Abstract
To investigate the predictors of toxicity and quality of life after stereotactic body radiation therapy (SBRT) for prostate cancer, the American Association of Physicists in Medicine Working Group on Biological Effects of Hypofractionated Radiotherapy/SBRT performed a comprehensive review of studies on prostate SBRT published between 2001 and 2018. Studies that analyzed factors associated with late urinary, bowel, or sexual toxicity and/or quality of life were included. Most studies were conducted in patients with low-intermediate risk prostate cancer and included mild/moderate (eg, grade 1–2) toxicity as endpoints. Normal tissue complication probability modelling was performed on studies that contained detailed dose/volume outcome data. Dosimetric and non-dosimetric factors associated with urinary, bowel, and sexual side effects after prostate SBRT are discussed in this article. While current data do not offer firm guidance on organ at risk tolerance doses, this report provides an overview and a summary of the constraints used in trials.
1. Clinical Significance
There are multiple, efficacious guideline-recommended treatment options for localized prostate cancer, including radical prostatectomy, external beam radiation, and brachytherapy. However, it is important to understand the different toxicity profiles of these distinct treatment modalities.1 Lack of information regarding side effects from treatment contributes to decisional regret among men receiving treatment for prostate cancer.2 Therefore, understanding the risks of different treatments is a key factor in determining which treatment is best for a patient.
SBRT is an emerging modality in the treatment of low-intermediate risk prostate cancer, and its use within the United States is increasing.3 Indeed, a recent ASTRO / ASCO / AUA guideline included recommendations regarding use of ultrafractionation (eg, SBRT) and moderate hypofractionation for prostate cancer.4 SBRT allows for delivery of high doses of radiation in a limited number of fractions in a highly-conformal manner. 5-year results from the HYPO-RT-PC randomized trial comparing a 7-fraction SBRT-like regimen to conventional fractionation demonstrated similar rates of cancer control and toxicity.5 Advantages of SBRT include patient convenience as well as delivery of high biologically effective doses, but steep dose gradients require judicious consideration of adjacent normal tissues. It is thus important for physicians to have accurate knowledge of dose constraints for normal tissues during the SBRT treatment planning process. The purpose of this study is to evaluate published data to determine associations between normal tissue dose and other factors with toxicity.
2. Endpoints
Prostate SBRT can cause urinary and bowel side effects, and in the published literature these are measured using physician-scored toxicity and/or patient-reported quality of life (QOL). The most commonly used physician-scored toxicity scales are the Radiation Therapy Oncology Group (RTOG) and Common Terminology Criteria for Adverse Events (CTCAE) version 3 or 4. Both scales objectively quantify bowel and urinary symptoms on a 0–5 scale with higher numbers representing worse severity grades, but the two scales may provide different results. A study of prostate brachytherapy patients that scored patient toxicity using both RTOG and CTCAE version 3 showed a higher rate of grade 1–2 urinary toxicities with the CTCAE scale.6
The most commonly used patient-reported QOL instruments are the Expanded Prostate Cancer Index Composite (EPIC)7 and the International Prostate Symptom Score (IPSS). The EPIC reports bowel, sexual, and urinary QOL domains, the latter of which is divided into the urinary incontinence and urinary obstruction/irritation subscales. The IPSS is specific to urinary side effects. For sexual QOL, the most commonly used instruments include the EPIC and Sexual Health Inventory for Men (SHIM), both of which incorporate questions regarding sexual desire, erection frequency, duration and quality, along with overall satisfaction.
It is difficult to compare patient-reported QOL outcomes to physician-scored toxicity due to the different scales used. This heterogeneity in measurement scales, as well as the different cut-points used in different publications to define “clinically meaningful” side effects, makes a pooled analysis of data across published studies challenging. Furthermore, there is substantial variation in the time points of assessment, with “late” effects defined anywhere from 3 months to several years post-treatment. Due to the overall low incidence of serious (eg, ≥ grade 3) complications and the expectation that many patients will develop some degree of low-grade toxicity, most studies analyzed factors correlated with QOL changes or mild-moderate (eg, grade 1–2) toxicity.
3. Challenges Defining and Segmenting Anatomic Volumes
Segmentation of target and organ at risk (OAR) volumes for prostate SBRT is critically important due to the close proximity of OARs to target, the high dose gradients employed, and organ motion. At a minimum, bladder, urethra, and rectum should be segmented; penile bulb and neurovascular bundles can also be considered. Both the bladder and rectum are interrelated distensible organs with substantial potential for both inter-fraction and intra-fraction variation. Therefore, the degree to which the position and volume of OARs at simulation is representative of actual treatment conditions is likely influenced by features of daily setup (eg, maintenance of consistent bladder filling, the use of a rectal balloon/enema, and/or urinary catheter; and more recently, absorbable hydrogel spacers placed between the prostate and rectum under ultrasound guidance).8 MRI imaging with its improved visualization of soft tissue detail reduces contoured volumes of the prostate compared to CT,9 and may also permit contouring of the urethra, neurovascular bundle, and penile bulb.10
Methods for OAR delineation are not always clearly defined in prostate SBRT studies. Historically, rectal volume constraints have commonly been expressed by percent volume of the rectal wall or entire rectum as a solid organ. However, there is a move toward using absolute volume constraints, which may make the superior and inferior border definitions less critical. The urethra may be defined with Foley catheter insertion at simulation or with the use of a fused MRI,11,12 while the urinary bladder has been variably defined as a single organ (with or without bladder filling)13 or as the bladder wall only.14 Because the bladder is highly distensible and its volume non-reproducible, determining a true representative bladder DVH is challenging.15 OAR delineation should factor in contouring uncertainty, variable filling, and organ motion in order to assure that SBRT is delivered safely; some institutions use a planning risk volume (PRV) expansion. Despite these challenges, current OAR contouring practices, motion management, and dose limits have enabled most institutions to avoid excessive toxicity as evidenced by the consistently low rates of high-grade toxicity in published studies. Several contouring references now exist, including the RTOG Contouring Atlases,16,17 ESTRO ACROP guidelines,18 American Society for Radiation Oncology eContouring sessions, eContour, and others.19
4. Review of Outcomes Data
A systematic PubMed search was performed for reports published between 2001 and 2018 using the search term “(prostate OR prostate cancer) AND (stereotactic OR SBRT OR hypofractionated OR hypofractionation).” Both linear accelerator and robotic (eg, CyberKnife) SBRT (defined as 5 or fewer fractions) were commonly used, and we included for initial review all studies reporting late urinary, bowel, and/or sexual toxicity or QOL. Our primary goal was to include studies that analyzed associations between dose-volume or other factors with late (generally ≥ 3 months post-RT) endpoints, and these are summarized in Table 1 (urinary),20–32 Table 2 (bowel),20,22,26,33–35 and Table 3 (sexual).22,36–38 We excluded studies that contained fewer than 40 patients (other than series reporting on sexual outcomes), utilized SBRT as a boost, were not in English, or reported only acute toxicity. Consistent with the other HyTEC (High Dose per Fraction, Hypofractionated Treatment Effects in the Clinic) reports, we did not include proton series because they were not felt to be comparable with photon studies. We also attempted to include only the most recent analysis of patients at each institution, and only included duplicate patients when unique endpoints or results were reported.
Table 1.
Selected studies analyzing factors associated with late urinary side effects
| Study | n | Treatment details | Med. f/u (yrs) | Urinary endpoint | Dosimetric factors associated* with worse urinary outcomes | Non-dosimetric factors associated* with worse urinary outcomes | |
|---|---|---|---|---|---|---|---|
| King 201220 (Stanford) | 67 | 36.25 Gy (5 fx), QD/QOD | 2.7 | RTOG G2: 5.0% G3: 3.5% |
|
||
| Bolzicco 201321 (Vicenza) | 100 | 35 Gy (5 fx), QD | 3.0 | RTOG G2: 3.0% G3: 1.0% |
|
||
| Elias 201422 (Sunnybrook) | 84 | 35 Gy (5 fx), QW | 4.2 | EPIC QOL MID: 17.9% |
|
|
|
| Katz 201423 (Flushing) | 515 | 35–36.25 Gy (5 fx), QD | 6.0 | RTOG G2:9.1% G3: 1.7% |
|
|
|
| Bernetich 201424 (Drexel) | 142 | 79%: 35–36.25 Gy (5 fx), QOD 21%: 37.5 Gy (5 fx), QOD | 3.0 | CTCAE v3 G2: 14% G3: 2% |
|
||
| Gurka 201525 (Georgetown) | 208 | 35–36.25 Gy (5 fx), QOD |
4.0 | CTCAE v4 ≥G2 bleed: 2.4% |
|
||
| Gomez 201526 (UCLA) | 75 | 40 Gy (5 fx), QOD | 1.0 | EPIC QOL (Avg of Obs/irrit and Incont) |
|
|
|
| Seymour 201527 (UCSF) | 56 | 38 Gy (4 fx), QD/QOD | 3.0 | CTCAE v4 G2: 19.6% G3: 3.6% |
|
|
|
| Qi 201628 (UCLA) | 86 | 40 Gy (5 fx), QOD | 1.0 | EPIC QOL Obs/irrit MID: 46% Incont MID: 28% |
|
|
|
| Kole 201629 (Georgetown) | 216 | 35–36.25 Gy (5 fx), QOD | 4.0 | IPSS Late urinary flare: 13% |
|
|
|
| Helou 201730 (Sunnybrook) | 259 | 32%: 35 Gy (5 fx), QW 39%: 40 Gy (5 fx), QW 29%: 40 Gy (5 fx), QOD |
3.2 | RTOG G2: 32.6% G3: 1.9% |
|
|
|
| Zhang 201731 (UCSF) | 78 | 38 Gy (4 fx), QD/QOD | 3.0 | CTCAE v4 G2: 19.2% G3: 2.6% |
|
|
|
| Jackson 201832 (Multicenter) | 66 | 37 Gy (5 fx), QOD | 3.1 | EPIC QOL Obs/irrit MID: 25% Incont MID: 24% |
|
||
Abbreviations: QW; every week, QOD; every other day, QD; every day, MID; minimally important difference, Obs/irrit, obstruction/irritation domain; Incont, incontinence domain.
Included if reported as trend or significant on univariate or multivariate analysis
Table 2.
Selected studies analyzing factors associated with late bowel side effects
| Study | n | Treatment details | Med. f/u (yrs) | Bowel endpoint | Dosimetric factors associated* with worse bowel outcomes | Non-dosimetric factors associated* with worse bowel outcomes |
|---|---|---|---|---|---|---|
| King 201220 (Stanford) | 67 | 36.25 Gy (5 fx), QD/QOD | 2.7 | RTOG G2: 2.0% |
|
|
| Kim 201433 (Multicenter) | 91 | 16%:45 Gy (5 fx), QOD 16%: 47.5 Gy (5 fx), QOD 67%: 50 Gy (5 fx), QOD |
2.0 | CTCAE v3 G2:23.1% G3:3.3% G4: 2.2% |
|
|
| Elias 201422 (Sunnybrook) | 84 | 35 Gy (5 fx), QW | 4.2 | EPIC QOL MID: 26.2% |
|
|
| Gomez 201526 (UCLA) | 75 | 40 Gy (5 fx), QOD | 1.0 | EPIC QOL |
|
|
| Musunuru 201634 (Sunnybrook) | 258 | 33%: 35 Gy (5 fx), QW 67%: 40 Gy (5 fx), QW or QOD | 2.5 | CTCAE v3 G2 bleed: 16.2% G3 bleed: 1.6% G4 bleed: 1.6% |
|
|
| Miszczyk 201735 (Gliwice) | 400 | 36.25 Gy (5 fx), QOD | 1.3 | RTOG G1:4.7% G2: 0.6% G3: 0.3% |
|
Abbreviations: QW; every week, QOD; every other day, QD; every day, MID; minimally important difference, SV; seminal vesicle.
Included if reported as trend or significant on univariate or multivariate analysis
Table 3.
Selected studies analyzing factors associated with late sexual side effect
| Study | n | Treatment details | Med. f/u (yrs) | Sexual endpoint | Dosimetric factors associated* with worse sexual outcomes | Non-dosimetric factors associated* with worse sexual outcomes |
|---|---|---|---|---|---|---|
| Wiegner 201036 (Stanford) | 32 (no ADT) | 36.25 Gy (5 fx), QD/QOD | 3.0 | EPIC QOL 20M impotency: 61% (23% if potent at baseline) | (PB not associated) |
|
| Obayomi-Davies 201337 (Georgetown) | 97 (Potent at baseline, no ADT) | 35–36.25 Gy (5 fx), QOD | 2.7 | EPIC QOL 2Y impotency: 45.6% |
(PB not associated) |
|
| Elias 201422 (Sunnybrook) | 84 (4% ADT) | 35 Gy (5 fx), QW | 4.2 | EPIC QOL MID: 37.5% |
|
|
| Dess 201838 (Georgetown) | 373 (no ADT) | 35–36.25 Gy (5 fx), QOD | 4.7 | EPIC QOL 2Y impotency: 66% (43% if potent at baseline) | (PB not analyzed) |
|
Abbreviations: ADT; androgen deprivation therapy, QW; every week, QOD; every other day, QD; every day, MID; minimally important difference, PB; penile bulb
Included if reported as trend or significant on univariate or multivariate analysis
In this section, we review dosimetric factors that are associated with outcomes. Section 5 (Factors Affecting Outcomes) reviews non-dosimetric factors. Four studies contained detailed data that permitted dose/volume outcome modeling, presented in Section 6 (Mathematical/Biological Models).28,29,31,33 Of note, all of the studies discussed in this section utilized 5-fraction SBRT, with the exception of the UCSF group, which utilized 4-fraction SBRT.
Urinary Domain (Table 1)
The literature review revealed a relationship between urinary side effects and bladder, urethra, and prescription doses. Three studies found that higher prescription doses were associated with increased urinary side effects.23,24,30 For example, in an analysis from Sunnybrook, the rate of RTOG ≥ grade 2 late urinary toxicity was 5% in patients receiving 35 Gy vs. 48% in patients receiving 40 Gy.30 Further, the UCSF group reported that heterogeneous treatment plans that delivered greater “hotspots” also resulted in more CTCAE grade 2–3 urinary toxicity.27,31 Of note, similar results regarding heterogeneity have also been reported in the setting of moderate hypofractionation.39 This issue may be more pertinent for CyberKnife plans, which are typically more heterogeneous and prescribed to lower prescription isodose lines.
The relationship between urinary side effects and doses to the bladder and urethra was shown in multiple studies. “High dose” bladder parameters (eg, high doses delivered to even small volumes) were found to be associated with worse EPIC urinary QOL by the Sunnybrook group (D5cc > 34 Gy), UCLA group (V40Gy > 5.5 cc, D2cc), and a multicenter analysis (Dmax).22,26,28,32 Similarly, the Georgetown group reported an association between bladder D12.7% > 33.5 Gy and increased rates of late (IPSS-defined) urinary symptom flare.29 Other studies also reported associations between urinary toxicity and/or QOL with “low dose” bladder parameters (eg, low/moderate doses delivered to large volumes).27,28,32 Perhaps due to challenges in delineation, we only found one group that analyzed urethral doses: a UCSF analysis found that urethra V42Gy > 2 cc was associated with increased CTCAE urinary toxicity.31
Bowel Domain (Table 2)
Similar to bladder side effects, higher prescription dose was also associated with an increased risk of bowel side effects. A study from Sunnybrook Hospital reported a >20% vs. 8% rate of CTCAE grade 2 or higher hematochezia in patients treated to 40 Gy vs. 35 Gy, respectively.40
High doses to the rectum were consistently found to be important in published studies. In an analysis of late bowel side effects of a multicenter dose-escalation trial, 6 of 61 patients (10%) treated to 50 Gy in 5 fractions (the highest dose level of this trial) experienced high grade (CTCAE grade 3–4) rectal toxicity, 5 of whom required temporary or permanent colostomy. Dosimetric analysis showed that late bowel toxicity was associated with rectum V50Gy >3 cc and percent rectum circumference (estimated at mid-prostate) V39Gy >35%.33 In another study from Sunnybrook Hospital of 258 patients, CTCAE grade 2 or higher hematochezia was observed in 19.4% of patients and was strongly associated with rectum V38Gy >2 cc.34 Other studies have also demonstrated a relationship between high-dose parameters and EPIC bowel QOL, including results from Sunnybrook (D1cc > 35 Gy) and UCLA (V40Gy > 1.5 cc, V36Gy > 4.2 cc).22,26
Sexual Domain (Table 3)
Sexual side effects are well-described after prostate SBRT and appear to occur at comparable rates to other radiation treatment modalities.38 However, few studies have examined possible associations between erectile function and dose to potentially important OARs. A study from Sunnybrook Hospital reported an association between penile bulb V20Gy and V35Gy with EPIC sexual QOL,22 but there was no association between penile bulb dose and sexual function found in analyses from Georgetown (penile bulb mean dose, p=0.75) and Stanford (non-significant correlation between penile bulb V29.5Gy and erectile function).36,37 Thus, it is currently unclear how dosimetric factors contribute to sexual function outcomes after SBRT.
5. Factors Affecting Outcomes
In addition to OAR dose, several other factors were found to be associated with urinary and bowel side effects. These include:
Patients with urinary symptoms at baseline or worse baseline urinary QOL tended to have a higher likelihood of urinary toxicity after SBRT.27,30,32
Patients with large prostate volumes or history of trans-urethral resection of the prostate (TURP) before SBRT have been found to have worse urinary side effects.21,23,25 For example, Katz et al reported in 336 patients that the rate of late grade 2–3 urinary toxicity was 15% vs. 8% in patients with prostate volume > vs. ≤ 60 cc, respectively.23 In addition, the Georgetown group reported that prior TURP was the greatest risk factor for hematuria; the rate of late grade 2–3 hematuria in those with vs. without prior TURP was 21% vs. 2%, respectively.25
Urinary and bowel toxicity may be worse with shorter treatment schedules such as daily (QD) vs. every other day (QOD).20,30,31 In a Stanford series, SBRT patients treated in earlier years received QD treatment, and later years QOD. QD was associated with increased rates of late grade 1–2 urinary (56% vs. 17%) and bowel (44% vs. 5%) toxicity.20 However, a possible confounder may be the institutional learning curve in earlier years of SBRT. The ASTRO / ASCO / AUA guideline recommended QOD treatment when using ultrafractionation, albeit based on overall low-quality evidence.
Anticoagulant use has been associated with both late hematuria25 and hematochezia.34 The Sunnybrook group reported that ≥ grade 2 late hematochezia was observed in 47% vs. 18% of patients taking vs. not taking anticoagulants, respectively.34
Prostate SBRT appears to have a similar potency preserving rate vs. other radiotherapy techniques – 45% at 5 years in one contemporary series.38 In contrast to urinary and bowel toxicity, sexual side effects of prostate SBRT have not been conclusively linked to OAR dose. Rather, post-radiation sexual function appears to be dependent on baseline patient factors. For example, in 373 patients who received prostate SBRT, younger age, higher baseline sexual function, and lower body mass index were associated with an improved probability of longer-term (2–5 years post-SBRT) potency preservation.38 Others studies have similarly reported associations between potency outcomes and patient factors, but not OAR dose.36,37
6. Mathematical/Biological Models
To perform normal tissue complication probability (NTCP) modeling, dosimetric and outcome data are ideally needed for individual patients. Of the reviewed studies, only four provided either results of the authors’ own NTCP modeling or individual patient data that allowed us to perform NTCP modeling. These four studies are summarized in this section. In studies where individual patient data were published, we presented odds ratios to indicate the strength of the association. Confidence intervals of the model parameters were estimated using the profile likelihood method. Because they do not follow a Gaussian distribution, these confidence intervals are not symmetric. Consistent with the available data, these models only apply to patients treated with 4–5 fractions. All specified doses refer to physical 5-fraction dose, with the exception of one 4-fraction study where physical dose was converted to a 5-fraction equivalent dose using the LQ model and α/β=3 Gy.
Bladder Dose and Late Urinary Flare (Kole et al, Figure 1A)
Figure 1.
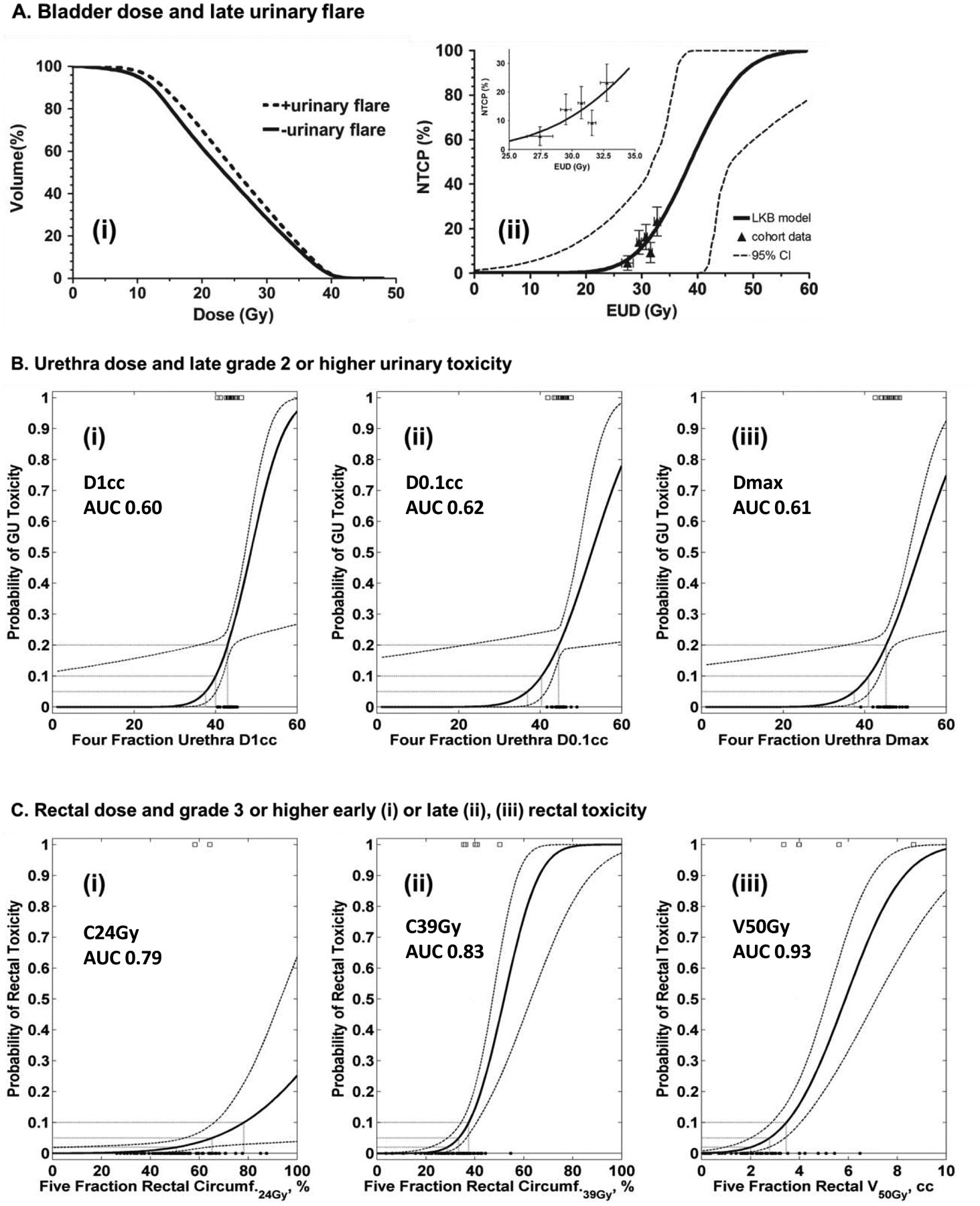
A. 5-fraction bladder dose and late urinary flare (Kole et al). (i) Mean cumulative whole bladder DVH’s for patients with (dashed line) and without (solid line) late urinary flare. (ii) LKB model of the probability of late urinary flare vs. bladder EUD. The figures in both panels were taken from the original publication.
B. 4-fraction urethra dose and late ≥ grade 2 urinary toxicity (Zhang et al). Probit models of the probability of late ≥ grade 2 urinary vs. different dose metrics to urethra: (i) D1cc, (ii) D0.1cc and (iii) Dmax. Patients with (marked by open squares) and without (black circles) toxicity are shown at the top and bottom of the plot, respectively. Fitting of the data to the probit model was performed by the authors of the current report using individual patient DVHs provided by the original publication, with a conversion to 5-fraction equivalent dose.
C. 5-fraction rectum dose and late ≥ grade 3 bowel toxicity (Kim et al). Probit models of the probability of late ≥ grade 3 rectal toxicity vs. different rectum circumference doses: (i) C24Gy, (ii) C39Gy and (iii) volume of rectal wall V50Gy. Patients with (marked by open squares) and without (black circles) toxicity are shown at the top and bottom of the plot, respectively. The fitting of the data to the probit model was performed by the authors of the present report using the data shown in Fig. 1 of the original publication.
Kole et al analyzed late urinary flare in 216 patients treated to 35–36.25 Gy in 5 fractions.29 29 patients experienced late urinary flare, defined as an increase in IPSS score ≥ 5 above post-treatment baseline, with an absolute IPSS score > 15, followed by a return to baseline within 2 years. Cumulative empty bladder DVH’s of patients with vs. without urinary flare are shown in Figure 1A (left panel), and the Lyman Kutcher Burman (LKB)-modeled dose-toxicity curve is shown in Figure 1A (right panel). Both figures were taken from the original publication. Using the endpoint of late urinary flare, the LKB NTCP model for bladder equivalent uniform dose (EUD)41 resulted in parameter estimates (95% CI) of TD50 = 38.7 Gy (31.1–46.4 Gy), m = 0.19 (CI 0–0.47), and n = 0.13 (−0.14–0.41).
Urethra Dose and Late ≥ Grade 2 Urinary Toxicity (Zhang et al, Figure 1B)
Zhang et al analyzed late (≥ 3 months) CTCAE ≥ grade 2 urinary toxicity in 78 patients treated to 38 Gy in 4 fractions.31 Although the original publication did not report a significant relationship between urethra Dmax and urinary toxicity (using t-test alone, p=0.29), the authors provided raw data allowing us to perform additional statistical and modelling analyses. Of note, three patients developed grade ≥3 early or late genitourinary complications in this study; 4-fraction urethra D1cc in these three patients were 43 Gy, 44 Gy, and 45 Gy
Appendix Figure 1 was generated using data provided by the publication and shows urethral DVH’s for patients with vs. without late ≥ grade 2 urinary toxicity. Probit-modeled dose-toxicity curves were derived for the D1cc, D0.1cc and Dmax dose metrics and are shown in Figure 1B. All physical doses were converted to a 5-fraction equivalent dose using the LQ model and α/β=3 Gy. The probit model parameter values for urethra D1cc resulted in parameter estimates (68% CI) of TD50 = 48.6 Gy (46.0–108.6) and m = 0.14 (0.08–0.77). Similarly, for urethra D0.1cc the parameter values were TD50 = 52.6 Gy (48.3-) and m = 0.18 (0.09-) (No CI upper limit in this model due to few events and clustering). Finally, for urethra Dmax the parameter values were TD50 = 53.5 Gy (49.7–147.6) and m = 0.18 (0.10–0.88). When the model parameters of D1cc, D0.1cc, and Dmax were applied to the dataset, the area under the ROC curve (AUC) was 0.60, 0.62, and 0.61, respectively. There was a significant association (p=0.04) between urethra Dmax ≥ 47 Gy and late ≥ grade 2 urinary toxicity (OR 4.2, 95% CI: 1.2–15.0). For grade 2 urinary complications, the 20% risk level corresponded to D1cc = 47 Gy, D0.1cc = 49 Gy, and Dmax = 50 Gy (in 5 fractions).
Rectum Dose and Late ≥ Grade 3 Bowel Toxicity (Kim et al, Figure 1C)
Kim et al analyzed late (occurring or persisting ≥ 270 days) CTCAE bowel toxicity in 91 patients treated to 45–50 Gy in 5 fractions in the context of a dose escalation trial.33 Figure 1C shows the probit-modeled circumferential dose-toxicity curve derived based on the data for 91 patients (and 6 events) with discernible data points (Plots from the original publication are shown in Appendix Figure 2). Using the endpoint of late ≥ grade 3 bowel toxicity, the Probit NTCP model for percent rectal circumference (estimated at mid-prostate) receiving at least 24 Gy (C24Gy) resulted in parameter estimates (68% CI) of TD50 = 123.8% (92.8–440.5) and m = 0.29 (0.19–0.48). Similarly, for rectal circumference C39Gy the parameters were TD50 = 51.9% (47.0–63.0) and m = 0.22 (0.16–0.31). Finally, for rectal wall volume V50Gy the parameters were TD50 = 5.9cc (5.1–7.1) and m = 0.32 (0.25–0.41). When the model parameters for C24Gy, C39Gy, and V50Gy were applied to the dataset, the AUC was 0.79, 0.83, and 0.93, respectively. There was a significant association (p=0.02) between rectal circumference C39Gy ≥ 40% and ≥ grade 3 bowel toxicity (OR 8.7, 95% CI: 1.3–59.4).
Bladder/Rectum Dose and Patient-reported QOL MID (Qi et al, Appendix Figure 3)
Qi et al analyzed late (12 month) EPIC QOL changes in 86 patients receiving 40 Gy in 5 fractions, treated with full bladder and empty rectum.28 The endpoint was defined as a minimally important difference (MID) in QOL: 6 point decrement for urinary incontinence, 5 point decrement for urinary obstruction/irritation, and 4 point decrement for bowel. Plots were provided in the publication’s supporting data which display, for each QOL domain, the normalized mean OAR dose (mean OAR dose divided by prescription dose) vs. normalized OAR-PTV overlap (overlap volume divided by OAR volume) for patients with MID decrement in QOL, no change, and improvement. From these plots we extracted individual mean bladder and rectum doses (all patients were treated to 40 Gy, thus mean OAR dose was 40 times the normalized mean OAR dose) and used the endpoint of MID decrement in QOL to create Probit NTCP models for each QOL domain.
For urinary incontinence QOL, bladder parameter estimates (68% CI) were TD50 = 18.6 Gy (15.8–23.4 Gy) and m = 0.79 (0.61–1.08). Applied to the dataset, these parameters produced an AUC of 0.63, with a significant association (p=0.008) between bladder mean dose ≥ 17 Gy and MID decrement in QOL (OR 7.7, 95% CI: 1.3–45.0; 17 Gy was chosen because it was the bladder mean dose that maximized the OR of the model) (Appendix, Figure 3). The best-fit probit models were not significant for urinary obstruction/irritation QOL (p=0.36) or bowel QOL (p=0.40).
7. Special Situations
Many other situations can affect the normal tissue complication probability for patients receiving prostate SBRT. Patient factors such as polymorphisms in genes related to oxidative stress, obesity, diabetes, and tobacco use may increase risk of toxicity with conventionally fractionated radiation.42–44 It is unknown how these risks translate to patients receiving SBRT. Patients with inflammatory bowel disease are known to have increased bowel side effects from radiation treatment.45,46
Another potential modifier of toxicity risk is prior radiation to the prostate. Emerging data suggest that SBRT may be a feasible option for salvage treatment after prior radiotherapy, and use of SBRT in this setting may increase in the future.47,48 In addition, as randomized data have demonstrated that a combination of conventionally-fractionated external beam radiotherapy plus brachytherapy boost vs. external beam radiotherapy alone improves long-term disease-free survival in patients with more aggressive prostate cancer,49 some are exploring the use of SBRT boost instead of brachytherapy.50,51 Normal tissue dose constraints for SBRT as salvage therapy after prior RT, or as a boost, are not well defined.
The use of absorbable hydrogel spacers that physically separate the rectum from the prostate has been shown to reduce rectal toxicity for conventionally fractionated radiation.52 These devices could also potentially be used for prostate SBRT to reduce rectal toxicity risk.
8. Recommended Dose/Volume Objectives
Most of the reviewed studies noted largely mild/moderate toxicity (eg, grade 2 or lower); and as the studies did not follow uniform dose/volume guidelines, there appear to be a range of acceptable and safe dose/volume constraints. Based on the limited data available to review and model as well as inter-study variation in OAR dosimetric parameters and endpoints reported, firm dose/volume recommendations generally cannot be made, consistent with the 2018 ASTRO / ASCO / AUA guideline.4 However, use of published planning objectives is recommended. Table 4 summarizes example sets of dose/volume objectives used in two clinical trials and at several of the authors’ institutions, all of which we feel are reasonable based on our review of the literature.53–56 Most significant dosimetric parameters were high-dose, and thus dosimetric goals for OAR’s should include at minimum Dmax or other similar high-dose constraint. Volume effects likely contribute, and thus a moderate-dose constraint is also reasonable.
Table 4.
Examples of dose constraints used in the authors’ institutions and on clinical trials
| Georgetown* (35–36.25 / 5 fx, over 2 weeks) | UNC (NCT 00643617)* (38 Gy / 4 fx over 4 days) | RTOG 093855 (36.25 Gy / 5 fx over 2.5 weeks) | NRG GU-00556 (36.25 Gy / 5 fx over 2.5 weeks) | |
|---|---|---|---|---|
| PTV | ≤ 125% prescription dose | D0.03cc ≤ 38.78 Gy (Linac) D0.03cc ≤ 43.5 Gy (CyberKnife) |
Dmax ≤ 38.78 Gy | |
| Prostatic urethra | V42Gy ≤ 0.03 cc | Dmax < 40 Gy | D0.03cc ≤ 38.78 Gy | D0.03cc ≤ 38.78 Gy |
| Bladder | V37Gy ≤ 5 cc | Dmax < 45.6 Gy D10cc < 41.8 Gy |
D1cc ≤ 38.06 Gy D10% ≤ 32.63 Gy D50% ≤ 18.13 Gy |
D0.03cc ≤ 38.06 Gy D10% ≤ 18.12 Gy |
| Rectum | V36Gy ≤ 1 cc | Dmax < 38 Gy | D1cc ≤ 38.06 Gy D3cc ≤ 34.4 Gy D10% ≤ 32.63 Gy D20% ≤ 29 Gy D50% ≤ 18.13 Gy |
D0.03cc ≤ 38.06 Gy D3cc ≤ 34.4 Gy D10% ≤ 32.63 Gy D20% ≤ 29 Gy D50% ≤ 18.13 Gy |
| Penile bulb | V29.5 Gy ≤ 3 cc | Dmax ≤ 36.25 Gy D3cc ≤ 20 Gy |
D0.03cc ≤ 36.25 Gy D3cc ≤ 19.9 Gy |
Abbreviations: OAR; organ at risk, fx; fractions
Note: Bladder and rectum volumes are defined as solid organs. For RTOG 0938 and NRG GU-005, the rectum is defined as extending 15cm from the anus or to the rectosigmoid flexure.
Dosimetric parameters currently used at authors’ institutions
For bowel side effects, toxicity may be reduced by limiting the rectum Dmax to the prescription dose. Furthermore, the reproducibility of rectal filling (and thus accuracy of OAR avoidance) may be improved with pre-simulation and daily pre-treatment enema. For urinary side effects, there is an important contribution of patient factors (eg, prostate size, baseline symptoms, prior TURP) that should be weighed equally with OAR doses. Bladder filling instructions could also be considered to increase reproducibility. With regards to prescription dose, all reviewed studies (other than earlier dose-escalation trials) utilized prescription doses of 35–40 Gy in 4–5 fractions. Though the ASTRO / ASCO / AUA guideline recommended against prescription doses above 36.25 Gy based on several studies showing higher grade 1–2 toxicity with higher prescription doses,4 the risk of severe (eg, grade 3 or higher) toxicity appears to be low with doses up to 40 Gy. Factors including OAR dose and prescription isodose line may be equally important as prescription dose in this regard.
9. Future Studies
Continued research to refine our understanding of organ tolerance to prostate SBRT is needed. Large studies that prospectively collect physician-assessed toxicity and/or patient-reported QOL, and with detailed dose-volume histogram data, will help address this knowledge gap. Future studies should investigate whether volume effects in NTCP modeling are better addressed through the calculation of generalized EUD or by modelling specific OAR regions such as rectum/bladder wall vs. entire volume. Several pertinent examples exist in the setting of conventionally-fractionated prostate radiation, including one study suggesting that bladder trigone dose may drive genitourinary toxicity, and another examining anal and anorectal wall EUD and bowel toxicity.57,58
All the studies we reviewed employed daily image guidance for target motion management. These include Cyberknife (which tracks implanted fiducials throughout the duration of each daily treatment), Calypso beacons on conventional linac,32 and pre-treatment imaging of implanted fiducials. OAR motion management could be important, with significant daily anatomic variability that can result in significant differences between planned and delivered OAR dose.59 Studies should continue to explore the impact on patient outcomes of OAR management practices including but not limited to bladder and bowel filling instructions, pre-treatment enema, and ultrasound-guided placement of hydrogel spacers. Further, should MRI’s be used to contour difficult-to-visualize OAR’s such as the urethra and penile bulb or even replace Foley catheters entirely for urethral delineation? Though MRI may be a useful supplement, we caution that OAR contours on image fusions should generally be cross-referenced to the base planning scan to generate a combined PRV. Additional questions include the comparative cancer-control efficacy and side effects of SBRT vs. more conventionally-fractionated RT (ongoing trials PACE [NCT01584258] and NRG-GU005 [NCT03367702]), optimal dose fractionation for prostate SBRT, and frequency of SBRT delivery (QD vs. QOD vs. weekly). Novel uses of SBRT as boost or salvage therapy after prior RT also require research to establish safety and efficacy.
10. Reporting Standards for Outcomes
Currently, side effects associated with prostate SBRT are reported using varying measures in different studies: RTOG and CTCAE physician-scored toxicity scales; EPIC, IPSS, SHIM and other patient-reported QOL instruments. Although this variation unfortunately limits the ability to compare outcomes across studies, all of these instruments are validated and it is not possible to recommend any one in particular that is “preferred.”
Additional issues remain related to variation in follow-up time and reporting of OAR dose-volume metrics and other clinical variables. We encourage authors to include raw data containing individual patient dose-volume / outcome data along with manuscript submissions, in order to facilitate future data pooling and modeling efforts.60 Though complete dose-volume data (eg, in manuscript appendices or electronic supplements) are preferred, at least OAR maximum and D1cc should be reported to assist in the analysis of severe late toxicity given the high doses per fraction utilized during SBRT. The absolute volume (as opposed to percent volume) of OAR receiving a clinically significant dose, eg V15–30Gy, should also be reported to aid in analyses of volume effect. Detailed descriptions of NTCP model parameters and their uncertainties are also needed if such modeling is conducted. Finally, clinical variables that may influence toxicity should be consistently reported, such as age, use of androgen deprivation therapy, history of urinary procedures such as TURP, prostate/target volume, treatment schedule, and baseline symptoms.
Supplementary Material
Funding:
AJ and EY were supported in part by NIH institutional core grant P30CA008748.
Acronyms:
- SBRT
Stereotactic body radiation therapy
- QOL
Quality of life
- RTOG
Radiation Therapy Oncology Group
- CTCAE
Common Terminology Criteria for Adverse Events
- EPIC
Expanded Prostate Cancer Index Composite
- IPSS
International Prostate Symptom Score
- SHIM
Sexual Health Inventory for Men
- OAR
Organ at risk
- PRV
Planning risk volume
- HyTEC
High Dose per Fraction, Hypofractionated Treatment Effects in the Clinic
- TURP
Trans-urethral resection of the prostate
- QD
Daily
- QOD
Every other day
- NTCP
Normal tissue complication probability
- LKB
Lyman Kutcher Burman
- EUD
Equivalent uniform dose
- MID
Minimally important difference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen RC, Clark JA, Talcott JA. Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J Clin Oncol. 2009;27(24):3916–3922. [DOI] [PubMed] [Google Scholar]
- 2.Albkri A, Girier D, Mestre A, Costa P, Droupy S, Chevrot A. Urinary Incontinence, Patient Satisfaction, and Decisional Regret after Prostate Cancer Treatment: A French National Study. Urol Int. 2018;100(1):50–56. [DOI] [PubMed] [Google Scholar]
- 3.Weiner J, Schwartz D, Shao M, Osborn V, Choi K, Schreiber D. Stereotactic radiotherapy of the prostate: fractionation and utilization in the United States. Radiat Oncol J. 2017;35(2):137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated Radiation Therapy for Localized Prostate Cancer: An ASTRO, ASCO, and AUA Evidence-Based Guideline. J Clin Oncol. 2018:JCO1801097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394(10196):385–395. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida K, Yamazaki H, Nakamara S, et al. Comparison of common terminology criteria for adverse events v3.0 and radiation therapy oncology group toxicity score system after high-dose-rate interstitial brachytherapy as monotherapy for prostate cancer. Anticancer Res. 2014;34(4):2015–2018. [PubMed] [Google Scholar]
- 7.Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology. 2010;76(5):1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alongi F, Cozzi L, Arcangeli S, et al. Linac based SBRT for prostate cancer in 5 fractions with VMAT and flattening filter free beams: preliminary report of a phase II study. Radiat Oncol. 2013;8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hentschel B, Oehler W, Strauss D, Ulrich A, Malich A. Definition of the CTV prostate in CT and MRI by using CT-MRI image fusion in IMRT planning for prostate cancer. Strahlenther Onkol. 2011;187(3):183–190. [DOI] [PubMed] [Google Scholar]
- 10.Wright JL, Newhouse JH, Laguna JL, Vecchio D, Ennis RD. Localization of neurovascular bundles on pelvic CT and evaluation of radiation dose to structures putatively involved in erectile dysfunction after prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2004;59(2):426–435. [DOI] [PubMed] [Google Scholar]
- 11.Zakian KL, Wibmer A, Vargas HA, et al. Comparison of Motion-Insensitive T2-Weighted MRI Pulse Sequences for Visualization of the Prostatic Urethra During MR Simulation. Pract Radiat Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kataria T, Gupta D, Goyal S, et al. Simple diagrammatic method to delineate male urethra in prostate cancer radiotherapy: an MRI based approach. Br J Radiol. 2016;89(1068):20160348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loblaw A, Cheung P, D’Alimonte L, et al. Prostate stereotactic ablative body radiotherapy using a standard linear accelerator: toxicity, biochemical, and pathological outcomes. Radiother Oncol. 2013;107(2):153–158. [DOI] [PubMed] [Google Scholar]
- 14.Boike TP, Lotan Y, Cho LC, et al. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol. 2011;29(15):2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viswanathan AN, Yorke ED, Marks LB, Eifel PJ, Shipley WU. Radiation dose-volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76(2):361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gay HA, Barthold HJ, O’Meara E, et al. Pelvic normal tissue contouring guidelines for radiation therapy: a Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83(3):e353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salembier C, Villeirs G, De Bari B, et al. ESTRO ACROP consensus guideline on CT- and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother Oncol. 2018;127(1):49–61. [DOI] [PubMed] [Google Scholar]
- 19.Harris VA, Staffurth J, Naismith O, et al. Consensus Guidelines and Contouring Atlas for Pelvic Node Delineation in Prostate and Pelvic Node Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015;92(4):874–883. [DOI] [PubMed] [Google Scholar]
- 20.King CR, Brooks JD, Gill H, Presti JC, Jr. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82(2):877–882. [DOI] [PubMed] [Google Scholar]
- 21.Bolzicco G, Favretto MS, Satariano N, Scremin E, Tambone C, Tasca A. A single-center study of 100 consecutive patients with localized prostate cancer treated with stereotactic body radiotherapy. BMC Urol. 2013;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elias E, Helou J, Zhang L, et al. Dosimetric and patient correlates of quality of life after prostate stereotactic ablative radiotherapy. Radiother Oncol. 2014;112(1):83–88. [DOI] [PubMed] [Google Scholar]
- 23.Katz AJ, Kang J. Quality of Life and Toxicity after SBRT for Organ-Confined Prostate Cancer, a 7-Year Study. Front Oncol. 2014;4:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernetich M, Oliai C, Lanciano R, et al. SBRT for the Primary Treatment of Localized Prostate Cancer: The Effect of Gleason Score, Dose and Heterogeneity of Intermediate Risk on Outcome Utilizing 2.2014 NCCN Risk Stratification Guidelines. Front Oncol. 2014;4:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurka MK, Chen LN, Bhagat A, et al. Hematuria following stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer. Radiat Oncol. 2015;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez CL, Xu X, Qi XS, et al. Dosimetric parameters predict short-term quality-of-life outcomes for patients receiving stereotactic body radiation therapy for prostate cancer. Pract Radiat Oncol. 2015;5(4):257–262. [DOI] [PubMed] [Google Scholar]
- 27.Seymour ZA, Chang AJ, Zhang L, et al. Dose-volume analysis and the temporal nature of toxicity with stereotactic body radiation therapy for prostate cancer. Pract Radiat Oncol. 2015;5(5):e465–472. [DOI] [PubMed] [Google Scholar]
- 28.Qi XS, Wang JP, Gomez CL, et al. Plan quality and dosimetric association of patient-reported rectal and urinary toxicities for prostate stereotactic body radiotherapy. Radiother Oncol. 2016;121(1):113–117. [DOI] [PubMed] [Google Scholar]
- 29.Kole TP, Tong M, Wu B, et al. Late urinary toxicity modeling after stereotactic body radiotherapy (SBRT) in the definitive treatment of localized prostate cancer. Acta Oncol. 2016;55(1):52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helou J, D’Alimonte L, Quon H, et al. Stereotactic ablative radiotherapy in the treatment of low and intermediate risk prostate cancer: Is there an optimal dose? Radiother Oncol. 2017;123(3):478–482. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Johnson J, Gottschalk AR, et al. Receiver operating curves and dose-volume analysis of late toxicity with stereotactic body radiation therapy for prostate cancer. Pract Radiat Oncol. 2017;7(2):e109–e116. [DOI] [PubMed] [Google Scholar]
- 32.Jackson WC, Dess RT, Litzenberg DW, et al. A multi-institutional phase 2 trial of prostate stereotactic body radiation therapy (SBRT) using continuous real-time evaluation of prostate motion with patient-reported quality of life. Pract Radiat Oncol. 2018;8(1):40–47. [DOI] [PubMed] [Google Scholar]
- 33.Kim DW, Cho LC, Straka C, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1–2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2014;89(3):509–517. [DOI] [PubMed] [Google Scholar]
- 34.Musunuru HB, Davidson M, Cheung P, et al. Predictive Parameters of Symptomatic Hematochezia Following 5-Fraction Gantry-Based SABR in Prostate Cancer. Int J Radiat Oncol Biol Phys. 2016;94(5):1043–1051. [DOI] [PubMed] [Google Scholar]
- 35.Miszczyk L, Namysl Kaletka A, Napieralska A, et al. Cyberknife Radioablation of Prostate Cancer - Preliminary Results for 400 Patients. Asian Pac J Cancer Prev. 2017;18(4):1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiegner EA, King CR. Sexual function after stereotactic body radiotherapy for prostate cancer: results of a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2010;78(2):442–448. [DOI] [PubMed] [Google Scholar]
- 37.Obayomi-Davies O, Chen LN, Bhagat A, et al. Potency preservation following stereotactic body radiation therapy for prostate cancer. Radiat Oncol. 2013;8:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dess RT, Hartman HE, Aghdam N, et al. Erectile function after stereotactic body radiotherapy for localized prostate cancer. BJU Int. 2018;121(1):61–68. [DOI] [PubMed] [Google Scholar]
- 39.McDonald AM, Baker CB, Popple RA, Cardan RA, Fiveash JB. Increased radiation dose heterogeneity within the prostate predisposes to urethral strictures in patients receiving moderately hypofractionated prostate radiation therapy. Pract Radiat Oncol. 2015;5(5):338–342. [DOI] [PubMed] [Google Scholar]
- 40.Musunuru HB, Quon H, Davidson M, et al. Dose-escalation of five-fraction SABR in prostate cancer: Toxicity comparison of two prospective trials. Radiother Oncol. 2016;118(1):112–117. [DOI] [PubMed] [Google Scholar]
- 41.Reporting Niemierko A. and analyzing dose distributions: a concept of equivalent uniform dose. Med Phys. 1997;24(1):103–110. [DOI] [PubMed] [Google Scholar]
- 42.Ahn J, Ambrosone CB, Kanetsky PA, et al. Polymorphisms in genes related to oxidative stress (CAT, MnSOD, MPO, and eNOS) and acute toxicities from radiation therapy following lumpectomy for breast cancer. Clin Cancer Res. 2006;12(23):7063–7070. [DOI] [PubMed] [Google Scholar]
- 43.Kalakota K, Liauw SL. Toxicity after external beam radiotherapy for prostate cancer: an analysis of late morbidity in men with diabetes mellitus. Urology. 2013;81(6):1196–1201. [DOI] [PubMed] [Google Scholar]
- 44.Steinberger E, Kollmeier M, McBride S, Novak C, Pei X, Zelefsky MJ. Cigarette smoking during external beam radiation therapy for prostate cancer is associated with an increased risk of prostate cancer-specific mortality and treatment-related toxicity. BJU Int. 2015;116(4):596–603. [DOI] [PubMed] [Google Scholar]
- 45.Chen AB, D’Amico AV, Neville BA, Earle CC. Patient and treatment factors associated with complications after prostate brachytherapy. J Clin Oncol. 2006;24(33):5298–5304. [DOI] [PubMed] [Google Scholar]
- 46.Murphy CT, Heller S, Ruth K, et al. Evaluating toxicity from definitive radiation therapy for prostate cancer in men with inflammatory bowel disease: Patient selection and dosimetric parameters with modern treatment techniques. Pract Radiat Oncol. 2015;5(3):e215–222. [DOI] [PubMed] [Google Scholar]
- 47.Fuller DB, Wurzer J, Shirazi R, Bridge SS, Law J, Mardirossian G. High-dose-rate stereotactic body radiation therapy for postradiation therapy locally recurrent prostatic carcinoma: Preliminary prostate-specific antigen response, disease-free survival, and toxicity assessment. Pract Radiat Oncol. 2015;5(6):e615–623. [DOI] [PubMed] [Google Scholar]
- 48.Leroy T, Lacornerie T, Bogart E, Nickers P, Lartigau E, Pasquier D. Salvage robotic SBRT for local prostate cancer recurrence after radiotherapy: preliminary results of the Oscar Lambret Center. Radiat Oncol. 2017;12(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris WJ, Tyldesley S, Rodda S, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):275–285. [DOI] [PubMed] [Google Scholar]
- 50.Mercado C, Kress MA, Cyr RA, et al. Intensity-Modulated Radiation Therapy with Stereotactic Body Radiation Therapy Boost for Unfavorable Prostate Cancer: The Georgetown University Experience. Front Oncol. 2016;6:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richardson M, Sidhom M, Gallagher S, et al. PROstate Multicentre External beam radioTHErapy Using a Stereotactic boost: the PROMETHEUS study protocol. BMC Cancer. 2018;18(1):588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamstra DA, Mariados N, Sylvester J, et al. Continued Benefit to Rectal Separation for Prostate Radiation Therapy: Final Results of a Phase III Trial. Int J Radiat Oncol Biol Phys. 2017;97(5):976–985. [DOI] [PubMed] [Google Scholar]
- 53.Chen LN, Suy S, Uhm S, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol. 2013;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuller DB, D. FA, Crabtree T, et al. Phase 2 Multicenter Trial of Heterogeneous-dosing Stereotactic Body Radiotherapy for Low- and Intermediate-risk Prostate Cancer: 5-year Outcomes. Eur Urol Oncol. 2018. ( 10.1016/j.euo.2018.06.013). [DOI] [PubMed] [Google Scholar]
- 55.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016;2(12):1607–1616. [DOI] [PubMed] [Google Scholar]
- 56.Robbins ME, Brunso-Bechtold JK, Peiffer AM, Tsien CI, Bailey JE, Marks LB. Imaging radiation-induced normal tissue injury. Radiat Res. 2012;177(4):449–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghadjar P, Zelefsky MJ, Spratt DE, et al. Impact of dose to the bladder trigone on long-term urinary function after high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2014;88(2):339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peeters ST, Hoogeman MS, Heemsbergen WD, Hart AA, Koper PC, Lebesque JV. Rectal bleeding, fecal incontinence, and high stool frequency after conformal radiotherapy for prostate cancer: normal tissue complication probability modeling. Int J Radiat Oncol Biol Phys. 2006;66(1):11–19. [DOI] [PubMed] [Google Scholar]
- 59.Wahl M, Descovich M, Shugard E, et al. Interfraction Anatomical Variability Can Lead to Significantly Increased Rectal Dose for Patients Undergoing Stereotactic Body Radiotherapy for Prostate Cancer. Technol Cancer Res Treat. 2017;16(2):178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackson A, Marks LB, Bentzen SM, et al. The lessons of QUANTEC: recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


