Abstract
Background
The United States Preventative Services Task Force (USPSTF) guideline on Prostate Specific Antigen (PSA)-based prostate cancer screening evolved both in 2008 (Grade I for men < 75 years and Grade D for men > 75 years) and in 2012 (Grade D for all ages).
Materials and methods
A statewide cancer registry operated by the Pennsylvania Department of Health was accessed to analyze over a 15-year period prostate cancer rates across different categories including age, stage, and geographic distribution.
Results
Local prostate cancer rates decreased significantly when comparing before and after USPSTF's guideline changes: 2002–2008 vs. 2009–2012 vs. 2013–2016 (p < 0.005). Conversely, the distant cancer rates increased significantly in Caucasian men (but not in African American men) (p = 0.0078). In age group analysis, distant cancer rates increased significantly in all age ranges, most notably in younger men (50–59 years). No observed difference in the trend of distant cancer rates when considering rural versus urban counties.
Conclusions
Incident prostate cancer cases diagnosed in Pennsylvania have decreased over the past 15 years with a recent rise in distant carcinomas potentially attributable to the USPSTF recommendations against PSA-based screening. Although the USPSTF revised their PSA-based prostate cancer screening guideline in 2018 (Grade C for men 55–69 years and Grade D for men > 70 years), the implications of the aforementioned observations on mortality outcomes merit further follow-up.
Keywords: Prostate Cancer, Screening, State Cancer Registry, USPSTF
1. Introduction
In 2018, prostate cancer is estimated to continue to have the highest incidence and 2nd leading cause of cancer mortality in men in the United States.1 The use of Prostate Specific Antigen (PSA)-based prostate cancer screening for early detection and intervention has been controversial with somewhat differing data highlighted by two large scaled randomized trials: the European Randomized Study of Screening for Prostate Cancer trial and the US Prostate, Lung, Colorectal and Ovarian trial.2,3
The United States Preventive Services Task Force (USPSTF) has issued several statements over the past 10 years based on these data. Specifically, in 2008, it deemed that the evidence was insufficient to assess the benefits and harms of prostate cancer screening in men younger than 75 years while recommending against prostate cancer screening in men aged 75 years and older. The well-documented Grade D recommendation in 2012 furthered this statement against PSA-based prostate cancer screening citing a lack of benefit of the prostate cancer screening with associated harms. Specifically, these potential harms included psychologic distress attributable to a false-positive test and discomfort and morbidity of prostate biopsy.4
Not surprisingly, the Grade D recommendation resulted in rates of men undergoing PSA-based screening, prostate biopsy and resultant prostate cancer incidence decreasing in all age groups in the United States.5 Associated with these observations was an increased incidence of adverse pathologic and disease characteristics, thereby prompting an appropriate re-evaluation of the USPSTF recommendations and an issuance of a Grade C revision in 2018 for men aged 55–69 years.6
Herein, we review the prostate cancer incidence and distribution of disease trends over this time frame across a large statewide registry. We seek to better characterize the implications of such USPSTF policy statements across the state with analysis of differential impact based on sociodemographic groups and geographic locations.
2. Materials and methods
2.1. Data extraction
Age-adjusted rates per 100,000, and percent stage distribution of newly diagnosed prostate cancers were extracted from the Pennsylvania Department of Health Enterprise Data Dissemination Informatics Exchange (EDDIE: https://www.phaim1.health.pa.gov/EDD/). Cancer incidence data were available for years 1990–2016.
2.2. Definitions
In the EDDIE database, prostate cancer at the time of diagnosis was staged based on the Surveillance Epidemiology and End Results (SEER) Summary Staging definitions (in situ, local, regional, and distant). In short, the cancer was staged in situ when confined within the basement membrane of the epithelial tissue involved, local when confined entirely to the organ of origin, regional when the cancer extends to nearby lymph nodes, organs, or tissue, and distant when the cancer extends to distant organs or distant lymph nodes.
Rural and urban counties were divided based on population density (population per square mile). The definition was adapted from the release of urbanized data from the U.S. Census Bureau in 2013. The USPSTF's Grade definitions are summarized in Table 1.
Table 1.
The USPSTF's Grade definitions.
| Grade | Definition |
|---|---|
| A | The USPSTF recommends the service (substantial net benefit) |
| B | The USPSTF recommends the service (moderate net benefit) |
| C | The USPSTF recommends selectively offering or providing the service (small net benefit) |
| D | The USPSTF recommends against the service (no net benefit) |
| I | The current evidence is insufficient |
USPSTF, United States Preventive Services Task Force.
2.3. Data groups
The data was grouped into three distinct groups by year: 2002–2008 vs. 2009–2012 vs. 2013–2016 to assess the impact of Prostate, Lung, Colorectal and Ovarian and European Randomized Study of Screening for Prostate Cancer trials along with USPSTF's recommendation in 2008 and the newer USPSTF's recommendation in 2012.
2.4. Statistical analysis
Two-sample T test were used to assess differences between groups with a P value of <0.05 considered significant.
3. Results
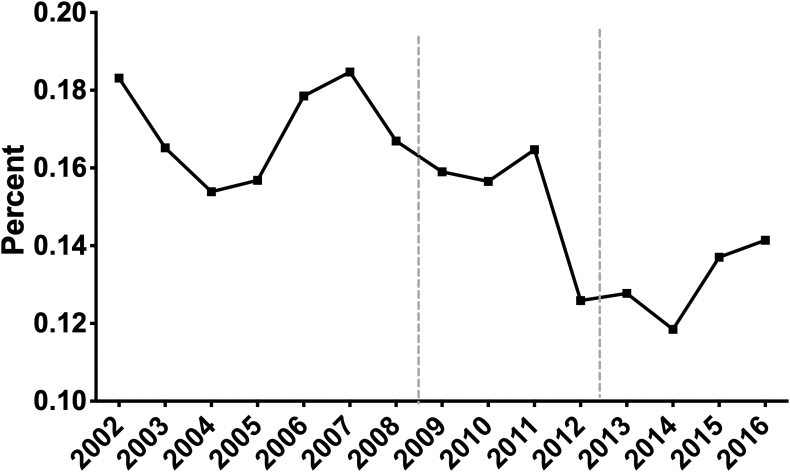
Fig. 1 summarizes the annual incidence of newly diagnosed prostate cancer in Pennsylvania adjusted for the population. Although yearly fluctuations were noted over this time frame a notable decrease was observed when comparing the earliest time cohort of 2002–2008 versus the latest 2013–2016 (P = 0.005). While a variety of factors may contribute to this observation, the evolution in USPSTF recommendations in both 2008 and 2012 are likely related to these changes.
Fig. 1.
The annual incidence of newly diagnosed prostate cancer over the last 15 years in Pennsylvania adjusted for the population (prostate cancer incidence/population∗100). Two dotted lines indicate the release of USPSTF's recommendation on PSA-based prostate cancer screening. PSA, Prostate Specific Antigen; USPSTF, United States Preventive Services Task Force.
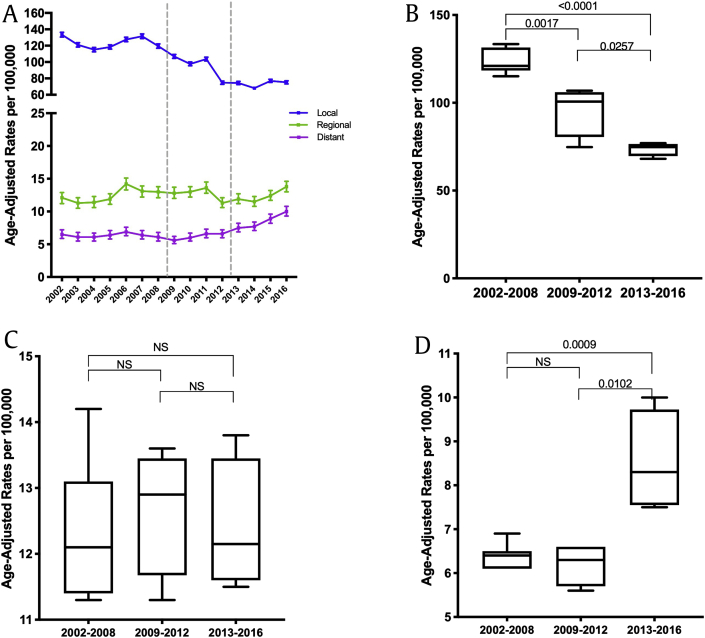
Fig. 2a summarizes the stage distribution of prostate cancer observed over this interval. In this analysis, a significant decrease in localized prostate cancer (Fig. 2b) with a corresponding increase in distant disease (Fig. 2d) was noted without a significant change in regional stage prostate malignancy (Fig. 2c). Specifically, the percent distribution of distant disease increased from 3.9% in 2008 to 8.6% in 2016 (P = 0.01).
Fig. 2.
(A) The stage distribution of prostate cancer in age-adjusted rate per 100,000 over the last 15 years in Pennsylvania; Local, Regional, Distant; two segmental y-axis was implemented to visualize the changes in regional and distant cancer rates. (B–D) The localized (2B), regional (2C), distant (2D) prostate cancer rates in 2002–2008 vs. 2009–2012 vs. 2013–2016; p values were determined by nonpaired, two-tailed, Student t-test for homogenous variance; <0.05 was considered significant.
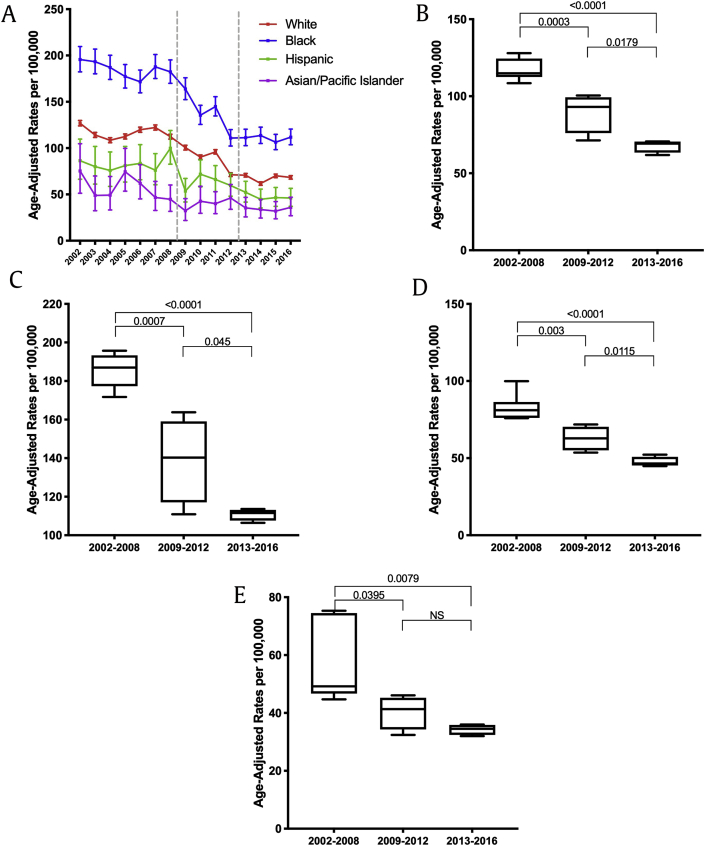
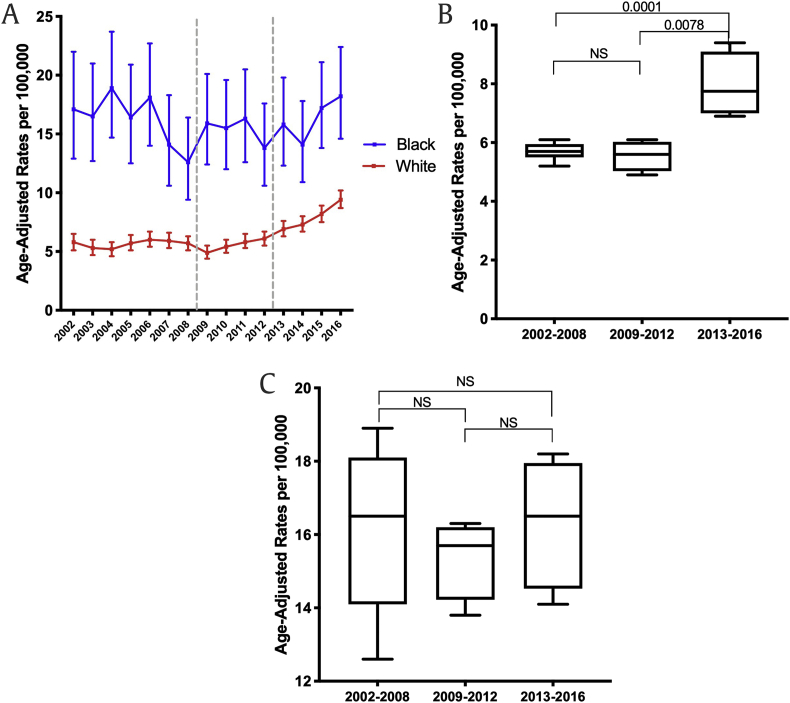
It is well described that African Americans are genetically more susceptible to developing prostate cancer compared with other races. In addition, income status and access to health care potentially may further impact the prostate cancer care of African American men when compared with other races.7,8 As shown in Fig. 3a, the incidence of localized prostate cancer in African American men exceeds that of other races throughout the study period. Furthermore, the localized cancer rates over the past 15 years significantly decreased irrespective of race (Fig. 3b [Caucasian], 3c [African American], 3d [Hispanic], and 3e [Asian]). However, when considering the distant cancer rates stratified by race (Fig. 4a), an increase in distant cancer rates over the recent years was only observed in the Caucasian population (Fig. 4b, P = 0.0078) but not in African American men (Fig. 4c).
Fig. 3.
(A) The localized prostate cancer rates in different races: Caucasian, African American, Hispanic, and Asian over the last 15 years in Pennsylvania. (B–D) The localized prostate cancer rates in Caucasian (3B), African American (3C), Hispanic (3D) and Asian (3E) in 2002–2008 vs. 2009–2012 vs. 2013–2016; p values were determined by nonpaired, two-tailed, Student t-test for homogenous variance; <0.05 was considered significant.
Fig. 4.
(A) The distant prostate cancer rates in Caucasian and African American men over the last 15 years in Pennsylvania. (B–D) The distant prostate cancer rates in Caucasian (4B) and African American (4C) in 2002–2008 vs. 2009–2012 vs. 2013–2016; p values were determined by nonpaired, two-tailed, Student t-test for homogenous variance; <0.05 was considered significant.
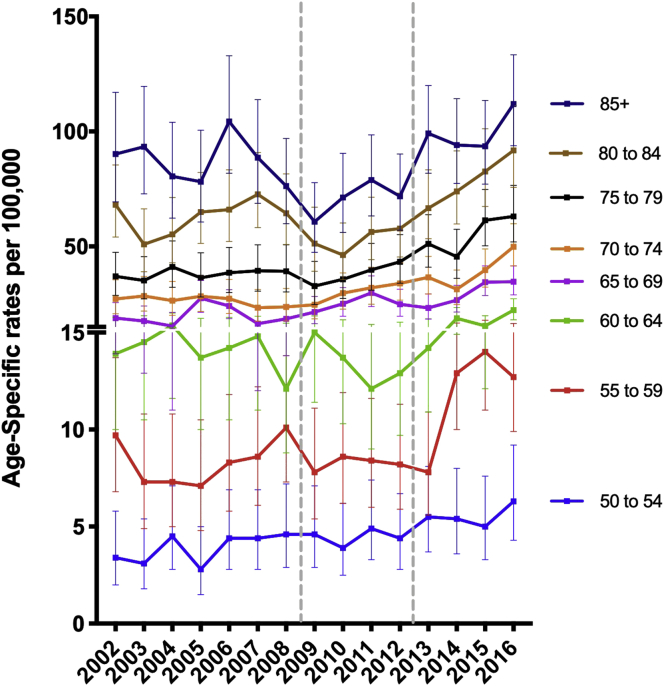
Fig. 5 demonstrates the trends of distant prostate cancer rate stratified by age groups. Notably, the younger age groups: 50–54 (P = 0.0041), 55–59 (P = 0.0413) along with the older age groups: 75–79 (P = 0.0107), 80–84 (P = 0.0051), 85+ (P = 0.0022) showed a significant increase when comparing 2009–2012 and 2013–2016. This younger age cohort is of particular concern, given the likelihood of such men experiencing subsequently experiencing prostate cancer specific mortality.
Fig. 5.
The distant prostate cancer percent over the past 15 years in Pennsylvania; two segmental y-axis was implemented to visualize the changes in distant rates in younger men aged 64 years and younger.
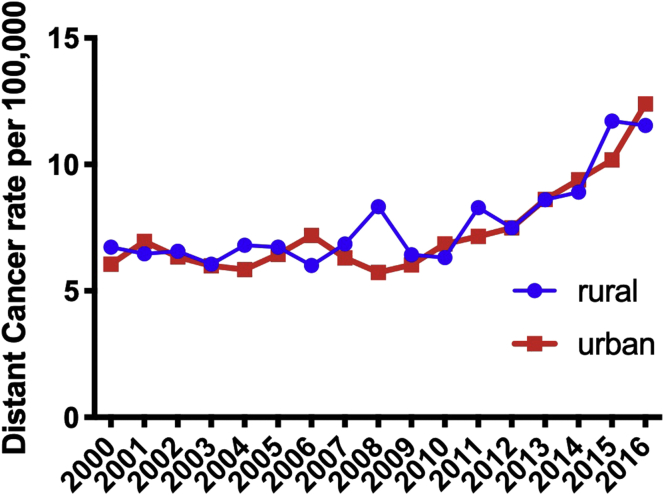
Finally, when considering geospatial distribution of prostate cancer across the state, there was no observed difference in the trend of distant cancer rates when considering rural versus urban counties (Fig. 6).
Fig. 6.
The distant prostate cancer rates per 100,000 in rural vs. urban counties; Urban counties: Allegheny, Beaver, Berks, Bucks, Chester, Cumberland, Dauphin, Delaware, Erie, Lackawanna, Lancaster, Lebanon, Lehigh, Luzerne, Montgomery, Northampton, Philadelphia, and York.
4. Discussion
Statewide registry data over the 15 years noted an overall decrease of prostate cancer incidence in Pennsylvania. Rates of localized prostate cancer demonstrated a similar decreasing trend, which was noted in all race categories. Conversely, the distant prostate cancer rate has increased most notably from 2013 to 2016 with all age groups of patients experiencing this rise including younger men less than 55 years of age. The observed increase in distant prostate cancer rates was similar in both rural and urban counties but was preferentially observed in Caucasian (versus African American) men.
African American men show overall higher rates of prostate cancer incidence at all time points perhaps reflecting a well characterized risk factor for prostate cancer. The etiology for the racial differentiation in distant prostate cancer rate changes is multifactorial including genetics, access to care, and income-related considerations. To explain the increased rates of distant cancer in Caucasian versus African American men, we hypothesize Caucasian men may be a population who are more likely to obtain routine PSA-based prostate cancer screening and therefore were more negatively impacted by the USPSTF recommendation changes.
In age group analysis, when comparing 2002–2008 to 2009–2012, older populations (aged 65 and older) demonstrated increased distant cancer rates. Furthermore, when comparing 2002–2008 to 2013–2016, the distant cancer rates were significantly increased in all of the age groups. This may reflect the incremental changes with the USPSTF's recommendations: Grade D for men aged 75 years and older in 2008 and Grade D for men of all ages in 2012. Of particular concern, however, is the rising rates of distant disease in younger patients with a longer anticipated life expectancy. Indeed, prostate cancer screening studies implicate that screening is most effective in this patient population owing to a lower likelihood of dying from competing causes of mortality in the subsequent 7–10 years.9
Our results support data from a large multicenter cohort of more than 19,000 prostatectomy cases evaluating the impact of decreased PSA-based screening on pathologic outcomes. These authors noted that following the USPSTF Grade D recommendation, higher rates of high grade (Gleason score ≥ 8+) prostate cancer, seminal vesical, and lymph node invasion were noted on final pathology. Associated with these adverse pathologic features was an increased risk of biochemical recurrence. Our study further highlights that distant metastatic prostate cancer rates have significantly increased with potential differential racial associations.18
The USPSTF statements from 2008 and 2012 lacked a key element of shared decision-making for prostate cancer screening. Indeed, it is better understood that accurate counseling of such patients is a balance between the anticipated risks of prostate cancer morbidity and mortality compared with the potential side effects associated with therapy. The American Urologic Association clinical practice guideline highlights this latter point in the cohort of men aged 55–69 years. Additional groups have advocated for a similar approach in the management of male patients including the American Cancer Society, the American College of Physicians, and the American Society of Clinical Oncology.10, 11, 12
It is important to recognize that screening for prostate cancer does not imply therapy for prostate cancer. Multiple studies underscore the increased adoption of active surveillance and the ability to establish a diagnosis with safe monitoring in the absence of therapy.13,14 On the other end of the spectrum, therapies for metastatic and advanced prostate cancer have improved albeit at a significant cost. Both the LATITIDE and CHAARTED trials have shown prolonged survival for metastatic, castration-sensitive prostate cancer using either antiandrogens or systemic chemotherapy along with conventional androgen deprivation.15,16 These trials, however, present a challenging cost conundrum when confronted with an increasing number of patients with metastatic prostate cancer. Indeed, as therapies improve, life expectancy increases but the cumulative treatment burden to the patient and society becomes substantial.17
We acknowledge some limitations of this work. Admittedly, this is a retrospective review of statewide cancer registry data to look at the potential impact of the USPSTF's guideline changes on prostate cancer incidence rates. The biggest limitation of this study is the arbitrary grouping of data to compare the impact of the USPSTF's recommendation changes. Considering the nature of primary prostate cancer, there can be a lag time to see increased rate of metastatic prostate cancer attributable to screening. Furthermore, prostate cancer mortality attributable to screening changes will not be available for years. Finally, these data are all based on previously extracted data without the granularity to examine specifics regarding the management and presentation of patients.
5. Conclusions
Incident prostate cancer cases diagnosed in Pennsylvania have decreased over the past 15 years with a recent rise in distant carcinomas likely reflecting recommendations of USPSTF against PSA screening testing. Rising rates of distant disease notably were observed in all age distributions (particularly younger men aged 50–54 years), as well as those of Caucasian race. Vigilant attention is requisite to better understand the implications of evolving screening recommendations for prostate cancer.
Conflicts of interest
No conflicts of interest associated with this manuscript.
Acknowledgments
These data were provided by the Division of Health Informatics, Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions. This project was funded by The Ken and Bonniey Shockey Fund for Urologic Cancer Research at Penn State Health (Hershey, PA, USA). The authors thank the Division of Outcomes, Research, and Quality in the Department of Surgery at Penn State Health for assistance with manuscript preparation and submission.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Andriole G.L., Crawford E.D., Grubb R.L., Buys S.S., Chia D., Church T.R. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schröder F.H., Hugosson J., Roobol M.J., Tammela T.L.J., Ciatto S., Nelen V. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 4.Moyer V.A., USPST Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 5.Fleshner K., Carlsson S.V., Roobol M.J. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol. 2017;14:26–37. doi: 10.1038/nrurol.2016.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grossman D.C., Curry S.J., Owens D.K., Bibbins-Domingo K., Caughey A.B., Davidson K.W. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. J Am Med Assoc. 2018;319:1901–1913. doi: 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- 7.Ziehr D.R., Mahal B.A., Aizer A.A., Hyatt A.S., Beard C.J., D’Amico A.V. Income inequality and treatment of African American men with high-risk prostate cancer. Urol Oncol. 2015;33 doi: 10.1016/j.urolonc.2014.09.005. 18.e17-18.e13. [DOI] [PubMed] [Google Scholar]
- 8.Mahal B.A., Ziehr D.R., Aizer A.A., Hyatt A.S., Sammon J.D., Schmid M. Getting back to equal: The influence of insurance status on racial disparities in the treatment of African American men with high-risk prostate cancer. Urol Oncol. 2014;32:1285–1291. doi: 10.1016/j.urolonc.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Barry M.J., Simmons L.H. Prevention of Prostate Cancer Morbidity and Mortality: Primary Prevention and Early Detection. Med Clin North Am. 2017;101:787–806. doi: 10.1016/j.mcna.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Qaseem A., Barry M.J., Denberg T.D., Owens D.K., Shekelle P., CGCotACo Physicians. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013;158:761–769. doi: 10.7326/0003-4819-158-10-201305210-00633. [DOI] [PubMed] [Google Scholar]
- 11.Basch E., Oliver T.K., Vickers A., Thompson I., Kantoff P., Parnes H. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology Provisional Clinical Opinion. J Clin Oncol. 2012;30:3020–3025. doi: 10.1200/JCO.2012.43.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith R.A., Andrews K.S., Brooks D., Fedewa S.A., Manassaram-Baptiste D., Saslow D. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2019;69:184–210. doi: 10.3322/caac.21557. [DOI] [PubMed] [Google Scholar]
- 13.Loeb S., Byrne N., Makarov D.V., Lepor H., Walter D. Use of Conservative Management for Low-Risk Prostate Cancer in the Veterans Affairs Integrated Health Care System From 2005-2015. J Am Med Assoc. 2018;319:2231–2233. doi: 10.1001/jama.2018.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loeb S., Folkvaljon Y., Curnyn C., Robinson D., Bratt O., Stattin P. Uptake of Active Surveillance for Very-Low-Risk Prostate Cancer in Sweden. JAMA Oncol. 2017;3:1393–1398. doi: 10.1001/jamaoncol.2016.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fizazi K., Tran N., Fein L., Matsubara N., Rodriguez-Antolin A., Alekseev B.Y. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 16.Sweeney C.J., Chen Y.-H., Carducci M., Liu G., Jarrard D.F., Eisenberger M. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737–746. doi: 10.1056/nejmoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norum J., Nieder C. Treatments for Metastatic Prostate Cancer (mPC): A Review of Costing Evidence. Pharmacoeconomics. 2017;35:1223–1236. doi: 10.1007/s40273-017-0555-8. [DOI] [PubMed] [Google Scholar]
- 18.Ahlering T., Huynh L.M., Kaler K.S., Williams S., Osann K., Jospeh J. Unintended consequences of decreased PSA-based prostate cancer screening. World J Urol. 2019;37(3):489–496. doi: 10.1007/s00345-018-2407-3. [DOI] [PubMed] [Google Scholar]