Abstract
Background and purpose:
Multiple large trials have established the non-inferiority of hypofractionated radiotherapy compared to conventional fractionation. This study will determine real-world hypofractionation adoption across different geographic regions for breast, prostate, cervical cancer, and bone metastases, and identify barriers and facilitators to its use.
Materials and methods:
An anonymous, electronic survey was distributed from January 2018 through January 2019 to radiation oncologists through the ESTRO-GIRO initiative. Predictors of hypofractionation were identified in univariable and multivariable regression analyses.
Results:
2316 radiation oncologists responded. Hypofractionation was preferred in node-negative breast cancer following lumpectomy (82·2% vs. 46·7% for node-positive; p < 0.001), and in low- and intermediate-risk prostate cancer (57·5% and 54·5%, respectively, versus 41·2% for high-risk (p < 0.001)). Hypofractionation was used in 32·3% of cervix cases in Africa, but <10% in other regions (p < 0.001). For palliative indications, hypofractionation was preferred by the majority of respondents. Lack of long-term data and concerns about local control and toxicity were the most commonly cited barriers. In adjusted analyses, hypofractionation was least common for curative indications amongst low- and lower-middle-income countries, Asia-Pacific, female respondents, small catchment areas, and in centres without access to intensity modulated radiotherapy.
Conclusion:
Significant variation was observed in hypofractionation across curative indications and between regions, with greater concordance in palliation. Using inadequate fractionation schedules may impede the delivery of affordable and accessible radiotherapy. Greater regionally-targeted and disease-specific education on evidence-based fractionation schedules is needed to improve utilization, along with best-case examples addressing practice barriers and supporting policy reform.
Keywords: Radiotherapy, Dose fractionation, Breast neoplasms, Prostatic neoplasms, Uterine cervical neoplasms, Global health
Many clinical trials have established the equivalence of conventionally fractionated and hypofractionated radiotherapy in terms of tumour control and long-term toxicity [1–7]. In the curative setting of breast and prostate cancer, both among the most common cancers and often requiring radiotherapy [8,9], a strong body of evidence supporting hypofractionation has informed professional society guidelines [10–12]. Within prostate cancer, three non-inferiority trials with over 30,000 combined patient-years of follow-up found that moderate hypofractionation was non-inferior to conventionally fractionated treatment for 5-year biochemical or clinical failure [5–7]. In breast cancer, large Canadian and United Kingdom trials have shown no difference between conventional and hypofractionated treatment in local recurrence, overall survival, or cosmetic outcome at 10 years [1,4]. Most recently, the FAST-Forward trial established the non-inferiority of a 5-fraction regimen for breast radiotherapy, as compared to 15-fractions [13].
Hypofractionation is especially relevant in the palliative setting to alleviate symptoms of advanced disease. Over the last 20 years, there have been 9 trials of over 4000 patients with bone metastases, which found no differences in pain relief or medication requirements between single fraction and multi-fraction radiotherapy regimens [14]. This is especially relevant in low- and middle-income countries (LMICs), where availability of machines is limited and the presentation of patients with disease is often delayed [9,15]. Adopting hypofractionation has also been found to be the most efficient treatment option by reducing treatment time and reducing costs associated with daily treatment [16]. Shorter treatment courses also liberates machine time, thereby improving access to radiotherapy for a greater number of patients. Moreover, since the onset of COVID-19, delivering shorter radiotherapy courses has also been advocated to mitigate the risk of infection to patients and healthcare workers by decreasing the time patients spend in hospitals [17–19].
Despite the evidence base for hypofractionation, the extent to which this knowledge is accepted amongst oncologists and translated into clinical practice at a global level remains unknown. The European Society for Radiotherapy and Oncology’s Global Impact of Radiotherapy in Oncology (ESTRO-GIRO) initiative, which has a mandate to drive evidence-based policy solutions to improve access to radiotherapy, launched an international patterns-of-care study to determine the extent of hypofractionation adoption in breast cancer, prostate cancer, cervical cancer, and bone metastases. Although the evidence on hypofractionation in cervical cancer is more limited, this cancer site was included due to its high burden in resource-constrained settings [20]. The objective of this study was to identify the clinical circumstances in which hypofractionation is used and to identify the barriers and facilitators to hypofractionation across different geographic regions and resource settings.
Materials and methods
Participants
Radiation oncologists who had completed their training were invited to participate. The survey was disseminated from January 2018 to January 2019 through the membership database of ESTRO and through the liaisons of several national and regional professional societies globally (see Appendix p9 for a list of professional societies engaged in survey distribution).
Survey design
An anonymous, electronic survey of hypofractionation practice patterns was developed using SurveyMonkey software, which could be answered only once from any single device (Appendix p1–8). The survey was designed to take 10 to 15 minutes to complete and consisted of 5 sections with a total of 28 questions. The first section focused on demographics, clinical experience, and available technology within respondents’ departments. The other four sections focused on clinical scenarios related to breast, prostate and cervical cancer, and bone metastases. For each disease site, only respondents who indicated that they treated at least one patient per month were subsequently surveyed on their practice patterns.
Multiple clinical scenarios were presented per disease site, asking for: (1) the use of conventional fractionation [≤2 Gray (Gy) per fraction], hypofractionation (>2Gy per fraction), or both; (2) the proportion of hypofractionated cases if “both” was selected; and (3) the preferred hypofractionated dose and fractionation. Respondents using hypofractionation were asked to justify their selection from a series of possible options, with the opportunity to indicate a free-text answer. Respondents not using hypofractionation were similarly asked about barriers to its use.
The questionnaire was written and initially assessed by 3 investigators (DR, OM, YL) from two different countries and was translated from English to Spanish, Japanese, and Mandarin. A panel of 4 radiation oncologists (SG, MLY, EZ, FYM) from 4 other countries pilot-tested the survey to establish face and content validity, ease of understanding, and completion time. The survey was revised based on the panel’s comments, who reviewed the survey again after each round of revisions. The survey was considered validated when the panel offered no further revisions. No incentives were provided for participation. This study received institutional review board exemption.
Statistical analysis
Descriptive statistics were reported as proportions, medians, and ranges for categorical variables and as means with standard deviations (SD) for continuous variables. Continuous variables were compared using the t test and categorical variables were compared using the Chi-square or Fisher’s exact test. Analyses were stratified by the following geographic regions based on the World Bank classification system: (1) North America, (2) Latin America and the Caribbean (“Latin America”), (3) Europe and Central Asia (“Europe”), (4) Middle East and North Africa (“Middle East”), (5) Sub-Saharan Africa (“Africa”), and (6) South Asia, and East Asia and Pacific (“Asia-Pacific”) [21]. Justifications and barriers were analyzed by geographic region and disease site and were grouped into the following categories: clinical evidence, economic and resource impact, professional culture, and patient considerations. Free-text responses were brief and not mandatory and were therefore not analyzed.
Univariable and multivariable logistic regression analyses measured the association between hypofractionation use and respondent characteristics using odds ratios (OR) and 95% confidence intervals. All factors significant or associated with hypofractionation (p ≤ 0.10) were entered into two distinct multivariable models for curative and palliative indications, respectively. Palliative indications included palliative symptom control for breast, prostate, and cervical cancer, as well as bone metastases. Hypofractionation use was defined as a dichotomous variable and included respondents who preferred hypofractionation for >75% of their patients within each disease site and in >50% of clinical scenarios overall, stratified by curative versus palliative indications. This definition was applied to evaluate respondents who expressed a consistent preference for hypofractionation in the majority of patients. The distribution of responses to the proportion of patients who hypofractionate is presented for each clinical scenario in the Appendix (p17–19). Independent variables evaluated in the univariable model included: sex, age, years in practice, region and World Bank income group, university-affiliation, size of patient catchment area, and available technology. All analyses were conducted using R (version 3.6.1), using 2-sided statistical testing at the 0.05 significance level.
Results
A total of 2316 radiation oncologists responded to the survey (see Appendix p9–15 for country representation). Overall, 40.1% of respondents were female, 58.1% were affiliated with a university, with the majority using linear accelerators (93.3%), CT-based 3D-planning (90.9%) and IMRT (85.0%) (Table 1). Over half of the total sample (54.3%) were from Europe; 36.3% were from LMICs.
Table 1.
Characteristics of respondents.
| Number (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Europe (N = 1259) |
Asia-Pacific (N = 438) |
Africa (N = 64) |
Latin America (N = 285) |
North America (N = 145) |
Middle East (N = 125) |
Total (N = 2316) |
P-value | |
| Female | 625 (49·6%) | 127 (29·0%) | 20 (31·3%) | 78 (27·4%) | 43 (29·7%) | 36 (28·8%) | 929 (40·1%) | <0·001 |
| Age | ||||||||
| 18–34 | 246 (19·5%) | 107 (24·4%) | 9 (14·1%) | 55 (19·3%) | 27 (18·6%) | 35 (28·0%) | 479 (20·7%) | <0·001 |
| 35–44 | 383 (30·4%) | 150 (34·2%) | 21 (32·8%) | 112 (39·3%) | 44 (30·3%) | 46 (36·8%) | 756 (32·6%) | |
| 45–54 | 361 (28·7%) | 103 (23·5%) | 18 (28·1%) | 54 (18·9%) | 36 (24·8%) | 33 (26·4%) | 605 (26·1%) | |
| >55 | 269 (21·4%) | 78 (17·8%) | 16 (25·0%) | 64 (22·5%) | 38 (26·2%) | 11 (8·8%) | 476 (20·6%) | |
| Years in practice | ||||||||
| <5 | 410 (32·6%) | 104 (23·7%) | 19 (29·7%) | 73 (25·6%) | 44 (30·3%) | 44 (35·2%) | 694 (30·0%) | 0·032 |
| 6–10 | 227 (18·0%) | 106 (24·2%) | 16 (25·0%) | 61 (21·4%) | 29 (20·0%) | 26 (20·8%) | 465 (20·1%) | |
| 11–20 | 311 (24·7%) | 113 (25·8%) | 16 (25·0%) | 85 (29·8%) | 29 (20·0%) | 29 (23·2%) | 583 (25·2%) | |
| >20 | 311 (24·7%) | 115 (26·3%) | 13 (20·3%) | 66 (23·2%) | 43 (29·7%) | 26 (20·8%) | 574 (24·2%) | |
| Income group | ||||||||
| Low | 0 (0·0%) | 8 (1·8%) | 11 (17·2%) | 0 (0·0%) | 0 (0·0%) | 6 (4·8%) | 25 (1·1%) | <0·001 |
| Lower-Middle | 20 (1·6%) | 190 (43·4%) | 31 (48·4%) | 14 (4·9%) | 0 (0·0%) | 54 (43·2%) | 309 (13·3%) | |
| Upper-Middle | 172 (13·7%) | 40 (9·1%) | 22 (34·4%) | 235 (82·5%) | 0 (0·0%) | 38 (30·4%) | 507 (21·9%) | |
| High | 1067 (84·7%) | 200 (45·7%) | 0 (0·0%) | 36 (12·6%) | 145 (10·00%) | 27 (21·6%) | 1475 (63·7%) | |
| Region of training | ||||||||
| North America | 12 (1·0%) | 3 (0·7%) | 1 (1·6%) | 14 (4·9%) | 129 (89·0%) | 17 (13·6%) | 176 (7·6%) | <0·001 |
| Latin America | 3 (0·2%) | 0 (0·0%) | 1 (1·6%) | 246 (86·3%) | 1 (0·7%) | 2 (1·6%) | 253 (10·9%) | |
| Asia-Pacific | 5 (0·4%) | 417 (95·2%) | 2 (3·1%) | 4 (1·4%) | 3 (2·1%) | 5 (4·0%) | 436 (18·8%) | |
| Europe | 1233 (97·9%) | 16 (3·7%) | 12 (18·8%) | 21 (7·4%) | 9 (6·2%) | 22 (17·6%) | 1313 (56·7%) | |
| Middle East | 6 (0·5%) | 1 (0·2%) | 3 (4·7%) | 0 (0·0%) | 2 (1·4%) | 76 (60·8%) | 88 (3·8%) | |
| Africa | 0 (0%) | 1 (0·2%) | 45 (70·3%) | 0 (0·0%) | 1 (0·7%) | 3 (2·4%) | 50 (2·2%) | |
| University affiliation | 822 (65·3%) | 196 (44·7%) | 31 (48·4%) | 103 (36·1%) | 123 (84·8%) | 70 (56·0%) | 1345 (58·1%) | <0·001 |
| Scope of practice* | ||||||||
| Public | 521 (41·4%) | 169 (38·6%) | 25 (39·1%) | 124 (43·5%) | 26 (17·9%) | 62 (49·6%) | 927 (40·0%) | <0·001 |
| Private | 171 (13·6%) | 157 (35·8%) | 19 (29·7%) | 171 (60·0%) | 19 (13·1%) | 31 (24·8%) | 568 (24·5%) | |
| Public-Private | 92 (7·3%) | 38 (8·7%) | 10 (15·6%) | 77 (27·0%) | 4 (2·8%) | 14 (11·2%) | 235 (10·0%) | |
| Catchment population | ||||||||
| < 100,000 | 531 (42·2%) | 187 (42·7%) | 14 (21·9%) | 73 (25·6%) | 9 (6·2%) | 41 (32·8%) | 855 (36·0%) | <0·001 |
| 100,000–500,000 | 83 (6·6%) | 24 (5·5%) | 4 (6·3%) | 18 (6·3%) | 27 (18·6%) | 6 (4·8%) | 162 (7·0%) | |
| 500,000–1,000,000 | 285 (22·6%) | 73 (16·7%) | 5 (7·8%) | 39 (13·7%) | 30 (20·7%) | 14 (11·2%) | 446 (19·3%) | |
| >1,000,000 | 360 (28·6%) | 154 (35·2%) | 41 (64·1%) | 155 (54·4%) | 79 (54·5%) | 64 (51·2%) | 853 (36·8%) | |
| Available technology* | ||||||||
| Cobalt-60 | 83 (6·6%) | 102 (23·3%) | 24 (37·5%) | 48 (16·8%) | 11 (7·6%) | 39 (31·2%) | 307 (13·3%) | <0·001 |
| Linear Accelerator | 1212 (96·3%) | 379 (86·5%) | 46 (71·9%) | 266 (93·3%) | 145 (100·0%) | 112 (89·6%) | 2160 (93·3%) | <0·001 |
| 2D-planning | 431 (34·2%) | 213 (48·6%) | 36 (56·3%) | 124 (43·5%) | 76 (52·4%) | 63 (50·4%) | 943 (40·7%) | <0·001 |
| CT-based 3D-planning | 1169 (92·9%) | 402 (91·8%) | 37 (57·8%) | 255 (89·5%) | 141 (97·2%) | 102 (81·6%) | 2106 (90·9%) | <0·001 |
| 3D-conformal therapy | 1171 (93·0%) | 378 (86·3%) | 38 (59·4%) | 261 (91·6%) | 138 (95·2%) | 102 (81·6%) | 2088 (90·2%) | <0·001 |
| IMRT | 1141 (90·6%) | 367 (83·8%) | 16 (25·0%) | 221 (77·5%) | 143 (98·6%) | 80 (64·0%) | 1968 (85·0%) | <0·001 |
Responses were not mutually exclusive. Abbreviations 2D-planning, two-dimensional planning CT-based 3D-planning, computed tomography three-dimensional planning 3D-conformal therapy, three-dimensional conformal therapy IMRT, intensity-modulated radiation therapy
Responses for each clinical scenario are reported by region in Fig. 1 (Appendix p16). Hypofractionation was preferred by 82.2% in the node-negative setting following lumpectomy, with the highest proportion of hypofractionation users in Europe (88·5%) and North America (97.3%); the lowest in Africa (40.0%) (p < 0.001). Hypofractionation was significantly reduced post-mastectomy, with the highest utilization in the Middle East (70.4%) and the lowest in Latin America and Asia-Pacific (38.5% and 36.2%, respectively; p = 0.002). Similar findings were observed for node-positive disease. In prostate cancer, the highest hypofractionation utilization rates were in low- and intermediate-risk disease at 57.5% and 54.5%, respectively, compared to 41.9% in high-risk disease and 23.6% when pelvic nodes were treated. The highest rates were in North America (94.3% low-risk, 87.8% intermediate-risk), and the lowest were in the Middle East (31.5% for low- and intermediate-risk) and Africa (18.8% for low-risk, 22.6% for intermediate-risk) (p < 0.001).
Fig. 1.
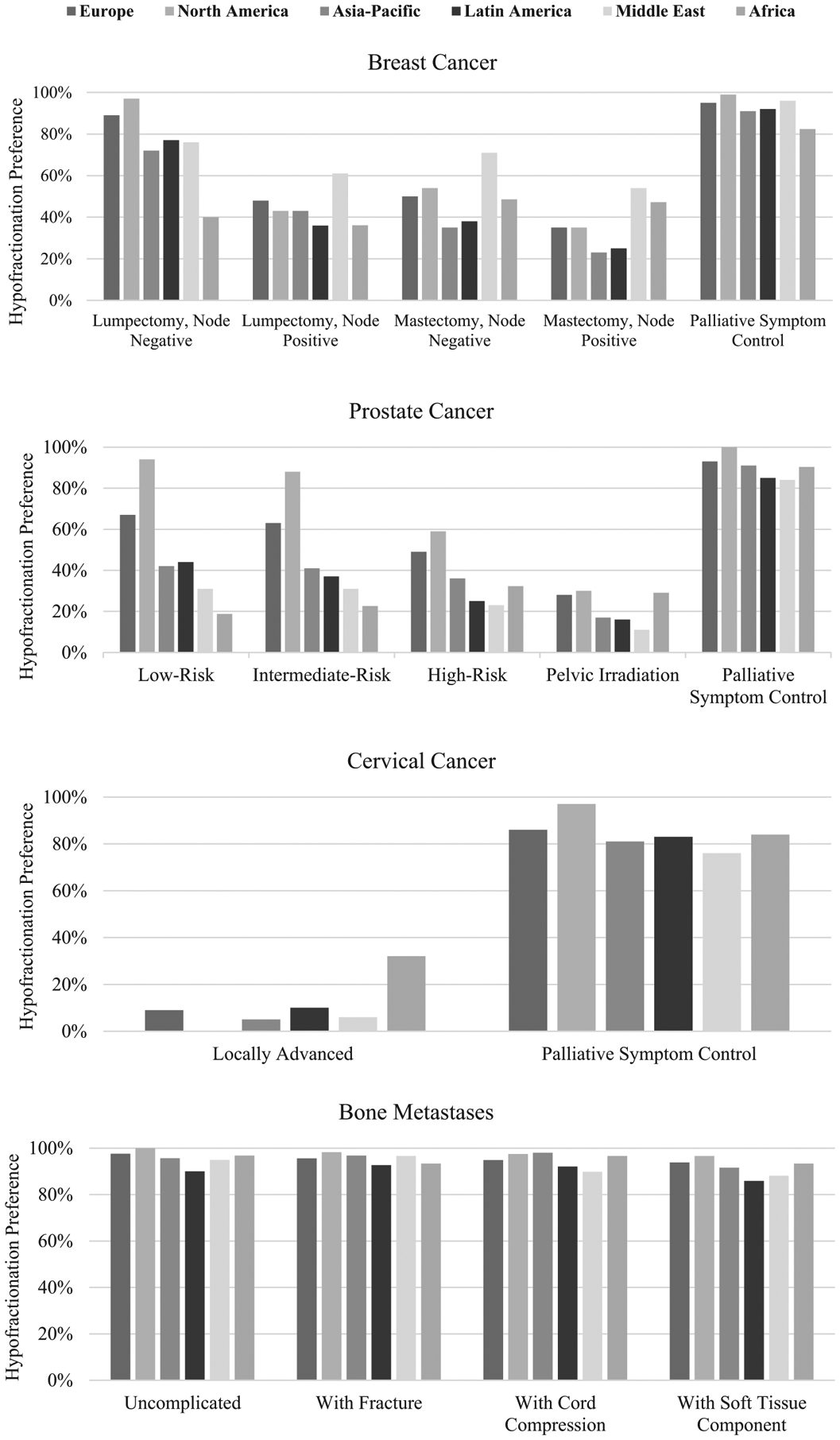
Hypofractionation practices by region and disease site.
Fewer than 10% of respondents outside of Africa favoured hypofractionation for locally advanced cervical cancer, compared with 32.3% in Africa (p < 0.001). By contrast, 84.3% of respondents favoured hypofractionation for palliative symptom control, ranging from 76.5% in the Middle East to 96.7% in North America (p = 0.04). High rates of hypofractionation for palliation of breast and prostate cancer were similarly reported. For bone metastases, ≥85% of respondents preferred hypofractionation in all scenarios, with a difference of 10% or less between regions.
Barriers and justifications for hypofractiontion are presented by disease in Fig. 2 and by region in Fig. 3. Across disease sites, clinical evidence (75.8%) and equivalence in local control (71.7%) were most frequently cited as their justification for hypofractionation. Reimbursement was the least frequently cited (5.4%), but resource optimization for improved machine availability and lower cost were reported by over half of respondents (66.7% and 52.2%, respectively). Those who reported barriers to hypofractionation most frequently cited lack of long-term data (35.0%) and concerns about acute and late toxicity (30.3% and 36.4%, respectively). Lack of technology was cited by only 14.0% overall, but varied across sites, being reported in 8.4% of respondents treating breast cancer and 23.2% of those treating prostate cancer. In the regional analysis, technology was most frequently cited as a barrier in the Middle East and Latin America (22.7% and 24.2%, respectively), but in only 3.2% of respondents in North America. Reimbursement was reported as a barrier by 15.1% and 14.3% of Latin American and Asia-Pacific respondents, respectively, but by ≤8.1% elsewhere.
Fig. 2.
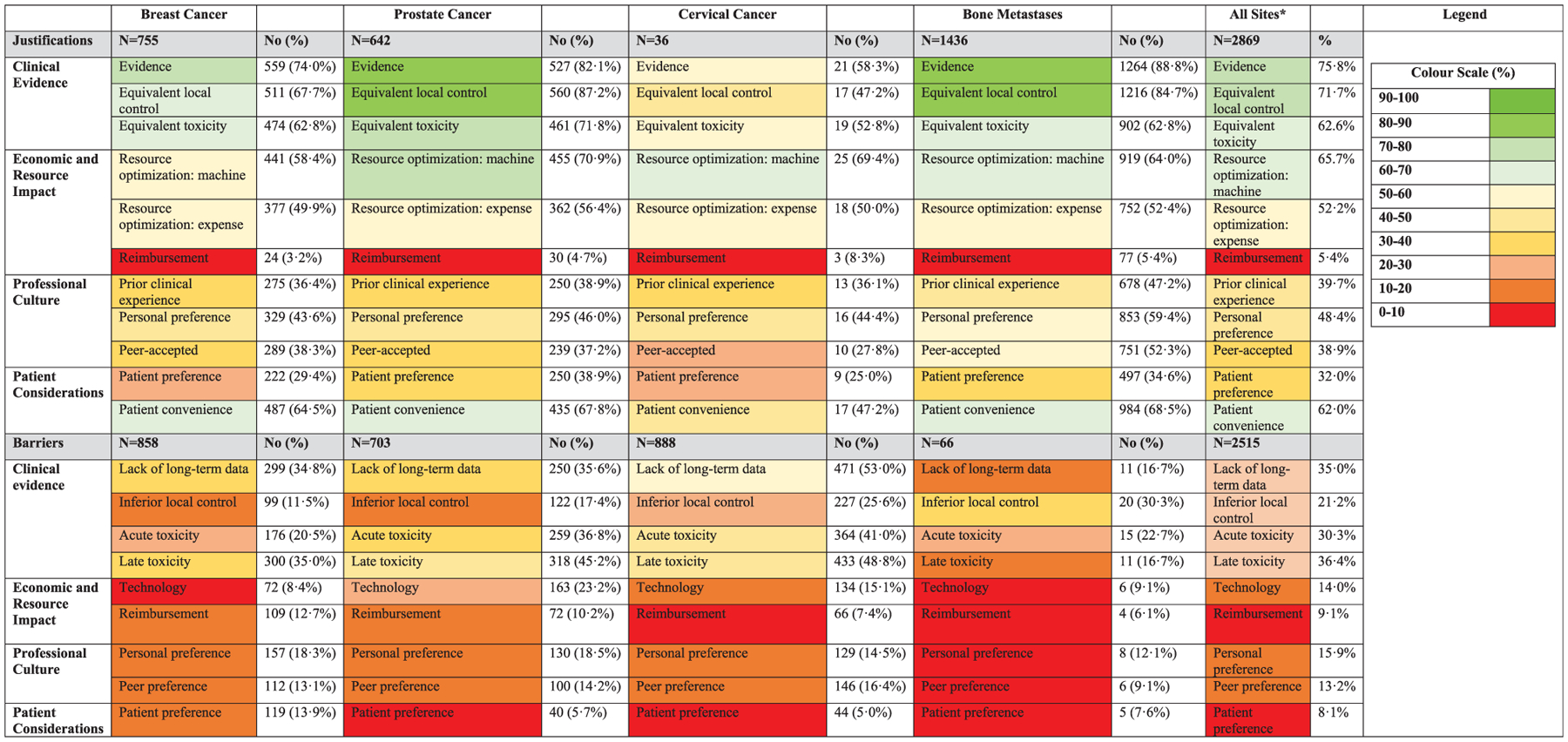
Justifications for and barriers to hypofractionation by disease site. * The values reported for all disease sites reflect the average value of responses for each disease site.
Fig. 3.
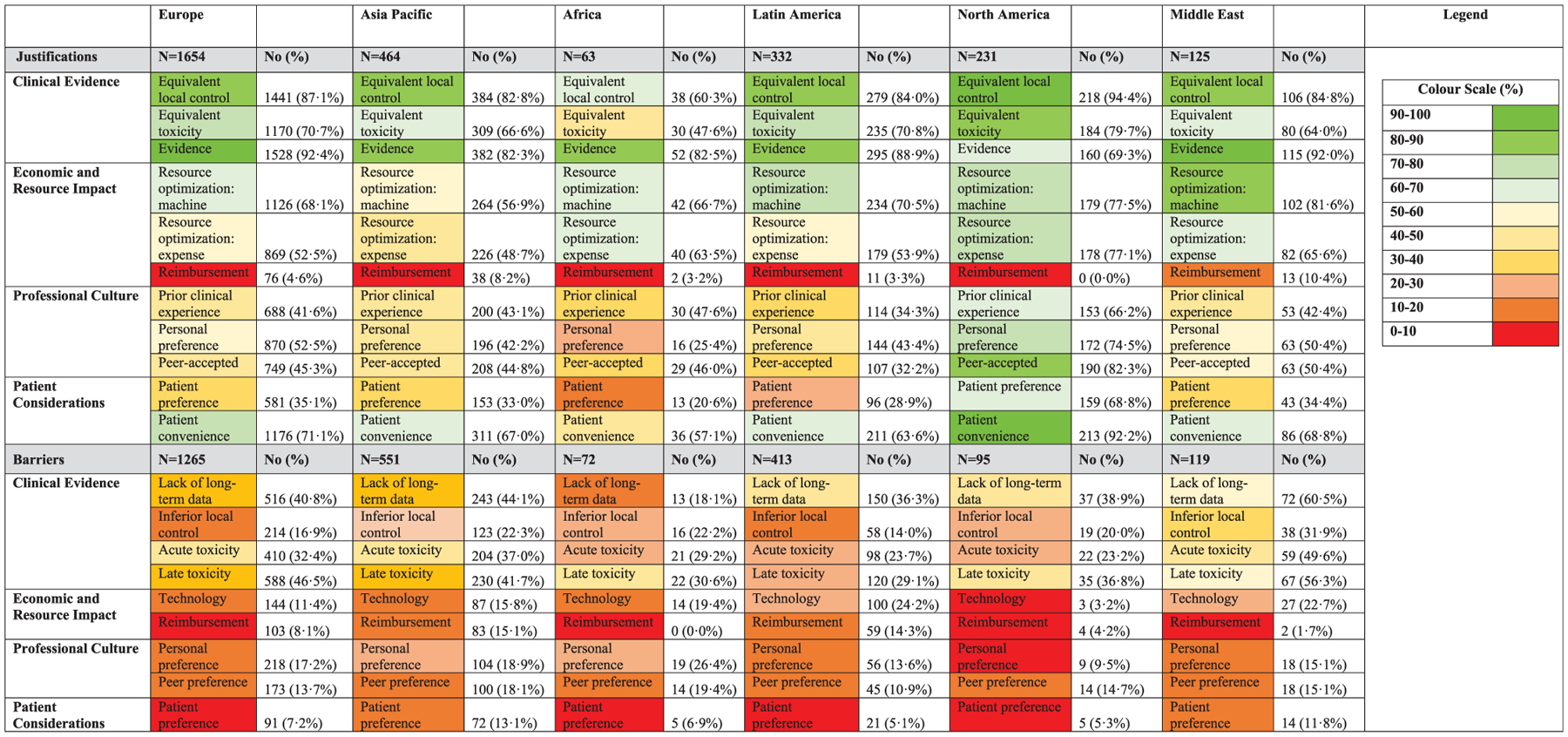
Justifications and barriers for hypofractionation by geographic location.
Predictors of hypofractionation are presented in Table 2. For curative indications, univariable regression identified practice in North America or in a high-income country, university affiliation, large catchment area (>1 million population), and use of IMRT as significantly associated with hypofractionation. Respondents who practiced in Asia-Pacific or Latin America, in a LMIC, and those who used Cobalt-60 were significantly less likely to use hypofractionation. On multivariable regression, however, only practice in Asia-Pacific and in a low- or lower-middle-income country remained significantly associated with decreased hypofractionation use; IMRT remained associated with increased hypofractionation. Further, women were 25% less likely to use hypofractionation.
Table 2.
Univariable and Multivariable Logistic Regression Analysis of Provider Characteristics Associated with Hypofractionation Use.
| Curative (N = 1,550) | Palliative (N = 1,693) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Sex | ||||||||||||
| Male | .. | .. | .. | .. | .. | .. | .. | .. | ||||
| Female | 0.84 | (0.68–1.03) | 0·09 | 0.75 | (0.6–0.95) | 0.014 | 0.83 | (0·58–1·19) | 0.3 | |||
| Age (years) | ||||||||||||
| <45 | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. |
| 45–54 | 1.11 | (0.87–1.41) | 0·39 | 1.07 | (0.75–1.52) | 0.84 | 1.94 | (0·66–1·63) | 0.87 | 0.86 | (0.54–1.37) | 0.52 |
| >55 | 0.78 | (0.60–1.01) | 0·06 | 0.77 | (0.48–1.26) | 0.71 | 0.64 | (0·42–0·97) | 0.04 | 0.49 | (0.32–0.77) | 0.002 |
| Years in Practice | ||||||||||||
| <5 | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. |
| 6–10 | 1.01 | (0.75–1.36) | 0.93 | 1.03 | (0.75–1.52) | 0.84 | 1.63 | (0·89–2·97) | 0.11 | |||
| 10–20 | 1.03 | (0.79–1.35) | 0.82 | 1.00 | (0.69–1.46) | 0.97 | 1.02 | (0·63–1·67) | 0.92 | |||
| >20 | 0.77 | (0.59–1.01) | 0.06 | 0.75 | (0.45–1.24) | 0.26 | 0.77 | (0·59–1·22) | 0.26 | |||
| Region of Practice | ||||||||||||
| Europe | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. |
| Asia-Pacific | 0.46 | (0.35–0.61) | < 0.001 | 0.47 | (0.33–0.65) | <0.001 | 0.52 | (0·33–0·81) | 0.004 | 0.65 | (0.38–1.12) | 0.12 |
| Africa | 0.53 | (0.26–1.08) | 0.08 | 1.02 | (0.44–2.31) | 0.96 | 0.52 | (0·18–1·51) | 0.23 | 1.32 | (0.38–4.55) | 0.66 |
| Latin America | 0.44 | (0.31–0.61) | < 0.001 | 0.74 | (0.48–1.13) | 0.17 | 0.50 | (0·30–0·82) | 0.006 | 0.71 | (0.38–1.33) | 0.29 |
| North America | 2.18 | (1.42–3.36) | 0.003 | 1.64 | (0.99–2.73) | 0.06 | 4.04 | (0·97–15·72) | 0.054 | 2.32 | (0.55–9.74) | 0.25 |
| Middle East | 1.19 | (0.73–1.92) | 0.49 | 1.39 | (0.80–2.41) | 0.25 | 0.74 | (0·31–1·79) | 0.51 | 1.03 | (0.39–2.68) | 0.95 |
| Income Group | ||||||||||||
| High | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. |
| Upper-Middle | 0.38 | (0.29–0.50) | <0.001 | 0.69 | (0.45–1.07) | 0.10 | 0.43 | (0·27–0·69) | <0.001 | 0.62 | (0.36–1.09) | 0.10 |
| Low and Lower-Middle | 0.54 | (0.40–0.73) | <0.001 | 0.37 | (0.26–0.52) | <0.001 | 0.53 | (0·34–0·80) | <0.001 | 0.61 | (0.31–1.19) | 0.15 |
| University Affiliation | ||||||||||||
| No | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. |
| Yes | 1.46 | (1.19–1.79) | <0.001 | 1.14 | (0.90–1.42) | 0.27 | 1.32 | (0·92–1·89) | 0.13 | |||
| Catchment Area | ||||||||||||
| <100.000 | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. |
| 100.000–500.000 | 1.15 | (0.83–1.58) | 0.40 | 0.93 | (0.66–1.31) | 0.67 | 2.31 | (1·38–3·87) | <0.001 | 1.99 | (1.15–3.43) | 0.01 |
| 500·000–1·000·000 | 1.55 | (1.1–2.19) | 0.01 | 1.35 | (0.93–1.97) | 0.11 | 1.99 | (1·16–3·41) | 0.01 | 1.76 | (1.00–3.00) | 0.05 |
| >1·000·000 | 1.46 | (1.07–1.98) | 0.02 | 1.64 | (1.17–2.31) | 0.004 | 2.26 | (1·4–3·65) | <0.001 | 2.50 | (1.51–4.15) | <0.001 |
| Available Technologyα | ||||||||||||
| Cobalt-60 | 0.68 | (0.49–0.94) | 0.02 | 1.16 | (0.78–1.73) | 0.46 | 0.54 | (0·33–0·87) | 0.02 | 1.03 | (0.57–1.87) | 0.91 |
| IMRT | 2.37 | (1.71–3.27) | <0.001 | 1.99 | (1.36–2.91) | <0.001 | 2.42 | (1·56–3·71) | 0.001 | 1.65 | (0.97–2.82) | 0.06 |
| Linear Accelerator | 1.16 | (0.74–1.82) | 0.52 | 2.44 | (1·31–4·55) | 0.005 | 1.63 | (0.72–3.66) | 0.24 | |||
| 3D-conformal therapy | 1.12 | (0.78–1.61) | 0.55 | 1.88 | (1·09–3·23) | 0.03 | 1.04 | (0.50–2.17) | 0.92 | |||
| CT-based 3D-planning | 1·16 | (0.79–1.72) | 0.45 | 1.78 | (0·99–3·22) | 0.07 | ||||||
| 2-D planning | 0.99 | (0.8–1.21) | 0.91 | 1.08 | (0·75–1·56) | 0.69 | ||||||
Notes: A hypofractionation user was defined as a provider who preferred hypofractionation for >75% of their patients within each disease site and in >50% of clinical scenarios overall. All p-values significant at p ≤ 0.05 are displayed in bold font. Practice setting (private, public, or mixed) was not included in the regression due to large number of missing responses (N = 592).
The reference category for each variable under available technology was “no/no access”.
Abbreviations: OR, Odds ratio; CI, confidence interval; IMRT, intensity-modulated radiation therapy.
For palliative indications, univariable analysis similarly revealed that practice in Asia-Pacific and Latin America, practice in low- and lower-middle-income countries, and use of Cobalt-60 were associated with decreased hypofractionation use; in addition, age > 55 was associated with decreased use. Use of IMRT, as well as use of a linear accelerator and 3D-conformal therapy, and practice in a catchment area > 100,000 were associated with increased hypofractionation use. On multivariable regression, only age > 55 remained associated with decreased use and practice in catchment areas of >1 million population remained associated with increased use.
Discussion
This international study on hypofractionation is the first to measure practice patterns across geographic regions, demonstrating significant variability in the adoption of hypofractionation across curative indications and much greater use and concordance in the palliative setting. Although over half of respondents cited resource optimization as a justification for hypofractionation, respondents in low- and lower-middle-income countries were significantly less likely to hypofractionate than their peers in high-income countries. These findings are especially relevant in the context of the ongoing COVID-19 pandemic in which minimizing infection risk to patients and staff and preservation of hospital resources have become important drivers of clinical and health-system decision-making.
In North America, almost all respondents reported using hypofractionation for early-stage breast cancer following lumpectomy. This contrasts sharply from an earlier US study that reported hypofractionation in 13·6% of patients in 2009–2010 [22]. In 2013, the American Society of Radiation Oncology included conventional fractionation for early-stage breast cancer in its Choosing Wisely list of low-value interventions [23,24]. Findings from the present survey suggest changing attitudes, although over half (61%) of North American respondents in this study were Canadian. A 2015 Canadian study found that 75% of patients with ductal carcinoma in-situ or early-stage breast cancer received hypofractionated treatment post-lumpectomy and 40% post-mastectomy. This compares to 50% in our survey who reported using hypofractionated chest wall radiotherapy [25]. Similar trends of increasing breast hypofractionation have also been reported in other countries, including Australia and Spain [26,27].
The recently-published FAST-Forward trial reported the 5-year results of randomising older women with low-risk disease to either moderate hypofractionation (40 Gy in 15 fractions) or ultra-hypofractionated radiotherapy (26–27 Gy in 5 fractions over 1 week) [13]. Both regimens demonstrated equivalent disease control, with no difference in normal tissue effects between 26 Gy and 40 Gy. Although questions remain unanswered, including late effects beyond 5 years [28], this trial has already been endorsed as a standard-of-care regimen by an international panel of experts during COVID-19 [18] and has indeed been adopted by several centres and jurisdictions [17]. In our study, concern about late toxicity was the most commonly cited barrier to hypofractionation in breast cancer, which raises the question about whether FAST-Forward and other accelerated and ultra-hypofractionated regimens will continue to be adopted post-pandemic. Further, patient preference was most commonly cited as a barrier to hypofractionation in breast cancer. In that regard, prior studies in other disease sites have found that, when patients are presented with the available evidence, many express a preference for more fractionated schedules [29].
With the exception of Africa, prostate hypofractionation was used up to two-thirds less frequently in patients who had pelvic irradiation compared to patients with low-risk disease. This is in keeping with published guidelines [10], as the clinical trials did not include pelvic lymph node treatment. However, there was also a significant drop in hypofractionation for patients with high-risk disease, and concerns about toxicity were noted as a barrier by a significant proportion of respondents. While the evidence is strongest in low- and intermediate-risk, there is evidence supporting hypofractionation in high-risk groups. The CHHiP trial did not find a significant interaction between treatment effect and risk group (p = 0.17) [6]. Further, the HYPRO study, which enrolled predominantly high-risk patients, did not find evidence of significant heterogeneity across subgroups (p = 0.95) [30]. In Africa, however, acceptance of prostate hypofractionation overall was low overall, but increased for high-risk and pelvic lymph node indications, raising concerns about knowledge gaps. Meanwhile, consensus guidelines for radiation during COVID-19 have recommended hypofractionation for localized disease and moderate hypofractionation postprostatectomy [19]. Even in the absence of image-guidance, moderate 20-fraction hypofractionation was recommended.
Hypofractionation in cervical cancer is less well studied than in other disease areas and over half of respondents reported the lack of long-term data as a barrier to hypofractionation. Recently, the Cervix Cancer Research Network, founded by the Gynecologic Cancer Intergroup to increase patient access – especially in LMICs – to high-quality clinical trials [20], launched two phase II trials. These chemoradiation trials randomize patients to conventionally fractionated (50 Gy or 45 Gy in 25 fractions) or hypofractionated treatment (40 Gy in 16 fractions), followed by definitive radical hysterectomy in one trial and brachytherapy in the other [20]. If these studies demonstrate similar efficacy and toxicity profiles, hypofractionation use may increase patient access to radiotherapy and limit patients’ time away from home.
Although 86% of respondents overall did not perceive technology as a barrier, use of IMRT was one of the strongest predictors of hypofractionation use in curative disease, while technology was most frequently cited in prostate cancer (23.2%) as a barrier to hypofractionation. Although modern trials have failed to establish an improved toxicity profile in prostate cancer patients treated with hypofractionation and modulated treatment techniques [10,31], trials using conventionally fractionated regimens with IMRT have been associated with a greater than 50% reduction in toxicity [6]. This suggests that treatment quality, including margin reduction with appropriate image-guidance, and modulated treatment with lower hot spots on organs at risk, may be more significant factors than fractionation schedule [6].
In 2015, the Global Task Force on Radiotherapy for Cancer Control (GTFRCC) published an investment framework, demonstrating the health and economic benefits of scaling up radiotherapy in LMICs [9]. This framework was modelled using the mean number of fractions per treatment course needed for each indication and tumour type, favouring the lower number of fractions when two regimens were of equal efficacy. The findings of this survey, however, suggest that some of the lowest uptake of curative hypofractionation are in regions with significant issues in access. Achieving the results produced by the GTFRCC, and delivering affordable and accessible radiotherapy, will require greater adherence to evidence-based guidelines of practice.
Given the large body of high-level evidence in support of hypofractionation for bone metastases, it is reassuring to note such a high degree of acceptance, although the proportion using single-fraction versus multi-fraction radiotherapy was not analysed. Reimbursement was infrequently cited as a barrier to hypofractionation, but the reimbursement system was not evaluated. In an earlier European study, fee-for-service reimbursement predicted for lower uptake of hypofractionation in uncomplicated bone metastases [32]. A recent reimbursement survey conducted by the ESTRO-HERO (Health Economics in Radiation Oncology) project found that all but 5 of the 25 responding European countries reported lower reimbursement for hypofractionation compared with conventional fractionation [33]. While some countries support specific techniques for ultra-hypofractionation (such as stereotactic body radiotherapy) with additional reimbursement, there are still financial disincentives to adopt shorter fractionation schedules. Applying provider payment models that link reimbursement with performance, which are already used by several countries for specialist care to incentivize adherence to evidence-based practice [34], could provide an opportunity to move away from fee-per-fraction and increase hypofractionation use.
This study must be considered in the context of its strengths and limitations. The survey was administered through professional society membership databases in order to survey a large sample of international radiation oncologists. As a result, however, sample size could not be accurately estimated, and selection bias may be present. Further, survey responses were not correlated with actual utilization and there may be incomplete adjustment or unknown confounders in the multivariable regression analysis. This study’s generalizability to other disease sites such as head and neck or lung cancer, where hypofractionation is also being applied, is unclear. Further, while translating evidence into clinical practice and changing well-entrenched habits is complex and time-intensive, further research is needed to identify the most effective means of promoting knowledge translation [35].
In conclusion, this international survey of hypofractionation identified progress in adoption and concordance of hypofractionation for palliative indications, but significant variability across curative clinical indications and between geographic regions and income groups. These findings underscore the need to develop more effective clinical decision-support and targeted clinician and patient education to address knowledge gaps, entrenched practices, and patient expectations, with a focus on low- and lower-middle-income countries. Improving global adoption of hypofractionation is an important step toward increasing availability, access, and affordability of treatment.
Supplementary Material
Acknowledgements
The authors wish to thank Chiara Gasparotto and Gabriella Axelsson (European Society of Radiotherapy and Oncology) for their support in administering the survey. CG and GA are employees of ESTRO, but ESTRO did not participate in the study design, data analysis, data interpretation, or writing of the report. The authors also gratefully acknowledge the fellowship support receive by DR from the Canadian Association of Radiation Oncology, the Royal College of Physicians and Surgeons of Canada, and The Commonwealth Fund, as well as the support received by SG from the Mentored Patient Oriented Career Research Development Award (1-K08CA230170-01A1), during the conduct of this study. None of these organisations had a role in study design, data collection, data analysis, data interpretation, or writing of the report.
Funding source
There was no funding source for this study, but ESTRO provided logistical support to carry out the survey. The corresponding author had full access to all of the data and had final responsibility for the decision to submit for publication.
Footnotes
Conflict of interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.radonc.2021.01.003.
References
- [1].Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513–20. [DOI] [PubMed] [Google Scholar]
- [2].Coles CE, Griffin CL, Kirby AM, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, noninferiority trial. Lancet 2017;390:1048–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hickey BE, James ML, Daly T, Soh FY, Jeffery M. Hypofractionation for clinically localized prostate cancer. Cochrane Database Syst Rev 2019;9:CD011462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086–94. [DOI] [PubMed] [Google Scholar]
- [5].Catton CN, Lukka H, Gu CS, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol 2017;35:1884–90. [DOI] [PubMed] [Google Scholar]
- [6].Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 2016;17:1047–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol 2016;34:2325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [9].Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol 2015;16:1153–86. [DOI] [PubMed] [Google Scholar]
- [10].Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated radiation therapy for localized prostate cancer: an ASTRO, ASCO, and AUA evidence-based guideline. J Clin Oncol 2018;36:3411–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Smith BD, Bellon JR, Blitzblau R, et al. Radiation therapy for the whole breast: executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol 2018;8:145–52. [DOI] [PubMed] [Google Scholar]
- [12].Freitas NMA, Rosa AA, Marta GN, et al. Recommendations for hypofractionated whole-breast irradiation. Rev Assoc Med Bras 2018;64:770–7. [DOI] [PubMed] [Google Scholar]
- [13].Murray Brunt A, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020;395:1613–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lutz ST, Chow EL, Hartsell WF, Konski AA. A review of hypofractionated palliative radiotherapy. Cancer 2007;109:1462–70. [DOI] [PubMed] [Google Scholar]
- [15].Elmore SNC, Grover S, Bourque J-M, et al. Global palliative radiotherapy: a framework to improve access in resource-constrained settings. Ann Palliat Med 2019;8:274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hunter D, Mauldon E, Anderson N. Cost-containment in hypofractionated radiation therapy: a literature review. J Med Radiat Sci 2018;65:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koch CA, Lee G, Liu ZA, et al. Rapid adaptation of breast radiation therapy use during the coronavirus disease 2019 pandemic at a large Academic Cancer Center in Canada. Adv Radiat Oncol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Coles CE, Aristei C, Bliss J, et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol (R Coll Radiol) 2020;32:279–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zaorsky NG, Yu JB, McBride SM, et al. Prostate cancer radiotherapy recommendations in response to COVID-19. Adv Radiat Oncol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ager BJ, Gallardo-Rincón D, de León DC, et al. Advancing clinical research globally: cervical cancer research network from Mexico. Gynecol Oncol Rep 2018;25:90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed Jan 1, 2019).
- [22].Jagsi R, Falchook AD, Hendrix LH, Curry H, Chen RC. Adoption of hypofractionated radiation therapy for breast cancer after publication of randomized trials. Int J Radiat Oncol Biol Phys 2014;90:1001–9. [DOI] [PubMed] [Google Scholar]
- [23].Choosing Wisely. 2020. https://www.choosingwisely.org/ (accessed November 1, 2019).
- [24].Pramesh CS, Chaturvedi H, Reddy VA, et al. Choosing Wisely India: ten low-value or harmful practices that should be avoided in cancer care. Lancet Oncol 2019;20:e218–23. [DOI] [PubMed] [Google Scholar]
- [25].Chan S, Sutradhar R, Yao Z, et al. Fractionation in adjuvant radiotherapy for invasive breast cancer and ductal carcinoma in situ in Ontario, Canada from 2009 to 2015. Breast J 2019. [DOI] [PubMed] [Google Scholar]
- [26].Delaney GP, Gandhidasan S, Walton R, Terlich F, Baker D, Currow D. The Pattern of Use of Hypofractionated Radiation Therapy for Early-Stage Breast Cancer in New South Wales, Australia, 2008 to 2012. Int J Radiat Oncol Biol Phys 2016;96:266–72. [DOI] [PubMed] [Google Scholar]
- [27].Prades J, Algara M, Espinas JA, et al. Understanding variations in the use of hypofractionated radiotherapy and its specific indications for breast cancer: a mixed-methods study. Radiother Oncol 2017;123:22–8. [DOI] [PubMed] [Google Scholar]
- [28].Levy A, Rivera S. 1-week hypofractionated adjuvant whole-breast radiotherapy: towards a new standard?. Lancet 2020;395:1588–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shakespeare TP, Lu JJ, Back MF, Liang S, Mukherjee RK, Wynne CJ. Patient preference for radiotherapy fractionation schedule in the palliation of painful bone metastases. J Clin Oncol 2003;21:2156–62. [DOI] [PubMed] [Google Scholar]
- [30].Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019;394:385–95. [DOI] [PubMed] [Google Scholar]
- [31].Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol 2016;17:464–74. [DOI] [PubMed] [Google Scholar]
- [32].Lievens Y, Van den Bogaert W, Rijnders A, Kutcher G, Kesteloot K. Palliative radiotherapy practice within Western European countries: impact of the radiotherapy financing system?. Radiother Oncol 2000;56:289–95. [DOI] [PubMed] [Google Scholar]
- [33].Lievens Y, Defourny N, Corral J, Gasparotto C, Grau C, Borras JM. How public health services pay for radiotherapy in Europe: an ESTRO-HERO analysis of reimbursement. Lancet Oncol 2020;21:e42–54. [DOI] [PubMed] [Google Scholar]
- [34].Cashin C, Chi Y-L, Smith PC, Borowitz M, Thomson S, editors. Paying for performance in health care. Open University Press; 2014. [Google Scholar]
- [35].Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med 2011;104:510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


