1. Introduction
Elevated VTE rates have been reported in COVID-19 patients [1,2]. Although ICU patients in general have an elevated VTE risk [3] we recently reported a higher incidence of VTE in ICU compared to ward patients with COVID-19 [4]. As the optimal approach for thromboprophylaxis is unknown, we implemented intermediate dose anticoagulation in COVID-19 ICU patients. We hypothesized that intermediate dose heparin prophylaxis would be associated with lower incidences of symptomatic VTE, death, or a composite outcome of both in COVID-19 ICU patients.
2. Methods
Data were retrospectively collected on COVID-19 patients admitted to BWH or BWFH requiring ICU care. Participants were entered into the RRS-SARS-CoV-2 (IRB approved) from March 7th to June 1st, 2020, and followed until June 27th, 2020. We implemented intermediate dose prophylaxis for COVID-19 ICU patients on April 24th, 2020. Readmissions were not included. SARS-CoV-2 positivity was determined by reverse-transcription polymerase chain reaction on nasopharyngeal or endotracheal samples.
ICU admission criteria, laboratory monitoring, and treatment protocols were previously described [4]. Participants placed on standard (enoxaparin 40 mg daily, unfractionated heparin 5000 IU twice or three times daily) or intermediate (enoxaparin 40 mg twice daily, adjusted for extremes of weight (0.5 mg/kg twice daily) or 7500 IU unfractionated heparin three times daily) dose prophylactic anticoagulation at the time of ICU admission, adjusted for renal failure and thrombocytopenia, were included in the primary analysis. Propensity scores were calculated (MatchIt R package) and patients were matched based on age, sex, body-mass index, DVT history, ferritin, creatinine, activated partial thromboplastin time, C-reactive protein, history of hematologic malignancy, total length of hospital stay, need for vasopressors within 24 h of ICU admission, and type of respiratory support (no intubation, HFNC, BiPAP, intubation). Data on those receiving treatment dose anticoagulation (heparin continuous infusion) were also collected to compare event and bleeding rates, but were not part of the primary analysis.
The primary outcomes were time-to-death, symptomatic radiographically-confirmed VTE [4], and a composite of death and symptomatic VTE. No surveillance imaging was performed. We assessed the association of a binary variable indicating standard (0) or intermediate (1) dose prophylactic anticoagulation with each outcome. Follow up time was measured from time of hospital admission until death, symptomatic VTE, discharge, or 28 days. Major bleeding events were defined using ISTH criteria [5].
All analyses were performed in R version 4.0.3 (https://www.r-project.org). Variables were compared with Student t-tests, Wilcoxon, ANOVA, or Kruskal-Wallis tests, as appropriate. Bleeding rates were compared with Pearson Chi-squared statistic. KM analyses were performed using the survival R package; curves were compared using log-rank tests, and p-values were considered significant if below a Bonferroni-adjusted threshold. Cox proportional hazard models were used to estimate hazard ratios and 95% confidence intervals. Proportional hazard assumptions were assessed by Schoenfeld residual plots and tests.
3. Results
We collected data on 205 COVID-19 patients who were placed on standard (n = 144) or intermediate (n = 61) dose prophylactic anticoagulation at the time of ICU admission. After propensity score matching, we included 47 patients in each group (total n = 94). Characteristics of original and matched populations are shown in Table 1 . Groups had similar anthropomorphic features, laboratory measurements, need for renal replacement therapy, vasopressor requirements, ventilatory supports, and comorbidities. Median follow up times were 19 days [interquartile range (IQR): 9.5–28 days] in the standard and 16 days [IQR: 9.0–22 days] in the intermediate dose groups (p = 0.1). Compared to the standard dose group, the intermediate dose group demonstrated a trend toward higher symptomatic VTE rates (23.4% versus 14.9%, p = 0.4). We observed a trend toward higher ISTH major bleeding rates in the intermediate dose group (10.6% vs. 4.3%, p = 0.03), though this result is not significant after adjusting for multiple comparisons. In a secondary analysis of those placed on therapeutic dose anticoagulation (n = 42), 15 (35.7%) died and 3 (7.1%) had symptomatic VTE; 9 patients (21%) experienced major bleeding.
Table 1.
Characteristics of participants matched on age, sex, body-mass index, DVT history, ferritin, creatinine, partial thromboplastin time, history of hematologic malignancy, total length of hospital stay, need for vasopressors within 24 hours of ICU admission, and type of respiratory support (no intubation, HFNC, BiPAP, intubation).
| Characteristic | Overall population |
Matched population |
||||
|---|---|---|---|---|---|---|
| Standard dose | Intermediate dose | p | Standard dose | Intermediate dose | p | |
| n | 144 | 61 | 47 | 47 | ||
| Age (mean (SD)) | 61.90 (15.9) | 57.02 (12.0) | 0.03 | 58.43 (15.2) | 58.09 (12.3) | 0.9 |
| Sex (M, No. %) | 82 (57.3) | 43 (70.5) | 0.108 | 34 (72.3) | 30 (63.8) | 0.5 |
| Race (No. %) | 0.01 | 0.11 | ||||
| Asian | 4 (2.8) | 5 (8.2) | 2 (4.3) | 5 (10.6) | ||
| Black Hispanic | 6 (4.2) | 3 (4.9) | 3 (6.4) | 3 (6.4) | ||
| Black Non-Hispanic | 42 (29.4) | 11 (18.0) | 16 (34.0) | 9 (19.1) | ||
| Hispanic | 19 (13.3) | 1 (1.6) | 6 (12.8) | 1 (2.1) | ||
| Native | 0 (0.0) | 1 (1.6) | 0 (0.0) | 1 (2.1) | ||
| Other | 15 (10.5) | 10 (16.4) | NA | NA | ||
| Unavailable | 7 (4.9) | 0 (0.0) | 1 (2.1) | 6 (12.8) | ||
| White Hispanic | 8 (5.6) | 5 (8.2) | 3 (6.4) | 3 (6.4) | ||
| White Non-Hispanic | 42 (29.4) | 25 (41.0) | 16 (34.0) | 19 (40.4) | ||
| Body-mass index (kg/m2) (mean (SD)) | 29.6 (7.2) | 30.9 (7.1) | 0.2 | 30.6 (9.2) | 30.3 (6.7) | 0.9 |
| Coronary artery disease (No. %) | 25 (17.5) | 9 (14.8) | 0.8 | 9 (19.1) | 7 (14.9) | 0.8 |
| Prior myocardial infarction (No. %) | 11 (7.7) | 6 (9.8) | 0.8 | 3 (6.4) | 4 (8.5) | 1 |
| Atrial fibrillation (No. %) | 9 (6.3) | 8 (13.1) | 0.2 | 2 (4.3) | 5 (10.6) | 0.4 |
| Prior DVT (No %) | 4 (2.8) | 1 (1.6) | 1 | 1 (2.1) | 1 (2.1) | 1 |
| Autoimmune disease history (No. %) | 10 (7.0) | 2 (3.3) | 0.5 | 3 (6.4) | 2 (4.3) | 1 |
| Hematologic malignancy history (No. %) | 6 (4.2) | 3 (4.9) | 1 | 3 (6.4) | 2 (4.3) | 1 |
| Hemoglobin (g/dL) (mean (SD)) | 12.5 (2.1) | 12.5 (2.3) | 1 | 12.2 (2.0) | 12.3 (2.4) | 0.8 |
| Platelets (K/μL) (mean (SD)) | 231.2 (103.8) | 225.6 (113.9) | 0.7 | 247.5 (107.4) | 232.3 (117.3) | 0.5 |
| Prothrombin time (s) (mean (SD)) | 14.2 [13.4, 15.1] | 14.3 [13.4, 15.5] | 0.7 | 14.3 [13.4, 15.1] | 14.3 [13.4, 15.2] | 0.7 |
| Partial thromboplastin time (s) (median [IQR]) | 33.4 [30.5, 39.6] | 35.1 [30.7, 40.2] | 0.3 | 34.9 [29.3, 39.5] | 33.6 [30.6, 39.8] | 0.4 |
| Creatinine (g/dL) (median [IQR]) | 1.04 [0.80, 1.42] | 1.12 [0.91, 1.47] | 0.2 | 0.96 [0.83, 1.40] | 1.09 [0.86, 1.52] | 0.3 |
| C-reactive protein (mg/dL) (mean (SD)) | 149.9 (97.5) | 157.5 (104.0) | 0.6 | 160.1 (98.5) | 152.5 (104.4) | 0.7 |
| Lactate dehydrogenase (U/L) (median [IQR]) | 428 [326, 556] | 428 [337, 573] | 0.5 | 416 [342, 555] | 427 [336, 601] | 0.6 |
| Ferritin (ng/mL) (mean (SD)) | 1555.50 (2340.43) | 1810.50 (2031.55) | 0.5 | 2073.36 (3168.00) | 1819.60 (2043.57) | 0.6 |
| ICU length of stay (days) (median [IQR]) | 10.00 [5.50, 22.00] | 11.00 [3.00, 21.00] | 0.4 | 18.00 [7.50, 26.00] | 11.00 [3.50, 16.50] | 0.03 |
| Total length of stay (days) (mean (SD)) | 22.8 (15.7) | 24.7 (16.4) | 0.4 | 27.8 (19.1) | 23.8 (17.3) | 0.3 |
| Intubated (No. %) | 114 (79.7) | 44 (72.1) | 0.3 | 39 (83.0) | 36 (76.6) | 0.6 |
| Time intubated (days) (median [IQR]) | 14.00 [7.00, 21.00] | 13.50 [7.00, 27.25] | 0.7 | 20.00 [9.50, 24.00] | 13.00 [7.00, 22.25] | 0.2 |
| Renal replacement therapy (No. %) | 24 (16.8) | 6 (9.8) | 0.3 | 8 (17.0) | 4 (8.5) | 0.4 |
| D-dimer nearest time of VTE (ng/μL) (median [IQR]) | 4000 [2913, 4000] | 4000 [3176, 4000] | 0.4 | 4000 [2973, 4000] | 3362 [2277, 4000] | 0.7 |
| Required vasopressors on ICU admission (No. (%)) | 64 (44.4) | 29 (47.5) | 0.8 | 26 (55.3) | 22 (46.8) | 0.5 |
| Respiratory support (No. (%)) | 0.1 | |||||
| No intubation, HFNC, or BiPAP | 30 (20.8) | 16 (26.2) | 8 (17.0) | 11 (23.4) | ||
| HFNC | 2 (1.4) | 3 (4.9) | 1 (2.1) | 1 (2.1) | ||
| BiPAP | 0 (0.0) | 1 (1.6) | NA | NA | ||
| Intubated | 112 (78.3) | 41 (67.2) | 38 (80.9) | 35 (74.5) | ||
| Outcomes | ||||||
| Symptomatic VTE (No. %) | 12 (8.3) | 18 (29.5) | <0.001 | 7 (14.9) | 11 (23.4) | 0.4 |
| Death (No. %) | 44 (30.8) | 16 (26.2) | 0.629 | 13 (27.7) | 12 (25.5) | 1 |
| Composite outcome | 52 (36.4) | 28 (45.9) | 0.262 | 19 (40.4) | 20 (42.6) | 1 |
| ISTH bleeding event (No. %) | ||||||
| Gastrointestinal | 2 (1.4) | 5 (8.2) | 0 (0.0) | 4 (8.5) | ||
| Intracranial hemorrhage | 2 (1.4) | 1 (1.6) | 2 (4.3) | 0 (0.0) | ||
| Other | 1 (0.7) | 2 (3.3) | 0 (0.0) | 1 (2.1) | ||
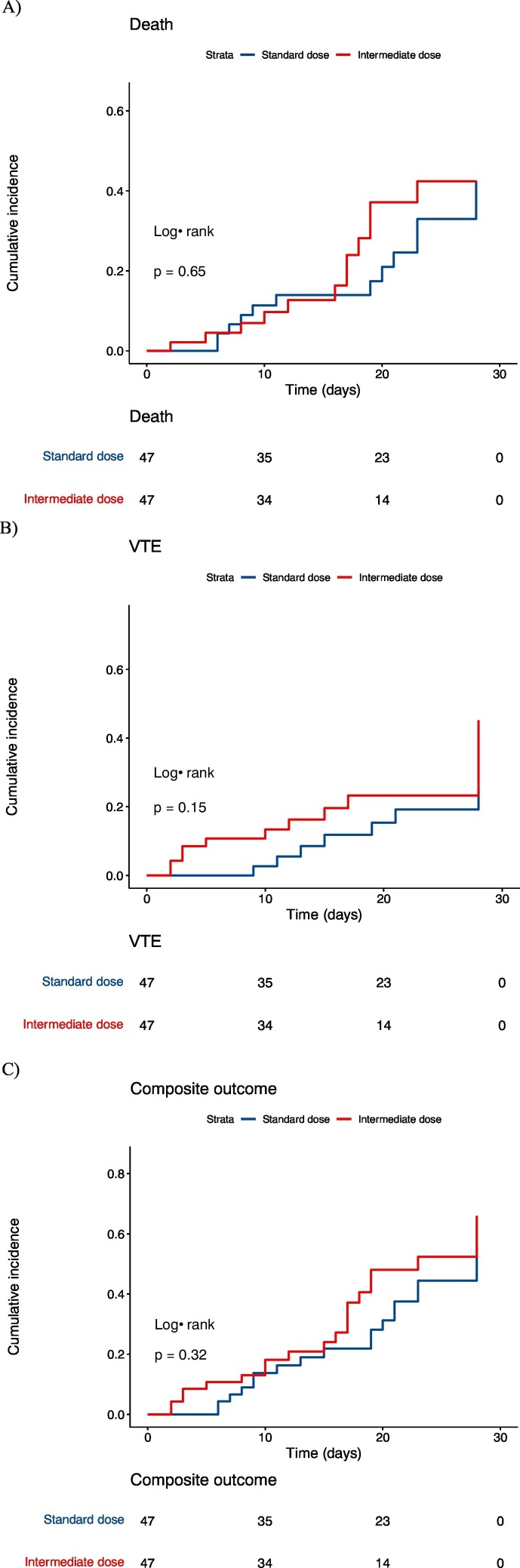
The 14-day cumulative incidences in the standard dose anticoagulation group were 14% [95% CI: 6.5%–29%] for death, 8.5% [95% CI: 2.8%–24%] for symptomatic VTE, and 19% [95% CI: 9.9%–35%] for the composite outcome. The cumulative incidences in the intermediate dose group were 13% [95% CI: 5.4–28%] for death, 16% [95% CI: 8–31%] for symptomatic VTE, and 21% [95% CI: 11–36%] for the composite outcome. Cumulative incidence curves based on KM analyses are shown in Fig. 1 . For each outcome, the cumulative incidence curves were not statistically significantly different (all p > 0.017 [Bonferroni-adjusted threshold: 0.05/3 = 0.017]). There was a trend toward higher VTE rates in the intermediate dose group. The hazard ratio for being in the intermediate group was 1.2 (95% CI: 0.54–2.3, p = 0.7) for death, 2.0 (95% CI: 0.8–5.2, p = 0.2) for symptomatic VTE, and 1.4 (95% CI: 0.73–2.6, p = 0.3) for the composite outcome.
Fig. 1.
Cumulative incidence of death (A), symptomatic VTE (B), and a composite of death and symptomatic VTE (C) in matched patients on standard (blue) and intermediate (red) dose prophylaxis. Patients were followed for 28 days or until death, symptomatic VTE, or discharge. Shaded areas represent 95% confidence bands. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In this propensity score-matched analysis of 94 COVID-19 ICU patients at a large U.S. tertiary-care academic hospital, there was no significant difference in death, symptomatic VTE, or a composite of death and VTE in those on intermediate compared to standard dose heparin prophylaxis. A trend toward more bleeding in the intermediate dose group was seen. We observed higher bleeding rates in a secondary analysis of those on therapeutic dose anticoagulation, consistent with prior reports [6]. These results suggest that the thrombo-inflammatory state of critically-ill COVID-19 patients may not be addressed by intermediate dose heparin alone.
Retrospective studies comparing therapeutic to standard dose heparin prophylaxis have yielded conflicting results for mortality, with several reports of elevated bleeding rates in those on therapeutic anticoagulation [[7], [8], [9]]. The ACTIV-4a trial [10], comparing therapeutic to standard dose prophylaxis, was halted in critically-ill patients as there was no improvement in the endpoint of organ dysfunction at 21 days; however, a recent press release indicates benefit in moderately-ill hospitalized patients, though results of other endpoints, such as bleeding, are not yet available [11]. The INSPIRATION randomized trial of intermediate versus standard dose prophylaxis results were published after we submitted our work, and also found no difference in mortality and thrombotic outcomes, however event rates were very low in comparison to other reported rates [12]. Recent data suggest that VTE events are still high in the second wave despite lower mortality [13]. Given the continued urgency and remaining uncertainty about optimal treatment, we utilized a propensity score-matched analysis, comparing intention-to-treat with intermediate versus standard dose heparin prophylaxis in critically-ill COVID-19 patients. A recent time-varying exposure analysis demonstrated improvement in thrombotic complications for those on intermediate or equivalent to therapeutic dose versus standard dose heparin prophylaxis [14]; the degree to which those on therapeutic dose drove these results are unclear. Taken together, our findings suggest that the optimal combination of patient characteristics and VTE prophylaxis strategy for critically-ill COVID-19 patients requires additional investigation.
Our findings suggest that, in critically-ill patients, intermediate dose heparin alone may not counteract the profound immune responses leading to VTE and death in COVID-19 patients. While the causal relationships between immune dysregulation and critical illness remain unclear, cytokine levels have been reported to predict COVID-19 severity and survival [15], and trials targeting specific cytokines have been undertaken [16]. Our data highlight the importance of understanding mechanisms of disease pathogenesis and targeting critical pathways.
The observed symptomatic VTE rates were similar to VTE rates reported in a meta-analysis of ICU patients (12.7% [95% CI: 8.7–17.5%]) [3]. This result might reflect that our definition of symptomatic VTE provides a more conservative estimate of VTE rates compared to other COVID-19 VTE studies [1]. Further, this comparison highlights that VTE rates in the ICU population might be unacceptably high, and further research into optimal thromboprophylaxis for critically-ill patients is needed. We observed higher symptomatic VTE in the intermediate dose heparin group in the original study population, alluding to potential reverse confounding. When we implemented intermediate dose prophylaxis, critical care clinicians were acutely aware of the elevated VTE risk in COVID-19 patients, which may have led to more testing for VTE. However, in the matched population, the trend was not significant, suggesting adequate matching for measures of severity. Perhaps a combination of heparin, anti-platelets, direct thrombin inhibitors, or other agents are required to reduce VTE events without markedly increasing bleeding rates; these are important questions for future studies.
Strengths of this study are the timely clinical question, a well-characterized cohort with detailed outcome information, and use of propensity score-matched analysis, which reduces the effects of confounding. The intention-to-treat aspect accounts for the real-world issue of time-variant heparin dosing strategies. Limitations include a single center cohort and modest sample size. While propensity score-matching is a rigorous statistical method, results are not always confirmed by randomized trials. However, until randomized trials address this question, our study demonstrates that intermediate dose heparin may not fully mitigate thrombotic risks in COVID-19 ICU patients. There was a trend toward higher bleeding in the intermediate dose group, but this result does not pass correction for multiple comparisons.
In conclusion, intermediate dose heparin was not associated with a lower incidence of death, symptomatic VTE, or a composite of death and VTE compared to standard dose prophylaxis. Replication of these findings, as well as meta-analyses, is urgently needed.
Funding
MM is supported by NIH T32HL007427, U01 HL089897, and U01 HL089856 from the National Heart, Lung, and Blood Institute.
BDH is supported by NIH K08HL136928 and R01 HL089856.
MHC is supported by NIH R01HL137927 and R01HL135142.
CRediT authorship contribution statement
Study Design: MM, RLZ, KWS, VC, MAM, JMC.
Acquisition, analysis, or interpretation of the data: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: MM, MAM, MHC, JMC.
Obtained funding: JMC, MHC, AEW.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
JMC: Personal fees from Bristol-Myer Squibb, Abbott, Portola, Pfizer. Research funding to the institution from CSL Behring.
RMB serves on the Advisory Boards for Genentech and Merck.
MHC: Personal fees from AstraZeneca and Illumina. Research funding to institution from GSK and Bayer.
RLZ is a consultant and stockholder for Amagma Therapeutics.
LEF: Research funding to the institution from Bayer, outside the submitted work.
AH is a consultant for Olix Pharmaceuticals and Deep Track Capital.
References
- 1.Nopp S., Moik F., Jilma B., Pabinger I., Ay C. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res. Pract. Thromb. Haemost. 2020 doi: 10.1002/rth2.12439. published online Sept 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abou-Ismail M.Y., Diamond A., Kapoor S., Arafah Y., Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb. Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malato A., Dentali F., Siragusa S., et al. The impact of deep vein thrombosis in critically ill patients: a meta-analysis of major clinical outcomes. Blood Transfus. 2015;13:559–568. doi: 10.2450/2015.0277-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moll M., Zon R.L., Sylvester K.W., et al. Venous thromboembolism in COVID-19 ICU patients. Chest. 2020 doi: 10.1016/j.chest.2020.07.031. published online July 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulman S., C Kearon S on C of A of the S and SC of the IS on T and H Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 6.Lynn L., Reyes J.A., Hawkins K., et al. The effect of anticoagulation on clinical outcomes in novel coronavirus (COVID-19) pneumonia in a U.S. cohort. Thromb. Res. 2021;197:65–68. doi: 10.1016/j.thromres.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijaya I., Andhika R., Huang I. The use of therapeutic-dose anticoagulation and its effect on mortality in patients with COVID-19: a systematic review. Clin. Appl. Thromb. Hemost. 2020;26 doi: 10.1177/1076029620960797. 107602962096079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tremblay D., van Gerwen M., Alsen M., et al. Impact of anticoagulation prior to COVID-19 infection: a propensity score-matched cohort study. Blood. 2020;136:144–147. doi: 10.1182/blood.2020006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadkarni G.N., Lala A., Bagiella E., et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIH ACTIV Trial of blood thinners pauses enrollment of critically ill COVID-19 patients. https://www.nih.gov/news-events/news-releases/nih-activ-trial-blood-thinners-pauses-enrollment-critically-ill-covid-19-patients
- 11.Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients
- 12.Bikdeli B., Talasaz A.H., Rashidi F., et al. Intermediate versus standard-dose prophylactic anticoagulation and statin therapy versus placebo in critically-ill patients with COVID-19: rationale and design of the INSPIRATION/INSPIRATION-S studies. Thromb. Res. 2020;196:382–394. doi: 10.1016/j.thromres.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutch COVID & Thrombosis Coalition, Kaptein F.H.J., Stals M.A.M., et al. Incidence of thrombotic complications and overall survival in hospitalized patients with COVID-19 in the second and first wave. Thromb. Res. 2021;199:143–148. doi: 10.1016/j.thromres.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tacquard C., Mansour A., Godon A., et al. Impact of high dose prophylactic anticoagulation in critically ill patients with COVID-19 pneumonia. Chest. 2021 doi: 10.1016/j.chest.2021.01.017. Published online Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Valle D.M., Kim-Schulze S., Huang H.-H., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salama C., Han J., Yau L., et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N. Engl. J. Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]