Abstract
Background
Disease progression in castrate-resistant prostate cancer (PCa) is most commonly driven by the reactivation of androgen receptor (AR) signaling and involves AR splice variants including ARV7.
Materials and methods
We used the ARV7-positive PCa cell line, 22Rv1, to study the relationship of the PCa marker α-methylacyl-CoA racemase (AMACR), AR, and ARV7 in PCa.
Results
Docetaxel addition but not AMACR inhibition decreased the proliferation of 22Rv1 cells. The combination of AMACR inhibition and docetaxel treatment resulted in a maximum reduction of cell proliferation. The Western blotting analysis revealed that both AR and ARV7 expression were significantly decreased with the use of charcoal-stripped serum following AMACR inhibition and docetaxel treatment. AMACR inhibition and docetaxel treatment in the charcoal-stripped serum condition reduced the proliferation of 22Rv1, possibly via the downregulation of the heat shock protein 27.
Conclusion
Using cell proliferation and Western blot analysis, we demonstrated that AMACR inhibition and docetaxel treatment, under androgen deprivation conditions, significantly reduced the proliferation of ARV7 positive cancer cells and decreased the levels of AR and ARV7 expression, possibly via downregulation of heat shock protein 27.
Keywords: AMACR, ARV7, Docetaxel, HSP27, Prostate cancer
Abbreviations: PCa, prostate cancer; CRPC, castrate-resistant prostate cancer; AR, androgen receptor; AMACR, α-methylacyl-CoA racemase; FBS, fetal bovine serum; CSS, charcoal-stripped serum; HSP27, heat shock protein 27
1. Introduction
Androgen deprivation therapy (ADT) is widely considered as the primary treatment for metastatic prostate cancer (PCa). Although patients are initially responsive to ADT, remission occurs after only 2–3 years1. PCa progresses into castration-resistant PCa (CRPC), which is refractory to ADT. Identifying targetable mechanisms underlying CRPC progression is essential for improving the survival of PCa patients with advanced disease.
CRPC progression is most commonly driven by the re-activation of androgen receptor (AR) signaling2. This has led to the clinical development and approval of potent AR-targeted therapies for patients with metastatic CRPC3. However, in the majority of the cases, treatment with potent AR-targeted therapies results in an activation of the AR signaling and CRPC progression. This can occur through secondary alterations involving the AR gene (e.g., amplification or activating point mutations, AR splice variants), AR bypass, or crosstalk mechanisms (e.g., the glucocorticoid receptor or the PI3K/AKT pathway activation)4.
ARV7 is a major AR splice variant expressed in human PCa and is associated with the development and progression of CRPC5,6. However, the relationship between AR and ARV7 is not well defined.
The α-methylacyl-CoA racemase (AMACR) is a peroxisomal and mitochondrial enzyme capable of racemizing the α-carbon of various α-methylacyl-CoA derivatives7. In the context of urological cancer, AMACR is typically negative or weakly positive in urothelial carcinoma8, whereas Ha YS et al. reported the first case of AMACR-expressing urachal adenocarcinoma arising in the abdominal wall9. Overexpression of AMACR has been identified in PCa and is now used as an immunohistochemical marker such as prostate specific antigen10. However, the function of AMACR in PCa, particularly in CRPC, is yet to be characterized.
In light of this, we decided to examine the functions of ARV7 and AMACR to better understand the mechanism of CRPC progression. We investigated the relationship between AR, ARV7, and AMACR in vitro using chemically synthesized AMACR siRNA and docetaxel treatment.
2. Materials and Methods
2.1. Cell lines
The human PCa cell lines 22Rv1, LNCaP, PC3, and DU145 were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). The human PCa cell line C4-2 was obtained from Dr. L.W. Chung (University of Virginia, Charlottesville, VA, USA). Cells were maintained in RPMI1640 supplemented with 10% fetal bovine serum (FBS) (Life Technologies, Burlington, ON, Canada) or charcoal/dextran-treated FBS (CSS) (Thermo Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin (Life Technologies) at 37°C in a humidified atmosphere containing 5% CO2.
2.2. siRNA transfection in 22Rv1 cells
22Rv1 cells were grown in RPMI1640 medium supplemented with 10% FBS or CSS without antibiotics overnight (Day 0). Chemically synthesized AMACR siRNA or negative control siRNA (B-bridge International, Mountain View, CA, USA) diluted in Opti-MEM I with Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) were added to each well and incubated for 4 h (Day 1). The medium was then changed to RPMI1640 medium supplemented with 10% FBS or CSS for cell assays or Western blotting analysis.
2.3. Cell growth assay
22Rv1 cells (5 × 10³ per well) were seeded into 96-well plates in RPMI1640 medium supplemented with 10% FBS or CSS containing 1% penicillin/streptomycin (Day 1). Cells were cultured for 2 and 4 days, respectively, at 37°C in a humidified atmosphere containing 5% CO2. Cell proliferation was evaluated using the cell counting kit-8 (Dojindo Laboratories, Japan) according to the manufacturer's instructions. The absorbance was measured at 450 nm using a spectrophotometer.
For monitoring cell growth in AMACR siRNA-transfected cells, 22Rv1 cells (1 × 105 per well) were seeded into 6-well plates in RPMI1640 medium supplemented with 10% FBS or CSS without antibiotics overnight (Day 0). Transfection was done with 10 nM of AMACR siRNA or negative control siRNA (Day 1), and cell proliferation was evaluated on Days 2 and 4.
For monitoring cell growth in cells treated with the combination therapy of AMACR siRNA and docetaxel treatment, 22Rv1 cells (1 × 105 per well) were seeded into 6-well plates in RPMI1640 medium supplemented with 10% FBS or CSS without antibiotics overnight (Day 0). At Day 1, 10 nM of AMACR siRNA or negative control siRNA were transfected. On the second day, 1, 2.5, or 10 nM of docetaxel (Sigma-Aldrich, St. Louis, MO, USA) was added to each plate. Cell proliferation was evaluated on Days 3 and 5.
2.4. Migration assay
22Rv1 cells (5 × 105 per well) were seeded in a 100-mm dish and maintained in RPMI1640 medium supplemented with 10% FBS or CSS without antibiotics overnight (Day 0). Transfection with 10 nM of AMACR siRNA or negative control siRNA was performed (Day 1). At Day 3, 1 × 105 22Rv1 cells were cultured in 500 μL of serum-free medium and added to the upper chambers of Transwell Migration Plates (8 μm pore size; Corning, Lowell, MA, USA). Medium supplemented with 20% FBS (1 mL) was added to each lower chamber. After 24 h, the culture inserts were washed with PBS, and cells were fixed with 70% ethanol for 30 min and stained with Giemsa at room temperature for 30 min. For each insert, at least five randomly selected fields were observed using optical microscopy, and the number of migrating cells in each field was counted.
2.5. Western blotting
For the characterization of 22Rv1, LNCaP, DU145, PC3, and C4-2 cells, 20x105 cells from each cell line were seeded into 100-mm dishes in RPMI1640 medium supplemented with 10% FBS and incubated for 48 h.
For the immunoblotting of 22Rv1 lysates after AMACR siRNA transfection, 22Rv1 cells (20 × 105 per well) were seeded in a 100-mm dish and maintained in RPMI1640 medium supplemented with 10% FBS or CSS without antibiotics overnight (Day 0). The cells were harvested at Day 3 for the AMACR siRNA transfection protocol alone and at Day 4 for the combination therapy of AMACR siRNA transfection and docetaxel treatment (10 nM). The cells were collected and lysed using the RIPA buffer (25 mM Tris, 0.1 M NaCl, 1% Triton X-100, 0.5% deoxycholic acid, 0.1% SDS, pH 7.4). Expression changes at the protein level were determined by Western blotting. 40 μg of the total protein from each sample was loaded on NuPAGETM 4%-12% Bis-Tris Protein Gels (Invitrogen). The primary antibodies were as follows: ARV7 (1:1000; RevMAb Biosciences, San Francisco, CA, USA), AMACR (1:500; OriGene Technologies, Inc., Rockville, MD, USA). AR (1:1000), HSP27 (heat-shock protein 27) (1:1000), and β-actin (1:1000) were purchased from Abcam in Cambridge, UK. Images were captured and analyzed using a LuminoGraph I (ATTO, Tokyo, Japan).
2.6. Statistical analysis
All values have been represented as the mean ± SD. Statistical comparison of results was performed using the Student t test. Western blot analyses were performed twice, and all other in vitro experiments were repeated at least in triplicate and analyzed.
3. Results
3.1. Characteristics of 22Rv1 cells
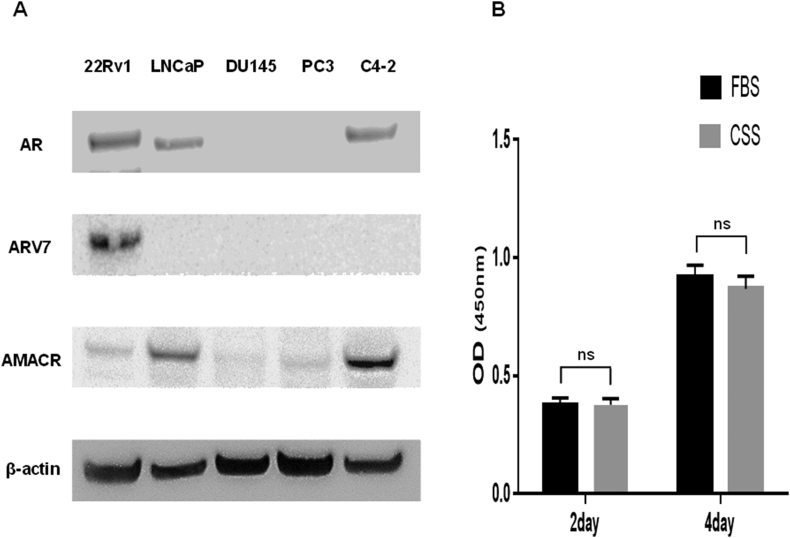
To investigate the characteristics of PCa cell lines, Western blot analysis was performed (Fig. 1A). AR expression was not observed in DU145 or PC3 cells, but it was observed in 22Rv1, LNCaP, and C4-2 cells, whereas ARV7 expression was observed only in 22Rv1 cells. The cell proliferation of 22Rv1 cells in CSS medium was not inhibited on Days 2 or 4 confirming that 22Rv1 was a CRPC cell line (Fig. 1B).
Fig. 1.
Characteristics of 22Rv1 cells (A) Whole cell lysates from 22Rv1, LNCaP, DU145, PC3, and C4-2 cells were subjected to immunoblotting with antibodies against AR, ARV7, AMACR, and β-actin (B) The proliferation of 22Rv1 with the RPMI1640 medium supplemented with 10% FBS or CSS was assessed on Days 2 and 4 using the cell counting kit-8. Histograms represent the mean ± SD (ns: not significant). AMACR, α-methylacyl-CoA racemase; CSS, charcoal-stripped serum; FBS, fetal bovine serum.
3.2. AMACR knockdown by siRNA in 22Rv1 cells
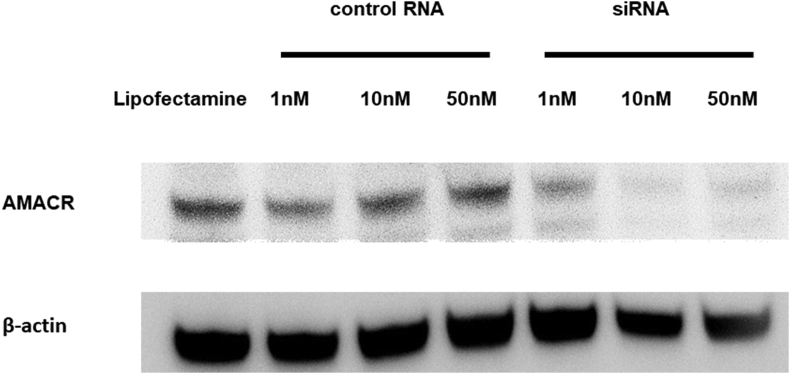
Transfection of chemically synthesized AMACR siRNA into 22Rv1 cells was performed to investigate the biological role of AMACR and ARV7. Western blot analysis was used to determine the specificity and potency of AMACR siRNA in inhibiting the AMACR protein levels in 22Rv1 cells. We treated 22Rv1 cells with AMACR siRNA or negative control siRNA at concentrations of 1 nM, 10 nM, and 50 nM. AMACR expression was stably suppressed at AMACR siRNA concentrations 10 nM and 50 nM as compared with the treatment of the negative control (Fig. 2).
Fig. 2.
Knockdown of AMACR with siRNA transfection in 22Rv1 cells. Western blot analysis of 22Rv1 cells transfected with the chemically synthesized AMACR siRNA or the negative control siRNA at 1 nM, 10 nM, and 50 nM. AMACR, α-methylacyl-CoA racemase.
3.3. Unaffected cell growth of 22Rv1 cells after the treatment of AMACR inhibition
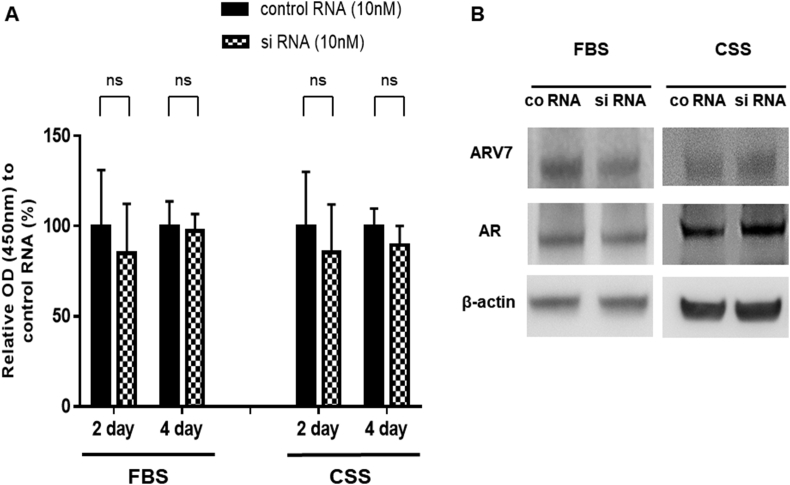
Firstly, we examined the influence of AMACR inhibition on the cell growth of 22Rv1 cells. In medium containing 10% FBS or CSS, 22Rv1 cell growth ratio (Days 2 and 4) after treatment with AMACR siRNA was compared with that of cells transfected with the negative control siRNA. Using either serum, there was no significant difference in 22Rv1 cell growth between the treatment with AMACR siRNA and negative control siRNA on both Days 2 and 4 (Fig. 3A). Western blotting analysis on Day 3 demonstrated that both AR and ARV7 expression were not affected after AMACR inhibition in 22Rv1 cells grown in either FBS or CSS medium (Fig. 3B).
Fig. 3.
Effect of AMACR transfection in 22Rv1 cells (A) The proliferation of 22Rv1 cells transfected with AMACR siRNA (10 nM) or negative control siRNA (10 nM) was assessed on Days 2 and 4 using the cell counting kit-8. The percentage of cells with AMACR siRNA in each group has been expressed as relative proliferation activity with negative control siRNA. Histograms represent the mean ± SD (ns: not significant) (B) Representative pictures of Western blot analysis with 22Rv1 cells after the transfection of AMACR siRNA or negative control siRNA. AR, androgen receptor; AMACR, α-methylacyl-CoA racemase; CSS, charcoal-stripped serum; FBS, fetal bovine serum.
3.4. Effects of AMACR inhibition on 22Rv1 cell migration
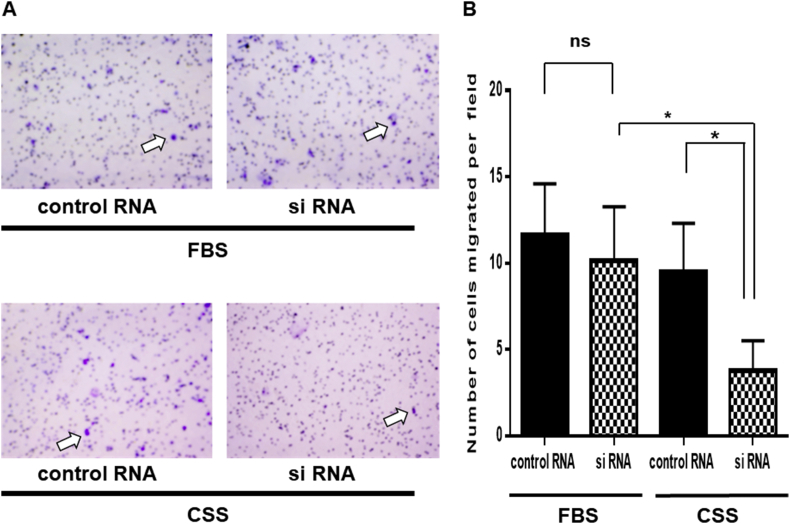
We next evaluated 22Rv1 cell migration on Day 4 after treatment with AMACR siRNA or negative control siRNA. In the Transwell assay, the suppression of 22Rv1 cell migration was only observed in the CSS medium after AMACR inhibition (Fig. 4A). There was a significant difference in 22Rv1 cell migration between the AMACR siRNA and negative control siRNA treatments in the CSS medium. Furthermore, the number of migrating 22Rv1 cells in the CSS medium after AMACR inhibition were significantly decreased compared with the number in the FBS medium (Fig. 4B). These results indicated that AMACR inhibition suppressed the migration of 22Rv1 cells under androgen-deprivation conditions.
Fig. 4.
Effects of AMACR inhibition on 22Rv1 cell migration (A) Migration after treatment with AMACR siRNA or negative control siRNA in the FBS or CSS medium. White arrows indicate 22Rv1 migrated cells (B) Number of 22Rv1 cells that migrated per field after treatment with AMACR siRNA or negative control siRNA in the FBS or CSS medium. Histograms represent the mean ± SD (ns: not significant; ∗P < 0.05). AMACR, α-methylacyl-CoA racemase; CSS, charcoal-stripped serum; FBS, fetal bovine serum.
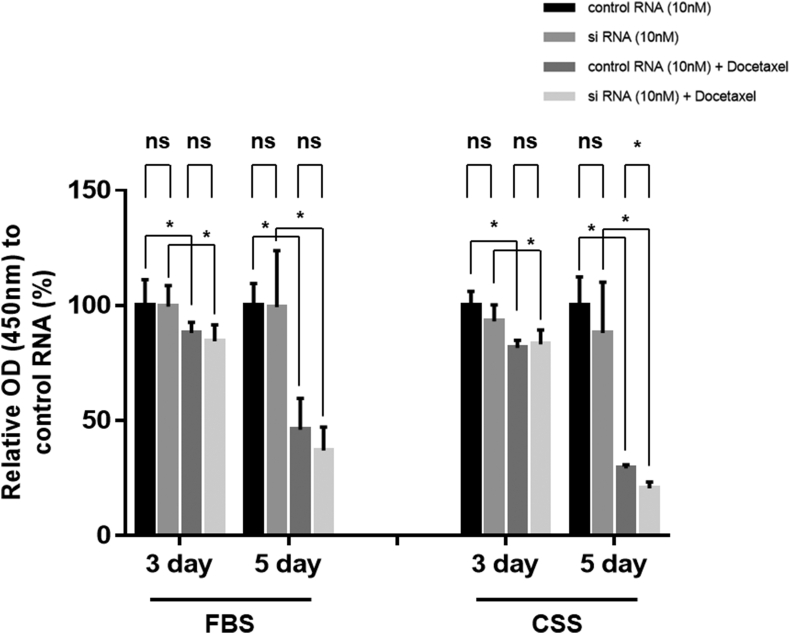
3.5. Reduction of cell numbers in 22Rv1 after combined AMACR inhibition and docetaxel treatment
We then evaluated the cell growth of 22Rv1 cells after combination therapy with AMACR siRNA and docetaxel. One day after the treatment with AMACR siRNA and the negative control siRNA, 10 nM docetaxel was added to the medium containing 10% FBS or CSS, and the cell growth assay was performed on Days 3 and 5. As expected, on both Days 3 and 5, there was no significant difference in cell growth between 22Rv1 cells treated with AMACR siRNA and the negative control siRNA in either FBS or CSS medium. The cell growth of 22Rv1 cells, in both FBS and CSS media, after docetaxel treatment was significantly reduced. Moreover, cell proliferation significantly decreased after docetaxel treatment using only 10% CSS medium in 22Rv1 cells treated with AMACR siRNA compared with 22Rv1 cells treated with the negative control siRNA (Fig. 5).
Fig. 5.
Effect of the combination therapy of AMACR transfection and docetaxel treatment in 22Rv1 cells. The proliferation of 22Rv1 cells with AMACR transfection alone or the combination therapy of AMACR transfection and docetaxel treatment were assessed on Days 3 and 5 using the cell counting kit-8. The percentage of cells with 22Rv1 in each group has been expressed as relative proliferation activity with the negative control siRNA alone. Histograms represent the mean ± SD (ns: not significant; ∗P < 0.05). AMACR, α-methylacyl-CoA racemase; CSS, charcoal-stripped serum; FBS, fetal bovine serum.
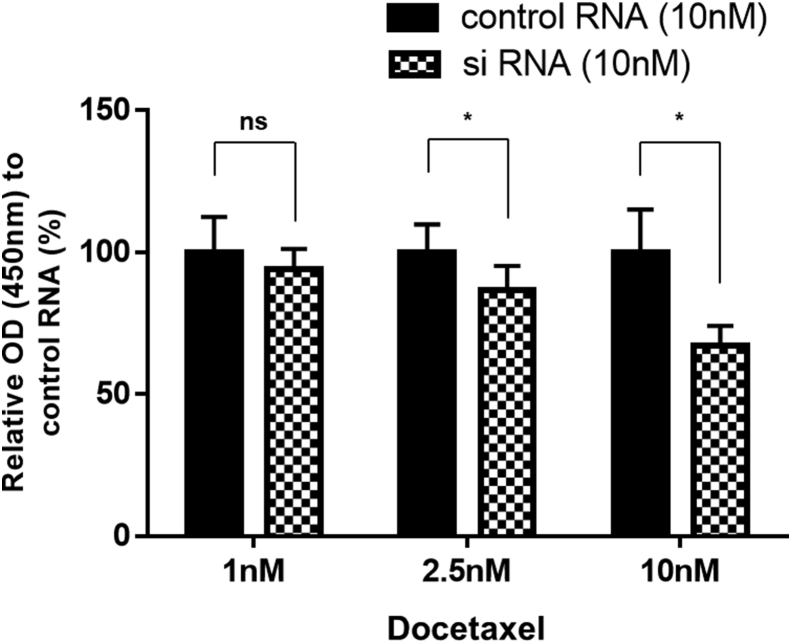
3.6. Docetaxel dose-dependent cell reduction of 22Rv1 after AMACR inhibition
To support the result of the significantly reduced growth of 22Rv1 cells after the combination therapy of AMACR inhibition and docetaxel treatment at 5 days in 10% CSS medium, another experiment was performed. The experiment used different concentrations of docetaxel (1, 2.5, 10 nM) and 10 nM of AMACR siRNA or negative control siRNA. After the combination therapy of AMACR inhibition and docetaxel treatment, a docetaxel dose-dependent cell reduction of 22Rv1 growth was observed. There was a significant difference between AMACR siRNA and the negative control siRNA using 2.5 nM and 10 nM of docetaxel (Fig. 6). These results suggested that the inhibition of 22Rv1 growth in the CSS medium involved the synergistic effect between AMACR inhibition and docetaxel treatment.
Fig. 6.
The synergistic effect between AMACR inhibition and docetaxel treatment in 22Rv1 cells. The proliferation of 22Rv1 cells with different concentrations of docetaxel (1, 2.5, and 10 nM) and 10 nM of AMACR siRNA or negative control siRNA were assessed in the CSS medium on Day 5 using the Cell Counting Kit-8. The percentage of cells with 22Rv1 in each group is expressed as relative proliferation activity with the negative control siRNA. Histograms represent the mean ± SD (ns: not significant; ∗P < 0.05). AMACR, α-methylacyl-CoA racemase.
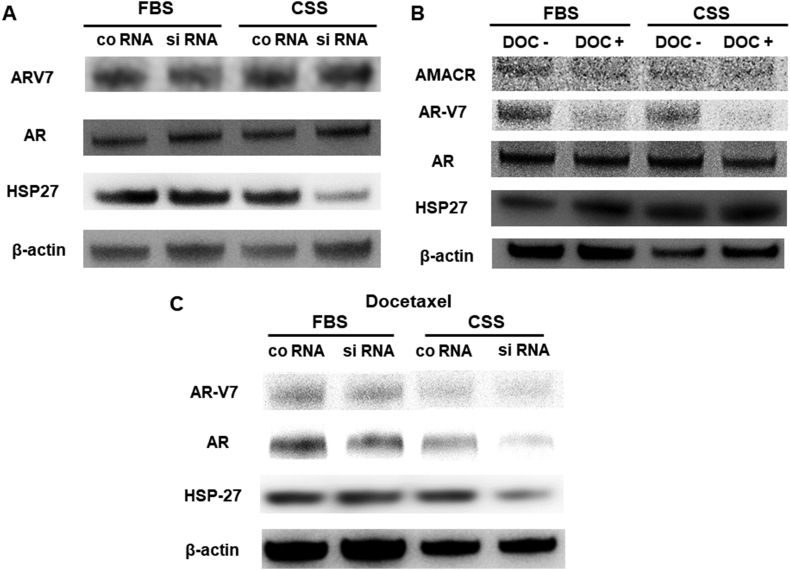
3.7. Decreased expression of AR and ARV7, possibly via HSP27 reduction, in CSS medium after combination therapy of AMACR inhibition and docetaxel treatment
To understand the changes in 22Rv1 cell proliferation following a combination therapy of AMACR inhibition and docetaxel treatment (10 nM), the expression of AMACR, HSP27, AR, and ARV7 were examined by Western blot analyses. Both ARV7 and AR expression levels were not changed in FBS or CSS medium in the presence of AMACR inhibition, whereas only HSP27 expression in CSS medium was decreased as compared with other conditions (Fig. 7A). When 22Rv1 cells were treated with docetaxel, the expression of AMACR, AR, and HSP27 was not affected. However, ARV7 expression was decreased compared with no treatment in FBS or CSS medium (Fig. 7B). The expression of ARV7, AR, and HSP27 was decreased after the combination therapy of AMACR inhibition and docetaxel treatment in the CSS medium (Fig. 7C). Our cell proliferation assays and Western blotting results suggested that 22Rv1 cell growth was reduced by the combination therapy of AMACR inhibition and docetaxel treatment with a decreased expression of AR and ARV7, possibly via the HSP27 pathway, under androgen-deprivation conditions.
Fig. 7.
Representative pictures of Western blot analysis of 22Rv1 cells (A) Western blot analysis of 22Rv1 cells after AMACR siRNA transfection alone (B) Western blot analysis of 22Rv1 cells after docetaxel treatment alone (C) Western blot analysis of 22Rv1 cells after the combination therapy of AMACR siRNA transfection and docetaxel treatment. AMACR, α-methylacyl-CoA racemase
4. Discussion
Patients with CRPC currently receive next-generation hormone treatments such as an AR-targeted therapy (abiraterone or enzalutamide)11 or chemotherapies such as those with docetaxel12,13. In recent years, the role of AR mutations in the development of PCa has been demonstrated in a number of studies14. Resistance to ADT in PCa has been associated with alternative splicing of the AR and the expression of truncated and structurally active ARV7. ADT-induced AR gene transcription and the subsequent recruitment of splicing factors to the AR pre-mRNA contribute to an ARV7 splicing in PCa cells15. Moreover, an increased AR gene copy number was associated with an increased ARV7 mRNA expression in CRPC metastases16. However, the relationship between AR and ARV7, especially in CRPC, is not well understood. In this study, we focused on the PCa marker AMACR and investigated its relationship with AR and ARV7 in CRPC cells.
A previous study has demonstrated that a decrease in the level of the AMACR expression results in the decreased proliferation of prostate adenocarcinoma cells and that the function and expression of AMACR are independent of the AR-mediated signaling. However, the growth inhibition of PCa can be accelerated by androgen ablation to achieve an additional growth-inhibitory effect17, following the unclear function of AMACR in PCa cells. We have previously reported that AMACR inhibition might induce an increase in the expression of AR and the conversion of PCa cells from being hormone-independent to being hormone-dependent18.
Docetaxel and cabazitaxel are used as standard chemotherapeutics for the treatment of patients with naïve CRPC13,19, 20, 21, 22. Despite the clinical availability of docetaxel, cabazitaxel, and newer-generation taxane chemotherapeutics, CRPC often develops resistance to these agents20,23. Therefore, combination therapies consisting of docetaxel/cabazitaxel and other targeting agents should be tested for patients with CRPC.
We performed a combination therapy of AMACR inhibition and docetaxel treatment in CRPC cells, focusing on the expression of AR and ARV7. In the present study, the growth of the ARV7-positive cell line, 22Rv1, was not changed by AMACR inhibition alone in either FBS or androgen-depleted CSS medium. AR and ARV7 expression levels remained constant as well. However, our migration assay revealed that AMACR inhibition suppressed the migration of 22Rv1 cells under androgen-deprivation conditions. We next evaluated the growth of 22Rv1 cells after combination therapy with AMACR siRNA and docetaxel. The proliferation of 22Rv1 cells was significantly decreased after treatment with docetaxel alone. Of note, the growth of 22Rv1 cells after the combination therapy of AMACR inhibition and docetaxel treatment did not change as compared with the docetaxel treatment alone in the normal FBS medium, although cell growth was significantly reduced in a docetaxel dose-independent manner in the CSS medium.
To further elucidate these findings observed in our cell proliferation assays and Western blotting results, we then analyzed the expression of HSP27 in 22Rv1 cells. The overexpression of HSP27, a cytoprotective chaperone protein, has been identified in many cancers including PCa. The overexpression of HSP27, which is a chaperone protein important for AR to remain stable, is also associated with a poor prognosis and castration resistance. The role of HSP27 in AR signaling has recently been investigated24,25. Kiliccioglu et al. reported that the expression of AR and AR-V7 was significantly reduced when HSP27 was inhibited together with NF-κB, suggesting that targeting ARV7, the NF-κB pathway, and the HSP27 together had a great potential in the treatment of an advanced stage PCa24.
In our Western blotting analyses, HSP27 expression in 22Rv1 cells was not decreased by docetaxel treatment but by an AMACR inhibition, especially in the presence of an androgen-depleted CSS medium. In contrast, ARV7 expression in 22Rv1 cells was not decreased by an AMACR inhibition but by docetaxel treatment in both normal FBS and CSS media. However, the expression of AR after docetaxel treatment was not affected regardless of the medium conditions. The expression of ARV7, AR, and HSP27 was decreased, especially after the combination therapy of AMACR inhibition and docetaxel treatment in the CSS medium. These Western blotting analyses strongly support our results of proliferation assays. They demonstrated that a significant difference in 22Rv1 cell reduction between the treatment with AMACR siRNA and the negative control siRNA was observed after the docetaxel treatment using only CSS medium. With respect to the previous report by Kiliccioglu et al. which demonstrated that the activations of HSP27 and NF-κB pathways were associated with AR/ARV7 expression in PCa cells, our results of Western blot analyses were consistent with those findings. Again, because HSP27 expression only decreased in the androgen-depleted CSS medium after an AMACR inhibition in 22Rv1 cells, the cell proliferation was significantly reduced with decreasing levels of AR and ARV7 expression, possibly via downregulation of HSP27, when performing the combination therapy of AMACR inhibition and docetaxel treatment in the androgen-deprivation condition.
It remains unclear why the expression of HSP27 was decreased only under androgen-deprivation conditions after an AMACR inhibition. However, considering that AMACR may be an important factor whose inhibition induces the conversion of PCa cells from being hormone independent to being hormone dependent18, the characteristics of HSP27 might have changed as well as AMACR inhibition, following the significant decrease in expression under androgen-deprivation conditions.
In conclusion, our in vitro experiments demonstrated that cell proliferation of ARV7 positive cancer cells was significantly reduced through decreased levels of AR and ARV7 expression, possibly via downregulation of HSP27, through AMACR inhibition and docetaxel treatment under androgen-deprivation conditions. Although further studies are needed, the combination therapy of AMACR inhibition and docetaxel treatment may be useful for CRPC patients.
Conflicts of interest
The authors disclose no potential conflicts of interest.
References
- 1.Kassi E., Moutsatsou P. Glucocorticoid receptor signaling and prostate cancer. Cancer Lett. 2011;302:1–10. doi: 10.1016/j.canlet.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Watson P.A., Arora V.K., Sawyers C.L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–711. doi: 10.1038/nrc4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham L., Schweizer M.T. Targeting persistent androgen receptor signaling in castration-resistant prostate cancer. Med Oncol. 2016;33:44. doi: 10.1007/s12032-016-0759-3. [DOI] [PubMed] [Google Scholar]
- 4.Vlachostergios P.J., Puca L., Beltran H. Emerging Variants of Castration-Resistant Prostate Cancer. Curr Oncol Rep. 2017;19:32. doi: 10.1007/s11912-017-0593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornberg E., Ylitalo E.B., Crnalic S., Antti H., Stattin P., Widmark A. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu R., Lu C., Mostaghel E.A., Yegnasubramanian S., Gurel M., Tannahill C. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–3462. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferdinandusse S., Denis S., L I.J., Dacremont G., Waterham H.R., Wanders R.J. Subcellular localization and physiological role of alpha-methylacyl-CoA racemase. J Lipid Res. 2000;41:1890–1896. [PubMed] [Google Scholar]
- 8.Suh N., Yang X.J., Tretiakova M.S., Humphrey P.A., Wang H.L. Value of CDX2, villin, and alpha-methylacyl coenzyme A racemase immunostains in the distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Mod Pathol. 2005;18:1217–1222. doi: 10.1038/modpathol.3800407. [DOI] [PubMed] [Google Scholar]
- 9.Ha Y.S., Kim Y.W., Min B.D., Lee O.J., Kim Y.J., Yun S.J. Alpha-methylacyl-coenzyme a racemase-expressing urachal adenocarcinoma of the abdominal wall. Korean J Urol. 2010;51:498–500. doi: 10.4111/kju.2010.51.7.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J., Stolk J.A., Zhang X., Silva S.J., Houghton R.L., Matsumura M. Identification of differentially expressed genes in human prostate cancer using subtraction and microarray. Cancer Res. 2000;60:1677–1682. [PubMed] [Google Scholar]
- 11.Attard G., Parker C., Eeles R.A., Schroder F., Tomlins S.A., Tannock I. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 12.Petrylak D.P., Tangen C.M., Hussain M.H., Lara P.N., Jr, Jones J.A., Taplin M.E. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 13.Tannock I.F., de Wit R., Berry W.R., Horti J., Pluzanska A., Chi K.N. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 14.Brooke G.N., Bevan C.L. The role of androgen receptor mutations in prostate cancer progression. Curr Genomics. 2009;10:18–25. doi: 10.2174/138920209787581307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho Y., Dehm S.M. Androgen Receptor Rearrangement and Splicing Variants in Resistance to Endocrine Therapies in Prostate Cancer. Endocrinology. 2017;158:1533–1542. doi: 10.1210/en.2017-00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henzler C., Li Y., Yang R., McBride T., Ho Y., Sprenger C. Truncation and constitutive activation of the androgen receptor by diverse genomic rearrangements in prostate cancer. Nat Commun. 2016;7:13668. doi: 10.1038/ncomms13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zha S., Ferdinandusse S., Denis S., Wanders R.J., Ewing C.M., Luo J. Alpha-methylacyl-CoA racemase as an androgen-independent growth modifier in prostate cancer. Cancer Res. 2003;63:7365–7376. [PubMed] [Google Scholar]
- 18.Takahara K., Azuma H., Sakamoto T., Kiyama S., Inamoto T., Ibuki N. Conversion of prostate cancer from hormone independency to dependency due to AMACR inhibition: involvement of increased AR expression and decreased IGF1 expression. Anticancer Res. 2009;29:2497–2505. [PubMed] [Google Scholar]
- 19.de Bono J.S., Oudard S., Ozguroglu M., Hansen S., Machiels J.P., Kocak I. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 20.Galletti G., Leach B.I., Lam L., Tagawa S.T. Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat Rev. 2017;57:16–27. doi: 10.1016/j.ctrv.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Higano C.S., Crawford E.D. New and emerging agents for the treatment of castration-resistant prostate cancer. Urol Oncol. 2011;29:S1–S8. doi: 10.1016/j.urolonc.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Paller C.J., Antonarakis E.S. Cabazitaxel: a novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Des Devel Ther. 2011;5:117–124. doi: 10.2147/DDDT.S13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hongo H., Kosaka T., Oya M. Analysis of cabazitaxel-resistant mechanism in human castration-resistant prostate cancer. Cancer Sci. 2018;109:2937–2945. doi: 10.1111/cas.13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiliccioglu I., Konac E., Dikmen A.U., Sozen S., Bilen C.Y. Hsp-27 and NF-kappaB pathway is associated with AR/AR-V7 expression in prostate cancer cells. Gene. 2019;697:138–143. doi: 10.1016/j.gene.2019.02.055. [DOI] [PubMed] [Google Scholar]
- 25.Rocchi P., So A., Kojima S., Signaevsky M., Beraldi E., Fazli L. Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res. 2004;64:6595–6602. doi: 10.1158/0008-5472.CAN-03-3998. [DOI] [PubMed] [Google Scholar]