Abstract
The chemical synapse is the principal form of contact between neurons of the central nervous system. These synapses are typically configured as presynaptic axon terminations onto postsynaptic dendrites or somata, giving rise to axo-dendritic and axo-somatic synapses, respectively. Beyond these common synapse configurations are less-studied, non-canonical synapse types that are prevalent throughout the brain and significantly contribute to neural circuit function. Among these are the axo-axonic synapses, which consist of an axon terminating on another axon or axon terminal. Here, we review evidence for axo-axonic synapse contributions to neural signaling in the mammalian nervous system and survey functional neural circuit motifs enabled by these synapses. We also detail how recent advances in microscopy, transgenics, and biological sensors may be used to identify and functionally assay axo-axonic synapses.
Keywords: Electron microscopy, dopamine, glutamate, GABA, serotonin, acetylcholine, norepinephrine
Introduction
In 1961, Dudel & Kuffler discovered that the major inhibitory neurotransmitter GABA acts on the presynaptic terminal of a glutamatergic neuron to decrease the release probability of this major excitatory neurotransmitter. Since then, a panoply of G-protein coupled receptors (GPCRs) and ionotropic heteroreceptors localized to axon terminals were identified to modulate neurotransmitter release (Atwood et al., 2014). Clearly most, if not all, axon terminals contain GPCR autoreceptors, which allows for autoregulatory negative feedback. Other sources of neurotransmitters exist to exert presynaptic regulatory action, including retrograde messengers sourced from postsynaptic membranes and astrocytic transmitters. Several studies now demonstrate that axon terminals synapsing on another axon terminal provide direct neural circuit routes for presynaptic modulation throughout the mammalian brain.
Among other functions, the axo-axonic synapse allows for neurotransmitter release from the postsynaptic axon terminal that does not require action potential activity that is generated at the postsynaptic neuron’s axon hillock. Such is the case for substantia nigra dopamine neurons, in which somatic action potential firing rates often fail to account for activity observed at the axon terminal (see Berke, 2018). Here, we survey structural evidence for axo-axonic synapses, discuss how this configuration may impact circuit function, and describe how recent technological advances may aid in identifying and functionally assessing these non-canonical synapses.
Structurally defining axo-axonic synapses
The gold standard in elucidating neural circuits, electron microscopy (EM), provides nanometer scale resolution critical for discerning the morphological characteristics of axon terminals and postsynaptic targets (Figure 1). Axo-dendritic and axo-somatic synapses describe interactions in which an axon synapses on the dendrite (Figure 1a) or cell body (Figure 1b) of the post-synaptic neuron. Synapses on in the axon hillock or initial segment directly regulate action potential generation of the postsynaptic neuron (Figure 1c). Axo-axonic synapses are characterized by a presynaptic element or varicosity that contains neurotransmitter-filled synaptic vesicles and forms one or more electron-dense junctions with a similarly vesicle-filled axon terminal (Figure 1d) (Peters & Palay, 1996). In some instances, axon terminals form a close parallel junction that lack the electron-dense synaptic specialization and are thus referred to as appositions (Figure 1e).
Figure 1. Canonical and non-canonical synapse types.
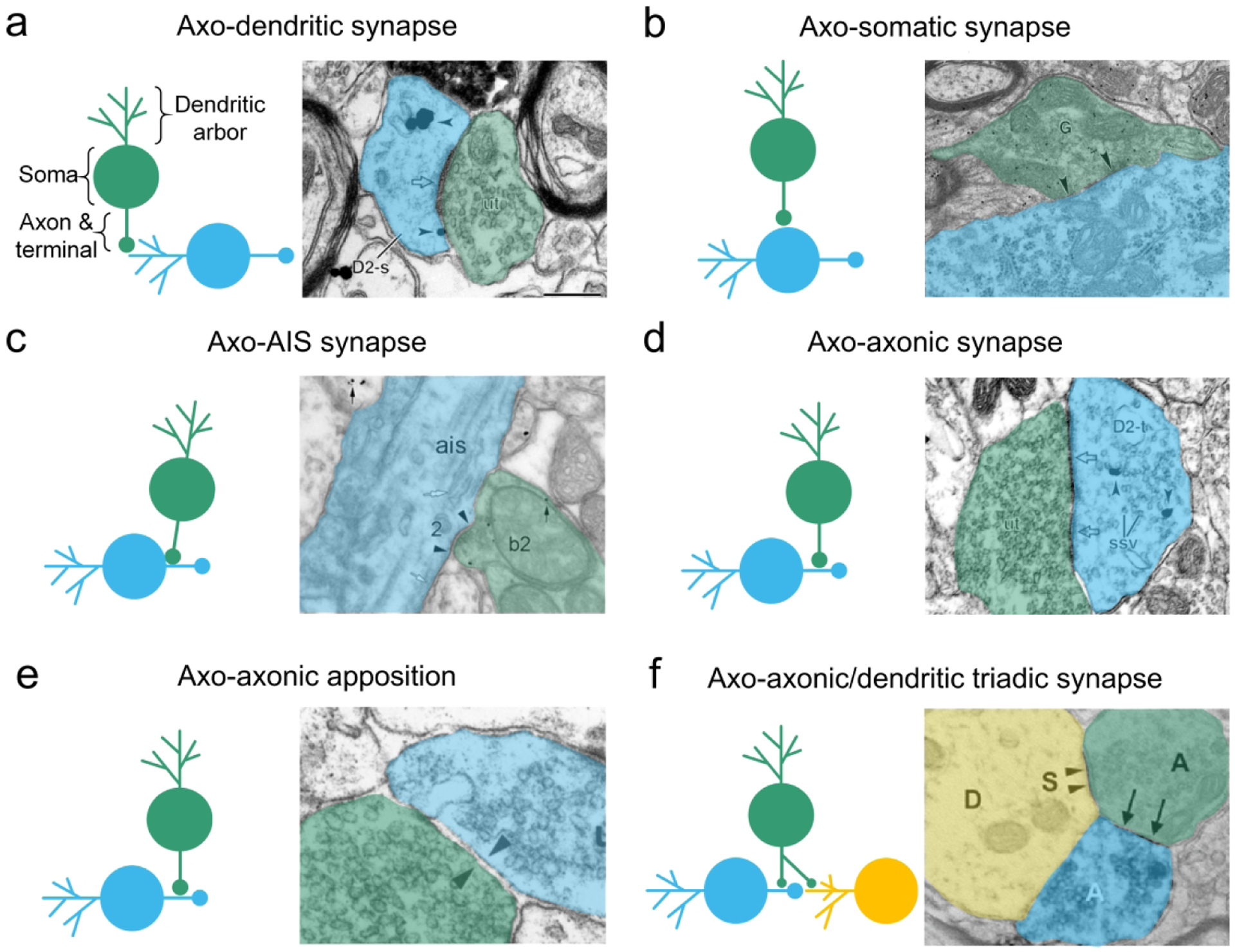
Four common synaptic arrangements between presynaptic (green) and postsynaptic (blue) neurons are depicted through schematic illustrations (left) and electron micrographs (right). a. The axo-dendritic synapse consists of the presynaptic axon terminal (green) synapsing on a dendritic element (i.e. spine or shaft) of the postsynaptic cell (blue). b. The axo-somatic synapse is comprised of a presynaptic axon (green) terminating on the cell body of the postsynaptic neuron (blue). c. An axon (green) may terminate on the axon initial segment (AIS) to modulate action potential generation of the postsynaptic neuron (blue). d. In an axo-axonic synapse, the presynaptic neuron (green) synapses on an axon or axon terminal of the postsynaptic cell (blue). e. Axo-axonic appositions describe closely apposed axon terminals with parallel membranes separated by a clear cleft and lack densities associated with synaptic elements. Note that the directionality of such synapses is often unclear, as is the case for the present example. f. The axo-axonic triad describes a configuration in which a neuron (green) synapses on both the presynaptic element (blue) and postsynaptic target (yellow) of an axo-dendritic or – somatic synapse. Electron micrographs are adapted from Wang & Pickel, 2002 (a, d), Lue, et al., 1997 (b), Takács et al., 2015 (c); Sesack & Pickel, 1990 (e), and Soiza-Reilly et al., 2013 (f).
Axo-axonic synapses have been observed from the earliest use of EM, including in the retina (Kidd, 1962), olfactory bulb (Hirata, 1964), and thalamic lateral geniculate nucleus (Colonnier & Guillery, 1964). Several approaches may be used to identify the participants in axo-axonic circuits. Neuronal degenerative agents can be injected prior to tissue collection to label a projection of interest. Antibodies for neurotransmitters or receptors facilitate the identification of projections or presynaptic localization of neurotransmitter receptors. The early development of an antibody for the beta-adrenergic receptor (Strader et al., 1983), for example, enabled the detection of these receptors localized to axons within the cortex (Aoki et al., 1987).
One limitation of EM, however, is that a given sample of tissue is limited to two or three labeling products. Consequently, it is common to observe axo-axonic synapses that lack identification for either afferent, efferent, or both partners. The ability to detect synapses is influenced by sectioning protocols. This is especially true for axo-axonic synapses. One study employing serial section reconstruction of the striatum noted that the distance of the synapse of the efferent axon may be greater than ten times the size of the afferent synapse (Kornhuber & Kornhuber, 1983)! Varicosity properties such as membrane contortions may also impede detection. Therefore, the incidence of axo-axonic synapses is certainly under-reported. The following examples of axo-axonic synapses by presynaptic neurotransmitter type are compiled from rodent tissue, unless otherwise stated. We also describe axo-axonic appositions in which functional evidence supports a role for presynaptic modulation. When referring to axo-axonic synapses and appositions collectively, we use the term axo-axonic contacts.
Glutamate
Glutamatergic projections form axo-axonic synapses or appositions in a range of brain structures. Axons arising from the prefrontal cortex (PFC) synapse on unidentified terminals in the habenula (Greatrex & Phillipson, 1982), whereas projections originating from the anterior cingulate cortex form axo-axonic synapses within the dorsal medial striatum (Wang & Pickel, 2002). Further characterization of these circuits is limited as the postsynaptic targets are presently unknown. However, a significant fraction of cortical boutons in the dorsal striatum contact putative dopaminergic terminals (Bouyer et al., 1984). Although the directionality of information flow at this synapse is unclear, physiological studies suggest that glutamate may suppress striatal dopamine release through mGluR1 receptors located on dopaminergic terminals (Paquet & Smith, 2003; Zhang & Sulzer, 2003).
An alternative approach to detecting axo-axonic synapses is through identification of heteroreceptors, or non-cognate receptors, located on axon terminals. The observation of NMDA receptors on non-glutamate releasing terminals may indicate the presence of a glutamatergic axo-axonic synapse. Indeed, presynaptically expressed NMDAR receptors are found throughout the brain to modulate a range of neurotransmitter systems (Bouvier et al., 2015; Gracy & Pickel, 1996). The bed nucleus of the stria terminalis (BNST) hosts such a configuration as NMDA receptors primarily localize to axons. Moreover, axons expressing NMDA receptors form axo-axonic appositions twice as frequently as traditional axo-dendritic or axo-somatic configurations (Gracy & Pickel, 1995). Primary sources of glutamatergic projections to the BNST arise from limbic regions such as the ventral subiculum and amygdala (Ch’ng et al., 2018). The axonal NMDA receptor expression is densest in the ventrolateral BNST, a region heavily innervated by noradrenergic projections (Freedman & Cassell, 1994), that may serve as the postsynaptic axon target. Here, adrenergic signaling induces anxiogenesis through hypothalamic-pituitary-adrenal stress axis activation and contributes to the aversive effects of drug withdrawal (Ch’ng et al., 2018). Thus, this non-canonical circuit may contribute to BNST function as an interface between stress and reward systems.
In the cortex, NMDA receptors are found on both excitatory and inhibitory axon terminals (Aoki et al., 1994; DeBiasi et al., 1996). Activation of these receptors on glutamatergic axons enhances excitatory transmission (Brasier & Feldman, 2008) and is necessary for time-dependent long-term depression (Sjöström et al., 2003). NMDA receptors located on cortical GABAergic interneurons provide a mechanism for excitatory circuits to augment local inhibitory signaling (De-May & Ali, 2013; Mathew & Hablitz, 2011). Axo-axonic glutamatergic signaling is not limited to NMDA receptors, however. Ionotropic glutamate receptors presumed to reside on GABAergic axon terminals allow cortical pyramidal neurons to inhibit neighboring pyramidal neurons through a pyramidal axon → interneuron terminal → pyramidal neuron di-synaptic circuit (Ren et al., 2007). Further investigation of glutamatergic axo-axonic synapses stands to reveal contributions of these circuits to cortical function and behavior.
GABA & Glycine
GABAergic interneurons in the dorsal horn primarily synapse on glutamatergic afferent fibers (Hughes et al., 2012). This provides modality-specific filtering of sensory information to suppress spurious activation of nociceptive circuits (Petitjean et al., 2015). A similar configuration is present in the trigeminal nuclei. Glutamatergic sensory afferents arising from the cat vibrissa (Moon et al., 2008) and tooth (Bae et al., 2005) receive GABAergic and glycinergic co-releasing terminals that gate orofacial sensory information. Glycinergic axon terminals synapsing on other axon terminals are also common in the dorsal horn (Lue et al., 1997), as well as in the trigeminal and cochlear nuclei (Clements et al., 1990).
In the dorsal raphe nucleus, a substantial proportion of GABAergic projections form synaptic triads with glutamatergic inputs. That is, for a given glutamatergic axo-dendritic synapse, inhibitory projections synapse on or appose both the glutamatergic afferent (axo-axonic) and the postsynaptic raphe dendrite (axo-dendritic) (see Figure 1f for illustration). This triadic configuration enables the presynaptic axon both pre- and post-synaptic modulatory control. In addition to directly inhibiting dorsal raphe nucleus neurons, GABA presynaptically regulates excitatory drive onto serotonergic neurons through either GABA-A receptor -mediated enhancement or GABA-B receptor -mediated inhibition of glutamatergic signaling (Soiza-Reilly et al., 2013).
Inhibitory axo-axonic regulation of glutamate signaling also occurs in the amygdala. Glutamatergic projections arising from the insular cortex primarily target GABAergic neurons of the lateral central amygdala. Local amygdalar GABAergic terminals frequently appose this excitatory projection (N. Sun & Cassell, 1993). Correspondingly, activation of presynaptic GABA-B receptors (McDonald et al., 2004) in the basolateral and lateral amygdala suppresses excitatory input (Nose et al., 1991; Yamada et al., 1999) and modulates potentiation of cortical synapses onto amygdalar pyramidal neurons (Pan et al., 2009). Inhibiting or genetically eliminating presynaptic GABA-B receptors prevents constraint of plasticity and results in generalization of conditioned fear (Pan et al., 2009; Shaban et al., 2006). Inhibitory signaling may regulate other circuits in the brain through axo-axonic synapses. Physiological studies indicate presynaptic GABA-B receptor -mediated effects in the thalamic medial geniculate nucleus (B. Luo et al., 2011) and nucleus accumbens (Manz et al., 2019).
Catecholamines
Projections containing dopamine or norepinephrine are traditionally identified in ultrastructural studies using tyrosine-hydroxylase immunostaining. Although this antibody labels all catecholamines, anatomical knowledge may be used to infer the present neurotransmitter. For brain regions innervated by both dopamine and norepinephrine projections, radiolabeled dopamine or dopamine beta-hydroxylase immunostaining can distinguish the two catecholamines. The following descriptions of catecholaminergic axo-axonic synapses follow this inferential approach.
Dopamine
In contrast to the previously described neurotransmitter systems, dopaminergic projections exhibit unique morphology that challenges traditional synaptic identification. Whereas dopamine synapses are traditionally presumed to occur en passant at varicosities along primarily unmyelinated axons, some studies fail to identify synapses at a given varicosity, whereas others find that these sites only account for a small proportion of dopaminergic synapses, especially in the striatum (Ducrot et al., 2020; Sesack, 2002). Ultrastructural examination throughout the brain reveals that a significant proportion of these projections fail to form synapses, suggesting that this neurotransmitter preferentially acts through volume transmission. However, dopamine terminals frequently appose other axons, often in a triad-like configuration with a shared dendritic target. Such configurations are common in the hippocampus (Sesack & Pickel, 1990) and striatum (Arluison et al., 1984; Pickel et al., 1981), where apposing terminals arise from the cortex (Bouyer et al., 1984). Triadic configurations are also present in the medial frontal cortex with dopamine terminals apposed to GABAergic axons and their targets (Verney et al., 1990). Dopaminergic axo-axonic appositions are also located in the amygdala (Asan, 1997), globus pallidus (squirrel monkey, Eid & Parent, 2015), dorsolateral BNST (Phelix et al., 1992), mediodorsal thalamus (macaque, Melchitzky et al., 2006), and cortex (Verney et al., 1990; macaque, Martin & Spühler, 2013). This pervasive parasynaptic configuration (Sesack, 2002) and the presence of presynaptic heteroreceptors (Feuerstein, 2008) suggests that dopamine may provide targeted presynaptic modulation despite lacking definitive ultrastructural evidence for synaptic contact.
Norepinephrine
Noradrenergic terminals forming definitive synapses of any configuration is a rarity in the cortex. Rather, a single norepinephrine neuron gives rise to over 300,000, largely asynaptic, axon terminals widely distributed across the cortex (Audet et al., 1988). These terminals frequently appose non-noradrenergic axonal processes (Séguéla et al., 1990). Whereas presynaptic-mediated noradrenergic modulation and behavioral effects are documented across a range of brain regions and synaptic mechanisms (Gilsbach & Hein, 2008), the characterization of presynaptic noradrenergic α1 receptor signaling in the PFC presents a compelling case for axo-axonic modulation. The α1 receptor is highly expressed in axon terminals that co-express vGluT1, suggesting localization to glutamatergic pyramidal axon terminals (Mitrano et al., 2012). α1 receptor activation induces a long-lasting suppression of pyramidal glutamate release probability onto GABAergic fast-spiking interneurons but not onto other pyramidal neurons (Wang et al., 2013). This circuit-specific presynaptic effect likely enhances PFC excitability (Luo et al., 2015). The behavioral consequence of such signaling is unclear; some studies report that PFC α1 receptor activation enhances spatial working memory (Hvoslef-Eide et al., 2015), whereas others demonstrate cognitive impairment (Arnsten et al., 1999). While these discrepancies may reside in the nuances of the cognitive tasks employed, they ultimately point towards a functional role for this synapse in cognition. Cortical excitability is also regulated through presynaptic α2 (Ohshima et al., 2017) and β1 (Luo et al., 2014) receptor signaling, offering additional venues for noradrenergic axo-axonic modulation.
The nucleus tractus solitarius serves as a junction for dense adrenergic and cardiovascular inputs that regulate the baroreceptor reflex. Axo-axonic synapses are readily observed in this region, with both noradrenergic (cat, Chiba & Doba, 1976) and non-catecholaminergic inputs (Kachidian & Pickel, 1993; Sumal et al., 1983) inputs synapsing on vagal terminals. Thus, this non-canonical synaptic configuration may serve to modulate blood pressure.
Serotonin
Like catecholaminergic fibers, serotonin projections exhibit a paucity of direct synaptic contacts. However, serotonergic terminals frequently appose unlabeled axons and terminals. This axo-axonic configuration accounts for approximately 30% of appositions in both the striatum (Soghomonian et al., 1989) and hippocampus (Oleskevich et al., 1991), as well as 19% in the cortex (Séguéla et al., 1989). Correspondingly, serotonin receptors are localized to presynaptic terminals throughout the brain (Feuerstein, 2008; Rodríguez et al., 1999; macaque, Jakab & Goldman-Rakic, 1998). 5-HT2A receptor activation, for example, induces glutamate release from projections impinging on pyramidal neurons in the PFC (Marek et al., 2001) and somatosensory cortex (Scruggs et al., 2000).
Beyond 5-HT2A, presynaptically -expressed 5-HT1B receptors are located on glutamatergic retinal terminals in the suprachiasmatic nucleus (SCN) and their activation suppresses optic transmission to reduce the magnitude of light-induced phase shifts (Pickard et al., 1999). Notably, these receptors are found in greater abundance on SCN GABAergic axons (presumed local interneurons) (Bosler, 1989). Here, 5-HT1B receptor activation provides a disinhibitory mechanism for SCN signaling by suppressing GABAergic transmission (Bramley et al., 2005), and in agreement, 5-HT1B receptor knock-out mice exhibit an attenuated light response (Sollars et al., 2006). Together, these two axo-axonic mechanisms provide means for serotonin to bidirectionally modulate photic regulation of circadian rhythms.
5-HT1B receptor -mediated presynaptic inhibition is also present at corticostriatal synapses, serving to persistently reduce striatal output activity (Mathur et al., 2011). Additionally, serotonergic presynaptic inhibition occurs at hippocampal CA1 local excitatory synapses (Winterer et al., 2011). This signaling bidirectionally modulates emotional memory (Eriksson et al., 2013).
Acetylcholine
Like the previously discussed modulatory neurotransmitters, the rates of cholinergic synaptic contacts are low, ranging from 14% in the parietal cortex (Umbriaco et al., 1994) to less than 10% in the hippocampus (Umbriaco et al., 1995) and striatum (Contant et al., 1996). However, there is a wealth of evidence for axo-axonically -mediated presynaptic actions of acetylcholine (Gilsbach & Hein, 2008). In the anterolateral BNST where glutamatergic and cholinergic axons form appositions, acetylcholine suppresses excitatory transmission through M2 receptor signaling on postsynaptic glutamatergic axon terminals (Guo et al., 2012). This mechanism is proposed to filter sensory input.
Conversely, activation of β2-containing nicotinic receptors located on thalamic terminals in the medial PFC induces glutamate release (Lambe et al., 2003). Arising from the midline and intralaminar thalamus, these cortical-terminating projections are suggested to participate in arousal, attention, and affective processing (Groenewegen & Berendse, 1994). Nicotine enhances such functions, suggesting that axo-axonic circuits contribute to these effects.
Neuropeptides
EM data demonstrate that neuropeptide-containing terminals serve as both afferents and efferents in axo-axonic configurations. Current knowledge of neuropeptide circuit function and directionality remains more limited compared to that of conventional neurotransmitters. Regardless, axo-axonic synapses exist between ghrelin and neuropeptide Y (NPY) -containing terminals in the arcuate nucleus (Guan et al., 2003). The SCN hosts similar configurations between somatostatin-positive and unidentified fibers (Buijs et al., 1995), and between serotonergic and NPY projections (Guy et al., 1987). NPY-releasing afferents also form axo-axonic synapses in the cat dorsal horn (Doyle & Maxwell, 1993).
Axo-axonic functional circuit motifs
Mounting evidence indicate axo-axonic contacts provide unique functional attributes to neural circuits. This configuration provides a “short circuit” by which an axon terminal synapsing on another axon terminal bypasses the somatodendritic processing (temporal and spatial summation) that occurs in traditional axo-somatic and -dendritic synapses (Figure 2a). For example, the SCN is dominated by intrinsic inhibitory signaling. GABAergic interneurons axo-axonically synapse onto other GABAergic terminals (Buijs et al., 1995; Castel & Morris, 2000), providing a direct mechanism to regulate inhibitory activity.
Figure 2. Axo-axonic synaptic contributions to neural circuit function.
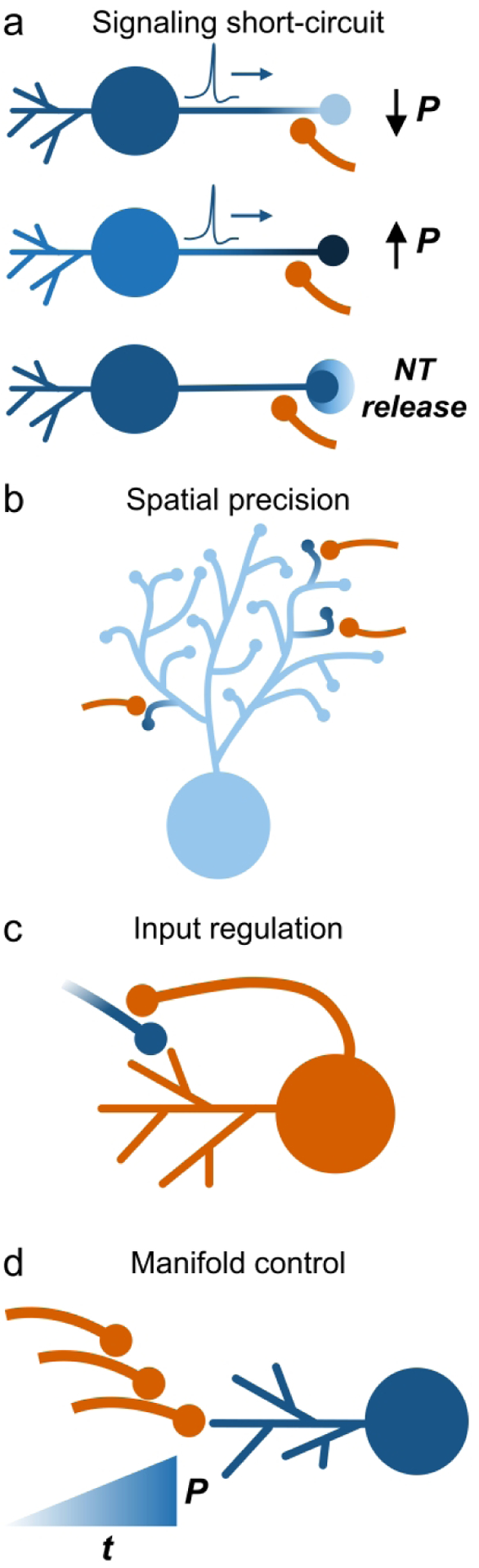
a. Axo-axonic synapses allow a presynaptic axon (orange) to negatively (top schematic) or positively (middle schematic) modulate neurotransmitter release probability (P) of the postsynaptic axon (blue). In addition, it is possible for an afferent to induce neurotransmitter (NT) release independently of the action potentials generated by the postsynaptic neuron (bottom schematic). b. In postsynaptic neurons with extensive axonal arborizations (light blue neuron), afferents synapsing axo-axonically (orange) provide spatially-precise neurotransmitter release or suppression. c. Axon collaterals from a neuron (orange) that synapse on impinging afferents (blue) provide a mechanism for that cell to regulate afferent input. d. Combinations of axo-axonic synapses either in convergence or serial formation (as shown) provide granular control of neurotransmitter release probability over time (t) for canonical axo-dendritic synapses.
Axo-axonic contacts may also facilitate spatially precise control of neurotransmitter release. Whereas somatic depolarization will generally influence neurotransmitter release from all downstream axon terminals from a particular neuron, axon terminals impinging directly on other axon terminals provide fine-grained modulation of specific efferent synapses (Figure 2b). This function is especially impactful for neurons with extensive axonal arbors. Substantia nigra dopamine neurons, for example, form unmyelinated axonal arbors that sum up to 1 cubic millimeter in the dorsal striatum (Matsuda et al., 2009). Striatal dopamine signaling critically regulates action initiation (Sheridan et al., 1987), yet somatically generated action potentials may not faithfully propagate along axons to confer terminal selectivity. However, activation of striatal cholinergic interneurons, or their upstream afferents, robustly elicits local dopamine release in a behaviorally significant manner (Cachope et al., 2012; Cover et al., 2019; Threlfell et al., 2012). Although existent striatal EM data only notes a high prevalence of ChAT-labeled axons juxtaposed to (but not synapsing on) unidentified striatal axon terminals (Contant et al., 1996), the presence of nicotinic acetylcholine receptors on nigrostriatal axon terminals supports a functional axo-axonic circuit (Jones et al., 2001).
Axo-axonic contacts may also provide input regulation. Whereas postsynaptic neurons are canonically limited to either retrograde signaling or indirect multi-synaptic feedback loops to modulate their presynaptic partners, axo-axonic synapses formed by a postsynaptic axon collateral synapsing on an axon terminal that synapses on that neuron’s own dendrite, allow for direct modulation of afferent inputs (Figure 2c). Such is the case for cholinergic neurons residing in the pedunculopontine nucleus and laterodorsal tegmentum. Cholinergic axon terminals appose projections to this area that express M2 heteroreceptors (Garzón & Pickel, 2006). Accordingly, M2 receptor activation decreases glutamatergic transmission onto cholinergic neurons (Ye et al., 2010).
Lastly, axo-axonic contacts confer an additional dimension of modulatory control over neural signaling. In canonical circuits, impinging afferents influence timing of postsynaptic action potential firing. The addition of axo-axonic configurations affords manifold control of this activity, providing granularity to neural systems. This function is magnified in more complex axo-axonic circuit configurations, such as two axons synapsing on a common postsynaptic axon (Bae et al., 2005). Serial formations of axo-axonic contacts also occur, which further amplify the modulatory resolution controlling downstream neurotransmitter release (Figure 2d). This configuration occurs in the cat trigeminal nucleus, where axo-axo-axonic di-synaptic circuits terminate on axon initial segments (Westrum, 1993).
Functional assessment of axo-axonic circuits
Despite a wealth of EM structural data, the functional differences between axo-axonic synapses and non-specific axonal appositions are unclear. Generally, it is assumed that appositions mediate “volume transmission” wherein diffusion of a neurotransmitter exerts slower, modulatory effects across many synapses, akin to that of hormones (Agnati et al., 1992). This may suggest that appositions mediate slow modulatory effects while axo-axonic synapses provide immediate control of neurotransmitter release and, therefore, behavior. However, in the dorsal striatum where cholinergic interneurons release acetylcholine to elicit local dopamine release from nigrostriatal terminals to support behavioral reinforcement (Cover et al., 2019), EM data only support an appositional relationship (Contant et al., 1996). This suggests that axo-axonal contacts may exert rapid and functionally significant events regardless of the classification as an axo-axonic synapse or apposition. Moreover, this example illustrates the need for improved methods to: 1) easily identify axo-axonic contacts and 2) assess the functional relevance of such non-canonical circuits.
Improving on EM, array tomography employs both immunofluorescence light microscopy and EM of ultrathin serial sections to allow for expanded labeling opportunities and improved z-axis resolution (Micheva & Smith, 2007). This technique facilitated the finding of GABA-glutamate terminal appositions in the dorsal raphe nucleus (Soiza-Reilly et al., 2013). Others have coupled immunofluorescence with confocal microscopy-enabled semi-automated 3D reconstruction to quantify serotonergic appositions to excitatory and inhibitory synapses throughout the limbic system (Belmer et al., 2017). Expansion microscopy, a process by which immunolabeled tissue is physically expanded, enables nanoscale resolution with light microscopy (Karagiannis & Boyden, 2018). With an expanded number of means to label presynaptic and postsynaptic-specific proteins, these approaches can be applied to identify axo-axonic circuits and provide a starting point for subsequent functional assessment.
The advent of viral and transgenic tools now allows more precise interrogation of the functional roles for axo-axonic contacts. One example arises from a mechanism by which oxytocin release into the nucleus accumbens induces a presynaptic 5-HT1B -mediated long-term depression of excitatory transmission onto nucleus accumbens medium spiny neurons (Mathur et al., 2011) that is required for social reward (Dölen et al., 2013). Although these findings were not confirmed with EM, the experimental results support an axo-axo-axo-dendritic configuration within the nucleus accumbens by which oxytocin released from hypothalamic projections into the nucleus accumbens activates oxytocin receptors present on serotonergic dorsal raphe terminals. The subsequent serotonin release activates 5-HT1B receptors present on glutamatergic terminals synapsing on medium spiny neuron dendrites. Thus, oxytocin-induced serotonin release activates 5HT1B receptors to suppress glutamate release onto medium spiny neurons. This study illustrates how viral tools may be combined with mouse transgenic models to elucidate and functionally evaluate such non-canonical circuits (Dölen et al., 2013).
Virally-expressed biosensors may also be leveraged to probe axo-axonic circuits. These membrane-bound sensors consist of a neurotransmitter binding site that, when occupied, changes the structure’s conformation to elicit photon release from a fluorophore (Leopold et al., 2019). Sensors for glutamate (GluSnFR) (Marvin et al., 2013), GABA (iGABASnFR) (Marvin et al., 2019), dopamine (dLight, GRABDA) (Patriarchi et al., 2018; F. Sun et al., 2018), acetylcholine (GRABACh) (Jing et al., 2020), or norepinephrine (GRABNE) (Feng et al., 2019) may be incorporated in ex vivo slice physiology studies or monitored in vivo though implanted photometric fibers in freely moving animals. This tool may also be coupled with rodent transgenic models to examine the afferent axonal neurotransmitter release onto sensor-expressing postsynaptic terminals. Using the presumed striatal cholinergic interneuron synapse onto dopamine terminals as an example, one may express a cre-dependent acetylcholine sensor in the substantia nigra of DAT-cre transgenic mice. Recording from the striatum, one can specifically monitor cholinergic signaling onto dopamine terminals and study how this axo-axonic signaling varies during behavior or under pharmacological manipulation. This approach may also be useful for identifying novel circuits or validating preliminary findings from microscopy studies. By expressing a cre-dependent neurotransmitter sensor in the postsynaptic axon and an optogenetic activator (or silencer) in the presynaptic axon terminal, one may investigate the connectivity of a proposed synapse as a first step toward functional validation.
Calcium sensors, such as GCaMP (Nakai et al., 2001), are commonly used to study neuronal activity and may be adapted to study non-canonical circuits in several ways. In addition to cre-dependent variants that allow for pathway-specific examination, the recent development of axon-targeting viral constructs (Broussard et al., 2018) enables activity monitoring of axon terminals arising from intrinsic neuronal populations such as GABAergic or cholinergic interneurons. Two-photon imaging also provides a means to study spatially discrete terminal activity with temporal resolution only limited by sensor kinetics. This approach was effectively employed to examine dopamine axonal activity in the context of movement (Howe & Dombeck, 2016). For appositions, coupling GCaMP with a red-shifted sensor like jrGECO (Dana et al., 2016), allows for simultaneous recording of two axon terminal populations. One may also pair a red-shifted calcium indicator with a neurotransmitter sensor to correlate neurotransmitter release from the presynaptic axon terminal with the activity of the postsynaptic axon terminal.
Whereas axo-axonic contacts have been consistently identified over the past sixty years of EM research, technological barriers hindered functional characterization of the circuits that arise from these interactions. The development of new tools to discern terminal-specific activity is finally opening doors to functionally understand the role of these non-traditional circuits in neuronal information processing and behavior expression. As these tools develop, it is apparent that a growing consideration for non-traditional synaptic configurations is warranted.
Acknowledgements
This work is supported by the National Institute on Alcohol Abuse and Alcoholism grant R01AA024845 (to B.N.M.) and National Institute of Drug Abuse grant F31DA047014 (to K.K.C.).
Footnotes
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- Agnati LF, Bjelke B, & Fuxe K (1992). Volume Transmission the Brain. American Scientist, 80(4), 362–373. [Google Scholar]
- Aoki C, Joh TH, & Pickel VM (1987). Ultrastructural localization of beta-adrenergic receptor-like immunoreactivity in the cortex and neostriatum of rat brain. Brain Research, 437(2), 264–282. 10.1016/0006-8993(87)91642-8 [DOI] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Go C, Mong J, & Dawson T (1994). Cellular and subcellular localization of NMDA-R1 subunit immunoreactivity in the visual cortex of adult and neonatal rats. The Journal of Neuroscience, 14(9), 5202–5222. 10.1523/JNEUROSCI.14-09-05202.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arluison M, Dietl M, & Thibault J (1984). Ultrastructural morphology of dopaminergic nerve terminals and synapses in the striatum of the rat using tyrosine hydroxylase immunocytochemistry: A topographical study. Brain Research Bulletin, 13(2), 269–285. 10.1016/0361-9230(84)90128-x [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Mathew R, Ubriani R, Taylor JR, & Li BM (1999). Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biological Psychiatry, 45(1), 26–31. 10.1016/s0006-3223(98)00296-0 [DOI] [PubMed] [Google Scholar]
- Asan E (1997). Ultrastructural features of tyrosine-hydroxylase-immunoreactive afferents and their targets in the rat amygdala. Cell and Tissue Research, 288(3), 449–469. 10.1007/s004410050832 [DOI] [PubMed] [Google Scholar]
- Atwood BK, Lovinger DM, & Mathur BN (2014). Presynaptic long-term depression mediated by Gi/o-coupled receptors. Trends in Neurosciences, 37(11), 663–673. 10.1016/j.tins.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet MA, Doucet G, Oleskevich S, & Descarries L (1988). Quantified regional and laminar distribution of the noradrenaline innervation in the anterior half of the adult rat cerebral cortex. The Journal of Comparative Neurology, 274(3), 307–318. 10.1002/cne.902740302 [DOI] [PubMed] [Google Scholar]
- Bae YC, Park KS, Bae JY, Paik SK, Ahn DK, Moritani M, Yoshida A, & Shigenaga Y (2005). GABA and glycine in synaptic microcircuits associated with physiologically characterized primary afferents of cat trigeminal principal nucleus. Experimental Brain Research, 162(4), 449–457. 10.1007/s00221-004-2022-y [DOI] [PubMed] [Google Scholar]
- Belmer A, Klenowski PM, Patkar OL, & Bartlett SE (2017). Mapping the connectivity of serotonin transporter immunoreactive axons to excitatory and inhibitory neurochemical synapses in the mouse limbic brain. Brain Structure & Function, 222(3), 1297–1314. 10.1007/s00429-016-1278-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD (2018). What does dopamine mean? Nature Neuroscience, 21(6), 787–793. 10.1038/s41593-018-0152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosler O (1989). Ultrastructural relationships of serotonin and GABA terminals in the rat suprachiasmatic nucleus. Evidence for a close interconnection between the two afferent systems. Journal of Neurocytology, 18(1), 105–113. 10.1007/BF01188429 [DOI] [PubMed] [Google Scholar]
- Bouvier G, Bidoret C, Casado M, & Paoletti P (2015). Presynaptic NMDA receptors: Roles and rules. Neuroscience, 311, 322–340. 10.1016/j.neuroscience.2015.10.033 [DOI] [PubMed] [Google Scholar]
- Bouyer JJ, Park DH, Joh TH, & Pickel VM (1984). Chemical and structural analysis of the relation between cortical inputs and tyrosine hydroxylase-containing terminals in rat neostriatum. Brain Research, 302(2), 267–275. 10.1016/0006-8993(84)90239-7 [DOI] [PubMed] [Google Scholar]
- Bramley JR, Sollars PJ, Pickard GE, & Dudek FE (2005). 5-HT1B receptor-mediated presynaptic inhibition of GABA release in the suprachiasmatic nucleus. Journal of Neurophysiology, 93(6), 3157–3164. 10.1152/jn.00770.2004 [DOI] [PubMed] [Google Scholar]
- Brasier DJ, & Feldman DE (2008). Synapse-Specific Expression of Functional Presynaptic NMDA Receptors in Rat Somatosensory Cortex. Journal of Neuroscience, 28(9), 2199–2211. 10.1523/JNEUROSCI.3915-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard GJ, Liang Y, Fridman M, Unger EK, Meng G, Xiao X, Ji N, Petreanu L, & Tian L (2018). In vivo measurement of afferent activity with axon-specific calcium imaging. Nature Neuroscience, 21(9), 1272–1280. 10.1038/s41593-018-0211-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, & Hou Y-X (1995). Colocalization of ?-aminobutyric acid with vasopressin, vasoactive intestinal peptide, and somatostatin in the rat suprachiasmatic nucleus. The Journal of Comparative Neurology, 358(3), 343–352. 10.1002/cne.903580304 [DOI] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang H-L, Morales M, Lovinger DM, & Cheer JF (2012). Selective Activation of Cholinergic Interneurons Enhances Accumbal Phasic Dopamine Release: Setting the Tone for Reward Processing. Cell Reports, 2(1), 33–41. 10.1016/j.celrep.2012.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel M, & Morris JF (2000). Morphological heterogeneity of the GABAergic network in the suprachiasmatic nucleus, the brain’s circadian pacemaker. Journal of Anatomy, 196 (Pt 1), 1–13. 10.1046/j.1469-7580.2000.19610001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, & Doba N (1976). Catecholaminergic axo-axonic synapses in the nucleus of the tractus solitarius (pars commissuralis) of the cat: Possible relation to presynaptic regulation of baroreceptor reflexes. Brain Research, 102(2), 255–265. 10.1016/0006-8993(76)90881-7 [DOI] [PubMed] [Google Scholar]
- Ch’ng S, Fu J, Brown RM, McDougall SJ, & Lawrence AJ (2018). The intersection of stress and reward: BNST modulation of aversive and appetitive states. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 87(Pt A), 108–125. 10.1016/j.pnpbp.2018.01.005 [DOI] [PubMed] [Google Scholar]
- Clements JR, Magnusson KR, & Beitz AJ (1990). Ultrastructural description of glutamate-, aspartate-, taurine-, and glycine-like immunoreactive terminals from five rat brain regions. Journal of Electron Microscopy Technique, 15(1), 49–66. 10.1002/jemt.1060150106 [DOI] [PubMed] [Google Scholar]
- Colonnier M, & Guillery RW (1964). SYNAPTIC ORGANIZATION IN THE LATERAL GENICULATE NUCLEUS OF THE MONKEY. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie (Vienna, Austria: 1948), 62, 333–355. 10.1007/BF00339284 [DOI] [PubMed] [Google Scholar]
- Contant C, Umbriaco D, Garcia S, Watkins KC, & Descarries L (1996). Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience, 71(4), 937–947. 10.1016/0306-4522(95)00507-2 [DOI] [PubMed] [Google Scholar]
- Cover KK, Gyawali U, Kerkhoff WG, Patton MH, Mu C, White MG, Marquardt AE, Roberts BM, Cheer JF, & Mathur BN (2019). Activation of the Rostral Intralaminar Thalamus Drives Reinforcement through Striatal Dopamine Release. Cell Reports, 26(6), 1389–1398.e3. 10.1016/j.celrep.2019.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman JP, Tsegaye G, Holt GT, Hu A, Walpita D, Patel R, Macklin JJ, Bargmann CI, Ahrens MB, Schreiter ER, Jayaraman V, Looger LL, Svoboda K, & Kim DS (2016). Sensitive red protein calcium indicators for imaging neural activity. ELife, 5. 10.7554/eLife.12727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBiasi S, Minelli A, Melone M, & Conti F (1996). Presynaptic NMDA receptors in the neocortex are both auto- and heteroreceptors: NeuroReport, 7(15), 2773–2776. 10.1097/00001756-199611040-00073 [DOI] [PubMed] [Google Scholar]
- De-May CL, & Ali AB (2013). Involvement of pre- and postsynaptic NMDA receptors at local circuit interneuron connections in rat neocortex. Neuroscience, 228, 179–189. 10.1016/j.neuroscience.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, & Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501(7466), 179–184. 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle CA, & Maxwell DJ (1993). Neuropeptide Y-immunoreactive terminals form axo-axonic synaptic arrangements in the substantia gelatinosa (lamina II) of the cat spinal dorsal horn. Brain Research, 603(1), 157–161. 10.1016/0006-8993(93)91315-j [DOI] [PubMed] [Google Scholar]
- Ducrot C, Bourque M-J, Delmas CVL, Racine A-S, Bello DG, Delignat-Lavaud B, Lycas MD, Fallon A, Michaud-Tardif C, Nanni SB, Herborg F, Gether U, Nanci A, Takahashi H, Parent M, & Trudeau L-E (2020). Dopaminergic neurons establish a distinctive axonal arbor with a majority of non-synaptic terminals [Preprint]. Neuroscience 10.1101/2020.05.11.088351 [DOI] [PubMed] [Google Scholar]
- Dudel J, & Kuffler SW (1961). Presynaptic inhibition at the crayfish neuromuscular junction. The Journal of Physiology, 155(3), 543–562. 10.1113/jphysiol.1961.sp006646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid L, & Parent M (2015). Morphological evidence for dopamine interactions with pallidal neurons in primates. Frontiers in Neuroanatomy, 9, 111. 10.3389/fnana.2015.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson TM, Alvarsson A, Stan TL, Zhang X, Hascup KN, Hascup ER, Kehr J, Gerhardt GA, Warner-Schmidt J, Arango-Lievano M, Kaplitt MG, Ogren SO, Greengard P, & Svenningsson P (2013). Bidirectional regulation of emotional memory by 5-HT1B receptors involves hippocampal p11. Molecular Psychiatry, 18(10), 1096–1105. 10.1038/mp.2012.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Zhang C, Lischinsky JE, Jing M, Zhou J, Wang H, Zhang Y, Dong A, Wu Z, Wu H, Chen W, Zhang P, Zou J, Hires SA, Zhu JJ, Cui G, Lin D, Du J, & Li Y (2019). A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine. Neuron, 102(4), 745–761.e8. 10.1016/j.neuron.2019.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein TJ (2008). Presynaptic receptors for dopamine, histamine, and serotonin. Handbook of Experimental Pharmacology, 184, 289–338. 10.1007/978-3-540-74805-2_10 [DOI] [PubMed] [Google Scholar]
- Freedmanand LJ, & Cassell MD (1994). Relationship of thalamic basal forebrain projection neurons to the peptidergic innervation of the midline thalamus. The Journal of Comparative Neurology, 348(3), 321–342. 10.1002/cne.903480302 [DOI] [PubMed] [Google Scholar]
- Garzón M, & Pickel VM (2006). Subcellular distribution of M2 muscarinic receptors in relation to dopaminergic neurons of the rat ventral tegmental area. The Journal of Comparative Neurology, 498(6), 821–839. 10.1002/cne.21082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsbach R, & Hein L (2008). Presynaptic metabotropic receptors for acetylcholine and adrenaline/noradrenaline. Handbook of Experimental Pharmacology, 184, 261–288. 10.1007/978-3-540-74805-2_9 [DOI] [PubMed] [Google Scholar]
- Gracy KN, & Pickel VM (1995). Comparative ultrastructural localization of the NMDAR1 glutamate receptor in the rat basolateral amygdala and bed nucleus of the stria terminalis. The Journal of Comparative Neurology, 362(1), 71–85. 10.1002/cne.903620105 [DOI] [PubMed] [Google Scholar]
- Gracy KN, & Pickel VM (1996). Ultrastructural immunocytochemical localization of the N - methyl- d -aspartate receptor and tyrosine hydroxylase in the shell of the rat nucleus accumbens. Brain Research, 739(1–2), 169–181. 10.1016/S0006-8993(96)00822-0 [DOI] [PubMed] [Google Scholar]
- Greatrex RM, & Phillipson OT (1982). Demonstration of synaptic input from prefrontal cortex to the habenula i the rat. Brain Research, 238(1), 192–197. 10.1016/0006-8993(82)90782-x [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, & Berendse HW (1994). The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends in Neurosciences, 17(2), 52–57. 10.1016/0166-2236(94)90074-4 [DOI] [PubMed] [Google Scholar]
- Guan J-L, Wang Q-P, Kageyama H, Takenoya F, Kita T, Matsuoka T, Funahashi H, & Shioda S (2003). Synaptic interactions between ghrelin- and neuropeptide Y-containing neurons in the rat arcuate nucleus. Peptides, 24(12), 1921–1928. 10.1016/j.peptides.2003.10.017 [DOI] [PubMed] [Google Scholar]
- Guo J-D, Hazra R, Dabrowska J, Muly EC, Wess J, & Rainnie DG (2012). Presynaptic muscarinic M(2) receptors modulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropharmacology, 62(4), 1671–1683. 10.1016/j.neuropharm.2011.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Bosler O, Dusticier G, Pelletier G, & Calas A (1987). Morphological correlates of serotonin-neuropeptide Y interactions in the rat suprachiasmatic nucleus: Combined radioautographic and immunocytochemical data. Cell and Tissue Research, 250(3), 657–662. 10.1007/BF00218960 [DOI] [PubMed] [Google Scholar]
- Hirata Y (1964). SOME OBSERVATIONS ON THE FINE STRUCTURE OF THE SYNAPSES IN THE OLFACTORY BULB OF THE MOUSE, WITH PARTICULAR REFERENCE TO THE ATYPICAL SYNAPTIC CONFIGURATIONS. Archivum Histologicum Japonicum = Nihon Soshikigaku Kiroku, 24, 293–302. 10.1679/aohc1950.24.293 [DOI] [PubMed] [Google Scholar]
- Howe MW, & Dombeck DA (2016). Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature, 535(7613), 505–510. 10.1038/nature18942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DI, Sikander S, Kinnon CM, Boyle KA, Watanabe M, Callister RJ, & Graham BA (2012). Morphological, neurochemical and electrophysiological features of parvalbumin-expressing cells: A likely source of axo-axonic inputs in the mouse spinal dorsal horn: Parvalbumin neurons in the mouse dorsal horn. The Journal of Physiology, 590(16), 3927–3951. 10.1113/jphysiol.2012.235655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvoslef-Eide M, Oomen CA, Fisher BM, Heath CJ, Robbins TW, Saksida LM, & Bussey TJ (2015). Facilitation of spatial working memory performance following intraprefrontal cortical administration of the adrenergic alpha1 agonist phenylephrine. Psychopharmacology, 232(21–22), 4005–4016. 10.1007/s00213-015-4038-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, & Goldman-Rakic PS (1998). 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proceedings of the National Academy of Sciences of the United States of America, 95(2), 735–740. 10.1073/pnas.95.2.735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing M, Li Y, Zeng J, Huang P, Skirzewski M, Kljakic O, Peng W, Qian T, Tan K, Zou J, Trinh S, Wu R, Zhang S, Pan S, Hires SA, Xu M, Li H, Saksida LM, Prado VF, … Li Y (2020). An optimized acetylcholine sensor for monitoring in vivo cholinergic activity. Nature Methods, 17(11), 1139–1146. 10.1038/s41592-020-0953-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IW, Bolam JP, & Wonnacott S (2001). Presynaptic localisation of the nicotinic acetylcholine receptor beta2 subunit immunoreactivity in rat nigrostriatal dopaminergic neurones. The Journal of Comparative Neurology, 439(2), 235–247. 10.1002/cne.1345 [DOI] [PubMed] [Google Scholar]
- Kachidian P, & Pickel VM (1993). Localization of tyrosine hydroxylase in neuronal targets and efferents of the area postrema in the nucleus tractus solitarii of the rat. The Journal of Comparative Neurology, 329(3), 337–353. 10.1002/cne.903290305 [DOI] [PubMed] [Google Scholar]
- Karagiannis ED, & Boyden ES (2018). Expansion microscopy: Development and neuroscience applications. Current Opinion in Neurobiology, 50, 56–63. 10.1016/j.conb.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd M (1962). Electron microscopy of the inner plexiform layer of the retina in the cat and the pigeon. Journal of Anatomy, 96, 179–187. [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J, & Kornhuber ME (1983). Axo-axonic synapses in the rat striatum. European Neurology, 22(6), 433–436. 10.1159/000115598 [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, & Aghajanian GK (2003). Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 28(2), 216–225. 10.1038/sj.npp.1300032 [DOI] [PubMed] [Google Scholar]
- Leopold AV, Shcherbakova DM, & Verkhusha VV (2019). Fluorescent Biosensors for Neurotransmission and Neuromodulation: Engineering and Applications. Frontiers in Cellular Neuroscience, 13, 474. 10.3389/fncel.2019.00474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue JH, Shieh WF, Chen SH, Shieh JY, & Wen CY (1997). Morphometric study of glycine-immunoreactive neurons and terminals in the rat cuneate nucleus. Journal of Anatomy, 191(3), 375–385. 10.1046/j.1469-7580.1997.19130375.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B, Wang H-T, Su Y-Y, Wu S-H, & Chen L (2011). Activation of presynaptic GABAB receptors modulates GABAergic and glutamatergic inputs to the medial geniculate body. Hearing Research, 280(1–2), 157–165. 10.1016/j.heares.2011.05.018 [DOI] [PubMed] [Google Scholar]
- Luo F, Tang H, & Cheng Z-Y (2015). Stimulation of α1-adrenoceptors facilitates GABAergic transmission onto pyramidal neurons in the medial prefrontal cortex. Neuroscience, 300, 63–74. 10.1016/j.neuroscience.2015.04.070 [DOI] [PubMed] [Google Scholar]
- Luo Fei, Guo N-N, Li S-H, Tang H, Liu Y, & Zhang Y (2014). Reduction of glutamate release probability and the number of releasable vesicles are required for suppression of glutamatergic transmission by β1-adrenoceptors in the medial prefrontal cortex. Neuropharmacology, 83, 89–98. 10.1016/j.neuropharm.2014.03.020 [DOI] [PubMed] [Google Scholar]
- Manz KM, Baxley AG, Zurawski Z, Hamm HE, & Grueter BA (2019). Heterosynaptic GABAB Receptor Function within Feedforward Microcircuits Gates Glutamatergic Transmission in the Nucleus Accumbens Core. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 39(47), 9277–9293. 10.1523/JNEUROSCI.1395-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Gewirtz JC, & Schoepp DD (2001). A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience, 105(2), 379–392. 10.1016/s0306-4522(01)00199-3 [DOI] [PubMed] [Google Scholar]
- Martin KAC, & Spühler IA (2013). The fine structure of the dopaminergic innervation of area 10 of macaque prefrontal cortex. The European Journal of Neuroscience, 37(7), 1061–1071. 10.1111/ejn.12124 [DOI] [PubMed] [Google Scholar]
- Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, Gordus A, Renninger SL, Chen T-W, Bargmann CI, Orger MB, Schreiter ER, Demb JB, Gan W-B, Hires SA, & Looger LL (2013). An optimized fluorescent probe for visualizing glutamate neurotransmission. Nature Methods, 10(2), 162–170. 10.1038/nmeth.2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin JS, Shimoda Y, Magloire V, Leite M, Kawashima T, Jensen TP, Kolb I, Knott EL, Novak O, Podgorski K, Leidenheimer NJ, Rusakov DA, Ahrens MB, Kullmann DM, & Looger LL (2019). A genetically encoded fluorescent sensor for in vivo imaging of GABA. Nature Methods, 16(8), 763–770. 10.1038/s41592-019-0471-2 [DOI] [PubMed] [Google Scholar]
- Mathew SS, & Hablitz JJ (2011). Presynaptic NMDA Receptors Mediate IPSC Potentiation at GABAergic Synapses in Developing Rat Neocortex. PLoS ONE, 6(2), e17311. 10.1371/journal.pone.0017311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur BN, Capik NA, Alvarez VA, & Lovinger DM (2011). Serotonin induces long-term depression at corticostriatal synapses. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(20), 7402–7411. 10.1523/JNEUROSCI.6250-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, & Kaneko T (2009). Single Nigrostriatal Dopaminergic Neurons Form Widely Spread and Highly Dense Axonal Arborizations in the Neostriatum. Journal of Neuroscience, 29(2), 444–453. 10.1523/JNEUROSCI.4029-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, & Muller JF (2004). Immunocytochemical localization of GABABR1 receptor subunits in the basolateral amygdala. Brain Research, 1018(2), 147–158. 10.1016/j.brainres.2004.05.053 [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Erickson SL, & Lewis DA (2006). Dopamine innervation of the monkey mediodorsal thalamus: Location of projection neurons and ultrastructural characteristics of axon terminals. Neuroscience, 143(4), 1021–1030. 10.1016/j.neuroscience.2006.08.056 [DOI] [PubMed] [Google Scholar]
- Micheva KD, & Smith SJ (2007). Array tomography: A new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron, 55(1), 25–36. 10.1016/j.neuron.2007.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DA, Schroeder JP, Smith Y, Cortright JJ, Bubula N, Vezina P, & Weinshenker D (2012). α−1 Adrenergic receptors are localized on presynaptic elements in the nucleus accumbens and regulate mesolimbic dopamine transmission. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 37(9), 2161–2172. 10.1038/npp.2012.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon YS, Paik SK, Seo JH, Yi HW, Cho YS, Moritani M, Yoshida A, Ahn DK, Kim YS, & Bae YC (2008). GABA- and glycine-like immunoreactivity in axonal endings presynaptic to the vibrissa afferents in the cat trigeminal interpolar nucleus. Neuroscience, 152(1), 138–145. 10.1016/j.neuroscience.2007.11.033 [DOI] [PubMed] [Google Scholar]
- Nakai J, Ohkura M, & Imoto K (2001). A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nature Biotechnology, 19(2), 137–141. 10.1038/84397 [DOI] [PubMed] [Google Scholar]
- Nose I, Higashi H, Inokuchi H, & Nishi S (1991). Synaptic responses of guinea pig and rat central amygdala neurons in vitro. Journal of Neurophysiology, 65(5), 1227–1241. 10.1152/jn.1991.65.5.1227 [DOI] [PubMed] [Google Scholar]
- Ohshima M, Itami C, & Kimura F (2017). The α2A -adrenoceptor suppresses excitatory synaptic transmission to both excitatory and inhibitory neurons in layer 4 barrel cortex. The Journal of Physiology, 595(22), 6923–6937. 10.1113/JP275142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleskevich S, Descarries L, Watkins KC, Séguéla P, & Daszuta A (1991). Ultrastructural features of the serotonin innervation in adult rat hippocampus: An immunocytochemical description in single and serial thin sections. Neuroscience, 42(3), 777–791. 10.1016/0306-4522(91)90044-O [DOI] [PubMed] [Google Scholar]
- Pan B-X, Dong Y, Ito W, Yanagawa Y, Shigemoto R, & Morozov A (2009). Selective gating of glutamatergic inputs to excitatory neurons of amygdala by presynaptic GABAb receptor. Neuron, 61(6), 917–929. 10.1016/j.neuron.2009.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet M, & Smith Y (2003). Group I metabotropic glutamate receptors in the monkey striatum: Subsynaptic association with glutamatergic and dopaminergic afferents. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(20), 7659–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong W-H, Folk RW, Broussard GJ, Liang R, Jang MJ, Zhong H, Dombeck D, von Zastrow M, Nimmerjahn A, Gradinaru V, Williams JT, & Tian L (2018). Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science, 360(6396), eaat4422. 10.1126/science.aat4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, & Palay SL (1996). The morphology of synapses. Journal of Neurocytology, 25(1), 687–700. 10.1007/BF02284835 [DOI] [PubMed] [Google Scholar]
- Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan L-Y, Ribeiro-da-Silva A, Braz JM, Basbaum AI, & Sharif-Naeini R (2015). Dorsal Horn Parvalbumin Neurons Are Gate-Keepers of Touch-Evoked Pain after Nerve Injury. Cell Reports, 13(6), 1246–1257. 10.1016/j.celrep.2015.09.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, & Paull WK (1992). Monoamine innervation of bed nucleus of stria terminalis: An electron microscopic investigation. Brain Research Bulletin, 28(6), 949–965. 10.1016/0361-9230(92)90218-m [DOI] [PubMed] [Google Scholar]
- Pickard GE, Smith BN, Belenky M, Rea MA, Dudek FE, & Sollars PJ (1999). 5-HT1B receptor-mediated presynaptic inhibition of retinal input to the suprachiasmatic nucleus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 19(10), 4034–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Beckley SC, Joh TH, & Reis DJ (1981). Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Research, 225(2), 373–385. 10.1016/0006-8993(81)90843-x [DOI] [PubMed] [Google Scholar]
- Ren M, Yoshimura Y, Takada N, Horibe S, & Komatsu Y (2007). Specialized Inhibitory Synaptic Actions Between Nearby Neocortical Pyramidal Neurons. Science, 316(5825), 758–761. 10.1126/science.1135468 [DOI] [PubMed] [Google Scholar]
- Rodríguez JJ, Garcia DR, & Pickel VM (1999). Subcellular distribution of 5-hydroxytryptamine2A and N-methyl-D-aspartate receptors within single neurons in rat motor and limbic striatum. The Journal of Comparative Neurology, 413(2), 219–231. [DOI] [PubMed] [Google Scholar]
- Scruggs JL, Patel S, Bubser M, & Deutch AY (2000). DOI-Induced activation of the cortex: Dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(23), 8846–8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguéla P, Watkins KC, Geffard M, & Descarries L (1990). Noradrenaline axon terminals in adult rat neocortex: An immunocytochemical analysis in serial thin sections. Neuroscience, 35(2), 249–264. 10.1016/0306-4522(90)90079-j [DOI] [PubMed] [Google Scholar]
- Séguéla Philippe, Watkins KC, & Descarries L (1989). Ultrastructural relationships of serotonin axon terminals in the cerebral cortex of the adult rat: 5-HT AXON TERMINALS IN CEREBRAL CORTEX. Journal of Comparative Neurology, 289(1), 129–142. 10.1002/cne.902890111 [DOI] [PubMed] [Google Scholar]
- Sesack SR (2002). Synaptology of Dopamine Neurons. In Chiara G. Di (Ed.), Dopamine in the CNS I: Vol. 154 / 1 (pp. 63–119). Springer Berlin Heidelberg. 10.1007/978-3-642-56051-4_4 [DOI] [Google Scholar]
- Sesack Susan R., & Pickel VM (1990). In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Research, 527(2), 266–279. 10.1016/0006-8993(90)91146-8 [DOI] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, & Lüthi A (2006). Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nature Neuroscience, 9(8), 1028–1035. 10.1038/nn1732 [DOI] [PubMed] [Google Scholar]
- Sheridan MR, Flowers KA, & Hurrell J (1987). Programming and execution of movement in Parkinson’s disease. Brain: A Journal of Neurology, 110 (Pt 5), 1247–1271. 10.1093/brain/110.5.1247 [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, & Nelson SB (2003). Neocortical LTD via Coincident Activation of Presynaptic NMDA and Cannabinoid Receptors. Neuron, 39(4), 641–654. 10.1016/S0896-6273(03)00476-8 [DOI] [PubMed] [Google Scholar]
- Soghomonian J-J, Descarries L, & Watkins KC (1989). Serotonin innervation in adult rat neostriatum. II. Ultrastructural features: A radioautographic and immunocytochemical study. Brain Research, 481(1), 67–86. 10.1016/0006-8993(89)90486-1 [DOI] [PubMed] [Google Scholar]
- Soiza-Reilly M, Anderson WB, Vaughan CW, & Commons KG (2013). Presynaptic gating of excitation in the dorsal raphe nucleus by GABA. Proceedings of the National Academy of Sciences, 110(39), 15800–15805. 10.1073/pnas.1304505110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollars PJ, Ogilvie MD, Simpson AM, & Pickard GE (2006). Photic entrainment is altered in the 5-HT1B receptor knockout mouse. Journal of Biological Rhythms, 21(1), 21–32. 10.1177/0748730405283765 [DOI] [PubMed] [Google Scholar]
- Strader CD, Pickel VM, Joh TH, Strohsacker MW, Shorr RG, Lefkowitz RJ, & Caron MG (1983). Antibodies to the beta-adrenergic receptor: Attenuation of catecholamine-sensitive adenylate cyclase and demonstration of postsynaptic receptor localization in brain. Proceedings of the National Academy of Sciences of the United States of America, 80(7), 1840–1844. 10.1073/pnas.80.7.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumal KK, Blessing WW, Joh TH, Reis DJ, & Pickel VM (1983). Synaptic interaction of vagal afferents and catecholaminergic neurons in the rat nucleus tractus solitarius. Brain Research, 277(1), 31–40. 10.1016/0006-8993(83)90904-6 [DOI] [PubMed] [Google Scholar]
- Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, Yong Z, Gao Y, Peng W, Wang L, Zhang S, Du J, Lin D, Xu M, Kreitzer AC, … Li Y (2018). A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell, 174(2), 481–496.e19. 10.1016/j.cell.2018.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, & Cassell MD (1993). Intrinsic GABAergic neurons in the rat central extended amygdala. The Journal of Comparative Neurology, 330(3), 381–404. 10.1002/cne.903300308 [DOI] [PubMed] [Google Scholar]
- Takács VT, Szőnyi A, Freund TF, Nyiri G, & Gulyás AI (2015). Quantitative ultrastructural analysis of basket and axo-axonic cell terminals in the mouse hippocampus. Brain Structure and Function, 220(2), 919–940. 10.1007/s00429-013-0692-6 [DOI] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, & Cragg SJ (2012). Striatal Dopamine Release Is Triggered by Synchronized Activity in Cholinergic Interneurons. Neuron, 75(1), 58–64. 10.1016/j.neuron.2012.04.038 [DOI] [PubMed] [Google Scholar]
- Umbriaco D, Garcia S, Beaulieu C, & Descarries L (1995). Relational features of acetylcholine, noradrenaline, serotonin and GABA axon terminals in the stratum radiatum of adult rat hippocampus (CA1). Hippocampus, 5(6), 605–620. 10.1002/hipo.450050611 [DOI] [PubMed] [Google Scholar]
- Umbriaco Denis, Watkins KC, Descarries L, Cozzari C, & Hartman BK (1994). Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: An electron microscopic study in serial sections. The Journal of Comparative Neurology, 348(3), 351–373. 10.1002/cne.903480304 [DOI] [PubMed] [Google Scholar]
- Verney C, Alvarez C, Geffard M, & Berger B (1990). Ultrastructural Double-Labelling Study of Dopamine Terminals and GABA-Containing Neurons in Rat Anteromedial Cerebral Cortex. The European Journal of Neuroscience, 2(11), 960–972. 10.1111/j.1460-9568.1990.tb00008.x [DOI] [PubMed] [Google Scholar]
- Wang H, & Pickel VM (2002). Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. The Journal of Comparative Neurology, 442(4), 392–404. 10.1002/cne.10086 [DOI] [PubMed] [Google Scholar]
- Wang H-X, Waterhouse BD, & Gao W-J (2013). Selective suppression of excitatory synapses on GABAergic interneurons by norepinephrine in juvenile rat prefrontal cortical microcircuitry. Neuroscience, 246, 312–328. 10.1016/j.neuroscience.2013.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrum LE (1993). Axon hillocks and initial segments in spinal trigeminal nucleus with emphasis on synapses including axo-axo-axonic contacts. Journal of Neurocytology, 22(9), 793–803. 10.1007/BF01181324 [DOI] [PubMed] [Google Scholar]
- Winterer J, Stempel AV, Dugladze T, Földy C, Maziashvili N, Zivkovic AR, Priller J, Soltesz I, Gloveli T, & Schmitz D (2011). Cell-type-specific modulation of feedback inhibition by serotonin in the hippocampus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(23), 8464–8475. 10.1523/JNEUROSCI.6382-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Saitow F, Satake S, Kiyohara T, & Konishi S (1999). GABA(B) receptor-mediated presynaptic inhibition of glutamatergic and GABAergic transmission in the basolateral amygdala. Neuropharmacology, 38(11), 1743–1753. 10.1016/s0028-3908(99)00126-4 [DOI] [PubMed] [Google Scholar]
- Ye M, Hayar A, Strotman B, & Garcia-Rill E (2010). Cholinergic modulation of fast inhibitory and excitatory transmission to pedunculopontine thalamic projecting neurons. Journal of Neurophysiology, 103(5), 2417–2432. 10.1152/jn.01143.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, & Sulzer D (2003). Glutamate spillover in the striatum depresses dopaminergic transmission by activating group I metabotropic glutamate receptors. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(33), 10585–10592. [DOI] [PMC free article] [PubMed] [Google Scholar]


