Abstract
Introduction
In the present study, we aim to provide more evidence about benefits of salvage radical prostatectomy (SRP). Our main objective is to assess prostatic-specific antigen control and postoperative urinary incontinence in open and robotic approaches as primary outcomes.
Materials and methods
After the Institutional Review Board approval (IRB00010193), we retrospectively analyzed 76 consecutive patients who underwent open or robot-assisted SRP for locally relapsed prostate cancer between 2004 and 2019 at the Urology Department of Hospital Italiano de Buenos Aires, Argentina. Data were collected from our electronic medical record and prospective database.
Postoperative variables, such as urinary incontinence, erectile function preservation, and vesicourethral anastomosis stricture development, were analyzed.
Results
Before SRP, 59 patients (76.6%) were treated with 3D external beam radiotherapy, 11 (14.3%) with brachytherapy, and 6 (7.8%) with intensity-modulated radiotherapy. Fifty patients underwent open SRP, and 26, robot-assisted SRP. Comparing surgical approaches, the global incontinence rate was 34.2% versus 9.1% in open versus robot-assisted approach, respectively (p: 0.01).
Vesicourethral anastomosis stricture occurred in six patients (8.7%), all in the open approach group (p: 0.07). Five patients of 69 (7.2%) preserved erectile function with/without use of phosphodiesterase 5 inhibitors. Two patients in the open approach group needed blood transfusion. Estimated 2-year biochemical recurrence–free survival rate in the open approach group and robot-assisted group was 67% (95% confidence interval: 53.7–80.3) and 60.9% (95% confidence interval: 40.5–81.3), respectively, with no statistical difference (log-rank test p: 0.873).
Conclusions
Robot-assisted SRP is a reliable procedure to treat local recurrences after external beam radiotherapy or brachytherapy, reducing the risk of anastomotic strictures and blood loss and improving continence outcomes.
Keywords: Urinary incontinence, Prostate cancer, Radiotherapy, Robotic surgery, Salvage radical prostatectomy
1. Introduction
Prostate cancer (PCa) represents the most commonly diagnosed noncutaneous cancer in men worldwide1. External beam radiotherapy (EBRT) or brachytherapy (BT) are well-known treatment modalities for newly diagnosed patients with clinically localized PCa. Nearly 30% to 60% of the patients will undergo biochemical recurrence (BCR) within the first 5–10 years after treatment2,3. In the absence of any salvage therapy after proven relapse, the median interval from BCR to development of distant metastases is approximately 3 years4; thus, these patients have an important opportunity for a definitive curative treatment. However, curative options are rarely contemplated as 90% of men with recurrence are treated with palliative systemic androgen deprivation therapy (ADT), experiencing related adverse effects and losing cancer control opportunity5.
Even more, most of them develop castration resistance at 5 years on average6,7.Salvage radical prostatectomy (SRP) is a technically demanding and challenging surgery. Historical open SRP series were associated with a higher morbidity rate; rectal injury could be observed in 19% of patients, urinary extravasations in 5%, bladder neck stricture in 40%, and persistent postprostatectomy urinary incontinence (UI) in 5-76.9% 8,9. Oncologic results were also discouraging, with positive surgical margin (PSM) rates ranging 70% and BCR rates at 10 years of 82%5.
Despite the widespread use of robotic surgery, at present only a small number of robotic SRP series have been published, showing significant improvements in functional outcomes and decreases in complications. A recent large multicenter study showed a higher degree of continence preservation, reaching 63.9%, and a reduced anastomotic stricture rate of 7.6%, all of them in the robotic surgery group5.
However, the European Association of Urology guidelines advise that strong recommendations regarding SRP cannot be made, as the available evidence for this treatment option is scarce and of very low quality10.
In the present study, we aim to provide more evidence about benefits of SRP.
Our main objective is to assess prostatic-specific antigen (PSA) control and postoperative UI in open and robotic approaches as primary outcomes.
2. Materials and Methods
After Institutional Review Board approval (IRB00010193), we retrospectively analyzed 76 consecutive patients who underwent open or robot-assisted SRP for locally relapsed PCa between August 2004 and March 2019 at the Urology Department of Hospital Italiano de Buenos Aires, Argentina. Our center has a mean caseload of 200 radical prostatectomies per year, and SRP was performed only by three experienced surgeons beyond their learning curve.
Data were collected from our electronic medical record and the prospective database. All patients underwent confirmatory prostate biopsy before SRP.
Postoperative variables, such as UI, erectile function preservation, and vesicourethral anastomosis stricture development, were analyzed.
2.1. Surgical approach
Open SRP was performed using the standard retropubic technique. Robot-assisted SRP was performed using the transperitoneal approach with the da Vinci Si HD Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). Extended lymph-node dissection was attempted in all cases. Preservation of the neurovascular bundles was attempted only with patients that were preoperatively potent, when it was oncologically feasible and in accordance with intraoperative findings. For neurovascular bundle preservation, dissection was always performed in an interfascial fashion.
2.2. Definitions
BCR after radiotherapy (RT) is defined by the American Society for Therapeutic Radiation and Oncology as a rise in serum PSA by 2 ng/ml from a nadir PSA. BCR after SRP was defined as PSA ≥0.2 ng/ml, followed by a subsequent confirmatory PSA value ≥ 0.2 ng/ml11.
The definition of continence was based on the response to “How many pads per day did you usually use to control urinary leakage?“. Continence was assessed at 12 months and defined as the use of no pads. Mild incontinence was defined as the use of 1 pad and moderate/severe more than 1 pad per day.
Potency was defined as the ability to achieve and maintain erections firm enough for sexual intercourse, with or without the use of phosphodiesterase 5 (PDE-5) inhibitors.
Intraoperative and postoperative complications within 30 days were rigorously recorded and scored as per the Clavien–Dindo system.
2.3. Statistical analysis
Continuous variables were presented as median and interquartile range (IQR) and for their comparison Mann–Whitney was used. Categorical variables were summarized as counts (frequency percentages), and they were compared with the Chi-square test or the Fisher's exact test when appropriate.
Univariate analysis for 1-year UI was performed by multinomial logistic regression because all patients were evaluated at this time. Regression results were expressed as the odds ratio (OR) with 95% confidence interval (CI 95%).
Survival curves were presented as Kaplan–Meier curves, and the log-rank test was used for comparison between groups. All of the analyses were considered significant at a two-tailed P-value of ≤0.05.
All statistical tests were performed using statistical software SPSS 23.0 TM for Microsoft (SPSS Inc; IBM, Chicago, IL) and STATA 8.0 TM version for Microsoft (Statacorp LP, College Station,TX).
3. Results
A total of 76 patients were included in the study, with a median age at time of salvage prostatectomy of 64.5 years (IQR: 60-68). Before SRP, 59 patients (77.6%) received 3D EBRT, 11 (14.5%) BT, and 6 (7.9%) intensity-modulated radiotherapy. Gleason score ≥7 was observed in 39 patients (51.3%) on preradiotherapy biopsy. Regarding the Gleason 7 group, 27 (84.3%) patients received a combination of ADT plus RT and five patients (15.6%) RT alone. In the Gleason 8 group, one patient received RT alone and six patients (85.7%) ADT plus RT.
Clinicopathological features of patients who underwent SRP are summarized in Table 1.
Table 1.
Clinicopathological features of patients who underwent SRP.
| Variable | Total (n:76) | Open (n:50) | Robot-Assisted (n:26) | p-Value |
|---|---|---|---|---|
| Age pre-RT, median (IQR) | 59 (55-62) | 60 (56-63) | 57 (54-62) | 0.073 |
| cTNM pre-SRP (%) | 0.659 | |||
| T1c | 43 (56.6) | 30 (60) | 13 (50) | |
| T2a | 9 (11.8) | 7 (14) | 2 (7.7) | |
| T2b | 17 (22.4) | 9 (18) | 8 (30.8) | |
| T2c | 5 (6.6) | 3 (6) | 2 (7.7) | |
| T3 | 2 (2.6) | 1 (2) | 1 (3.8) | |
| Radiotherapy subtype (%) | 0.207 | |||
| Brachytherapy | 11 (14.5) | 8 (16) | 3 (11.5) | |
| 3D EBRT | 59 (77.6) | 40 (80) | 19 (73.1) | |
| IMRT | 6 (7.9) | 2 (4) | 4 (15.4) | |
| Gleason sum pre-RT (%) | 0.524 | |||
| 6 | 37 (48.7) | 25 (50%) | 12 (46.2) | |
| 7 | 32 (42.1) | 22 (44) | 10 (38.5) | |
| 8 | 7 (9.2) | 3 (6) | 4 (15.4) | |
| Gleason sum pre-SRP (%) | 0.514 | |||
| 6 | 3 (3.9) | 3 (6) | 0 | |
| 7 | 33 (43.4) | 24 (48) | 9 (34.6) | |
| 8 | 26 (34.2) | 15 (30) | 11 (42.3) | |
| 9 | 14 (18.4) | 8 (16) | 6 (23.1) | |
| ESD pre SRP (%) | 17 (22.4) | 13 (26) | 4 (15.4) | 0.292 |
| PSA pre RT, median (IQR) | 8 (6.6-11) | 8.1 (6.1-10.8) | 8 (7.2-11) | 0.217 |
| PSA pre SRP, median (IQR) | 6.4 (4.1-8.4) | 6.1 (4-7.9) | 6.5 (4.2-10.3) | 0.350 |
| PSA DT median (IQR), months | 14.7 (8-25.7) | 16.5 (8.2-28.8) | 11.6 (7.4-21.8) | 0.359 |
| Post RT relapse time, median (IQR), months | 42 (24-60) | 42 (24.7-72) | 39 (24-60) | 0.564 |
EBRT: external beam radiotherapy; ESD: erectile dysfunction; IMRT, intensity-modulated radiotherapy; PSA DT: prostatic-specific antigen doubling time; RT: radiotherapy; SRP: salvage radical prostatectomy.
A large proportion of patients had high-risk PCa in pathological specimens after SRP. Gleason score 8 or greater (International Society of Urologic Pathologists "ISUP" grade 4/5) was observed in 68.4% and locally advanced disease (pT3a/pT3b) in 60%. PSMs were observed in 28.9% of the patients, overall. Extended lymph node dissection was attempted in all cases and achieved in 72 patients (94.7%). In the remaining four patients, lymph node dissection could not be performed because of extreme fibrosis in the surgical field. Involved lymph nodes were found in five patients (6.6%).
Postoperative complications Clavien–Dindo grade III-V were observed in seven patients (9.2%). Four patients developed urosepsis, and two patients developed atrial fibrillation with rapid ventricular response; all of them resolved with medical treatment. One patient developed hematuria and was treated with cystoscopy for clot evacuation.
With a median bladder catheterization time of 15 days, urinary leakeage was infrequent and observed only in one patient in the robotic group. Open reanastomosis was performed and patient recovered uneventfully.
Rectal injury was observed in one patient (3.8%) in the robotic group, p: 0.16. Injury was recognized intraoperatively and repaired primarily with two-layer rectal wall closure as patient had previous bowel preparation. Postoperative was uneventful.
Although bleeding is more frequent in open surgery, only two patients (4%) in the open group required blood transfusion. All perioperative results are summarized in Table 2.
Table 2.
Perioperative results.
| Variable | Total (n:76) | Open (n:50) | Robot-assisted (n:26) | p-value |
|---|---|---|---|---|
| pTNM (%) | 0.451 | |||
| pT2 | 25 (32.9) | 19 (38) | 6 (23.1) | |
| pT3a | 22 (28.9) | 13 (26) | 9 (34.6) | |
| pT3b | 24 (31.6) | 14 (28) | 10 (38.5) | |
| pT3b N1 | 5 (6.6) | 4 (8) | 1 (3.8) | |
| Gleason sum post-SRP (%) | 0.466 | |||
| 7 | 24 (31.6) | 18 (36) | 6 (23.1) | |
| 8 | 28 (36.8) | 18 (36) | 10 (38.5) | |
| 9 | 24 (31.6) | 14 (28) | 10 (38.5) | |
| Positive SM (%) | 22 (28.9) | 15 (30) | 7 (26.9) | 0.779 |
| Rectal injury (%) | 1 (1.3) | 0 | 1 (3.8) | 0.163 |
| Clavien-Dindo III-V (%) | 7 (9.2) | 4 (8) | 3 (11.5) | 0.613 |
| EBL median (IQR), ml | 150 (50-350) | 200 (50-400) | 150 (50-250) | 0.122 |
| Transfusion rate (%) | 2 (2.6) | 2 (4) | 0 | 0.301 |
| HS median (IQR), days | 3 (2-4) | 3 (2-4) | 2 (2-3) | 0.127 |
| Catheterization, days, median (IQR) | 15 (13-21) | 20 (15-21) | 13 (11-14) | 0.01 |
SM: surgical margin; pTNM: pathological tumor-node-metastasis; EBL: estimated blood loss; HS: hospital stay. Bold value indicates p-value less than 0.05 is statistically significant.
Median follow-up time was 47 months (IQR: 18.5-81); 68 patients had at least 1 year of follow-up. In this group, UI, erectile function, and development of vesicourethral anastomosis stricture were analyzed.
3.1. Urinary continence after SRP
All patients were continent before SRP. The global UI rate at 12 months was 26.4% (18 out of 68 patients). Comparing surgical approaches, the UI rate was 34.2% (16 patients: 10 mild; and 6 patients: moderate/severe grade) versus 9.1% (two patients, both mild grade) in the open versus robot-assisted approach, respectively (p: 0.01).
The UI OR in the robot-assisted versus open approach was 0.16 (CI 95%: 0.03–0.78, p: 0.023). History of BT as primary treatment also might result as a UI predictor (OR: 4.8, CI 95%: 1.1–20). Overall, three patients required an artificial urinary sphincter, with good functional outcomes.
Regarding severe UI cases, all of these occurred in the open surgery group (13%), OR: 7.2 (CI 95%: 0.3–134, p = 0.184). For this degree of UI, on univariate analysis, history of urethrovesical anastomosis stricture (OR: 7.2, CI 95%: 1–52, p 0.05) and time to bladder catheter removal (OR: 1.3, CI 95%: 1–1.7, p 0.05) may result also as predictors.
3.2. Erectile function after SRP
After SRP, 17 patients (22.1%) had erectile dysfunction. Interfascial neurovascular bundles preservation was attempted in 11 patients of 51 previously potent. Overall, five patients of 51 (9.8%) preserved their erectile function with/without use of PDE-5 inhibitors. In the nerve sparing and non-nerve sparing groups, three versus two patients preserved erectile function, respectively (p:0.027).
Stratifying patients into robot-assisted versus open approach, erectile function was conserved in one patient (4.5%) versus four (8.7%), respectively (p: 0.540), with the OR in the robot-assisted approach of 0.5 (CI 95%: 0.05–4.8, p:0.547).
3.3. Vesicourethral anastomosis stricture
This event occurred in six patients (8.8%); all of them were in open approach. p: 0.076.
3.4. Oncological outcomes
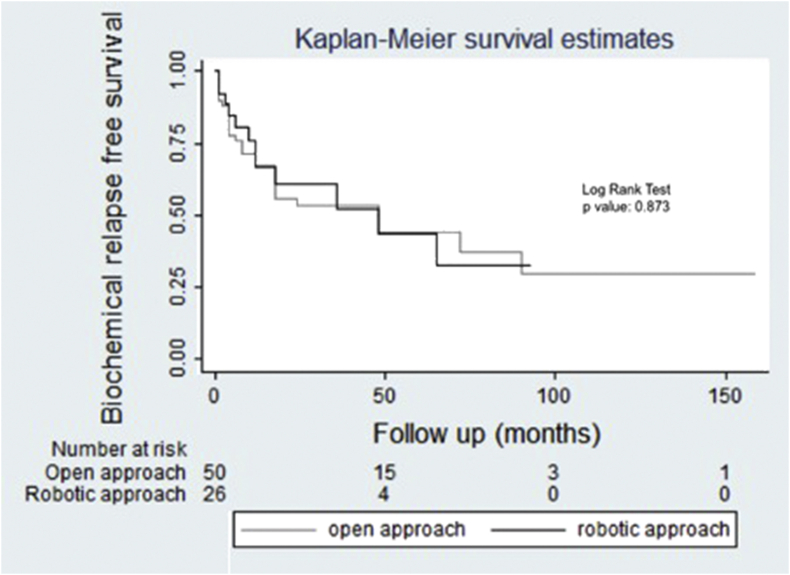
Estimated 2-year BCR free survival rate in the open approach group and robot-assisted group was 67% (CI 95%: 53.7–80.3) and 60.9% (CI 95%: 40.5–81.3), respectively, with no statistical difference (log-rank test p: 0.873), Fig. 1.
Fig. 1.
Kaplan–Meier survival curves expressing the estimated BCR-free survival rate.
Regarding the five patients with lymph node involvement (N+), four of them had a PSA value after SRP lower than 0.20 ng/ml. Three patients with N+ developed BCR and were treated with ADT. Mean time to ADT administration in this group was 25 months.
During follow-up, there were three deaths (two in the open versus one in the robot-assisted group) at 12, 39, and 65 months, respectively. The overall cancer-specific survival (CSS) was 95% at 5 years. We did not evidence any local recurrence after SRP.
4. Discussion
SRP has always been reserved for a minority group of patients after BCR because of high complications and postoperative morbidity rates11. For appropriately selected patients, SRP provides excellent cancer control without the addition of ADT. Therefore, SRP should be considered only for patients with low comorbidity, a life expectancy of at least 10 years, a pre-SRP PSA <10 ng/mL and biopsy ISUP grade <2/3, no lymph node involvement or evidence of distant metastatic disease pre-SRP, and those whose initial clinical staging was T1 or T210.
As robotic surgery techniques for prostate cancer treatment progressed, recent series started showing promising improvements regarding functional outcomes in the SRP setting. Until now, surgical outcomes reported in the literature on the open and robotic approach in SRP are limited. In Table 3, the most relevant published series are compared. To our knowledge, we are the first institution in Latin America in reporting a single tertiary cancer center experience in open and robot-assisted SRP and showing improvements in terms of continence and anastomotic stricture.
Table 3.
Comparison of published salvage radical prostatectomy series.
| Author | Year | Patients; n | Approach | BCR % | PSM% | LN +, % | Overall urinary incontience % | Overall anastomotic stricture % |
|---|---|---|---|---|---|---|---|---|
| Eandi21 | 2010 | 18 | Robotic | 67 | 28 | 5.5 | 67 | 17 |
| Heidenreich9 | 2010 | 55 | Open/lap | 87 | 11 | 20 | 19 | 11 |
| Chade17 | 2011 | 404 | Open | 37 | 25 | 16 | - | - |
| Zugor22 | 2014 | 13 | Robotic | 46 | 0 | - | 46 | 0 |
| Kenney23 | 2016 | 39 | Open/robotic | 30 | 15.3 | 12.8 | 25.6 | |
| Gontero5 | 2019 | 395 | Open/robotic | - | - | 15.7 | 42.5 | 11.85 |
| Present Study | 2020 | 76 | Open/robotic | 67 | 28.9 | 6.6 | 26.4 | 8.8 |
BCR: biochemical recurrence; PSM: positive surgical margin; LN+: lymph node involved; Lap: laparoscopic.
RT induces a wide variety of short- to long-term changes in the prostate and surrounding tissues, from neo-angiogenesis to fibrosis; thus, tissues are frailer, adhesions are more frequent, and healing becomes less effective, altering surgical planes and anatomical landmarks, than a nonirradiated pelvis15. As a consequence, postoperative complications and UI risk may be increased in SRP compared with first-line radical prostatectomy, reflecting the technical challenge and the high surgical complexity.
Regarding blood transfusions, only open SRP was associated with an increased requirement (4%), whereas none of the patients in the robotic group had significant blood loss. Recently, Gontero et al5 published a similar blood transfusion rates, 6.5% versus 2.7% in open versus robotic SRP, respectively.
Vesicourethral anastomosis stricture is one of the most feared complications, which needs, in the vast majority of the cases, additional surgical procedures, leading sometimes to severe incontinence that can only be controlled with an artificial urinary sphincter or urinary diversion12. Series describing functional outcomes of robotic SRP after primary treatment confirm that complications such as anastomotic stricture are frequent, ranging from 11% to 25.6%13 as shown in Table 3. Contrarily, Kaffenberger et al.14 reported a lower risk of anastomotic stricture in robotic SRP, ranging from 0 to 17% versus 11% to 30% in the open approach group. In our series, we reported a stricture incidence of 8.7%; all of them occurred in patients who underwent the open approach (p-value = 0.07). The surgeon should keep this issue in mind while performing the suture in these complicated cases. We recommend additional catheter days in these patients, to allow full tissue healing before applying tension on the anastomosis. Interestingly, no strictures were identified in the robot-assisted group. We believe that when performing robotic anastomosis, both the precise alignment between the bladder neck and the urethral stump as well as the nonischemic waterproof running suture used in this step could explain these findings.
UI continues to be a significant concern after SRP. For example, Gontero et al5 showed that 57.5% of patients had improved/unchanged continence at the last follow-up at 6 or 12 months whereas 24.6% were severely incontinent. On their multivariable analysis robotic-SRP was an independent predictor for continence preservation (OR: 0.411, CI 95%: 0.232–0.727, p = 0.022). In the present study, we observed a UI rate of 34.2% in the open group versus 9.1% in the robotic group, being the overall incontinence rate of 26.4%. This is an acceptable outcome, when comparing previous reports (Table 3). We also found that BT as primary treatment might result in a UI predictor (OR: 4.8, CI 95%: 1.1–20). Heidenreich et al.9 found an opposite result as they demonstrated a faster continence recovery in SRP after BT.
According to recent reviews, erectile function in SRP is poorly preserved with approximately less than 20% of patients maintaining erections with or without PDE-5 inhibitors administration15. Dissection and preservation of the neurovascular bundles in salvage setting is a complex and challenging surgical step due to tissue fibrosis and altered surgical planes explaining the lack of functional success when compared with first-line radical prostatectomy. Gontero et al5 showed that 8.1% of patients preserved spontaneous and/or PDE-5–assisted erection at 12 months and 15.5% who were potent before SRP had preserved erectile function. Our results demonstrate that 9.8% of previously potent patients preserved erectile function with/without use of PDE-5 inhibitors. Patients with potential locally advanced disease in the SRP setting must be identified to avoid nerve sparing surgery.
PSM rates vary from 13%16 to 45%17, and they are frequently located near the apex of the prostate. The importance of achieving R0 resection is underlined by Chade et al17, who described a trend toward an augmented risk of death from PCa for PSM (HR: 1.8, p = 0.068). We reported an overall PSM rate of 28.6%; 30% versus 25.9% in the open and robot-assisted approach, respectively. However, these relatively elevated rates might be due to the presence of approximately 60% of pT3a/pT3b disease in our cohort, denoting that radiorecurrent prostate cancer is often an aggressive and locally advanced disease.
Longer follow-up is necessary to estimate CSS and BCR-free survival, although many studies reported a 5-year BCR-free survival greater than 45%, as in Chade's17 and Mandel's18 series (48% and 48.7%, respectively). This correlates with our published data, where the estimated 2-year BCR-free survival rate in the open approach group and robot-assisted group was 67% (CI 95%: 53.7–80.3) and 60.9% (CI 95%: 40.5–81.3), respectively, with no statistical difference. In 2014, we observed an estimated 4-year BCR-free survival of 51.7%, although in this study sample size was smaller with longer follow-up time19. In a systematic review by Matei et al20 on SRP, better biochemical control and CSS rates were demonstrated with SRP than other salvage therapies. Moreover, salvage therapy can avoid or defer ADT20.
Based on our published outcomes and when oncologically indicated, we consider SRP should not be avoided because of the fear of poor outcomes18. Contrarily, its use should be recommended when we focus to achieve cancer-free status, considering that patient selection is of paramount importance.
This study has several strengths. First, median follow-up time was reasonable to measure our primary endpoints. Second, outcomes were analyzed and compared in two different surgical techniques. However, some limitations are worth mentioning. First, it is a retrospective analysis of prospectively collected data, and there is a potential selection bias. In addition, because of both the small sample size and small number of events, multivariate analysis was not performed. Further randomized studies are needed to confirm our findings, and patient selection should be of greatest importance in SRP setting to avoid major surgical complications.
5. Conclusions
The present study adds important information about contemporary outcomes of patients undergoing SRP for radiorecurrent prostate cancer. Open SRP and robot-assisted SRP have similar oncological outcomes with excellent cancer control. Robot-assisted SRP is a reliable procedure to treat local recurrences after EBRT or BT, reducing the risk of anastomotic strictures and blood loss and improving continence outcomes.
Conflicts of interest
All authors have no conflict of interest to declare.
References
- 1.Siegel R.L., Fedewa S.A., Miller K.D., Goding Sauer A., Pinheiro P., Martinez Tyson D. Cancer statistics for Hispanics/Latinos, 2015. CA. A Cancer Journal for Clinicians. 2015;65:457–480. doi: 10.3322/caac.21314. [DOI] [PubMed] [Google Scholar]
- 2.Yen-Chuan O.U., Sheng-Chun H., Li-Hua H., Chun-Kuang Y., Siu-Wan H., Min-Che T. Salvage Robotic-assisted Laparoscopic Radical Prostatectomy: Experience with 14 Cases. Anticancer Res. 2017;37:2045–2050. doi: 10.21873/anticanres.11550. [DOI] [PubMed] [Google Scholar]
- 3.Orré M., Piéchaud T., Sargos P., Richaud P., Roubaud G., Thomas L. Oncological and functional results of robotic salvage radical prostatectomy after permanent brachytherapy implants. Canc Radiother. 2017;21:119–123. doi: 10.1016/j.canrad.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Stephemson A., Scardino P., Bianco F., Jr., DiBlasio C., Fearn P., Eastham J. Morbidity and functional outcomes of salvage radical prostatectomy for locally recurrent prostate cancer after radiation therapy. J Urol. 2004;172(6):2239–2243. doi: 10.1097/01.ju.0000140960.63108.39. [DOI] [PubMed] [Google Scholar]
- 5.Gontero P., Marra G., Alessio P., Filippini C., Oderda M., Munoz F. Salvage Radical Prostatectomy for Recurrent Prostate Cancer: Morbidity and Functional Outcomes from a Large Multicenter Series of Open versus Robotic Approaches. J Urol. 2019;202:725–731. doi: 10.1097/JU.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 6.Stone H.B., Norman Coleman C., Anscher M.S., McBride W. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 7.Grossfeld G.D., Li Y.P., Lubeck D.P., Mehta S., Carroll P. Predictors of Secondary Cancer Treatment in Patients Receiving Local Therapy for Prostate Cancer: Data From Cancer of the Prostate Strategic Urologic Research Endeavor. J Urol. 2002;168:530–535. [PubMed] [Google Scholar]
- 8.Brenner P.C., Russo P., Wood D.P., Donat S., Fair W. Salvage radical prostatectomy in the management of locally recurrent prostate cancer after 125I implantation. Br J Urol. 1995;75:44–47. doi: 10.1111/j.1464-410x.1995.tb07230.x. [DOI] [PubMed] [Google Scholar]
- 9.Heidenreich A., Richter S., Thüer D., Pfister D. Prognostic Parameters, Complications, and Oncologic and Functional Outcome of Salvage Radical Prostatectomy for Locally Recurrent Prostate Cancer after 21st-Century Radiotherapy. Eur Urol. 2010;57:437–445. doi: 10.1016/j.eururo.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 10.Mottet N., Bellmunt J., Briers E., Bolla M., Bourke L., Cornford P. Publisher: EAU Guidelines Office. Place published: Arnhem; The Netherlands: 2020. members of the EAU – ESTRO – ESUR –SIOG Prostate Cancer Guidelines Panel. EAU – ESTRO – ESUR – SIOG Guidelines on Prostate Cancer; pp. 978–994. Edn. presented at the EAU Annual Congress Amsterdam. 92671-07-3. [Google Scholar]
- 11.Thompson I., Thrasher J.B., Aus G., Burnett A., Canby-Hagino E., Cookson M. Guideline for the Management of Clinically Localized Prostate Cancer: 2007 Update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Sivaraman A., Scardino P., Eastham J. Outcomes of salvage radical prostatectomy following more than one failed local therapy. Investig Clin Urol. 2018;59:152–157. doi: 10.4111/icu.2018.59.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albisinni S., Aoun F., Peltier A., van Velthoven R. The single knot running vesicourethral anastomosis after minimally invasive prostatectomy: review of the technique and its modifications, tips, and pitfalls. Prostate Cancer. 2016;2016:5. doi: 10.1155/2016/1481727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaffenberger S., Smith J. Salvage robotic radical prostatectomy. Indian J Urol. 2014;30:429. doi: 10.4103/0970-1591.142074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calleris G., Marra G., Dalmasso E., Falcone M., Karnes R., Morlacco A. Is it worth to perform salvage radical prostatectomy for radio-recurrent prostate cancer? A literature review. World J Urol. 2019;37:1469–1483. doi: 10.1007/s00345-019-02749-z. [DOI] [PubMed] [Google Scholar]
- 16.Chauhan S., Patel M.B., Coelho R., Liss M., Rocco B., Sivaraman A. Preliminary Analysis of the Feasibility and Safety of Salvage Robot-Assisted Radical Prostatectomy After Radiation Failure: Multi-Institutional Perioperative and Short-Term Functional Outcomes. J Endourol. 2011;25:1013–1019. doi: 10.1089/end.2010.0564. [DOI] [PubMed] [Google Scholar]
- 17.Chade D.C., Shariat S.F., Cronin A.M., Savage C., Karnes R.J, Blute M.L. Salvage Radical Prostatectomy for Radiation-recurrent Prostate Cancer: A Multi-institutional Collaboration. Eur Urol. 2011;60:205–210. doi: 10.1016/j.eururo.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandel P., Steuber T., Ahyai S., Kriegmair M., Schiffmann J., Boehm C. Salvage radical prostatectomy for recurrent prostate cancer: verification of European Association of Urology guideline criteria. BJU Int. 2016;117:55–61. doi: 10.1111/bju.13103. [DOI] [PubMed] [Google Scholar]
- 19.Martinez P.F., Billordo Peres N., Cristallo C., Isola M., Villamil W.A, Giudice C.R. Prostatectomia Radical de Rescate postradioterapia. Arch Esp Urol. 2014;67(4):313–322. 2014. [PubMed] [Google Scholar]
- 20.Matei D.V., Ferro M., Jereczek-Fossa B.A., Renne G., Crisan N., Bottero D. Salvage radical prostatectomy after external beam radiation therapy: a systematic review of current approaches. Urol Int. 2015;94:373–382. doi: 10.1159/000371893. [DOI] [PubMed] [Google Scholar]
- 21.Eandi J., Link B., Nelson R., Josephson D., Lau C., Kawachi M. Robotic assisted laparoscopic salvage prostatectomy for radiation resistant prostate cancer. J Urol. 2010;183(1):133–137. doi: 10.1016/j.juro.2009.08.134. [DOI] [PubMed] [Google Scholar]
- 22.Zugor V., Labanaris A.P, Porres D., Heidenreich A., Witt J.H. Robot-assisted radical prostatectomy for the treatment of radiation-resistant prostate cancer: surgical, oncological and short-term functional outcomes. Urol Int. 2014;92:20–26. doi: 10.1159/000351948. [DOI] [PubMed] [Google Scholar]
- 23.Kenney P., Nawaf C., Mustafa M., Wen S., Wszolek M., Pettaway C.A. Robotic-assisted laparoscopic versus open salvage radical prostatectomy following radiotherapy. Can J Urol. 2016;23(3):8271–8277. [PMC free article] [PubMed] [Google Scholar]