietal bones compared the growth of the skull. It is proposed that surgical interference with this normal process is responsible for the soft tissue component of late‐developing deformity that can occur following early (at 6–18 months of age) operations for the correction of trigonocephalic head shape associated with metopic synostosis.
Keywords: childhood, muscle growth, temporalis muscle
This study shows how, during childhood, increased masticatory demands upon temporalis muscle leads to an expansion in its bony origin from the temporal fossa that is out of proportion to the growth of the underlying skull.
![]()
1. INTRODUCTION
The temporalis muscle is the most powerful of the muscles of mastication. It is derived from the tissue of the first pharyngeal arch. It has a broad fan‐shaped origin from the squamous temporal, sphenoid, parietal, and frontal bones as well as from the deep surface of the temporal fascia. Its upper limit (where the temporal fascia fuses with pericranium; Tolhurst et al., 1991) is the superior temporal line whose anterior end is marked by the bony temporal crest. The muscle's anterior fibres run vertically, the posterior horizontally and the intermediate obliquely. Its tendinous attachment is to the coronoid process of the mandible and retro‐molar fossa. Its primary action is to elevate the mandible during biting and chewing.
The purpose of this study was to interrogate using 3D CT scan data from unaffected subjects the hypothesis that there is in normal children a previously undescribed age‐related extension of the bony origin of the temporalis muscle in response to the increasing masticatory demands placed upon it (Kamegai et al., 2005).
2. METHODS
Randomly selected CT scans of 50 children aged <15 years (providing a total of 100 temporalis muscles for analysis) investigated for disorders that did not affect the craniofacial skeleton or musculature were examined for this study. The age range of the subjects was from 0.6 to 15.0 years (mean: 7.1 years [range 0.6–15; SEM 0.37]). There were 29 males and 21 females.
All CT scans were obtained with a non‐contrast helical acquisition on the same CT scanner (Somatom Definition Flash 128‐slice CT scanner, Siemens Healthcare, Siemens) with X‐CARE organ‐based modulation to reduce radiation dose.
The scans were 3D reformatted with window widths and levels adjusted in each scan to provide the clearest delineation of temporalis muscle against the underlying bone. In order to allow for variations in the alignment of the subjects in the scanner, measurements were made after the image used had been rotated as necessary to display a true lateral projection.
Four measurements of the vertical and anterior‐posterior reach of both temporalis and the skull in the planes selected for the purpose of this study were undertaken independently by two observers (MCRE and GM) using the CT’s software's calibrated line ruler tool (Figure 1).
FIGURE 1.
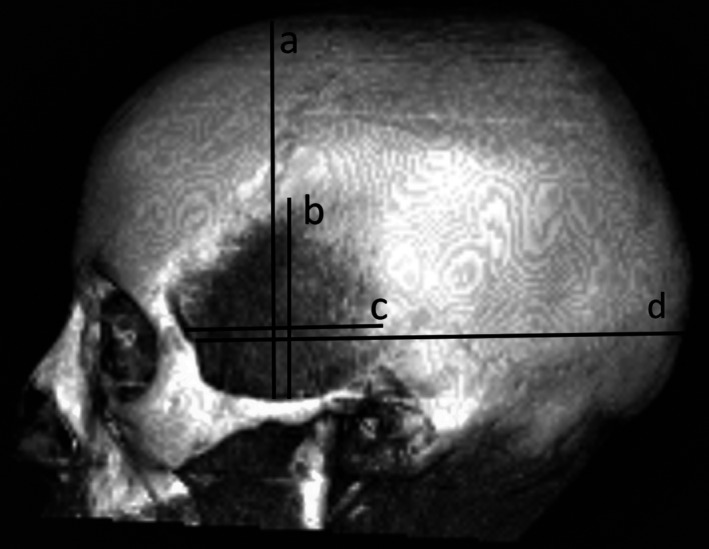
Lateral projection of a 3D CT skull showing lines a, b, c and d
Line a. (Skull vertical height) A perpendicular drawn from the midpoint of the superior border of the zygomatic arch to a horizontal line passing through the vertex.
Line b. (Temporalis vertical height/reach) A perpendicular drawn along the same plane as (a) to the superior limit of temporalis.
Line c. (Skull horizontal length) A line parallel to the upper border of the zygomatic arch drawn from the frontozygomatic suture to a horizontal line passing through the occipital bone.
Line d. (Temporalis horizontal length/reach) A line parallel to the upper border of the zygomatic arch drawn along the same plane as (d) to the posterior limit of temporalis.
These measurements allowed two ratios (b/a – the vertical height of 100 temporalis muscles to the vertical height of the skull along the same plane; and c/d – the horizontal length of 100 temporalis muscles to the anteroposterior skull length along the same plane) to be calculated and plotted against the age of the subject at the time of the scan.
The ratios so obtained were also analyzed for differences between right and left and male and female.
The presence or absence of the temporal crest (defined as a well‐demarcated, curved bony ridge that joins the SOR at the commencement of the zygomatic process of the frontal bone; Figure 2, right) was included in the study because of the role of temporalis muscle in its development (Washburn, 1947).
FIGURE 2.
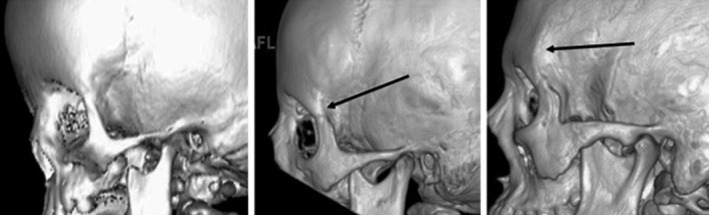
Grades of temporal crest development. (Left) Grade 0: No temporal crest (age 1.5 months); (Center) Grade 1: Rudimentary crest development (age 6 years); (Right) Grade 2: Fully developed temporal crest (age 15 years)
Its appearance was graded as follows:
Grade 0 = No temporal crest present (Figure 2, Left).
Grade 1 = An intermediate stage with a rudimentary crest (Figure 2, Center).
Grade 2 = Temporal crest (as defined above) present (Figure 2, Right).
2.1. Statistics
For the comparison of correlation of continuous variables (e.g. length), Pearson correlation tests were used and reported as a proportion of 1 (1 indicating perfect correlation, 0 none). For the assessment of inter‐observer reliability, the intraclass correlation coefficient (ICC) was calculated and reported as a proportion of 1 (1 indicating the exact correlation, 0 no correlation). For ICC calculations, a single‐rater, absolute agreement, two‐way random‐effects model was used. Finally, the comparison of mean age between categories, between right/left and between male/female differences were analyzed using the unpaired t‐test.
3. RESULTS
3.1. Inter‐observer agreement
To examine the degree of inter‐observer agreement between the two observers the ICC between their measurements of a, b, c and d were calculated. The results showed good to excellent agreement (Table 1).
TABLE 1.
Intraclass correlation coefficients (ICC) between the two observers’ measurements of lines a, b, c and d for right and left sides combined
| Measurement | a | b | c | d |
|---|---|---|---|---|
| CIC | 0.703 | 0.909 | 0.840 | 0.742 |
3.2. Measurements of temporalis
The average distance (of four—right and left by two observers) between the upper border of the zygomatic arch and superior limit of temoralis in the plane chosen for this study (b) increased from 31.1 mm at 0.6 years to 71.9 mm at 15 years.
The average horizontal length of the muscle along the plane chosen for this study (d) increased from 120.5 mm at 0.6 years to 144.5 mm at 15 years.
3.3. Vertical and Horizontal changes in the extent of temporalis with age
The ratios (b/a and c/d) derived from measurements made independently by the two observers were averaged to produce single age‐related figures for all 50 subjects. These were then plotted against the age of the subject at the time they were scanned.
There was a statistically significant age‐related increase along the plane studied of the vertical extent of temporalis muscle compared to that of the skull along the same plane (Pearson correlation coefficient R = 0.7826, p < .001; Figure 3).
FIGURE 3.
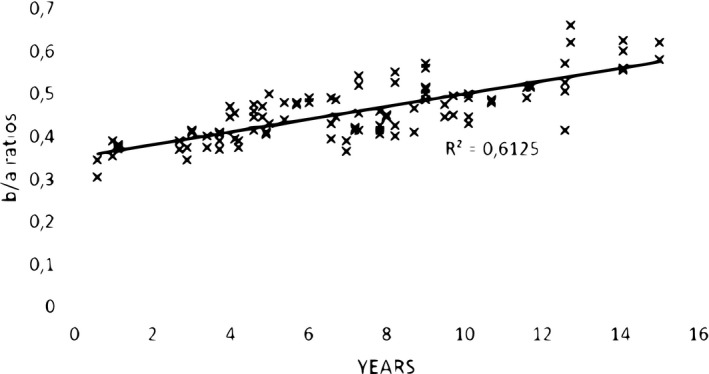
Ratios (b/a) of a vertical reach of 100 (50 right plus 50 left) temporalis muscles above the upper border of the zygomatic arch to skull height (upper border of the zygomatic arch to vertex) against age (years) at the scan
The age‐related relationship of the horizontal length of temporalis muscle (c/d) to that of the skull along the same plane was statistically significant, although less so than for the vertical (Pearson correlation coefficient: R = 0.5073, p < .001; Figure 4).
FIGURE 4.
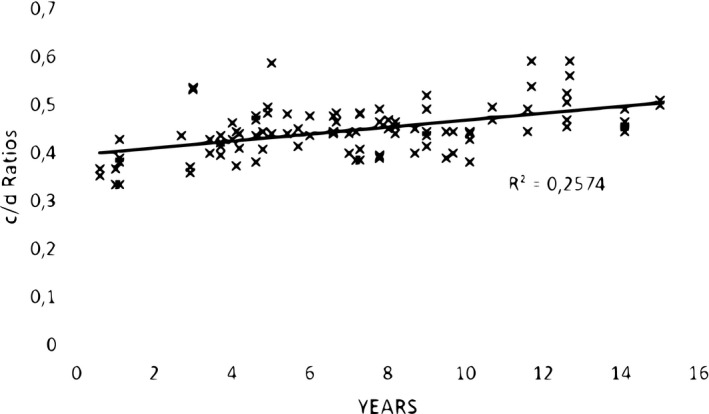
Ratios (c/d) of horizontal reach of 100 (50 right plus 50 left) temporalis muscles to horizontal skull length (from the frontozygomatic suture to occiput) against age (in years) at the scan
3.3.1. Right/left difference
There was no significant difference in results for either b/a or c/d between right and left sides (b/a: p = 0.9387; c/d: p = 0.3588).
3.3.2. Male/female difference
There was no significant difference in results for either b/a or c/d between females and males (b/a: p = 0.3814; c/d:p = 0.3922).
3.3.3. Temporal crest observations
The ICC for agreement between the two observers for their assessment of the 50 right‐side and 50 left‐side 3D images for the presence or absence of a temporal crest was 0.873 for the right side (good) and 0.562 for the left (reasonable).
The mean age (8.4 years) of 37 children who had at least one side rated grade 2 by at least one observer was statistically greater than that of the four children (mean age 1.0 year) with at least one side rated 0 by at least one observer (p < 0.001).
Because the number of children with no recorded crest development (grade 0) was so small, the mean age of the 12 children graded 2 by both observers was compared with the ages of the 38 remaining subjects. The mean age of the 12 subjects graded 2 by both observers (10.6 years) was significantly older (p < 0.001) than that of the 38 remaining subjects (6.0 years).
4. DISCUSSION
This study confirms that there is in normal children aged up to 15 years a statistically significant age‐related vertical and horizontal expansion of the bony origin of temporalis muscle (effectively the temporal fossa) that is disproportionate to the growth of the superior/posterior quadrant of the skull. Agreement between two observers acting independently was higher for the measurements of the muscle's vertical reach as both found the anteroposterior (horizontal) boundary of the muscle harder to define on the 3D CT scan lateral projections used for this investigation. No significant differences were found between right and left sides or between male and female in the 50 children studied.
The investigation was prompted by a previous study (Rodriguez‐Florez et al., 2019) into the late‐developing deformity that can complicate reconstructive operations carried out between 6 and 18 months of age for metopic synostosis (van der Meulen et al., 2008; Wes et al., 2014) that identified the absence of temporalis in the area normally occupied by its superior elements as the prime contributor to its soft tissue component. We hypothesized that this deformity (which typically becomes apparent some 3–4 years post‐surgery) was the consequence of surgical interference (the operations involve detachment and later re‐attachment of temporalis) with a previously undescribed childhood upward extension of the muscle by operations performed between 6 and 18 months of age.
It is a phenomenon that cannot be explained by proportionate muscle:skull growth. If that part of the head under examination is thought of as the superior/posterior quadrant of a sphere, any increase in that sphere's diameter that raised the height of its superior pole (in this example the vertex) by 2 cm (say) would have a negligible effect on the vertical position of a point (here the upper limit of temporalis) close to its equator—which would move outwards/laterally but not upwards. A similar argument can be applied to the increase in the muscle's horizontal reach.
The absence of these superior elements may also contribute to its bony component by the removal of the muscle's normal osteogenic stimulus (Rodriguez‐Florez et al., 2019).
The cells of temporalis, a striated muscle, do not divide after birth (Joubert, 1955; Pearson, 1990) and post‐natal growth occurs through the hypertrophy of existing muscle fibers. While their total number remains constant (Rowe & Goldspink, 1969) multiple enzymal and hormonal pathways (Schiaffino et al., 2013) control the protein synthesis needed to increase both the number of myofibrils within each muscle fibre and, through addition of sarcomeres to their ends, their length (Pearson, 1990). Proliferation and fusion of multipotent satellite cells may also contribute to the normal process (Schiaffino et al., 2013).
The result of this hypertrophy is that the junction between the temporal fascia and pericranium (the temporal line that defines the superior boundary of the temporal fossa; Tolhurst et al., 1991) extends upwards until, in older children, it reaches the level marked anteriorly by the development of the bony temporal crest.
Our study also shows that there is a statistically significant relationship between the presence or absence of this structure and the age of the children at which they were scanned (and significant agreement between both observers in their assessments despite the subjective nature of those assessments). This is not surprising. The temporal crest develops where the stresses generated by repeated contractions of temporalis effect a remodelling of the underlying bone mediated through, it has been suggested, the elastic properties of the Sharpey's fibres (Aaron, 2012) that connect the muscle to the bone. Its prominent anterior end marks where the (age‐related) pull of the muscle is concentrated, keystone‐like, at the junction of its superficial and inferior septations (O’Brien et al., 2013; Washburn, 1947).
The early phase of the age range studied here (0.5–15 years) is a busy one for a child's eating pattern and dentition due to the introduction of a more solid diet and the eruption of molar teeth that starts from 12 to 18 months and continues to full eruption at around two and a half years. It is a period when “Eating Efficiency” rapidly improves (Gisel, 1991) and the child's chewing pattern changes from the immature (more rotary or semi‐circular movement) to one that in order to mash and grind a more solid diet requires the greater input from the more anteriorly disposed (vertical) fibers of temporalis. It has been suggested, however, that this change may also represent “Refinement of the extant coordinative organization rather than from the potential effects of a wide variety of external factors” including diet (Green et al., 1997).
One would anticipate that the age‐related expansion of temporalis described here would be accompanied by an increase in its strength. Masticatory performance in children aged 5–15 years does indeed improve significantly with age (Toro et al., 2006) and is associated with an increase in body size. Masticatory performance has also been measured as “bite force” and several studies report it increasing during childhood, peaking at 12 years of age and stabilizing after 14 years of age (Chong et al., 2016; Kamegai et al., 2005; Le Révérend et al., 2014; Simione et al., 2018). Takaki et al. (2014) described how bite force increased from pre‐pubescence until age 20 following which stabilized but acknowledged the difficulty in measuring it accurately in young children due to issues with motivation and cooperation.
4.1. Limitations of this study
Our finding that there is a normal age‐related vertical and horizontal expansion of temporalis muscle disproportionate to the growth of the underlying skull from which it arises is based on only two—vertical and horizontal—measurements of the muscle and of the skull. It treats those elements of the muscle that lie above the zygomatic arch as a two‐dimensional structure and provides no information about its three‐dimensional bulk. The region covered by these measurements includes, however, not only the temporal fossa from which the muscle arises but those vertex and occipital points on the skull (as viewed on lateral 3D CT projections) reached by extrapolating the lines along which the muscle has been measured.
We submit, therefore, that the parameters chosen for our estimation of the age‐related increase in the reach of temporalis and the region of skull with which it is compared provide adequate and reproducible measurements upon which to base our conclusion—as illustrated by the examples selected for Figure 5.
FIGURE 5.
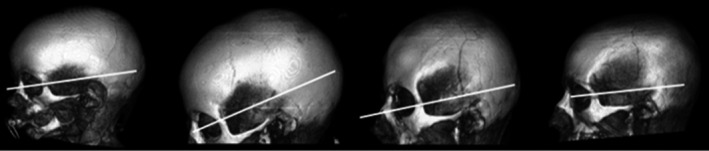
3D CT lateral images of four subjects aged, from left to right, 1 year; 5.4 years; 12.6 years; and 14 years demonstrating the increasing vertical and horizontal reach of temporalis muscle with age (reference white lines drawn parallel to the zygomatic arch at the level of the frontozygomatic suture)
Although variations in the alignment of the subjects in the scanner could in theory affect all distances measured, the 3D CT scan images used were rotated as needed by the observers to obtain a true lateral projection before measurements were made.
It could reasonably be argued that the superior ability of magnetic resonance imaging (MRI) to resolve soft tissue would be a more effective method for describing such age‐related changes in the muscle's anatomy. CT has, however, already proved to be an effective method for investigating temporalis—the relationship between its cross‐sectional area and the bite force it can generate, for example (Toro‐Ibacache et al., 2015). We have also chosen CT because, for a subsequent study of patients with post‐reconstructive surgery deformities, only CT data (essential for the preparation of the bespoke Polyetheretherketone (PEEK) (Ng & Nawaz, 2014) implants needed for their repair) is available and we considered it essential to use the same imaging modality for both studies.
5. CONCLUSION
Analysis of 3D CT scans from 50 children with no issues affecting the bone and soft tissues of the face and skull has identified a statistically significant, age‐related expansion of the upper limit of temporalis muscle that is disproportionate to the growth of that superior/posterior quadrant of the skull that contains its bony origin.
It is a process that coincides with an increase in the muscle's strength—its bite force—and the more mature chewing processes associated with the eruption of the molar teeth and a more solid diet. It affects right and left sides equally, is not sex‐related and concludes with the development of the temporal crest.
It is our opinion that interference with this process is responsible for the soft tissue component of the late‐occurring deformity that can complicate surgery for the management of metopic craniosynostosis—a hypothesis to be investigated in the light of the data presented here.
CONFLICT OF INTEREST
The authors declare no conflicts of interest in the preparation of this paper.
AUTHOR CONTRIBUTIONS
Moltoni & Rossi: Acquisition of data; D’Arco: Concept, preparation of data for acquisition; James: Statistical input; Hayward: Concept and design; data analysis; drafting of the manuscript. All authors approved the submitted draft.
[Correction added on 13 Jan 2021, after first online publication; a spelling error in the surname of Maria Camilla Rossi‐Espagnet has now been corrected]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aaron, J.E. (2012) Periosteal Sharpey’s fibers: A novel bone matrix regulatory system? Frontiers in Endocrinology, 3, 1–10. 10.3389/fendo.2012.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, X. , Khoo, C.D. , Goh, H. , Rahman, F. & Shoji, Y. (2016) Effect of age on bite force. Journal of Oral Science, 58, 361–363. [DOI] [PubMed] [Google Scholar]
- Gisel, E.G. (1991) Effect of food texture on the development of chewing of children between six months and two years of age. Developmental Medicine and Child Neurology, 33, 69–79. [DOI] [PubMed] [Google Scholar]
- Green, J.R. , Moore, C. , Ruark, J.L. , Rodda, P.R. , Morvée, W.T. & VanWitzenburg, M.J. (1997) Development of chewing in children from 12 to 48 months: longitudinal study of EMG patterns. Journal of Neurophysiology, 77, 2704–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert, D.M. (1955) Growth of muscle fibre in the fetal sheep. Nature, 175, 936–937. [DOI] [PubMed] [Google Scholar]
- Kamegai, T. , Tatsuki, T. , Nagano, H. , Mitsuhashi, H. , Kumeta, J. , Tatsuki, Y. et al. (2005) A determination of bite force in northern Japanese children. European Journal of Orthodontics, 27, 53–57. [DOI] [PubMed] [Google Scholar]
- Le Révérend, B.J.D. , Edelson, L.R. & Loret, C. (2014) Anatomical, functional, physiological and behavioural aspects of the development of mastication in early childhood. British Journal of Nutrition, 111, 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, Z.Y. & Nawaz, I. (2014) Computer‐designed PEEK implants: a peek into the future of cranioplasty? Journal of Craniofacial Surgery, 25, e55–e58. [DOI] [PubMed] [Google Scholar]
- O’Brien, J. , Dip, P. , Ashton, M. et al. (2013) New perspectives on the surgical anatomy and nomenclature of the temporal region: literature review and dissection study. Plastic and Reconstructive Surgery, 131, 510–522. 10.1097/PRS.0b013e31827c6ed6 [DOI] [PubMed] [Google Scholar]
- Pearson, A.M. (1990) Muscle growth and exercise. Critical Reviews in Food Science and Nutrition, 29(3), 167–196. 10.1080/10408399009527522 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Florez, N. , Florez‐Tapia, A. , Jeelani, N.U.O. , Schievano, S. , Dunaway, D.J. & Hayward, R.D. (2019) Investigating the cause of late deformity following fronto‐orbital remodelling for metopic synostosis using 3D CT imaging. Journal of Cranio‐Maxillo‐Facial Surgery, 47, 170–178. [DOI] [PubMed] [Google Scholar]
- Rowe, R.W.D. & Goldspink, G. (1969) Muscle fibre growth in five different muscles in both sexes of mice. I. Normal mice. Journal of Anatomy, 104, 519–530. [PMC free article] [PubMed] [Google Scholar]
- Schiaffino, S. , Dyar, K.A. , Ciciliot, S. , Blaauw, B. & Sandri, M. (2013) Mechanisms regulating skeletal muscle growth and atrophy. FEBS Journal, 280, 4294–4314. [DOI] [PubMed] [Google Scholar]
- Simione, M. , Loret, C. , Le Révérend, B. , Richburg, B. , Del Valle, M. , Adler, M. et al. (2018) Differing structural properties of foods affect the development of mandibular control and muscle coordination in infants and young children. Physiology & Behavior, 186, 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki, P. , Vieira, M. & Bommarito, S. (2014) Maximum bite force analysis in different age groups. International Archives of Otorhinolaryngology 18, 272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst, D.E. , Carstens, M.H. , Greco, R.J. & Hurwitz, D.J. (1991) The surgical anatomy of the scalp. Plastic and Reconstructive Surgery, 87, 603–612. [PubMed] [Google Scholar]
- Toro, A. , Buschang, P.H. , Throckmorton, G. & Roldán, S. (2006) Masticatory performance in children and adolescents with Class I and II malocclusions. European Journal of Orthodontics, 28, 112–119. [DOI] [PubMed] [Google Scholar]
- Toro‐Ibacache, V. , Zapata, V. , Munoz, V.Z. & O’Higgins, P. (2015) The predictability from skull morphology of temporalis and masseter muscle cross‐sectional areas in humans. The Anatomical Record, 298, 1261–1270. [DOI] [PubMed] [Google Scholar]
- van der Meulen, J.J.N.M. , Nazir, P.R.N. , Mathijssen, I.M.J. , van Adrichem, L.N.A. , Ongkosuwito, E. , Stolk‐Liefferink, S.A.H. et al. (2008) Bitemporal depressions after cranioplasty for trigonocephaly: A long‐term evaluation of (supra) orbital growth in 92 patients. Journal of Craniofacial Surgery, 19, 72–79. [DOI] [PubMed] [Google Scholar]
- Washburn, S.L. (1947) The relation of the temporal muscle to the form of the skull. Anatomical Record, 99, 239–248. [DOI] [PubMed] [Google Scholar]
- Wes, A.M. , Paliga, J.T. , Goldstein, J.A. , Whitaker, L.A. , Bartlett, S.P. & Taylor, J.A. (2014) An evaluation of complications, revisions, and long‐term aesthetic outcomes in nonsyndromic metopic craniosynostosis. Plastic and Reconstructive Surgery, 133, 1453–1464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


