Abstract
Objective
To report the first case of diabetic ketoacidosis (DKA) and its management in a patient with diet-controlled prediabetes and metastatic breast cancer treated with alpelisib, a PI3K (phosphatidylinosiotol-3-kinase) inhibitor.
Methods
Literature on the topic is reviewed. The case is that of a 66-year-old female with diet-controlled prediabetes and metastatic breast carcinoma who had initiated alpelisib 2 weeks prior to being admitted for diabetic ketoacidosis.
Results
Admission laboratory examination revealed a blood sugar of 1137 mg/dL, an anion gap of 25, large ketones in urine, and positive acetone in serum. The HbA1c level was 9.4% (79 mmol/mol) on admission, which had been 6.3% (45 mmol/mol) seven months earlier. She was discharged on subcutaneous insulin and instructed to discontinue alpelisib. Alpelisib was restarted 2 days later, which exacerbated her hyperglycemia within 24 hours. In the following months, her hyperglycemia was successfully managed with insulin and a SGLT 2 inhibitor. Unfortunately, her breast cancer progressed, ultimately leading to discontinuation of alpelisib. Blood sugar levels returned to a nondiabetic range upon discontinuation of alpelisib, and she is currently off all antihyperglycemic agents.
Conclusion
Although PI3KCA inhibitors remain a promising drug in patients with metastatic breast cancer who have not responded to previous treatment, patients must be closely monitored for adverse effects such as hyperglycemia. Hyperglycemia could be a potentially limiting side effect of alpelisib. The optimal management of hyperglycemia induced by alpelisib warrants further research.
Key words: alpelisib, type 2 diabetes, DKA, oncology
Abbreviations: Akt, protein kinase B; DKA, diabetic ketoacidosis; HbA1c, glycosylated hemoglobin; PI3K, phosphatidylinosiotol-3-kinase; SGLT2, sodium glucose cotransporter 2
Introduction
The phosphatidylinositol-3 kinase (PI3K) α inhibitor, alpelisib (Piqray), was approved in May 2019 for use in combination with fulvestrant in postmenopausal women with hormone receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer with PIK3CA mutations.1 There was an 11-month versus a 5.7-month progression-free survival when alpelisib plus fulvestrant was used, compared with placebo plus fulvestrant in the phase 3 randomized controlled clinical trial of alpelisib in breast cancer. Hyperglycemia was one of the most common adverse reactions and occurred in 63.7 % of the patients in the experimental group; this led to permanent discontinuation of alpelisib in 6.3% of the patients. Notably, 96% of the patients (n = 52) who discontinued alpelisib owing to hyperglycemia but continued treatment with fulvestrant saw fasting blood sugar return to baseline.2 We use this case report to illustrate the clinical presentation and discuss the proposed mechanism of action by which this agent causes hyperglycemia, and provide potential management options.
Case Report
We present the case of a 66-year-old female admitted for diabetic ketoacidosis (DKA) with a history of prediabetes and refractory breast cancer with metastasis to the liver and bone despite multiple previous treatments, including hormone receptor therapy. Two weeks before presenting to the emergency department, owing to evidence of progression of metastatic disease, her medication had been changed from doxorubicin, which she had been prescribed for the past 3 months, to alpelisib 300 mg daily with fulvestrant. During a routine oncology clinic follow-up, she complained of extreme fatigue, polyuria, and polydipsia, and was, therefore, instructed to present to the emergency department. Her last dose of alpelisib 300 mg had been the previous night. Outpatient medications included alpelisib 300 mg/day, cholecalciferol 2000 I.U./day, esomeprazole 40 mg/day, loratadine 10 mg/day, losartan 100 mg/day, prochlorperazine 10 mg as needed, and rizatriptan 10 mg as needed. On admission, the patient was alert and oriented, her blood pressure was 144/85 mm Hg, heart rate was 93 beats/min, and weight was 64.9 kg. Laboratory examination showed a blood sugar of 1137 mg/dL, an anion gap of 25 mmol/L, ketones in urine (80 mg/dL), and positive acetone in serum, consistent with the findings of DKA. A basic metabolic panel revealed a sodium level of 130 mmol/L, potassium level of 5.7 mmol/L, chloride level of 90 mmol/L, bicarbonate level of 90 mmol/L, blood urea nitrogen level of 43 mg/dL, creatinine level of 1.5 mg/dL, calculated osmolality of 330 mOsm/kg, glomerular filtration rate of 42 mL/min, glycosylated hemoglobin (HbA1c) level of 9.4% (79 mmol/mL), and C-peptide level of 13.3 ng/mL. Hemoglobin and hematocrit were 12.7 g/dL and 38.6%, respectively, without any recent blood transfusions. Two months prior to hospitalization, laboratory examination showed that electrolyte levels and renal function were in the normal range, with a blood urea nitrogen level of 19 mg/dL, creatinine level of 1.0 mg/dL, glomerular filtration rate > 60 mL/min, and random glucose level of 188 mg/dL. Seven months earlier her HbA1c level had been 6.3% (45 mmol/mol). Fructosamine was not measured. She was admitted to the intensive care unit and treated with intravenous hydration and electrolyte repletion; she required 166 units of insulin in the first 36 hours. She was transitioned to subcutaneous insulin on hospital day 2. Alpelisib was discontinued per oncology team recommendations. She was discharged on hospital day 3 and prescribed metformin, 38 units of insulin degludec at night, and 10 units of preprandial insulin lispro. In the days following her discharge, her insulin requirements continued to decrease to insulin degludec 25 units at night and 6 units insulin lispro with meals. Four days after discharge, oncology restarted alpelisib 250 mg daily with notable hyperglycemia within 24 hours. Two weeks after resuming alpelisib, she continued to have hyperglycemia despite 38 units of insulin degludec and 15 units of insulin lispro with meals along with low-dose correction; therefore, empaglifozin 25 mg was initiated. Continuous glucose monitoring showed notable hyperglycemia when alpelisib was increased to the 300 mg dose, and she ultimately required 60 units of insulin degludec, and 30 units insulin lispro for breakfast, 18 units for lunch, and 17 units for dinner while limiting her carbohydrate intake to 45 g with each meal. A rise in fingerstick blood glucose was noted to occur within 4 hours after taking alpelisib, and it lasted for 22 hours, with a significant drop in fingerstick blood glucose 23 to 24 hours after the administration of alpelisib (Fig. 1). Given the 22-hour hyperglycemic effect of alpelisib, our patient was switched to 60 units of detemir, which resolved hypoglycemia. However, she had persistent hyperglycemia within 20 hours after the administration of alpelisib (Fig. 2). Persistent hyperglycemia was later corrected by increasing the preprandial insulin lispro dose. Unfortunately, her breast cancer progressed and alpelisib was replaced by ixabepilone. Her blood glucose normalized within 14 days of discontinuation of alpelisib without further need for insulin or empagliflozin (Fig. 3). Three months after discontinuing alpelisib, her HbA1c is now 6.2% (44 mmol/mol).
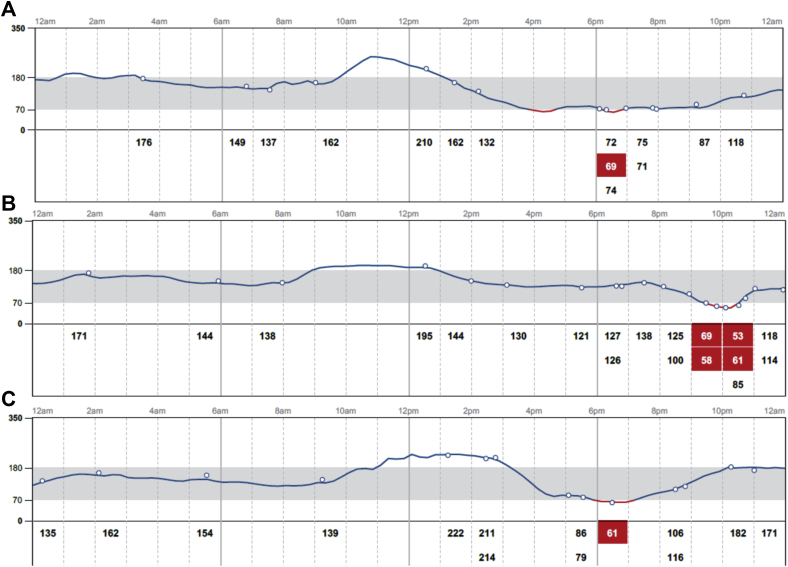
Fig 1.
Continuous glucose monitor tracing for 3 consecutive days while the patient was on lispro 30 units prior to breakfast (at 07:00 hours), 18 units prior to lunch (at 12:00 hours), and 17 units prior to dinner (at 16:00 hours) along with A, alpelisib 300 mg and 25 mg empagliflozin at 19:30 hours and degludec 60 units taken at 23:00 hours; B, alpelisib 300 mg and 25 mg empagliflozin 23:00 hours and degludec 60 units taken at 23:00 hours; and C, alpelisib 300 mg and 25 mg empagliflozin at 19:00 hours and degludec 60 units taken at 22:00 hours.
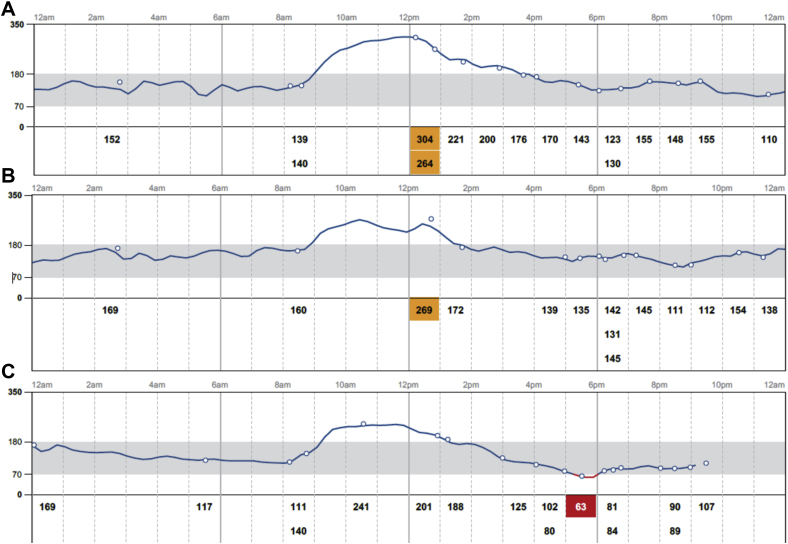
Fig 2.
Continuous glucose monitor tracing for 3 consecutive days while the patient was on lispro 32 units prior to breakfast (07:00 hours ), 18 units prior to lunch (12:00 hours ), and 18 units prior to dinner (16:00 hours) along with A, alpelisib 300 mg and 25 mg empagliflozin at 19:30 hours and detemir 60 units taken at 23:00 hours ; B, alpelisib 300 mg and 25 mg empagliflozin at 19:00 hours and detemir 60 units taken at 22:00 hours; C, alpelisib 300 mg and 25 mg empagliflozin at 19:15 hours and detemir 60 units taken at 22:00 hours.
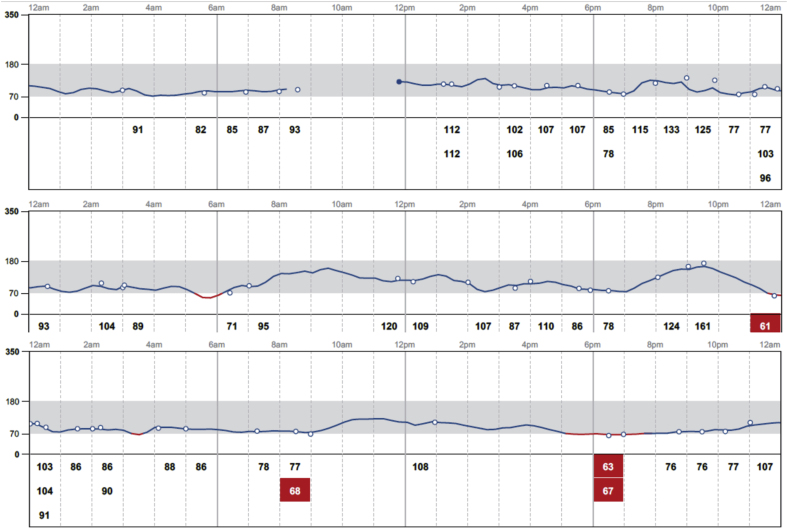
Fig 3.
Continuous glucose monitor tracing for 3 consecutive days after the patient had discontinued alpelisib, empagliflozin, and insulin therapy.
Discussion
The PI3K pathway is seen in nearly 44% of tumors, making it an excellent target for cancer therapies.3 PI3Ks are lipid kinases that play a central role in cellular growth, glucose homeostasis, lipid metabolism, and protein synthesis via phosphorylation of protein kinase B (Akt).4 Akt regulates apoptosis, cell survival, cell cycle progression, and metabolism.5 The most common PI3K gene mutation is PIK3CA, which is also the gene that encodes the isoform PI3K (p110α) that mediates insulin response in muscle, liver, and fat.6
Class I PI3Ks are heterodimers, consisting of the p110 catalytic subunit and the p85 regulatory subunit. P110 exists in various isoforms with p110α playing a critical role in the metabolic action of insulin. Under physiologic conditions, insulin secreted after a meal activates the PI3K/Akt signaling pathway, which increases glucose utilization, reduces gluconeogenesis in liver and muscle, and increases body lipid deposition. This is done by increasing insulin production in the pancreas, which then activates substrate proteins that bind to PI3K, ultimately leading to glucose uptake and glycogen synthesis by promoting translation and translocation of glucose transporters 1 and 4.7,8 Intracellularly, Akt stimulates the hexokinase that converts glucose to glucose-6-phosphate and produces cellular energy by regulating glucose-6-phosphate and glycogen synthase kinase 3 during glycolysis.5
Targeted inhibition of the PIK3CA-encoded enzyme, p110α, disrupts glucose metabolism in multiple tissues. The critical role of p110α was demonstrated when liver specific PI3Kα knockout mice showed decreased insulin sensitivity, increased gluconeogenesis, increased leptin and lipid levels, as well as impairment in insulin-sensitive tissues. Blocking insulin signaling promotes glycogen breakdown in the liver and prevents glucose uptake in skeletal muscle and adipose tissue. These knockout mice showed impaired insulin action that could not be rescued by p110β or alleviated by metformin.9 This was also highlighted in preclinical murine models in which increasing plasma concentrations of NVP-BYL719, a PI3Kα-selective agent, resulted in a proportionate increase in insulin levels.10
The phase 3 trial of alpelisib in breast cancer randomized 572 patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer to alpelisib-fluvestrant versus placebo-fluvestrant. The study only enrolled patients with controlled type 2 diabetes, which was defined as fasting plasma glucose ≤140 mg/dL (7.7 mmol/L) and HbA1c ≤6.4%. Grades of hyperglycemia were used to define the level of hyperglycemia experienced by patients and to categorize if they would receive metformin, metformin and pioglitazone, or a combination of these drugs plus insulin. The most common adverse effect was grade 3 or 4 hyperglycemia. In cases of grade 4 hyperglycemia, defined as fasting plasma glucose >500 mg/dL, the experimental drug was discontinued altogether. Patients with type 1 diabetes were not enrolled.2
In clinical trials, most p110α inhibitors inhibit both mutant and wild type p110α at therapeutic doses, thereby inducing acute insulin resistance and resulting in severe hyperglycemia, which, in turn, leads to severe hyperinsulinemia. The resultant hyperglycemia, caused by inhibition of PIK3CA, is usually transient, although hyperglycemia may be exacerbated or prolonged in patients with any degree of prior insulin resistance, as was seen in our patient who presented with a HbA1c of 9.4%. Existing insulin resistance in our patient was likely due to recent treatment with doxorubicin prior to initiation of alpelisib, given that she had been noted to have a fasting glucose level of 97 mg/dL to 188 mg/dL on routine bloodwork during doxorubicin therapy. This is thought to be because of compensatory insulin release from the pancreas, known as insulin feedback, which restores normal glucose homeostasis. It has also been postulated that PI3K signaling could be reactivated within a few hours in the muscles and the liver despite the presence of the drug.11
Our patient’s alpelisib was discontinued in the setting of DKA but was reintroduced successfully after careful management of her hyperglycemia with a low carbohydrate diet, metformin, insulin, and a sodium glucose cotransporter 2 (SGLT2) inhibitor. In murine models, the use of metformin with a PI3K inhibitor has been shown to reduce hyperglycemia by increasing insulin sensitivity, thereby reducing baseline glucose and insulin levels. Pretreatment with metformin alone had a minimal impact on PI3K inhibitor–induced elevation in blood glucose and insulin levels, whereas both SGLT2 inhibitors and the ketogenic diet decreased hyperglycemia and reduced the total amount of insulin released in response to PI3K inhibitors. Ketogenic diets deplete hepatic glycogen stores and limit acute release of glucose from the liver following PI3K inhibition. SGLT2 inhibitors work by inhibiting glucose transporters responsible for reabsorption of glucose in the kidney. SGLT2 inhibitors and the ketogenic diet also correlate with reduced glucose uptake in tumor cells, which results in smaller tumor volumes by reducing insulin levels, thereby decreasing insulin’s ability to activate insulin receptors in tumors.11
Hyperglycemia resulting from the inhibition of the downstream effects of PI3K is followed by increased insulin secretion, which further activates PI3K signaling in tumors and can lead to an increase in glucose uptake in malignant cells. Therefore, it has been hypothesized that treatment-induced hyperinsulinemia may be limiting the therapeutic effects of agents targeting the PI3K pathway. This is supported by data from murine models, which show an increase in glucose uptake in tumor cells immediately after PI3K inhibition, indicating that the spikes in insulin could be causing a transient increase in glucose uptake in these tumors, even in the presence of PI3K inhibitors.12
A prodrug of the PI3K inhibitor, which would only be activated in cancerous tissue, could also decrease adverse effects in target organs. The H1047R mutation in p110α has been shown to respond better to lower doses of PI3K inhibitors; this could allow for an even lower risk of hyperglycemia.13 Further research is needed to prevent the unwanted dose-limiting effects of these medications.
Conclusion
We report what is to our knowledge the first case of DKA in a patient being treated with PI3K inhibitors outside of a clinical trial. This case not only highlights the dose-limiting adverse effect (hyperglycemia) of alpelisib, but also a potential way of managing the unwanted and potentially therapy-limiting effects of this medication. Further investigation is needed to understand the best methods of managing of hyperglycemia without affecting alpelisib’s efficacy.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Novartis Pharmaceuticals Corporation. PIQRAY (alpelisib) tablets prescribing information. https://www.hcp.novartis.com/products/piqray/metastatic-breast-cancer/. Accessed May 15, 2019.
- 2.Andre F., Ciruelos E., Rubovsky G. Alpelisib for PIK3CA- mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 3.Millis S.Z., Jardim D.L., Albacker L. Phosphatidylinositol 3-kinase pathway genomic alterations in 60,991 diverse solid tumors informs targeted therapy opportunities. Cancer. 2019;125(7):1185–1199. doi: 10.1002/cncr.31921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang X., Liu G., Guo J. The PI3K/AKT pathway in obesity and type 2 diabetes. Intl J Biol Sci. 2018;14(11):1483–1496. doi: 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manning B.D., Toker A. AKT/PKB Signaling: navigating the network. Cell. 2017;169(3):381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallagher E.J., Fierz Y., Vijayakumar A., Haddad N., Yakar S., LeRoith D. Inhibiting PI3K reduces mammary tumor growth and induces hyperglycemia in a mouse model of insulin resistance and hyperinsulinemia. Oncogene. 2012;31(27):3213–3222. doi: 10.1038/onc.2011.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang S., Czech M.P. The GLUT4 glucose transporter. Cell Metab. 2007;5(4):237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Bentley J., Itchayanan D., Barnes K. Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface. J Biol Chem. 2003;278(41):39337–39348. doi: 10.1074/jbc.M305689200. [DOI] [PubMed] [Google Scholar]
- 9.Sopasakis V.R., Liu P., Suzuki R. Specific roles of the p110alpha isoform of phosphatidylinsositol 3-kinase in hepatic insulin signaling and metabolic regulation. Cell Metab. 2010;11(3):220–230. doi: 10.1016/j.cmet.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritsch C., Huang A., Chatenay-Rivauday C. Characterization of the novel and specific PI3Kα inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther. 2014;13(5):1117–1129. doi: 10.1158/1535-7163.MCT-13-0865. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins B.D., Pauli C., Du X. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560(7719):499–503. doi: 10.1038/s41586-018-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruman D.A., Chiu H., Hopkins B.D., Bagrodia S., Cantley L.C., Abraham R.T. The PI3K pathway in human disease. Cell. 2017;170(4):605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchanan C.M., Lee K.L., Shepherd P.R. For better or worse: the potential for dose limiting the on-target toxicity of PI3-kinase inhibitors. Biomolecules. 2019;9(9):402. doi: 10.3390/biom9090402. [DOI] [PMC free article] [PubMed] [Google Scholar]