Abstract
Background
This study investigated the inhibition of tumor growth in castrate-resistant prostate cancer (CRPC)-bearing mice by tumor necrosis factor–related apoptosis-inducing ligand (TRAIL)-overexpressing adipose-derived stem cells (ADSCs) (hTERT-ADSC.sTRAIL), which was enhanced by combined treatment with CPT-11.
Materials and methods
An hTERT-ADSC.sTRAIL cell line was established by transfection with a lentiviral vector (CLV-Ubic) encoding the human sTRAIL gene. Quantitative polymerase chain reaction and Western blots were performed to confirm gene overexpression. An invasion study for the selective migration ability toward PC3 cells was performed. In the in vivo study, the tumor volume in mice treated with ADSC. sTRAIL and CPT-11 was measured.
Results
Carboxylesterase was generated from hTERT-ADSCs. The gene expression of sTRAIL from hTERT-ADSC.sTRAIL was shown. The directional migration of ADSC.sTRAIL cells toward PC3 cells was significantly stimulated by PC3 cells in vitro (P < 0.05). In the in vitro study, the viability of PC3 cells significantly decreased in the presence of ADSC.sTRAIL (62.7 ± 2.0%) and CPT-11 compared with that of CPT-11 alone (83.0 ± 1.0%) at a cell ratio as low as 0.05 (PC3: ADSC.sTRAIL) (P < 0.05). The proportion of apoptotic PC3 cells significantly increased in the presence of ADSC.sTRAIL (37.2 ± 2.1%) and CPT-11 compared with that of CPT-11 alone (16.5 ± 1.0%) (P < 0.05). In the in vivo study, the inhibition of tumor growth in CRPC-bearing mice by TRAIL-overexpressing adipose stem cells was enhanced by combined treatment with the chemotherapeutic agent CPT-11 compared with that in the treatment with cpt-11 alone. Immunohistochemical staining of the removed tumors showed anti-TRAIL–positive cells and apoptotic bodies after hTERT-ADSC.sTRAIL treatment or combined treatment with hTERT-ADSC.sTRAIL and CPT-11.
Conclusions
Therapeutic stem cells expressing sTRAIL genes combined with CPT-11 can provide a new strategy for treating CRPC in clinical trials using the patients’ own ADSCs.
Keywords: Prostate cancer, TRAIL, CPT-11
1. Introduction
Prostate cancer (PC) is one of the most common cancers in men and is the second most common cause of death among all cancers(1). The current limitations in treating PC depend on the treatment of castrate-resistant prostate cancer (CRPC). Tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) induces apoptosis selectively in a variety of tumor cells with minimal effects on normal cells and tissues, suggesting it as an attractive cancer therapeutic candidate(2, 3). TRAIL and its death receptors have been introduced as targets for the development of tumoricidal agents since the 1990s(4).
However, most localized cancers, including PC and CRPC cell lines, PC3 and DU145(5), are resistant to TRAIL-induced apoptosis. To overcome this limitation of TRAIL-induced apoptosis, research has focused on developing compounds or combinations to improve TRAIL susceptibility(6). Recently, cell therapy has been used in many studies, especially stem cells in combination with TRAIL(7).
Because stem cells (SCs) migrate toward cancer, the introduction of genes into SCs is an attractive cell-based delivery strategy(8, 9). The homing and migration of SCs into tumors is well-known and has been demonstrated by many experimental studies(10). Our previous study also showed the efficacy of SCs as an effective PC treatment(11).
Adipose-derived SCs (ADSCs) have the potential for expanded applications because they are easy to obtain without ethical issues(12). The patient's own ADSCs can be obtained easily in their undifferentiated state without inducing an immune response, and using the patient's own ADSCs prevents immune rejection. Human ADSCs can be transduced with therapeutic genes with high efficiency and rapidly expanded to numbers required for therapeutic applications(13).
Recently, the chemotherapeutic agent CPT-11 (irinotecan), a derivative of the natural alkaloid camptothecin, was shown to enhance TRAIL-induced apoptosis in several cancer cell lines, including prostate, breast, and colon(4, 14). Combined treatment with TRAIL and CPT-11 showed enhanced apoptosis in PC cells by regulating BCL-2 family proteins and activating caspases(14). CPT-11 is activated by human liver carboxylesterase to generate SN-38 (7-ethyl-10-hydroxy-camptothecin), a topoisomerase I inhibitor that is 100- to1000-fold more potent than irinotecan in vitro and in vivo (15).
To overcome the previously known limitation of TRAIL resistance in PC treatment, this study innovated the strategy of treating with both ADSCs and CPT-11. This study was performed to investigate the inhibition of tumor growth in CRPC-bearing mice by TRAIL-overexpressing ADSCs in combination with the chemotherapeutic agent CPT-11. The cytotoxicity of the combined treatment was also investigated.
2. Materials and Methods
The general study design and methods were executed similar to our previous study protocol(11).
2.1. Cell culture
An ASC52TELO, hTERT-ADSC (ATCC® SCRC-4000™, ATCC, Manassas, VA, USA) cell line was purchased and cultured in Dulbecco's modified Eagle medium (DMEM) (GibcoBRL, Grand Island, NY, USA) supplemented with 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated fetal bovine serum (FBS, GibcoBRL).
The PC3 PC mouse cell line (Korean Cell Line Bank, Seoul, Korea) was cultured in DMEM supplemented with 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated FBS (GibcoBRL). All of the cells were propagated in 5% CO2 and 95% air at 37 °C in a humidified incubator and routinely passaged via trypsinization.
2.2. Generation of hTERT-ADSC.TRAIL lines
Recombinant lentivirus was generated from CLV-Ubic/sTRAIL containing the entire coding sequence of the rabbit CE gene. Briefly, transfection was completed via calcium phosphate coprecipitation. The medium was exchanged on the next day, and the supernatant was harvested 16 to 20 h later, which served as the recombinant lentivirus stock. The cells were infected with the aforementioned retroviruses at 37 °C for 4 to 6 h in the medium containing 8 μg/mL of polybrene (Sigma-Aldrich, St. Louis, MO, USA). The medium was replaced with fresh virus-free medium, and the cells were incubated for two days at 37 °C. The infected cells were selected by the addition of puromycin (Sigma) to a final concentration of 3 μg/mL for two weeks, and the puromycin-resistant cells were expanded for subsequent experiments. The hTERT-ADSC.sTRAIL cell line encoding the human TRAIL gene was established.
2.3. Analysis of hTERT-ADSC.CE
2.3.1. Reverse transcription-polymerase chain reaction -PCR
Total RNA was extracted from the selected cell clones using the TRIzol reagent (GIBCO). Reverse transcription (RT) was performed using the total RNA and random primers. RT-polymerase chain reaction (PCR) was performed using the following primers: forward: 5′- ATGGTGATTTGCATAGTGCTCC -3’; reverse: 5′- GCAAGCAGGGTCTGTTCAAGA-3’. GAPDH controls were used to confirm equal protein loading. The amplification program was as follows: a denaturation step of 3 min, followed by 35 cycles at 95 °C for 1 min, 63 °C for 1 min, and 72 °C for 1 min. The amplification products were examined by electrophoresis in 1.2% agarose 1X TAE gels with ethidium bromide staining.
2.3.2. Western blot analysis
The total proteins were extracted from the cell lysates using RIPA lysis buffer (Thermo Fisher Scientific, Waltham, MA, USA), followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to isolate the proteins. The isolated proteins were then transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA) for Western blotting. Five percent skim milk was used to block the membranes, followed by incubation with specific primary antibodies against TRAIL (Abcam, Cambridge, UK) and β-actin (Santa Cruz Biotechnology, Dallas, TX, USA). Later, peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) were used to target the proteins on the membrane. The bands were analyzed following their visualization using enhanced chemiluminescence reagent (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK).
2.3.3. Flow cytometry analysis
A Cyflow Cube 8 FACS instrument (SysmexPartec, Görlitz, Germany) was used for flow cytometry analysis, and the data were analyzed using standard FSC Express software (De Novo Software, Los Angeles, CA, USA). The hTERT-ADSC and hTERT-ADSC cells were subjected to flow cytometry analysis using anti-CD29 (OriGene Technologies, Rockville, MD, USA), CD 34 (Invitrogen, Carlsbad, CA, USA), CD 45 (LDBIO, Seattle, WA, USA), CD 90 (OriGene Technologies, Rockville, MD, USA), and CD 105 (OriGene Technologies, Rockville, MD, USA) antibodies. A two-color flow cytometry system (BD FACS Canto II; Becton-Dickinson, Franklin Lakes, NJ, USA) was used to analyze the immunostained cells. The percentage of stained cells was calculated by comparing the results to the corresponding negative controls.
2.4. Cell invasion assays of hTERT-ADSCs.TRAIL in PC
Matrigel-coated transwell cell culture chambers (8-μm pore size; Merck Millipore, Billerica, MA, USA) were used to assess the tropism of hTERT-ADSC.sTRAIL for tumor cells. Medium or 1.5 x 104 WPMY-1, human prostatic myofibroblasts (ATCC, Manassas, VA, USA), were loaded in the lower well of 24-well plates 24 h before the start of the experiment. The hTERT-ADSCs and hTERT-ADSC. sTRAIL (105) cells in serum-free medium were placed on the 8-Am pore-size inserts coated with ECMatrix. The lower wells containing the cells were washed with phosphate-buffered saline (PBS), filled with a serum-free medium, assembled for the migration assay, and incubated for 48 h. The noninvading cells and ECMatrix were wiped away from the inside of the insert, and the cells were stained for 20 min. The invading cells were photographed under a microscope. The results were evaluated by directly counting the number of migrated cells in five fields and calculating the mean.
2.5. In vivo migration toward prostate cancer
All procedures were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (2001) and approved by the Institutional Animal Care and Use Committee of the hospital. Adult male nude mice, each weighing 20 – 25 g were purchased (OrientBio, Seongnam, Korea). The animals were housed in a temperature-controlled environment with a 12:12-h day-night cycle. All mice ate regular chow and had free access to water. The mice were anesthetized with isoflurane (BKPharm, Goyang, Korea), and 1.0 × 106 PC3 cells (in 100 μl of PBS) were injected subcutaneously into the flank using a 30-gauge Hamilton syringe. The mice were anesthetized with isoflurane (BKPharm, Goyang, Korea) two weeks after PC3 inoculation. PBS or 1 x 106 hTERT.ADSC or hTERT.ADSC.sTRAIL cells mixed with 100 μl of saline were injected into the left ventricle using a 100-micrometer syringe with a 26-gauge needle. After two weeks, the mice were euthanized, and the prostate cancer tumor induced at the flank was removed.
To confirm the in vivo migration toward prostate cancer, RT-PCR for green fluorescent protein (GFP), sTRAIL, and β-actin was performed on the tumors induced at the flank by hTERT-ADSC.sTRAIL via intracardiac injection to determine whether the tumors demonstrated the presence of GFP and rabbit CE.
Quantitative RT-PCR (qRT-PCR) was performed using Maxima SYBR Green/ROX qPCR Master Mix (Thermo Fisher Scientific). The qRT-PCR reactions were run on a PikoReal 96 (Thermo Fisher Scientific) under cycling conditions of 95 °C for 15 seconds (denaturation), 55 °C for 30 seconds (annealing), and 72 °C for 60 seconds (extension) for 45 cycles. The gene expression level, normalized to β-actin, was then calculated using the 2-ΔΔ formula with reference to the hTERT-ADSC.sTRAIL. The primers are shown in Table 1.
Table 1.
PCR primer sequences.
| Gene | Sequence | Size (bp) |
|---|---|---|
| CE | Sense: 5′-TGCTGGGCTATCCACTCTCT-3′ | 237 |
| Antisense: 5′-CTCCAGCATCTCTGTGGTGA-3′ | ||
| SCF | Sense: 5′-ACTTGGATTCTCACTTGCATTT-3′ | 505 |
| Antisense: 5′- CTTTCTCAGGACTTAATGTTGAAG-3′ | ||
| c-kit | Sense: 5′- GCCCACAATAGATTGGTATTT-3′ | 332 |
| Antisense: 5′-AGCATCTTTACAGCGACAGTC-3′ | ||
| SDF-1 | Sense: 5′-ATGAACGCCAAGGTCGTGGTC-3′ | 200 |
| Antisense: 5′-GGCTGTTGTGCTTACTTGTTT-3′ | ||
| CXCR4 | Sense: 5′-CTCTCCAAAGGAAAGCGAGGTGGACAT-3′ | 733 |
| Antisense: 5′-AGACTGTACACTGTAGGTGCTGAAATCA-3′ | ||
| VEGF | Sense: 5′-AAGCCATCCTGTGTGCCCCTGATG-3′ | 541 |
| Antisense: 5′-GCTCCTTCCTCCTGCCCGGCTCAC-3′ | ||
| VEGFR1 | Sense: 5′-GCAAGGTGTGACTTTTGTTC-3′ | 512 |
| Antisense: 5′-AGGATTTCTTCCCCTGTGTA-3′ | ||
| VEGFR2 | Sense: 5′-ACGCTGACATGTACGGTCTAT-3′ | 438 |
| Antisense: 5′-GCCAAGCTTGTACCATGTGCG-3′ | ||
| VEGFR3 | Sense: 5′-AGCCATTCATCAACAAGCCT-3′ | 298 |
| Antisense: 5′-GGCAACAGCTGGATGTCATA-3′ | ||
| β-actin | Sense: 5′-GCA CCA CAC CTT CTA CAA TG -3 Antisense: 5′-TGC TTG CTG ATC CAC ATC TG -3 |
619 |
Carboxylesterase (CE); stem cell factor (SCF); stromal cell-derived factor 1 (SDF-1); C-X-C Motif Chemokine Receptor 4 (CECR4); Vascular endothelial growth factor (VEGF); Vascular endothelial growh factor receptor (VEGFR).
2.6. Confirmation of chemoattractant ligands and receptors
RT-PCR was used to determine the transcription of the ligand, stem cell factor, stromal cell–derived factor 1, and vascular endothelial growth factor (VEGF) in the PC3 prostate cancer cells and their corresponding receptors, c-kit, chemokine receptor 4 (CXCR4), VEGF receptor (VEGFR)-1, VEGFR2, and VEGFR3 in the hTERT-ADSC cells.
2.7. In vitro experiments
2.7.1. Antitumor effect of hTERT-ADSC.sTRAIL
Apoptosis and the viability of PC3 cells exposed to hTERT-ADSC.sTRAIL and CPT-11 were assessed. The hTERT-ADSC.sTRAIL cells (5 x 103 cells/well) were plated in 96-well plates (Falcon, Becton-Dickinson Co.). After 24 h, the cells were treated with 5 μM CPT-11 (Sigma-Aldrich). The cells were incubated at 37 °C for three days, and the cell viability was quantified. All experiments were conducted in quadruplicate. The cell viability was determined using a modified version of the MTT assay (Promega, Madison, WI, USA), which was based on the conversion of the tetrazolium salt of MTT to a formazan product by mitochondrial dehydrogenase. A total of 10 μl MTT solution was added to each well and incubated for 4 h at 37 ˚C. The color was extracted with dimethyl sulfoxide (Sigma-Aldrich) at 37 ˚C for 20 min. The formazan product was quantified by measuring the absorbance of the reaction at 570 nm using a microplate reader (Infinite F50; Tecan, Männedorf, Switzerland). The cell viability was expressed as the mean ± standard error (SE) percent of the control viability, which was set to 100.
2.7.2. Cytotoxicity of hTERT-ADSC.sTRAIL to PC3 cells
The hTERT-ADSC.sTRAIL and PC3 prostate cancer cells (1 x 104 cells) were plated. The ratios of hTERT-ADSC.sTRAIL and PC3 prostate cancer cells were 0, 0.1, and 0.01 in 96-well cell culture plates (Falcon, Becton-Dickinson Co.) in DMEM plus 3% FBS and incubated at 37 °C. After 24 h, the PC3 prostate cancer cells cocultured with hTERT-ADSC.sTRAIL cells were treated with 5 μM CPT-11. The microplates were incubated at 37 °C in a humidified atmosphere of 5% CO2 and 95% air for three days, and the cytotoxicity was measured by a colorimetric assay.
2.7.3. Propidium iodide/annexin V flow cytometry analysis
The proportion of apoptotic cells was determined by flow cytometry analysis. The hTERT-ADSC.sTRAIL cells were stained with annexin V–FITC and propidium iodide (FITC Annexin V Apoptosis Detection Kit; BD Pharmingen, Franklin Lakes, NJ, USA), and evaluated using a Cyflow Cube 8 FACS instrument (Sysmex Partec, Görlitz, Germany). The data were analyzed using standard FSC Express software (De Novo Software, Los Angeles, CA, USA).
2.8. In vivo experiments
The mice were anesthetized with isoflurane (BKPharm, Goyang, Korea). PC3 cells (1.0 × 106 in 100 μl PBS) were injected subcutaneously into the flank using a 30-gauge Hamilton syringe. One and two weeks after intracardiac injection of hTERT-ADSC.sTRAIL, the animals were treated with 1.7 mg/kg/day of CPT-11 diluted in 100 μl of PBS i.p. in two rounds of five consecutive days with a break of two days. At days 0 and 14 days after the injection, the tumor volumes were measured using calipers, and the volume was calculated based on the formula, volume = length × width2/2. To evaluate the effect of a high concentration compared with that of a low concentration of CPT-11, at weeks one and two after the intracardiac injection of hTERT-ADSC.sTRAIL, the animals were treated with 13.5 mg/kg/day of CPT-11 diluted in 100 μl of PBS intraperitoneal injection (i.p.) in two rounds of five consecutive days with a break of two days. On days 0 and 14 after the injection, the tumor volumes were measured. The results are expressed as the mean tumor volume ± SE. Fourteen days after the injection, the mice were euthanized, and the induced tumors were removed.
2.9. Immunohistochemistry
The removed tumors were analyzed immunohistopathologically for TRAIL to demonstrate the presence of the delivered TRAIL and its involvement in anticancer activity. We used a TRAIL antibody (1:100 ADI-AAP-470, Enzo Life Sciences INC, Farmingdale, NY, USA) and a DAB Detection IHC Kit (Abcam, Cambridge, UK). The TRAIL assay was performed on tumor tissues that were removed after two weeks of treatment with hTERT-ADSC.sTRAIL or the combination of hTERT-ADSC.sTRAIL and CPT-11. The stained sections were visualized using a microscope (Olympus, Tokyo, Japan). Low-power (X100) or high-power (X400) fields were examined.
To detect apoptosis, a terminal deoxynucleotidyl transferase–mediated dUTP nick end-labeling (TUNEL) assay was performed. We used a TdT apoptosis detection kit (Takara Bio Inc, Mountain View, CA, USA) and a DAB Detection IHC Kit (Abcam). The TUNEL assay was performed on tumor tissues that were removed after two weeks of treatment with hTERT-ADSC.sTRAIL or the combination hTERT-ADSC.sTRAIL and CPT-11. The stained sections were visualized using a microscope (Olympus, Tokyo, Japan). Low-power (X100) or high-power (X400) fields were examined.
2.10. Statistics
Two-way ANOVA and a posthoc Tukey test were used to determine the significant differences in cell viability and tumor volume. The data are presented as means ± SE. A P value of < 0.05 was considered statistically significant.
3. Results
3.1. Generation of hTERT-ADSC
The ASC52TELO, hTERT-ADSC (ATCC® SCRC-4000™) is an immortalized human adipose mesenchymal SC line containing hTERT. The cells were shown to express mesenchymal SC markers CD29, CD90, and CD105, but not CD34 or CD45 markers for hematopoietic SCs. The telomerase assay [telomerase repeat amplification protocol(TRAP)] showed that the cells expressed markers for telomerase activity (Product sheet from ATCC® SCRC-4000™, ATCC, Manassas, VA, USA).
3.2. Gene expression of TRAIL from hTERT-ADSC.sTRAIL
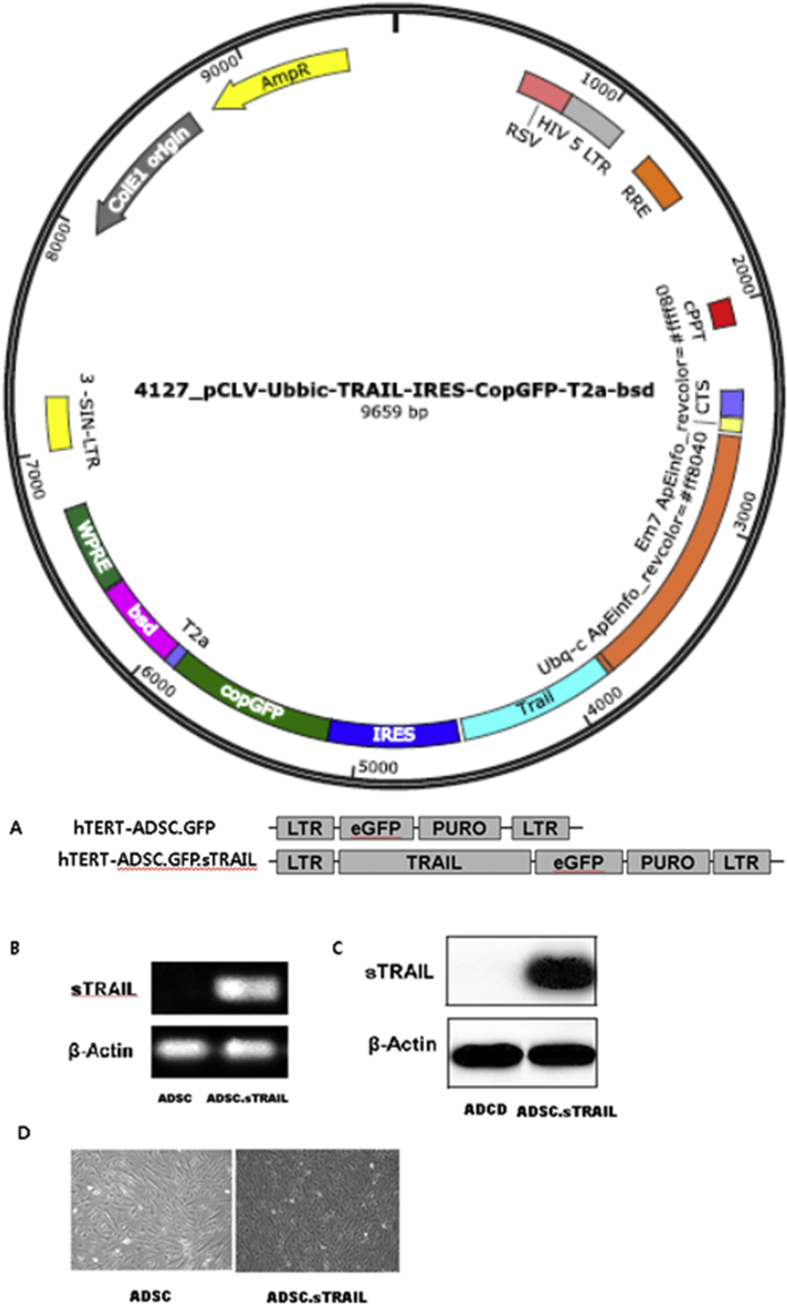
hTERT-ADSC.sTRAIL cells were produced by lentiviral transduction of the sTRAIL gene using a CLV-Ubic puromycin/sTRAIL vector. The expression of sTRAIL transcripts from hTERT-ADSC.sTRAIL was confirmed by RT-PCR. The expression of sTRAIL protein from hTERT-ADSC.sTRAIL was confirmed by Western blotting. sTRAIL transcripts and protein were demonstrated only in hTERT-ADSC.sTRAIL, but not in hTERT-ADSC cells (Fig. 1).
Fig. 1.
The sTRAIL-overexpressing hTERT immortalized human adipose stem cell line (hTERT-ADSC. sTRAIL). (A) hTERT-ADSC and hTERT-ADSC.sTRAIL were generated by the lentiviral transduction of GFP and the sTRAIL gene using the CLV-Ubic vector. (B), (C): The expression of the sTRAIL transcript or protein was confirmed by RT-PCR or Western blots, respectively. (D) Phase-contrast microscopy of the hTERT-ADSC.GFP line (left) and hTERT-hADSC.GFP. sTRAIL (right) line. ADCS = hTERT-hADSC, sTRAIL = secretable trimeric forms of tumor necrosis factor (TNF)-related apoptosis-inducing ligand, ADSC = adipose-derived stem cells, ADSC.sTRAIL = sTRAIL-overexpressing ADSC, RT-PCR = reverse transcription-polymerase chain reaction.
3.3. hTERT-ADSC.sTRAIL phenotype
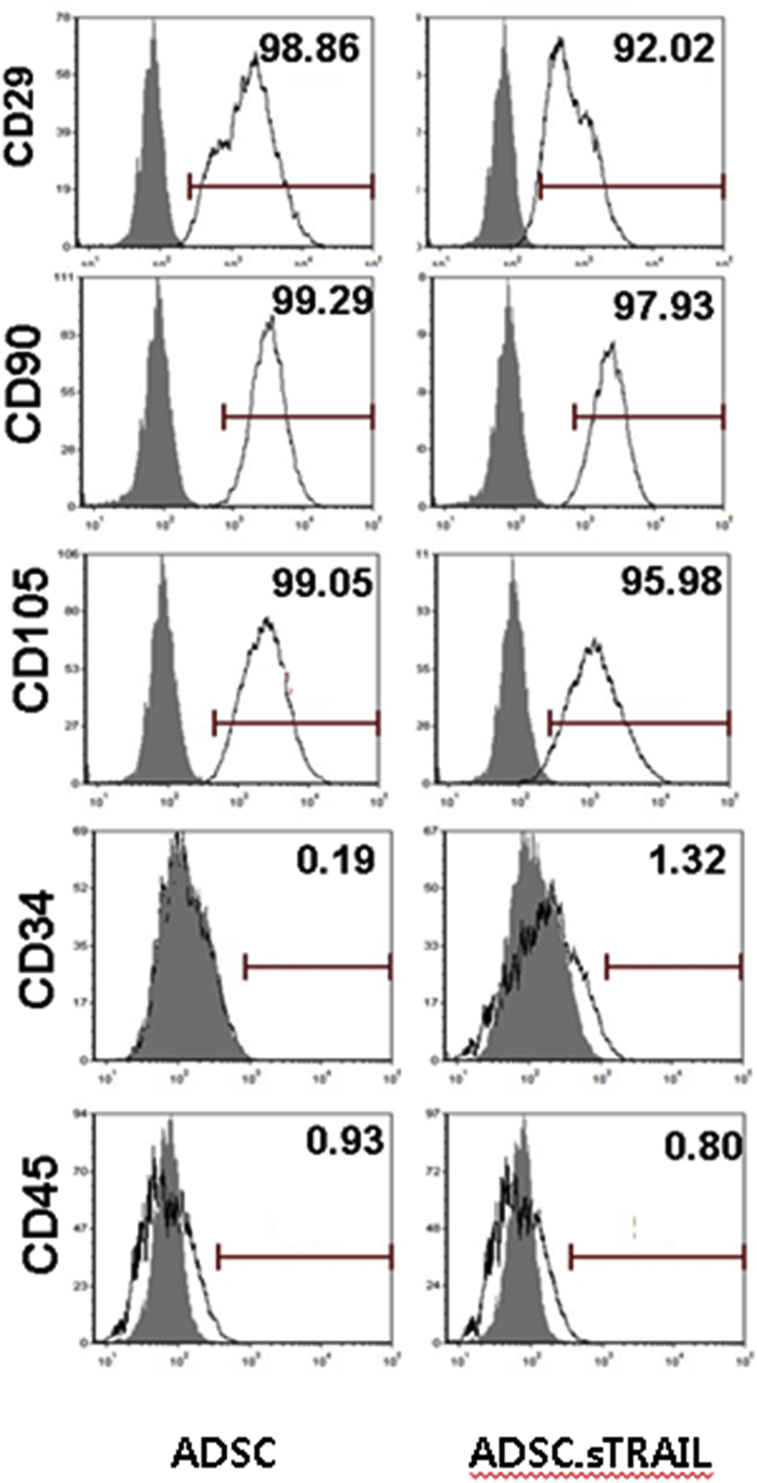
hTERT-ADSC and hTERT-ADSC.sTRAIL cells expressed cell type–specific markers for mesenchymal stem cell markers CD29, CD90, and CD105, but did not express CD34 or CD45 cell type markers for hematopoietic stem cells (Fig. 2).
Fig. 2.
Cell markers of hTERT-ADSC.GFP and hTERT-ADSC.GFP.sTRAIL cells. hTERT-ADSC cells express cell type–specific markers for mesenchymal stem cell markers CD29, CD90, and CD105, but do not express CD34 or CD45 cell type markers for hematopoietic stem cells. ADSC = hTERT immortalized human adipose-derived stem cells, ADSC.sTRAIL = sTRAIL-overexpressing ADSCs.
3.4. Migration study
3.4.1. Invasion study
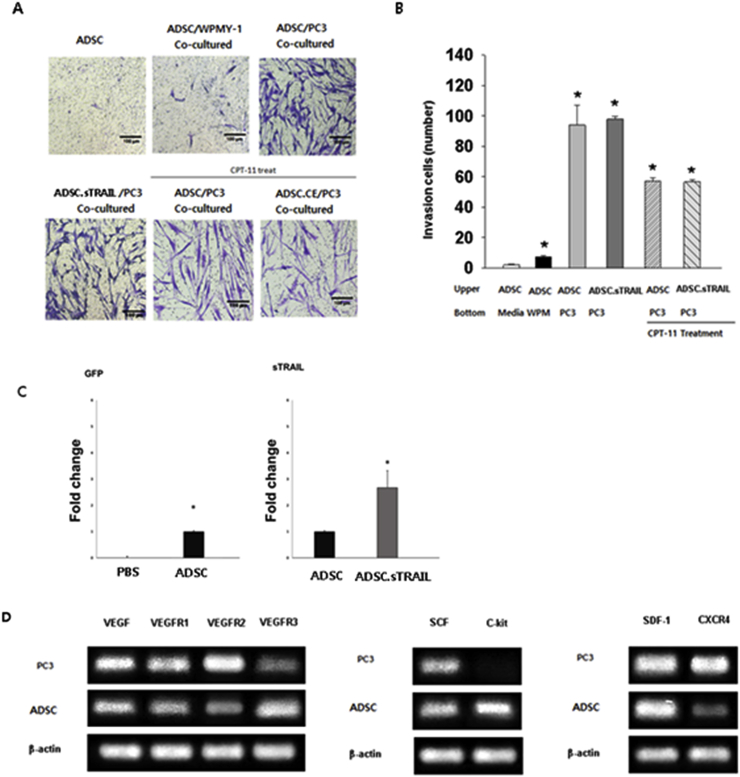
Whereas only a few hTERT-ADSC and hTERT-ADSC.sTRAIL cells migrated toward media or the WPMY-1 cells, the directional migration of hTERT-ADSC and hTERT-ADSC.sTRAIL cells toward PC3 cells was significantly stimulated by PC3 cells in vitro (P < 0.05). The migration capacity was not affected by sTRAIL transgene expression. The directional migration of hTERT-ADSC and hTERT-ADSC.sTRAIL cells toward PC3 cells was also significantly stimulated by PC3 cells treated with 5 μM CPT-11 in vitro (P < 0.05). The migration capacity of hTERT-ADSC.sTRAIL cells was not affected by treatment with 5 μM CPT-11 for 72 hours (P < 0.05) (Fig. 3A and B).
Fig. 3.
hTERT-ADSC.sTRAIL line targets prostate cancer. (A), (B) The invasion study shows the migration of hTERT-ADSC or hTERT-ADSC.sTRAIL to prostate cancer. (C) Intracardiac delivery of hTERT-ADSC targeting prostate cancer. qPCR demonstrated GFP and human sTRAIL from experimentally induced prostate cancer tissues removed from the mice. (D) Expression of chemoattractant factors. The presence of the ligands, SCF, SDF-1, and VEGF in PC3 cells and their corresponding receptors c-kit, CXCR4, VEGFR1, VEGFR2, and VEGFR3 in hTERT-ADSC cells. ADCS = hTERT-ADSC, stem cell factor, SCF = stromal cell–derived factor, SDF = vascular endothelial growth factor, VEGF = vascular endothelial growth factor, VEGFR = vascular endothelial growth factor receptor, ADSC = hTERT immortalized human adipose stem cell line, ADSC.sTRAIL = sTRAIL-overexpressing ADSCs. ∗P < 0.05.
3.4.2. In vivo migration study
RT-PCR demonstrated the presence of GFP and human sTRAIL from the induced prostate cancer tissue removed from the mice. GFP (fold) mRNA was not detectable in PBS. However, it was seen in the ADSCs (1.0 ± 0.1). The mRNA level of sTRAIL in the treated mice (2.7 ± 0.7) was higher than that in the ADSC-treated group (1.0 ± 0.1, P < 0.05) (Fig. 3C). Because neither GFP nor human sTRAIL exists in mouse tissue, the presence of GFP or human sTRAIL demonstrates the selective migration of hTERT-hADSC.sTRAIL toward the prostate cancer induced in the mice. The intracardiac delivery of hTERT-hADSC.sTRAIL toward targeted prostate cancer was confirmed.
3.4.3. Confirmation of chemoattractant ligands and receptors
The presence of ligands, stem cell factor, stromal cell–derived factor 1, and VEGF in PC3 prostate cancer cells and their corresponding receptors, c-kit, CXCR4, VEGFR1, VEGFR2, and VEGFR3 was confirmed in the hTERT-ADSC cells by RT-PCR (Fig. 3D).
3.5. In vitro antitumor effects of hTERT-ADSC.sTRAIL
3.5.1. In vitro suicide effect of hTERT-ADSC.sTRAIL
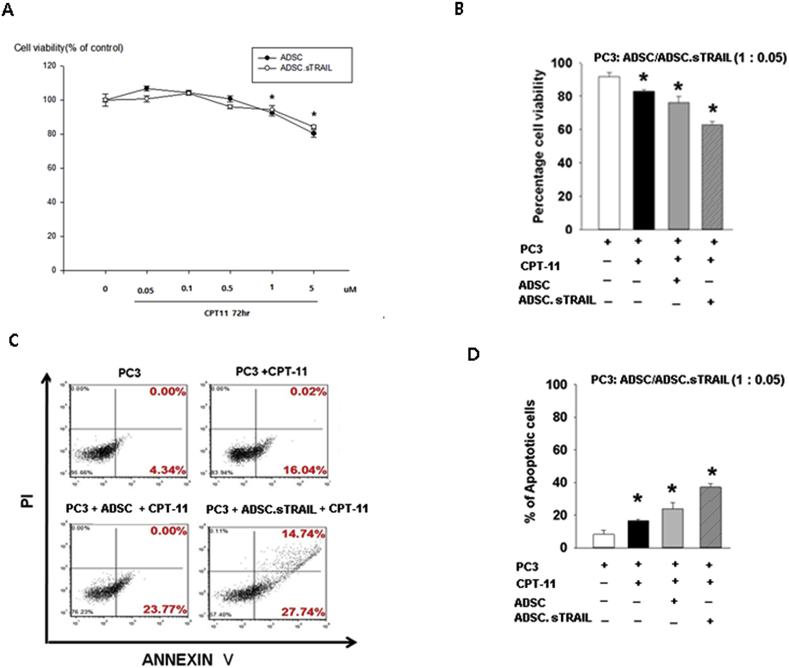
To analyze the in vitro cytotoxicity of CPT-11 on hTERT-ADSC.sTRAIL cells, we used a cell viability assay. hTERT-hADSC.sTRAIL cells were cultured under varying concentrations of CPT-11 (0.01 – 5 μM) for 72 h. The cell viability was not changed by treatment with CPT-11 from 0.01 to 0.5 μM. At 1 μM CPT-11, the viability of hTERT-hADSC cells (92.5 ± 1.8%) and hTERT-hADSC.sTRAIL cells (94.3 ± 2.4%) was lower than that of hTERT-hADSC (100.0 ± 3.5%) and hTERT-hADSC.sTRAIL (100.0 ± 1.1%) at 0 μM CPT-11. At a final concentration of 5 μM cpt-11, the hTERT-ADSC (80.3 ± 2.0%) and hTERT-ADSC.sTRAIL cell lines (84.4 ± 1.2%) showed lower cell viability at 72 hours than treatment with 0.1 μM cpt-11 alone (100.9 ± 1.1%, P < 0.05). A concentration of CPT-11 greater than 5 μM was required to achieve an effective in vitro suicide effect of hTERT-hADSC.sTRAIL by cpt-11 treatment (Fig. 4A).
Fig. 4.
In vitro effect of combined treatment with hTERT-ADSC.sTRAIL and cpt-11. (A) The suicide effect. At greater than 1 μM CPT-11, the viability of ADSC.sTRAIL cells was lower than at more than 0 μM CPT-11 (P < 0.05). (B) The cytotoxicity of ADSC.sTRAIL and 5 μM CPT-11 toward PC3 at cocultured ratios of 0.05. The cell viability of PC3 decreased significantly by treatment with both CPT-11 and ADSC.sTRAIL compared with CPT-11 treatment alone. (D) Flow cytometry for propidium iodide/annexin V staining showed that the percentage of annexin V–positive apoptotic PC3 cells increased in the presence of ADSC.sTRAIL and CPT-11 compared to CPT-11 treatment alone. (E) The percentage of apoptotic cells significantly increased in the ADSC.sTRAIL-treated group compared with those treated with cpt-11 alone (P < 0.05). ADSC = hTERT immortalized human adipose-derived stem cells, ADSC.sTRAIL = sTRAIL-overexpressing hTERT immortalized human adipose stem cells. ∗P < 0.05.
3.5.2. In vitro cytotoxicity of hTERT-ADSC.sTRAIL
The selective cytotoxicity toward PC3 prostate cancer cells was mediated by hTERT-ADSC.sTRAIL in the presence of 5 μM CPT-11 in vitro for 72 hours. The viability of the PC3 cells was significantly decreased by combined treatment with ADSC.sTRAIL and CPT-11 (62.7 ± 2.0%) compared with treatment with CPT-11 alone (83.0 ± 1.0%) at a cell ratio of 0.05 (PC3: ADSC.sTRAIL) (P < 0.05). The percentage of viable hTERT-ADSC.sTRAIL cells was significantly decreased compared with CPT-11 treatment alone (Fig. 4B).
3.5.3. In vitro apoptotic effect of ADSC.sTRAIL
Flow cytometry for annexin V/propidium iodide staining showed that the percentage of annexin V–positive apoptotic PC3 cells was increased in the ADSC.sTRAIL (42.5%) and the 5 μM CPT-11–treated group compared with the group treated with CPT-11 alone (16.06%) (Fig. 4C). The percentage of apoptotic PC3 cells significantly increased in the hTERT-ADSC.sTRAIL and CPT-11–treated group (37.2 ± 2.1%) compared with the group treated with CPT-11 alone (16.5 ± 1.0%, P < 0.05) (Fig. 4D).
3.6. Tumor growth inhibition by the systemic administration of hTERT-ADSC.sTRAIL in vivo
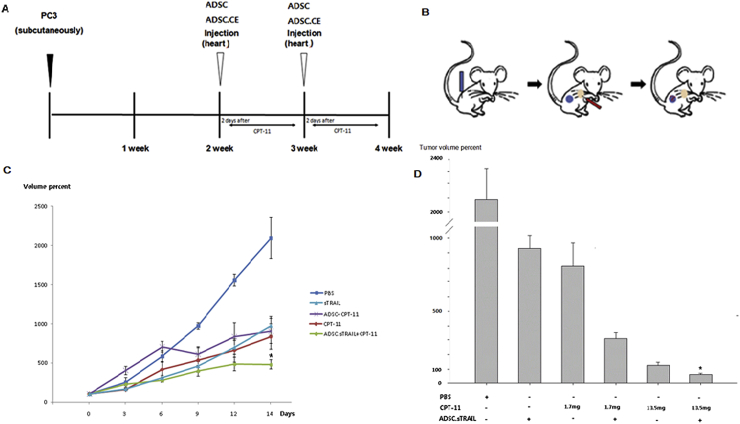
Prostate cancer was induced in mice (Fig. 5A and B). The systemic administration of hTERT-ADSC.sTRAIL in combination with CPT-11 reduced prostate cancer volume. Two weeks after CPT-11 treatment, the volume of the prostate cancer tumors was 2089.0 ± 264.9% (control group), 968.8 ± 118.6% (hTERT-ADSC.sTRAIL group), 830.1 ± 161.9% (CPT-11 group), 901.1 ± 158.4% (hTERT-ADSC + CPT-11 group), and 476.4 ± 59.5% (hTERT-ADSC.sTRAIL + CPT-11 group) of the baseline prostate cancer volume 0 weeks after CPT-11 treatment (Fig. 5C).
Fig. 5.
Treatment with hTERT-hADSC.sTRAIL cells and CPT-11 has a significant therapeutic effect in vivo. (A) The schematic summary of the treatment. (B) Illustration of the induction of prostate cancer using PC3 cells, the systemic injection of hTERT-ADSC.sTRAIL cells, and the migration of gene-modified stem cells toward prostate cancer. Blue: PC3; red: hTERT-ADSC.sTRAIL cells. (C) Administration of hTERT-hADSC.sTRAIL in combination with 1.7 mg/kg/day CPT-11 reduced prostate cancer tumor volumes in mice. The inhibitory effects of hTERT-ADSC.sTRAIL combined with CPT-11 were higher than those of hTERT-ADSC.sTRAIL or cpt-11 alone. (D) The inhibitory effects of hTERT-ADSC.CE combined with a high CPT-11 concentration (13.5 mg/kg/day) were better than those at a low CPT-11 concentration (17 mg/k/day). ADSC = hTERT immortalized human adipose stem cell line, hTERT-ADSC.sTRAIL = sTRAIL-overexpressing hTERT immortalized human adipose stem cells, H&E = hematoxylin & eosin. ∗P < 0.05.
After the systemic administration of hTERT-ADSC.sTRAIL combined with CPT-11, prostate cancer tumor growth was inhibited compared with the control group (P < 0.05). After systemic administration of hTERT-ADSC.sTRAIL in combination with CPT-11, prostate tumor growth was inhibited compared with the hTERT-ADSC.sTRAIL or CPT-11 monotherapy groups (P < 0.05). The inhibitory effects of ADSC.sTRAIL and CPT-11 were greater than those of CPT-11 alone.
Two weeks after the systemic administration of hTERT-ADSC.sTRAIL in combination with 13.5 mg/Kg/day CPT-11, the prostate cancer volume decreased up to 86.7 ± 4.2% (hTERT-ADSC.sTRAIL +13.5 mg/Kg/day CPT-11) and 140.2 ± 15.6% (13.5 mg/Kg/day CPT-11 monotherapy), compared with 100 ± 17.5% (baseline prostate cancer volume at 0 weeks) (P < 0.05) (Fig. 5D). The inhibitory effects of hTERT-ADSC.CE combined with 13.5 mg/Kg/day CPT-11 were better than those of 13.5 mg/Kg/day CPT-11 monotherapy and decreased the prostate cancer volume up to 61.8%.
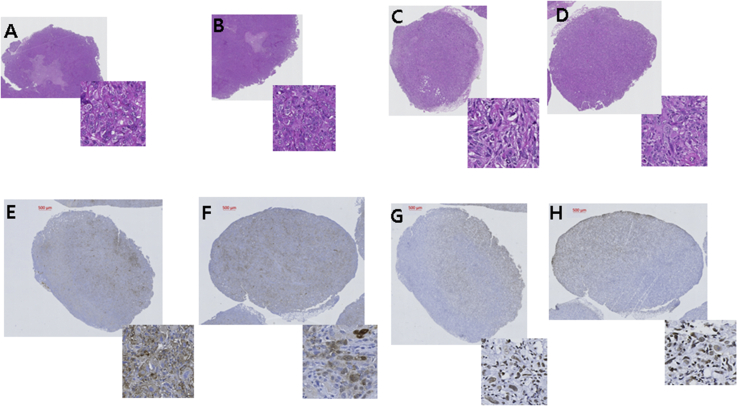
Hematoxylin and eosin staining of the transplanted PC3 cell tumor showed the induction of prostate cancer two weeks after the injection of PC3 prostate cancer cells into the flanks of the mice (Fig. 6A). The tumor was well-demarcated from dermal tissue and composed of closely packed cancer cells. The neoplastic cells remaining in the peripheral lesion were shrunken and had distinct borders. Geographic necrosis was found (Fig. 6A–D). Immunohistochemical staining showed anti-TRAIL–positive cells in the removed tumor by treatment with hTERT-ADSC.sTRAIL or the combination of hTERT-ADSC.sTRAIL and CPT-11 (Fig. 6E and F), and hyperchromatic nuclei were centrally located with condensed eosinophilic cytoplasm, which has apoptotic bodies (Fig. 6G and H).
Fig. 6.
Immunohistochemistry of removed prostate cancer tumors. (A)–(D) H&E staining of induced prostate cancer two weeks after the implantation of PC3 cells into the flanks of the mice. (A) Tumors in PC3-transplanted mice (X100), Inset: (X400). The tumor is well-demarcated from the dermal tissue and composed of closely packed cancer cells. (B) Tumors in PC3-transplanted mice after 13.5 mg/kg/day CPT-11 systemic treatment (X100). (C) Tumors in PC3-transplanted mice after hTERT-ADSC.sTRAIL systemic treatment (X100). (D) Tumors in PC3-transplanted mice after hTERT-ADSC.sTRAIL combined with 13.5 mg/kg/day CPT-11 systemic treatment (X100). Geographic necrosis was seen. The neoplastic cells remaining in the peripheral lesion were shrunken and had distinct borders. (E), (F) TRAIL immunohistochemical staining of tumors after treatment shows anti-TRAIL–positive cells. (E) Tumors in the PC3-transplanted mice after hTERT-ADSC.sTRAIL systemic treatment (X100), Inset: (X400). (F) Tumors in PC3-transplanted mice after hTERT-ADSC.sTRAIL combined with 13.5 mg/kg/day CPT-11 systemic treatment (X100), Inset: (X400) (G), (H) TUNEL immunohistochemical staining of tumors after treatment shows TUNEL-positive cells. (G) Tumors in PC3-transplanted mice after hTERT-ADSC.sTRAIL systemic treatment (X100), Inset: (X400). (H) Tumors in PC3-transplanted mice after hTERT-ADSC.sTRAIL combined with 13.5 mg/kg/day CPT-11 systemic treatment (X100), Inset: (X400). Hyperchromatic nuclei are centrally located with condensed eosinophilic cytoplasm, which have apoptotic bodies. Inset: Apoptotic cells with apoptotic bodies. Bar. 500 μm. H&E = hematoxylin & eosin, TUNEL = terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling.
4. Discussion
Although initial clinical trials using the death ligand TRAIL have shown disappointing results, TRAIL is still an attractive cancer therapeutic agent because it can induce remarkable apoptosis in tumors with minimal toxicity to normal tissues. However, several cancers, including PC and CRPC, are resistant to TRAIL-induced apoptosis. To overcome the known limitation of TRAIL resistance in PC treatment, we added several combinations to classic TRAIL, including secreted trimeric forms of TRAIL, ADSC, and CPT-11.
TRAIL induces apoptosis selectively in a variety of tumor cells, but not in normal cells or tissues, suggesting it as an excellent therapeutic candidate for cancer with reduced toxicity (2, 3). Because of low toxicity in normal tissue or cells and apoptosis by extrinsic and intrinsic pathways, TRAIL and anti-TRAIL antibodies could be promising antitumor agents(16). In this study, the sTRAIL transcript and protein were detected only in hTERT-ADSC.sTRAAIL, but not in hTERT-ADSC cells (Fig. 1), indicating that gene modification was successfully accomplished.
Gene therapy has several advantages compared with conventional drug therapies. First, the high-dose and long-term expression of a drug can be achieved relatively easily. The drug may be delivered locally and specifically to the target tissues. ADSCs can be used as delivery vehicles for therapeutic genes.
ASC52TELO, hTERT-ADSC (ATCC® SCRC-4000™) is an immortalized human adipose mesenchymal SC line containing hTERT. The cells express markers specific for mesenchymal SCs CD29, CD90, and CD105, but not hematopoietic SC markers CD34 or CD45. The telomerase assay showed the expression of the marker for telomerase activity. Therefore, the modification of ADSCs with hTERT did not alter the SC properties.
The major advantage of using SCs to treat cancer is their ability to migrate and infiltrate tumors. Moreover, this tumor-specific tropism may be exploited to target minor distant metastases and infiltrate malignant satellites after the complete removal of the main tumor. In this study, the directional migration of hTERT-ADSC.sTRAIL cells toward PC3 cells was significantly stimulated by PC3 cells in vitro. The migration ability was not affected by sTRAIL transgene expression or by treatment with 5 μM CPT-11 for 72 hours. In this study, the directional migration of hTERT-ADSC.sTRAIL cells toward PC3 cells was significantly stimulated by PC3 cells in vitro (Fig. 3). The migration capacity was not affected by sTRAIL transgene expression or treatment with 5 μM CPT-11 for 72 hours (Fig. 3). Neither gene modification using lentiviral transduction nor transgene expression affected SC differentiation, migration properties, or pluripotency transcription factor expression(10, 16, 17, 18). RT-PCR demonstrated the presence of GFP and human sTRAIL in the removed prostate cancer tissues induced by the injection of PC3 cells into the flanks of mice (Fig. 3C). Because artificially transfected human sTRAIL or GFP does not exist in normal mouse tissues, the presence of human sTRAIL or GFP in the experimentally induced prostate cancer tissues demonstrated the selective migration of hTERT-hADSC.sTRAIL cells toward induced prostate cancer in mice. The hTERT-ADSCs.sTRAIL cells migrated to the implanted tumor sites and reduced the tumor volume in mice exposed to CPT-11.
The combined treatment of TRAIL and CPT-11 potentiated the antitumor effect without the need to increase the concentration of CPT-11 and with reduced toxicity. The generation of TRAIL-overexpressing SCs triggered the persistent release of TRAIL at high concentrations from the SCs. CPT-11 is known to augment the TRAIL-induced apoptosis of cancer (14). In this study, the in vitro suicide effect of ADSC.sTRAIL was shown at a concentration of CPT-11 greater than 5 μM (Fig. 4A). A concentration of CPT-11 greater than 5 μM was required for the effective in vitro suicide effect of hTERT-hADSC.sTRAIL cells by treatment with cpt-11. Cytotoxicity toward PC3 prostate cancer cells was mediated by hTERT-ADSC.sTRAIL cells in the presence of 5 μM CPT-11 in vitro for 72 hours (Fig. 4B). hTERT-ADSC.sTRAIL cells exerted a strong selective cytotoxic effect on PC3 prostate cancer cells, even at a ratio of hTERT-ADSC.CE to PC3 cells of 0.05 to 1. In this study, hTERT-ADSC.sTRAIL cells induced apoptosis in vitro in the combined treatment with 5 μM CPT-11 for 72 hours (Fig. 4C). The percentage of apoptotic cells was significantly increased in the hTERT-ADSC.sTRAIL cells compared with the hTERT-ADSC cells (Fig. 4 D).
In the in vivo study, the systemic administration of hTERT-ADSC.sTRAIL in combination with 1.7 mg/Kg/day CPT-11 reduced the prostate cancer tumor volume. After the systemic administration of hTERT-ADSC.sTRAIL in combination with CPT-11, the PC tumor growth was significantly inhibited compared with that of CPT-11 treatment alone (Fig. 5D). The inhibition of castration-resistant prostate cancer tumor growth using TRAIL-overexpressing adipose stem cells was enhanced by the addition of the chemotherapeutic agent CPT-11. This suggests that the antitumor effect of the prolonged production of higher local concentrations of TRAIL released from hTERT-ADSC.sTRAIL and the potentiation by CPT-11 increased the therapeutic efficiency.
The inhibitory effects of hTERT-ADSC.CE combined with 13.5 mg/Kg/day CPT-11 were better than those of hTERT-ADSC.sTRAIL combined with 1.7 mg/Kg/day CPT-11 (Fig. 5E). The inhibitory effects of hTERT-ADSC.sTRAIL combined with 13.5 mg/Kg/day CPT-11 were better than those of 13.5 mg/Kg/day CPT-11 treatment alone and decreased the volume of the prostate cancer tumors up to 61.8% compared with 13.5 mg/Kg/day CPT-11 monotherapy (Fig. 5E). Because the treatment of hTERT-ADSC.sTRAIL cells in combination with CPT-11 decreased the effective concentration of CPT-11, enhanced efficacy of CPT-11 with reduced toxicity can be expected.
Immunohistochemical staining of the removed tumor showed anti-TRAIL–positive cells after hTERT-ADSC.sTRAIL or hTERT-ADSC.sTRAIL treatment combined with CPT-11 (Fig. 6E and F) and hyperchromatic nuclei were centrally located with condensed eosinophilic cytoplasm, which had apoptotic bodies (Fig. 6G and H). Immunohistopathological examination of the removed tumors for TRAIL demonstrated that the delivered TRAIL was present and involved in anticancer activity. This suggests that TRAIL-induced apoptosis in the prostate cancer and CPT-11 augmented TRAIL-induced apoptosis in prostate cancer.
Recently, several studies have tried to overcome the limitation of resistance to classical TRAIL-induced apoptosis in PC. Mohr et al.(7) showed that the combination of small-molecule drugs with SC.sTRAIL enhanced the sensitization of PC cells to TRAIL and reduced cytokine production related to the side effects of TRAIL. Chen at al.(19) showed that a novel combination of cyproterone acetate and TRAIL caused apoptosis in CRPC cell lines, including PC3 and DU145.
Although our study demonstrated remarkable findings using the novel combination of sTRAIL, ADSCs, and CTP-11, there were several limitations. First, our study did not include any results using full-length TRAIL. There are conflicting results on the efficacy differences between full-length TRAIL and sTRAIL. A second limitation was the lack of molecular analyses to define the crucial mechanism of hTERT-ADSC.sTRAIL in PC. To date, several detailed mechanisms of TRAIL in cancer have been reported. Besides the classical mechanism of DR5 upregulation, the sensitization of tumors or cancer SCs to SC-TRAIL–induced apoptosis; increases in antiapoptotic molecules; the activation of caspase-8, which induces caspase-3 via cleavage (extrinsic pathway) or caspase-3 activation (intrinsic pathway); and unfolded protein response induced by endoplasmic reticulum (ER) stress need to be elucidated in PC. Further characterization of the precise mechanisms by which the combined treatments enhanced the susceptibility to TRAIL-induced apoptosis is warranted. Third, in the in vitro and in vivo studies, our study did not demonstrate the apoptotic effect of hTERT-ADSC.sTRAIL or hTERT-ADSC on PC3 cells without CPT-11 and did not show an in vivo treatment effect of hTERT-ADSC.sTRAIL or hTERT-ADSC on PC3 cells without CPT-11. Lastly, other CRPC cells, such as 22RV1 or VCaP, should be included to demonstrate the universality of the combined treatment effect on CRPC.
5. Conclusions
Human ADSCs in combination with the sTRAIL transgene migrated to PC, potentiated cytotoxic effects against tumor cells in vitro and exerted a robust inhibition of tumor growth in vivo in combination with the prodrug CPT-11. These results indicate that therapeutic sTRAIL-overexpressing ADSCs combined with CPT-11 represents a new strategy for clinical trials for CRPC treatment using the patients’ own ADSCs.
Author contributions
SHL and YSS conceived and designed the experiments. HYL, JHK, SHD, WJY, EUO, JYH, and YSH performed the experiments. JHK, SHL, and YSS analyzed the data. SHL and YSS wrote the paper.
Conflicts of interest
There is no conflict of interest.
Acknowledgments
This research was supported by a grant from the Soonchunhyang University Research Fund and the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2018R1A2B6001693).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prnil.2020.07.002.
Contributor Information
Sang Hun Lee, Email: ykckss1114@nate.com.
Yun Seob Song, Email: yssong@schmc.ac.kr.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wong M.C., Goggins W.B., Wang H.H., Fung F.D., Leung C., Wong S.Y. Global Incidence and Mortality for Prostate Cancer: Analysis of Temporal Patterns and Trends in 36 Countries. Eur Urol. 2016;70(5):862–874. doi: 10.1016/j.eururo.2016.05.043. PubMed PMID: 27289567. [DOI] [PubMed] [Google Scholar]
- 2.Pitti R.M., Marsters S.A., Ruppert S., Donahue C.J., Moore A., Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271(22):12687–12690. doi: 10.1074/jbc.271.22.12687. Epub 1996/05/31. PubMed PMID: 8663110. [DOI] [PubMed] [Google Scholar]
- 3.Walczak H., Miller R.E., Ariail K., Gliniak B., Griffith T.S., Kubin M. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5(2):157–163. doi: 10.1038/5517. Epub 1999/02/04. PubMed PMID: 9930862. [DOI] [PubMed] [Google Scholar]
- 4.Roth W., Isenmann S., Naumann U., Kugler S., Bahr M., Dichgans J. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem Biophys Res Commun. 1999;265(2):479–483. doi: 10.1006/bbrc.1999.1693. PubMed PMID: 10558893. [DOI] [PubMed] [Google Scholar]
- 5.Voelkel-Johnson C., King D.L., Norris J.S. Resistance of prostate cancer cells to soluble TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) can be overcome by doxorubicin or adenoviral delivery of full-length TRAIL. Canc Gene Ther. 2002;9(2):164–172. doi: 10.1038/sj.cgt.7700420. PubMed PMID: 11857034. [DOI] [PubMed] [Google Scholar]
- 6.Newsom-Davis T., Prieske S., Walczak H. Is TRAIL the holy grail of cancer therapy? Apoptosis. 2009;14(4):607–623. doi: 10.1007/s10495-009-0321-2. PubMed PMID: 19194800. [DOI] [PubMed] [Google Scholar]
- 7.Mohr A., Chu T., Brooke G.N., Zwacka R.M. MSC.sTRAIL Has Better Efficacy than MSC.FL-TRAIL and in Combination with AKTi Blocks Pro-Metastatic Cytokine Production in Prostate Cancer Cells. Cancers. 2019;11(4) doi: 10.3390/cancers11040568. PubMed PMID: 31010082; PubMed Central PMCID: PMCPMC6521093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamada H., Kobune M., Nakamura K., Kawano Y., Kato K., Honmou O. Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapy. Canc Sci. 2005;96(3):149–156. doi: 10.1111/j.1349-7006.2005.00032.x. Epub 2005/03/18. PubMed PMID: 15771617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Studeny M., Marini F.C., Champlin R.E., Zompetta C., Fidler I.J., Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Can Res. 2002;62(13):3603–3608. Epub 2002/07/05. PubMed PMID: 12097260. [PubMed] [Google Scholar]
- 10.Studeny M., Marini F.C., Dembinski J.L., Zompetta C., Cabreira-Hansen M., Bekele B.N. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96(21):1593–1603. doi: 10.1093/jnci/djh299. Epub 2004/11/04. PubMed PMID: 15523088. [DOI] [PubMed] [Google Scholar]
- 11.Lee H.J., Doo S.W., Kim D.H., Cha Y.J., Kim J.H., Song Y.S. Cytosine deaminase-expressing human neural stem cells inhibit tumor growth in prostate cancer-bearing mice. Canc Lett. 2013;335(1):58–65. doi: 10.1016/j.canlet.2013.01.048. PubMed PMID: 23391716. [DOI] [PubMed] [Google Scholar]
- 12.Kucerova L., Altanerova V., Matuskova M., Tyciakova S., Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Can Res. 2007;67(13):6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. Epub 2007/07/10. PubMed PMID: 17616689. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.H., Lee H.J., Song Y.S. Stem cell based gene therapy in prostate cancer. BioMed Res Int. 2014;2014:549136. doi: 10.1155/2014/549136. Epub 2014/08/15. PubMed PMID: 25121103; PubMed Central PMCID: PMCPMC4120795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray S., Almasan A. Apoptosis induction in prostate cancer cells and xenografts by combined treatment with Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand and CPT-11. Can Res. 2003;63(15):4713–4723. Epub 2003/08/09. PubMed PMID: 12907654. [PubMed] [Google Scholar]
- 15.Kawato Y., Aonuma M., Hirota Y., Kuga H., Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Can Res. 1991;51(16):4187–4191. Epub 1991/08/15. PubMed PMID: 1651156. [PubMed] [Google Scholar]
- 16.Cha S.S., Kim M.S., Choi Y.H., Sung B.J., Shin N.K., Shin H.C. 2.8 A resolution crystal structure of human TRAIL, a cytokine with selective antitumor activity. Immunity. 1999;11(2):253–261. doi: 10.1016/s1074-7613(00)80100-4. PubMed PMID: 10485660. [DOI] [PubMed] [Google Scholar]
- 17.Schrepfer S., Deuse T., Reichenspurner H., Fischbein M.P., Robbins R.C., Pelletier M.P. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39(2):573–576. doi: 10.1016/j.transproceed.2006.12.019. Epub 2007/03/17. PubMed PMID: 17362785. [DOI] [PubMed] [Google Scholar]
- 18.Togel F., Yang Y., Zhang P., Hu Z., Westenfelder C. Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury. Am J Physiol Ren Physiol. 2008;295(1):F315–F321. doi: 10.1152/ajprenal.00098.2008. Epub 2008/05/16. PubMed PMID: 18480180; PubMed Central PMCID: PMCPMC4063418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L., Wolff D.W., Xie Y., Lin M.F., Tu Y. Cyproterone acetate enhances TRAIL-induced androgen-independent prostate cancer cell apoptosis via up-regulation of death receptor 5. BMC Canc. 2017;17(1):179. doi: 10.1186/s12885-017-3153-4. PubMed PMID: 28270124; PubMed Central PMCID: PMCPMC5341373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.