Abstract
Introduction:
In this study, we aimed to determine whether muscle transverse relaxation time (T2) magnetic resonance (MR) mapping results correlate with motor unit loss, as defined by motor unit recruitment patterns on electromyography (EMG).
Methods:
EMG and 3-Tesla MRI exams were acquired no more than 31 days apart in subjects referred for peripheral nerve MRI. Two musculoskeletal radiologists qualitatively graded T2-weighted, fat-suppressed sequences for severity of muscle edema- like patterns and manually placed regions of interest within muscles to obtain T2 values from T2-mapping sequences. Concordance was calculated between qualitative and quantitative MR grades and EMG recruitment categories (none, discrete, decreased) as well as interobserver agreement for both MR grades.
Results:
Thirty-four muscles (21 abnormal, 13 control) were assessed in 13 subjects (5 females and 8 males; mean age, 46 years) with 14 EMG-MRI pairs. T2-relaxation times were significantly (P < .001) increased in all EMG recruitment categories com- pared with control muscles. T2 differences were not significant between EMG grades of motor unit recruitment (P = .151-.702). T2 and EMG score concordance was acceptable (Harrell’s concordance index [c index]: rater A, 0.71; 95% confidence interval [CI], 0.51-0.87; rater B, 0.77; 95% CI, 0.57-0.91). Qualitative MRI and EMG score concordance was poor to acceptable (c index: rater A, 0.60; 95% CI, 0.50-0.79; rater B, 0.72; 95% CI, 0.55-0.89). T2 values had moderate-to-substantial ability to distinguish between absent vs incomplete (ie, decreased or discrete) motor unit recruitment (c index: rater A, 0.78; 95% CI, 0.50-1.00; rater B, 0.86; 95% CI, 0.57-1.00).
Discussion:
Quantitative T2 MR muscle mapping is a promising tool for noninvasive evaluation of the degree of motor unit recruitment loss.
Keywords: electromyography, MRI, muscle, musculoskeletal, T2 mapping
1. Introduction
Muscle denervation and changes in motor unit recruitment secondary to peripheral neuropathies are conventionally evaluated via needle electromyography (EMG), the results of which not only inform diagnosis but help to prognosticate recovery. Although EMG is considered the clinical “gold standard,” drawbacks include its invasive nature, the need to individually test each muscle, and inherent operator dependence associated with intra- and interobserver variability.1,2
Magnetic resonance imaging (MRI) can serve as a noninvasive complement to EMG for the diagnosis of peripheral neuropathies3 and evaluation of regional changes associated with muscle denervation and motor unit recruitment. MR signal intensity changes within denervated muscle can be attributed to increased muscle perfusion and capillary permeability, leading to extracellular fluid accumulation4 and, subsequently, increased transverse relaxation time (T2 prolongation). On conventional, fat-suppressed, T2-weighted qualitative MR images, actively denervated muscle and motor unit dropout manifest as diffusely homogeneous signal hyperintensity (hereafter referred to as an “edema pattern”).5 In chronic phases (>6 months) of denervation and loss of motor unit function, irreversible fatty infiltration of muscle (in addition to reduced bulk) may occur, which further increases T2 and reduces longitudinal relaxation time (T1). These long-term changes are most apparent on either T1-weighted or fat images derived from Dixon in/out-of-phase sequences.6-8
Correlation of EMG with MRI results may provide improved characterization of muscle denervation and quantification of motor unit function. To that end, qualitative MR signal intensity (SI) changes have been found to correlate with the level of motor unit recruitment on EMG.3,9 However, SI changes remain subjective, as MR SI is inherently dependent on hardware and software settings that are not quantitative in nature. To improve the quantitative aspect, one previous study attempted to calibrate SI levels across different scans on the same MRI magnet to evaluate denervated muscles in the hand.10 However, this method is semi-quantitative and neither repeatable nor reproducible across different scanners or other anatomical regions.
T2 mapping is a quantitative MR technique, unbiased by arbitrary SI levels, whereby the transverse relaxation time parameter, T2, is derived. T2 parameters provide information about the biochemical and structural characteristics of tissue.11,12 T2 mapping studies have been performed in different myopathies,7,13,14 but correlation with EMG findings in humans has yet to be reported. Correlation between T2 muscle values and EMG parameters has been reported in rodent models of nerve transection,15-17 but the evolution and pattern of denervation both in traumatic and nontraumatic peripheral neuropathies in humans are likely different.18 Given that T2 parameters are promising preclinical biomarkers of denervation and that published MRI studies of denervation in humans are sparse, there is strong motivation to evaluate the relationship between T2 values and the level of motor unit recruitment, secondary to denervation, measured on EMG.
In this study, we evaluated correlations between muscle T2-relaxation metrics and EMG in the setting of peripheral neuropathies and motor unit recruitment loss in humans. Our objective was to determine whether muscle T2 values could serve as reliable biomarkers of muscle motor unit function. We hypothesized that muscle T2 values would correlate with EMG scores of motor unit recruitment and be more sensitive than conventional MRI sequences (qualitative SI) to changes in motor unit recruitment.
2. Methods
This institutional review board–approved prospective study involved the analysis of data obtained between December 2015 and September 2017 in subjects with the clinical suspicion of peripheral neuropathy who were referred for both EMG and MRI evaluation and who provided informed consent. Patients with symptom onset beginning at least 2 years earlier, or in whom EMG and MRI studies were obtained more than 1 month apart, were excluded from analysis. Cases in which a control muscle (ie, normal motor unit recruitment on EMG) was not included within the imaged field of view (FOV) on the T2-mapping MR sequence were also excluded.
2.1. MRI Acquisition
In addition to standard-of-care MRI sequences (ie, axial proton density and T2-weighted fat-suppressed sequences), a quantitative T2- mapping sequence was prospectively obtained in all subjects. All MRI studies were acquired on a clinical 3-Tesla MRI system (Discovery MR750; GE Healthcare, Waukesha, Wisconsin). Axial, T2-weighted fat-suppressed sequences, which were qualitatively evaluated for the presence of an edema pattern, were obtained with the following imaging parameters: TE: 85 ms; repetition time (TR): 3500 to 5000 ms; readout bandwidth (RBW): ±62.5 kHz; number of excitations (NEX): two; acquisition matrix: 320 × 192; FOV: 12 to 20 cm; slice thickness 2.5 to 3.0 mm (no gap). In addition, axial T2-mapping sequences for quantitative muscle evaluation were acquired: TR: 1000 ms; TE: eight echoes between 0 and 90 ms; inter-echo spacing: 10 ms; RBW: ±62.5 kHz; NEX: one; acquisition matrix: 320 × 256; FOV: 10 to 20 cm; slice thickness: 2 mm (no gap).
2.2. MRI Analysis
Two musculoskeletal radiologists (rater A [D.B.S.]: 5 years of subspecialty musculoskeletal imaging experience; rater B [J.S.W.]: 1 year of subspecialty experience) independently graded the presence and severity of MRI muscle denervation edema pattern (T2-weighted signal hyperintensity relative to expected signal intensity of skeletal muscle) on a qualitative scale from 0 to 3 (0 = none; 1 = mild; 2 = moderate; 3 = severe). Postprocessing of acquired T2-mapping sequences and calculation of T2-relaxation times in affected muscles were performed on the vendor-provided software (AW Server version 3.2, ext 1.2; GE Healthcare). Each rater manually placed standardized regions of interest (25 mm2) within normal and affected muscles, being careful to exclude any central tendon slip or fascia that could bias measurements.
2.3. EMG Acquisition and Analysis
Needle EMGs were performed for each of the 13 patients, with all studies performed by one of five experienced practitioners. A single provider (J.H.F.) with 30 years of electrodiagnostic experience was responsible for the evaluation of 14 of the 21 involved muscles tested on EMG. The degree of motor unit recruitment, from highest to lowest (ie, full [normal], decreased [mild], discrete [moderate], and none [severe]), was obtained from EMG reports and recorded for each muscle interrogated on the T2-mapping MR sequence.
2.4. Statistical Analysis
Concordance between subjective MRI edema pattern scores, quantitative T2 values, and EMG motor unit recruitment scores were evaluated using Harrell’s concordance index (c index), obtained by fitting ordinal logistic regression models.19,20 EMG scores were also dichotomized to compare cases of absent with incomplete (decreased or discrete) motor unit recruitment. The rationale for this dichotomization was that management of patients in these two subgroups often differs, particularly in the setting of traumatic injury. The c indices were also obtained for dichotomized analyses. Analogous to an area under the receiver-operating characteristic curve, c indices were interpreted as follows: <0.5 indicative of no predictive ability; 0.5 to 0.7 considered poor discrimination; 0.7 to 0.8 considered acceptable discrimination; and 1.0 indicative of perfect discrimination.
Evaluation of the comparative ability of quantitative T2 values vs subjective MRI edema pattern scores to predict EMG scores was accomplished by calculating the differences in their respective c indices. Interobserver agreement for MRI edema pattern and quantitative T2 values was assessed with the ordinal-weighted Cohen’s kappa coefficient and intraclass correlation coefficient (ICC [2,1], two-way random effects, absolute agreement, single rater), respectively.21 Strength of agreement was interpreted as follows: <0.00 = poor; 0.00 to 0.20 = slight; 0.21 to 0.40 = fair; 0.41 to 0.60 = moderate; 0.61 to 0.80 = substantial; 0.81 to 1.00 = almost perfect.22
The c indices, differences in c indices, weighted kappa, and ICC (2,1) were reported as point estimates with cluster-robust, 95% percentile bootstrapped confidence intervals (CIs) calculated from 1000 resamples to account for the dependency from multiple muscles per subject.23 Statistical significance of the differences in c indices is implied by bootstrapped 95% CIs that exclude the null value of 0. All statistical analyses were performed with R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
A total of 13 subjects (5 females, 8 males; mean age, 46.0 years; age range, 29.0-67.0 years) met the inclusion criteria. Patients were evaluated based on the clinical suspicion of neuralgic amyotrophy (5), peroneal neuropathy (5), postganglionic L4 radiculopathy (1), axillary neuropathy (1), and posterior interosseous neuropathy (1). One patient had two separate sets of EMG and MRI studies of the brachial plexus that met the criteria, whereas all other patients had one set of EMG and MRI studies each, yielding a total of 14 EMG-MRI pairs. Within these data sets, 34 muscles (21 muscles with motor unit loss and 13 control muscles) were evaluated.
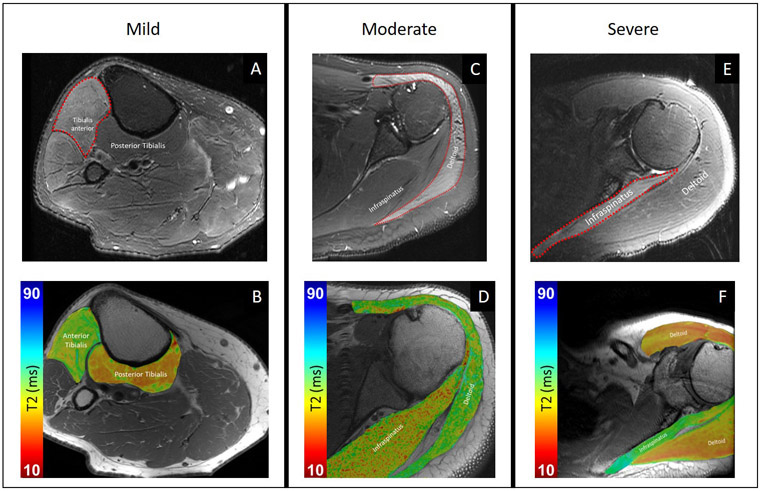
Average time between symptom onset and MRI acquisition was 8.8 months (median, 8.3 months; range, 8 days to 20 months). There was a median of 6 days between MRI and EMG. Of the 14 EMG-MRI pairs, 7 were of the upper extremity, including the brachial plexus (4), arm (2), humerus (1), and elbow (1), and 6 were of the lower extremity, including the knee (5) and lower leg (1). Table S1 provides a detailed breakdown of the subject demographics and abnormal muscles and their corresponding recruitment patterns. Of the 21 abnormal muscles evaluated with EMG, 5 had decreased recruitment, 9 had discrete recruitment, and 7 had no recruitment. Positive sharp waves and fibrillation potentials were detected in 17 of 21 abnormal muscles and all abnormal muscles had motor unit recruitment loss. All 13 control muscles had full EMG motor unit recruitment and no edema pattern on MRI. Figure 1 shows examples of three cases of varying severity of edema pattern, as graded by the radiologists, with corresponding T2 maps.
Figure 1:
Morphological, Dixon fat-suppressed T2-weighted images (top row) and T2 maps (bottom row), superimposed on proton-density weighted images, for patients with varying edema pattern severity by morphological grading. (A) 72-year-old man with recent onset right foot drop status post lumbar laminectomy two weeks prior, with mild edema pattern of the tibialis anterior muscle and normal signal intensity arising from the posterior tibialis. (B) Corresponding representative axial T2 mapping image demonstrates elevated T2 of the anterior tibialis. (C) 42-year-old man with weakness of shoulder abduction status post glenohumeral capsular repair surgery 1.5 years prior, with moderate edema pattern of all three deltoid muscle heads and normal signal intensity of the infraspinatus. (D) Corresponding representative axial T2 mapping image demonstrates elevated T2 of the deltoid. (E) 54-year-old man with spontaneous onset of severe pain followed by left shoulder abduction weakness, 8 months prior and attributed to neuralgic amyotrophy, with severe edema pattern of the infraspinatus muscle and normal signal intensity of the deltoid. (F) Corresponding representative axial T2 mapping image demonstrates elevated T2 of the infraspinatus.
Concordance between quantitative T2 values and EMG scores was considered acceptable for both readers; c indices were 0.71 (95% CI, 0.51-0.87) for rater A and 0.77 (95% CI, 0.57-0.91) for rater B. The concordance between subjective MRI edema pattern scores and EMG scores was poor for rater A at 0.60 (95% CI, 0.50-0.79), but acceptable for rater B at 0.72 (95% CI, 0.55-0.89). No statistically significant differences were found between c indices of quantitative T2 mapping and qualitative edema pattern scores for predicting EMG scores for either observer, with a difference of +0.11 (95% CI, −0.31 to 0.22) for rater A and +0.045 (95% CI, −0.29 to 0.25) for rater B.
Differences in T2-relaxation times between abnormal and control (normal) muscles were also evaluated as a means of normalizing data for intersubject comparisons (Supplemental Table 1). T2-relaxation times increased significantly (P < .001) in abnormal muscle for all EMG categories of motor unit recruitment (none, discrete, decreased) compared with normal control muscle. However, mean T2 differences (10.11-18.48 ms) (ie, abnormal muscle T2 – normal control muscle T2) between groups were not statistically significant.
Data were also dichotomized to compare two subgroups: cases of absent motor unit recruitment to decreased or discrete recruitment. Using these dichotomized data, the agreement between T2 mapping and EMG was stronger in both raters, with c indices of 0.78 (95% CI, 0.50-1.00) for rater A and 0.86 (95% CI, 0.57-1.00) for rater B, indicating an acceptable predictive ability of T2 mapping to distinguish between cases of absent vs some level of motor unit recruitment. In contrast, dichotomization resulted in the agreement between subjective edema pattern assessment and EMG becoming weaker for rater A and stronger for rater B, with c indices of 0.56 (95% CI, 0.50-0.85) and 0.79 (95% CI, 0.58-0.96) for the two respective raters.
The strength of interobserver agreement was moderate for both MRI techniques, with an ICC of 0.59 (95% CI, 0.30-0.75) for quantitative T2 mapping and an ordinal weighted Cohen’s kappa coefficient of 0.51 (95% CI, 0.19-0.77) for subjective edema pattern scoring.
4. Discussion
The results of this study suggest that quantitative T2 mapping may useful to evaluate the degree of motor unit recruitment loss in the setting of denervation. Loss of motor unit recruitment, an indicator of muscle function loss, can be secondary to denervation and/or neuropraxia. Similar to trends in animal studies,15-17 abnormal muscles exhibited prolonged T2 values when compared with control muscles. Although T2 values positively trended with the degree of muscle abnormality, as assessed both by qualitative MRI and EMG, no significant differences were found between quantitative and qualitative MR to distinguish individual EMG grades. However, when EMG grades were subsequently dichotomized, quantitative T2 mapping was better than qualitative MRI in discriminating complete from incomplete loss of motor unit recruitment on EMG. This suggests that our sample size was only adequate to coarsely resolve EMG grades (complete vs incomplete).
EMG results are severity- and time-dependent; it can take weeks for denervation to become apparent on EMG. In animal experiments, T2-weighted MRI has been shown to be sensitive to muscle changes as early as 24 hours after nerve injury,24,25 and these changes pre- ceded detection of abnormal spontaneous activity on EMG. Anecdotally, however, we have not witnessed these changes on T2-weighted pulse sequences in human, clinical exams. As T2-mapping results agreed more strongly than subjective MRI with EMG in this study, it is possible that T2 mapping could be more sensitive to muscle injury changes than T2-weighted morphological imaging, especially at earlier timepoints. Therefore, an objective T2-mapping biomarker may provide both reduced subjectivity and increased sensitivity to EMG and qualitative T2-weighted MRI, and afford longitudinal assessment of muscle in a more reliable, noninvasive manner. Previous research has shown that muscle MRI can be used as a tool for monitoring nerve regeneration over time, where changes in signal intensity serve to predict functional recovery.10 In addition, MRI can provide a single snapshot of multiple muscles as compared with EMG, which interrogates one muscle at a time. Moreover, qualitative MRI edema patterns can be occasionally confused with traumatic muscle injury, myopathy, compartment syndrome, and rhabdomyolysis. This is particularly so when these patterns are diffuse, as is seen in denervation.26,27 Herein a quantitative metric (T2 value rather than subjective T2-weighted pixel intensity) may provide a means to evaluate these pathologies.
We acknowledge several study limitations. Sample size was relatively small, and we therefore analyzed more than one abnormal muscle from the same exam when information about that muscle was available on both EMG and MRI. Muscle regions of interest were manually selected, which could have led to increased interrater variability. We did not analyze correlations of MRI against other electrodiagnostic measurements; one rationale is that the degree of motor unit recruitment is the most uniform measurement across the muscle and best reflects motor function. In comparison, spontaneous activities may vary more widely, which may be less reliable and may not correlate as well with MRI.
One reason for not performing T2 mapping more routinely is the relatively long scan time of the commercially available sequence used (approximately 10 times as long as nonquantitative T2-weighted MRI for the same spatial resolution). The T2-mapping sequence could benefit from accelerated techniques, including a more recently developed simultaneous multislice acquisition28 that interleaves k-space acquisition with echo times to reduce the number of echoes acquired for T2 mapping.29 Another limitation of T2 mapping is that its accuracy is likely biased by spatial nonuniformity in the transmit radiofrequency (B1+) field,30 which occurs inherently in MRI due to spatial variation in electrical conductivity and body habitus.31 This results in over-estimation of T2 values, but this could be corrected in subsequent studies by acquiring fast B1+ maps32 and using these to correct T2- value fitting.33 In this study, we also utilized a simple, linear regression method for fitting to obtain the T2 values. More robust fitting methods, as described elsewhere,11 could be implemented to improve accuracy and sensitivity to T2 changes.
In this study we also did not account for regions of fatty infiltration that may have been present in some chronically denervated muscles, although the patient cohort comprised mostly acute injuries (median time from injury to imaging = 4.2 months). Fat, similar to fluid, would increase T2 values of skeletal muscle.7,13 This effect could be obviated in the future by incorporating fat-suppression schemes34,35 during T2 mapping or by obtaining separate fat fraction maps, typically acquired using Dixon fat/water separation techniques,36,37 to determine the relative contribution of fat to the measured T2 value. Another promising biomarker is diffusivity, which could elucidate microstructural changes related to the caliber and permeability of muscle fibers, as well as the presence of fibrosis and edema.38 Diffusion MRI has also been previously investigated in other muscle denervation studies.16,39 We are currently developing a multiparametric neuromuscular MRI exam that would not only incorporate morphological and T2 mapping assessments but also generate fat fraction and diffusion measurements to more comprehensively characterize the states of muscle abnormality.
One logistic limitation we faced in our study was the time interval between EMG and MRI exams, which could confound results, as muscle denervation can improve or deteriorate depending on the nerve injury type.10,26 This problem was partially mitigated by excluding patients with EMG and MRI exams performed more than 1 month apart.
In conclusion, our study of T2 mapping and EMG in peripheral neuropathies suggests that quantitative T2 MR mapping may provide objective, reliable information about loss of motor function secondary to muscle denervation. Although we only evaluated muscles at a single timepoint, T2-mapping techniques could be used to longitudinally monitor muscle denervation and nerve recovery.
Supplementary Material
Acknowledgments:
This publication was made possible, in part, by Grant Number 1R21TR003033-01A1 from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- CI
confidence interval
- C-index
Harrell's concordance index
- EMG
electromyography
- FOV
field-of-view
- ICC
intraclass correlation coefficient
- MRI
magnetic resonance imaging
- NEX
number of excitations
- RBW
readout bandwidth
- SI
signal intensity
- T1
longitudinal relaxation time
- T2
transverse relaxation time
- TE
echo-time
- TR
repetition-time
Footnotes
Conflict of Interest: The authors report an institutional research agreement between Hospital for Special Surgery and General Electric Healthcare.
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Data Availability Statement
The anonymized data that support the findings of this study are avail- able from the corresponding author upon reasonable request.
References:
- 1.Narayanaswami P, Geisbush T, Jones L, et al. Critically re-evaluating a common technique: accuracy, reliability, and confirmation bias of EMG. Neurology. 2016;86:218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuglsang-Frederiksen A, Johnsen B, Vingtoft S, et al. Variation in per- formance of the EMG examination at six European laboratories. Elec- troencephalogr Clin Neurophysiol. 1995;97:444–450. [DOI] [PubMed] [Google Scholar]
- 3.McDonald CM, Carter GT, Fritz RC, Anderson MW, Abresch RT, Kilmer DD. Magnetic resonance imaging of denervated muscle: com- parison to electromyography. Muscle Nerve. 2000;23:1431–1434. [DOI] [PubMed] [Google Scholar]
- 4.Polak JF, Jolesz FA, Adams DF. Magnetic resonance imaging of skele- tal muscle. Prolongation of T1 and T2 subsequent to denervation. Invest Radiol. 1988;23:365–369. [DOI] [PubMed] [Google Scholar]
- 5.Kim SJ, Hong SH, Jun WS, et al. MR imaging mapping of skeletal mus- cle denervation in entrapment and compressive neuropathies. Radio- graphics. 2011;31:319–332. [DOI] [PubMed] [Google Scholar]
- 6.Khoury V, Cardinal E, Brassard P. Atrophy and fatty infiltration of the supraspinatus muscle: sonography versus MRI. AJR Am J Roentgenol. 2008;190:1105–1111. [DOI] [PubMed] [Google Scholar]
- 7.Mankodi A, Bishop CA, Auh S, Newbould RD, Fischbeck KH, Janiczek RL. Quantifying disease activity in fatty-infiltrated skeletal muscle by IDEAL-CPMG in Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jo C, Shin J. Changes in appearance of fatty infiltration and muscle atrophy of rotator cuff muscles on magnetic resonance imaging after rotator cuff repair: establishing new time-zero traits. Arthroscopy. 2013;29:449–458. [DOI] [PubMed] [Google Scholar]
- 9.Tepeli B, Karatas M, Coskun M, Yemisci OU. A comparison of mag- netic resonance imaging and electroneuromyography for denervated muscle diagnosis. J Clin Neurophysiol. 2017;34:248–253. [DOI] [PubMed] [Google Scholar]
- 10.Viddeleer AR, Sijens PE, van Ooijen PM, et al. Quantitative STIR of muscle for monitoring nerve regeneration. J Magn Reson Imaging. 2016;44:401–410. [DOI] [PubMed] [Google Scholar]
- 11.Raya JG, Dietrich O, Horng A, Weber J, Reiser MF, Glaser C. T2 mea- surement in articular cartilage: impact of the fitting method on accu- racy and precision at low SNR. Magn Reson Med. 2010;63:181–193. [DOI] [PubMed] [Google Scholar]
- 12.Argentieri EC, Sneag DB, Nwawka OK, Potter HG. Updates in muscu- loskeletal imaging. Sports Health. 2018;10:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinclair CD, Morrow JM, Janiczek RL, et al. Stability and sensitivity of water T2 obtained with IDEAL-CPMG in healthy and fat-infiltrated skeletal muscle. NMR Biomed. 2016;29:1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azzabou N, Loureiro de Sousa P, Caldas E, Carlier PG. Validation of a generic approach to muscle water T2 determination at 3T in fat- infiltrated skeletal muscle. J Magn Reson Imaging. 2015;41: 645–653. [DOI] [PubMed] [Google Scholar]
- 15.Wessig C, Koltzenburg M, Reiners K, Solymosi L, Bendszus M. Muscle magnetic resonance imaging of denervation and reinnervation: corre- lation with electrophysiology and histology. Exp Neurol. 2004;185: 254–261. [DOI] [PubMed] [Google Scholar]
- 16.Ha DH, Choi S, Kang EJ, Park HT. Diffusion tensor imaging and T2 mapping in early denervated skeletal muscle in rats. J Magn Reson Imaging. 2015;42:617–623. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Zhang G, Morrison B, Mori S, Sheikh KA. Magnetic reso- nance imaging of mouse skeletal muscle to measure denervation atro- phy. Exp Neurol. 2008;212:448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu P, Chawla A, Spinner RJ, et al. Key changes in denervated mus- cles and their impact on regeneration and reinnervation. Neural Regen Res. 2014;9:1796–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 20.Harrell FE Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–152. [DOI] [PubMed] [Google Scholar]
- 21.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1:30–46. [Google Scholar]
- 22.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 23.Huang FL. Using cluster bootstrapping to analyze nested data with a few clusters. Educ Psychol Meas. 2018;78:297–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendszus M, Wessig C, Solymosi L, Reiners K, Koltzenburg M. MRI of peripheral nerve degeneration and regeneration: correlation with electrophysiology and histology. Exp Neurol. 2004;188: 171–177. [DOI] [PubMed] [Google Scholar]
- 25.Bendszus M, Koltzenburg M, Wessig C, Solymosi L. Sequential MR imaging of denervated muscle: experimental study. AJNR Am J Neuroradiol. 2002;23:1427–1431. [PMC free article] [PubMed] [Google Scholar]
- 26.Kamath S, Venkatanarasimha N, Walsh MA, Hughes PM. MRI appear- ance of muscle denervation. Skeletal Radiol. 2008;37:397–404. [DOI] [PubMed] [Google Scholar]
- 27.Viddeleer AR, Sijens PE, van Ooyen PM, Kuypers PD, Hovius SE, Oudkerk M. Sequential MR imaging of denervated and reinnervated skeletal muscle as correlated to functional outcome. Radiology. 2012; 264:522–530. [DOI] [PubMed] [Google Scholar]
- 28.Gagoski BA, Bilgic B, Eichner C, et al. RARE/turbo spin echo imaging with simultaneous multislice wave-CAIPI. Magn Reson Med. 2015;73: 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilbert T, Sumpf TJ, Weiland E, et al. Accelerated T2 mapping com- bining parallel MRI and model-based reconstruction: GRAPPATINI. J Magn Reson Imaging. 2018;48:359–368. [DOI] [PubMed] [Google Scholar]
- 30.Poon CS, Henkelman RM. Practical T2 quantitation for clinical appli- cations. J Magn Reson Imaging. 1992;2:541–553. [DOI] [PubMed] [Google Scholar]
- 31.Sung K, Nayak KS. Measurement and characterization of RF non- uniformity over the heart at 3T using body coil transmission. J Magn Reson Imaging. 2008;27:643–648. [DOI] [PubMed] [Google Scholar]
- 32.Sacolick LI, Wiesinger F, Hancu I, Vogel MW. B1 mapping by Bloch- Siegert shift. Magn Reson Med. 2010;63:1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sumpf TJ, Petrovic A, Uecker M, Knoll F, Frahm J. Fast T2 mapping with improved accuracy using undersampled spin-echo MRI and model-based reconstructions with a generating function. IEEE Trans Med Imaging. 2014;33:2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bydder GM, Young IR. MR imaging: clinical use of the inversion recovery sequence. J Comput Assist Tomogr. 1985;9:659–675. [PubMed] [Google Scholar]
- 35.Haase A, Frahm J, Hanicke W, Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol. 1985;30:341–344. [DOI] [PubMed] [Google Scholar]
- 36.Ma J, Son JB, Bankson JA, Stafford RJ, Choi H, Ragan D. A fast spin echo two-point Dixon technique and its combination with sensitivity encoding for efficient T2-weighted imaging. Magn Reson Imaging. 2005;23:977–982. [DOI] [PubMed] [Google Scholar]
- 37.Hardy PA, Hinks RS, Tkach JA. Separation of fat and water in fast spin-echo MR imaging with the three-point Dixon technique. J Magn Reson Imaging. 1995;5:181–185. [DOI] [PubMed] [Google Scholar]
- 38.Berry DB, Regner B, Galinsky V, Ward SR, Frank LR. Relationships between tissue microstructure and the diffusion tensor in simulated skeletal muscle. Magn Reson Med. 2018;80:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holl N, Echaniz-Laguna A, Bierry G, et al. Diffusion-weighted MRI of denervated muscle: a clinical and experimental study. Skeletal Radiol. 2008;37:1111–1117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The anonymized data that support the findings of this study are avail- able from the corresponding author upon reasonable request.