Abstract
The generalist genes specialist environment model, when applied to developmental psychopathology, predicts that genetic influences should explain variance that is shared across internalizing and externalizing problems, whereas environmental influences should explain variance that distinguishes the two overarching problem types. The present study is a direct test of this hypothesis, leveraging a sample of 708 twins and siblings (aged 10–18 years, 93% White) from the United States. Measures of severity of symptoms, regardless of type, and of directionality of symptoms – whether the adolescent tended to exhibit more externalizing or internalizing problems – were subjected to ACE variance decompositions. As expected, severity of problems was under substantial genetic influence, but there were also significant shared and non-shared environmental influences. Contrary to the generalist genes specialist environment model, directionality of problem type was also under considerable genetic influence, with modest nonshared environmental influence. Findings corroborate existing evidence from other designs highlighting the role of familial influences (including generalist genes) in comorbidity of adolescent internalizing and externalizing problems, but suggest that the specialist environments hypothesis may not be the key factor in distinguishing problem type.
Keywords: Generalist Genes, Specialist Environments, Twin Study, Severity and Directionality, Comorbid Internalizing and Externalizing
In 1997, Thalia Eley proposed an innovation in developmental psychopathology that described genetic influences as general factors influencing the likelihood of youth developing problems, but environmental stressors as playing a critical role in determining which type of symptoms and therefore which class of problems youth would exhibit (considering anxiety and depressive symptoms). Later, Kovas and Plomin (2007) coined the terms ‘generalist genes’ and ‘specialist environments’ as a heuristic for understanding findings from studies that found that complex traits that are frequently comorbid tend to be influenced by the same set of genes (e.g., generalist genes), and that what tends to differentiate traits or abilities are mostly environmental in nature (e.g., specialist environments). Many multivariate genetic studies have tested this hypothesis as it relates to the expression of internalizing (depressive symptoms, general and specific anxieties) and externalizing problems (attention problems, oppositional defiant problems, conduct problems) during adolescence through examining genetic and environmental influences on hierarchical factor structures of psychopathology dimensions. However, a complementary method – examining the genetic and environmental influences on symptom severity versus directionality (e.g., Essex, Klein, Cho, & Kraemer, 2003) – has not been tested. The present study takes a new step towards testing whether the generalist genes specialist environments model is a generalizable heuristic for the comorbidity of internalizing and externalizing problems during adolescence (i.e., outside of the multivariate genetic methods used to test it thus far) by leveraging the severity and directionality model of psychopathology.
Generalist genes and Specialist Environments
Rhee, Lahey, and Waldman (2015) summarized the support for generalist genes and specialist environments hypothesis for the intersection of internalizing and externalizing problems during adolescence. They concluded that there was generally support for the model in the literature, based primarily on multivariate twin studies (e.g., Cosgrove et al., 2011; Lahey, Van Hulle, Singh, Waldman, & Rathouz, 2011). Two main types of models have provided this type of evidence: independent pathways models examine dimensions or symptoms as individual scores entered into the model, and create factors based on genetic and environmental commonalities or correlations among those scores. Independent pathways models including symptom scores encompassing internalizing and externalizing problems have found that a single genetic factor tends to emerge, as well as two additional non-shared environmental (e.g., non-genetic influence that leads to differences among family members) factors representing the differentiated internalizing and externalizing domains (Lahey et al., 2011; Pettersson, Anckarsater, Gillberg, & Lichtenstein, 2013; Spatola et al., 2007). In contrast, common pathways models first model the phenotypic similarity in the basic scores (e.g., the symptom or dimension scores) via a factor hierarchy (which has also been termed the ‘p-factor’; Caspi et al., 2014). Then, genetic and environmental influences are examined on the first (p-factor) and, if included second-order (dimensional) factors, as well as residuals from specific symptoms (Cosgrove et al., 2011; Pettersson, Lahey, Larsson, & Lichtenstein, 2018; Waldman, Poore, van Hulle, Rathouz, & Lahey, 2016). Here, too, there is support for genetic influence on the higher order factors, but often both genetic and non-shared environmental influences on the internalizing and externalizing factors.
Results from multivariate twin models and from recent data using measured genes (e.g., Brikell et al., 2018; Jones et al., 2018; Selzam, Coleman, Caspi, Moffitt, & Plomin, 2018) converge in finding support for generalist genes predicting commonalities among internalizing and externalizing forms of psychopathology in adolescence. Multivariate twin models also converge in finding evidence for specialist environments (non-shared environmental influences) differentiating internalizing versus externalizing problems (Lahey, Krueger, Rathouz, Waldman, & Zald, 2017). These studies have also found support for a smaller but sometimes significant role of shared environmental or familial influences (any environmental influence that serves to foster similarity among family members) on commonalities among internalizing and externalizing problems during adolescence (generalist environments), and a smaller but sometimes important role of genetic influences on problem specificity (specialist genes). However, the bulk of the variance in comorbidity consistently can be attributed to genetic influences, whereas the bulk of the variance in domain or dimension-specific problems consistently can be attributed to non-shared environmental influences.
Severity and Directionality
Thus far, twin studies have examined comorbidity by addressing the correlations among different problem types – in this case internalizing and externalizing problems. However, another way that comorbidity of internalizing and externalizing problems has been addressed in the literature is by considering severity and directionality of symptoms separately (Essex et al., 2003), which we will refer to as the severity-directionality model. Here, symptom severity reflects what two scores have in common, and amounts to an expression of how severe an individual’s problems are, regardless of symptom type. Symptom directionality is what differentiates the internalizing and externalizing scores – in other words, the individual’s tendency towards internalizing problems rather than externalizing problems regardless of severity. As a concrete example (see also Supplemental Table 1 for an illustration), an individual with 5 internalizing symptoms and 5 externalizing symptoms would have relatively high symptom severity, but score 0 for directionality – this individual is perfectly comorbid. However, an individual with 10 internalizing symptoms and 0 externalizing symptoms would have the same level of symptom severity as the first individual, but would have a directionality score far below zero (−10), indicating a tendency toward internalizing symptoms. Re-organizing data from correlated internalizing and externalizing scales into these completely orthogonal severity and directionality scores has the advantage of addressing the comorbidity in a way that allows researchers to investigate commonalities separately from differentiating factors for internalizing and externalizing problems. In this way, severity is not unlike a first-order factor or ‘p’.
The concept of directionality assumes that most people fall somewhere on the spectrum that includes both internalizing and externalizing symptoms, but leaves open the possibility that there will be individuals who exhibit ‘pure’ internalizing or ‘pure’ externalizing problems. It is an approach that explicitly models comorbidity from a within-person perspective, as opposed to the more traditional approach that models between-person correlations among problem types (i.e., that models whether individuals with higher internalizing scores relative to the rest of the sample also have higher externalizing scores relative to the sample). That is, since directionality is defined as essentially the number of internalizing relative to externalizing symptoms, it is a measure of an individual’s comorbidity (with scores near zero indicting balanced/comorbid problems and positive or negative scores indicating low comorbidity). Another way to think about directionality is that it is a data-driven approach that sorts individuals into roughly three directionality ‘profiles’: internalizing, comorbid, and externalizing. However, this approach does not require that individuals be clustered into profiles or groups, but rather allows them to be continuously distributed, while still providing the clarity of distinguishing how individuals fall on an internalizing-externalizing dimension of psychopathology at the within-person level. Thus using the severity-directionality model, different and complementary information is provided as compared to the predominant multivariate genetic analytic models used to test the generalist genes specialist environments conceptual model thus far. The severity-directionality model therefore informs our understanding about sources of variation pushing youth towards acting out vs. closing in types of behavior more generally, making results not directly comparable to the previous literature, instead adding new information from a different conceptual lens.
A growing body of literature has used the severity-directionality model to examine the development of psychopathology, often in relation to hypothalamic-pituitary-adrenal axis functioning (e.g., Essex et al., 2006; Essex et al., 2011; Luebbe, Elledge, Kiel, & Stoppelbein, 2012; Marceau, Ruttle, et al., 2015; Ruttle, Shirtcliff, Armstrong, Klein, & Essex, 2015; Shirtcliff & Essex, 2008). Severity and directionality scores show stability across adolescence: in a sample of adolescents over-sampled for pure internalizing, pure externalizing, and comorbid internalizing and externalizing problems, Pearson’s correlations over two years were r = .74 for severity and r = .56 for directionality (Marceau, Zahn-Waxler, et al., 2015). Early life stress (Essex et al., 2011) and an inability to resolve adolescent-parent conflicts (Marceau, Zahn-Waxler, et al., 2015) have been linked to increased symptom severity but not symptom directionality during adolescence. Further, in an adoption study, marital hostility was associated with symptom severity but not directionality in childhood (Neiderhiser et al., 2016). These influences are familial, and thus could reflect shared environmental influences that would be consistent with the generalist genes specialist environments model, although this is an open empirical question.
Fewer predictors of directionality have been identified in the literature. Neiderhiser et al., (2016), identified a pattern of interactions that predicted directionality: both genetic risk for substance abuse and marital hostility moderated the extent to which obstetric complications predicted a tendency toward internalizing problems relative to externalizing problems (directionality; Neiderhiser et al., 2016). These findings open an intriguing hypothesis that prenatal exposures may represent an important area for understanding directionality of symptoms in childhood and adolescence, and loosely conform to the specialist environments part of the generalist genes specialist environments model.
Applying the severity-directional model to the generalist genes specialist environments model
A novel way of testing the generalist genes specialist environments hypothesis is to leverage the severity-directionality model of internalizing and externalizing psychopathology. To illustrate how the generalist genes and specialist environments model interfaces with severity and directionality, imagine a plinko board (a game in which one drops a chip or ball down a nearly vertical board through a matrix of pegs; the chip/ball bounces off the pegs, landing in various bins distributed across the bottom, Figure 1). Next, imagine that the board is filled with several liquids of varied density (like water, corn syrup, and oil – they will separate into layers based on density). Each individual is represented by a ball that is dropped into the board. The distance the ball sinks down into the board corresponds to the severity of problems exhibited, with balls that progress further exhibiting higher severity of problems. The density of the ball influences how far down the ball will sink down into the board (it will sink through liquids less dense than the ball but float on the next layer of liquid that is denser, becoming trapped in the middle – many liquids of varied density would approach a continuous assessment of severity). In this metaphor, the density of the ball corresponds to generalist genes – higher genetic predisposition to psychopathology generally is reflected in a denser ball that would result in the ball traveling further down the board, or more severe symptomatology.
Figure 1. Plinko board representing the severity-directionality model.
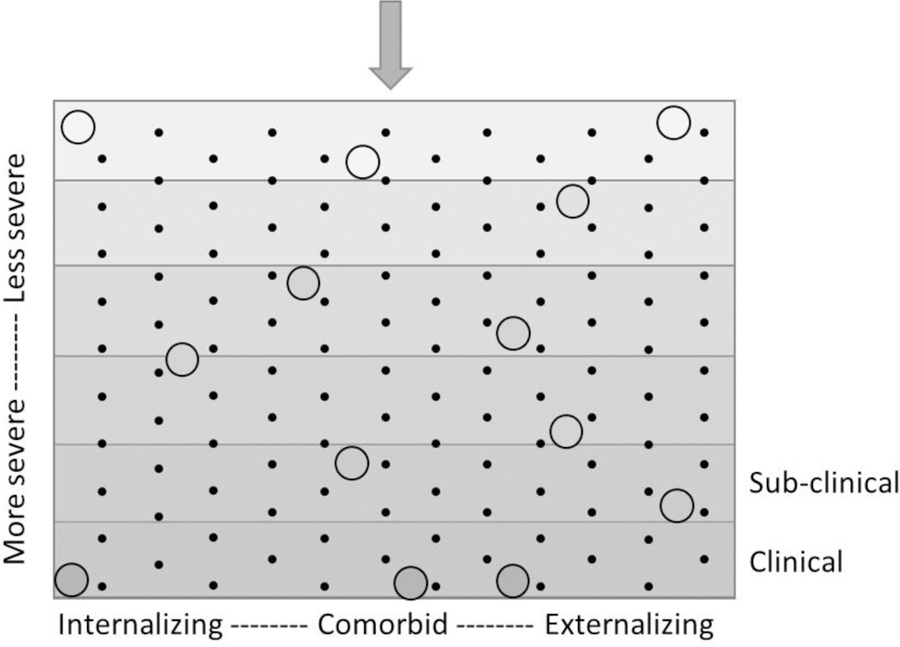
Circles represent individuals. The y-axis represents symptom severity, with gravity pulling balls downward to more severe symptomatology. The shaded gradations of the background correspond to the density of the liquid filling that part of the plinko board. The shade of the circle also represents density, which is key for determining the penetrance into symptom severity – for example, “generalist genes”. The x-axis represents symptom directionality. Black dots (e.g., the pegs) represent various (primarily non-shared environmental) influences that can serve as “specialist environments” pushing individuals towards internalizing or externalizing or comorbid problems, regardless of how deep into the board they sink.
A plinko board is also full of pegs. Each peg represents a particular influence that will push each ball to the left or the right. Here, a ball that comes to rest towards the left of the board is an individual that shows more internalizing symptoms than externalizing symptoms, a ball that comes to rest towards the right is an individual that shows more externalizing than internalizing symptoms, and a ball that stays suspended near the vertical center is an individual exhibiting some level of comorbidity. In this metaphor, the influence of the pegs in pushing balls to the left, right, or center corresponds to any influence that leads to differentiation of problem type – depending on which pegs the individual ball hits, they will tend towards internalizing, externalizing, or comorbid problems. Although most pegs are presumed to be specialist environments under the generalist genes specialist environments model (e.g., non-shared environmental influences; Kovas & Plomin, 2007), some few of them may also reflect specialist genes (e.g., based on findings suggesting that there were residual genetic influences on internalizing and externalizing factors after accounting for the general common genetic influence; Lahey et al., 2011). Together, the plinko board illustrates the orthogonality of severity and directionality of symptoms, as well as defines two possible influences on where individual balls would “land” based on the generalist genes specialist environments model.
Present Study
The present study leveraged the severity-directionality model (Essex et al., 2003) for examining comorbid internalizing (primarily depressive symptoms) and externalizing (primarily antisocial) psychopathology during adolescence in order to provide a novel test of the generalist genes specialist environment hypothesis in developmental psychopathology (Eley, 1997). We did this by applying univariate ACE decompositions of symptom severity and directionality scores in a sample of adolescent twins and siblings (the Nonshared Environment in Adolescent Development study; Neiderhiser, Reiss, & Hetherington, 2007). The severity-directionality model is a very different way of considering comorbidity between internalizing and externalizing psychopathology than has been employed in the behavior genetic literature thus far, and so there is no literature that perfectly guides our hypothesis generation. Nonetheless, we draw from findings based on the more common models of comorbidity to generate our hypotheses. We hypothesized that symptom severity would show primarily genetic influences with a small but significant role of shared environmental influences, consistent with the generalist genes hypothesis. We hypothesized that symptom directionality would show primarily nonshared environmental influences, consistent with the specialist environments hypothesis. We also hypothesized a small genetic contribution to symptom directionality consistent with findings of residual genetic influence on internalizing vs externalizing problems after accounting for generalist genetic influences (Lahey et al., 2011).
Methods
Participants
Participants included 708 families drawn from the adolescent assessment of the Nonshared Environment in Adolescent Development Study (NEAD; Neiderhiser et al., 2007; Reiss, Neiderhiser, Hetherington, & Plomin, 2000), a sample of 10–18 year old twins and siblings (~50% female; >93% White; mostly middle class). Families were recruited primarily through a national market survey of 675,000 families and supplemented by random digit dialing of 10,000 telephone numbers throughout the United States. For a family to be eligible, mothers, fathers, and two same-sex siblings who were within 4 years of age of each other and who lived in the same home at least half of the time all were required to participate. The sample included nondivorced families and stepfamilies, who were required to be together for at least 5 years. Zygosity was established via a well-validated and commonly used questionnaire measures about twins’ physical similarity (Goldsmith, 1991; Nichols & Bilbro, 1966). The analytic sample included 90 monozygotic (MZ) twin pairs, 93 dizygotic (DZ) twin pairs, and 87 full sibling (FI) pairs in nondivorced families, and 170 full sibling (FS) pairs, 102 half-sibling (HS) pairs, and 120 genetically unrelated sibling (US) pairs in stepfamilies who had data on zygosity, and both internalizing and externalizing problems in both siblings. Additional sample information can be found in Reiss et al. (2000) and Neiderhiser et al. (2007).
Measures
Internalizing and Externalizing Scores.
The present study used previously described and published internalizing and externalizing composite scores (Reiss et al., 2000) to derive measures of severity and directionality. The internalizing score consisted of depressive symptoms measured by both parents’ and adolescent report on the Behavior Problems Index (BPI; Zill, 1985), the Behavior Events Inventory (BEI; Hetherington & Clingempeel, 1992), and the Child Depression Inventory (Kovacs, 1985), as well as observer reports of depressed mood from video recordings of 10-minute videotaped discussions of familial disagreements in dyadic interactions between parents and adolescents (inter-coder reliability = .70; Hetherington & Clingempeel, 1992). The internalizing composite was created by first standardizing and then summing these scores (α = .85; for more detail see Reiss et al., 2000). Similarly, the externalizing score consisted of antisocial behavior measured by both parents’ and adolescent report on the BPI, BEI, and observer ratings of rude, disruptive, aggressive, and coercive behaviors in the same interactions (inter-coder reliability = .86). Again these scores were standardized and summed (α = .85). Internalizing and externalizing composite scores showed comorbidity, with correlations ranging from .19 to .52 across older/younger siblings and the various sibling types (see Supplemental Table S2; O’Connor, McGuire, Reiss, Hetherington, & Plomin, 1998). Adolescents in this sample are considered low-to-moderate risk for psychopathology.
Severity and Directionality Scores.
We used principal component analysis (PCA) to derive severity and directionality scores. Specifically, the internalizing and externalizing composites were simultaneously entered into a PCA and exactly two factor scores extracted and saved. These resulting scores are completely orthogonal, the first reflecting severity, or what the scores have in common, and the second directionality – what differentiates the scores. Prior to subjecting these data to the PCA, we adjusted for age, sex, and age difference between siblings by regressing the internalizing and externalizing scores on these covariates and saving the residual for use in the PCA. Finally, the PCA’s were run separately for sibling 1 and sibling 2, and separately within sibling type (e.g., MZ twins, DZ twins, etc.) in order to avoid small induced associations of severity and directionality within the sub-groups that could compromise the assumptions of the twin models, as the levels of comorbidity varied somewhat across birth order and type1.
Findings from the PCAs suggested that severity accounted for 59–75% of the variance in internalizing and externalizing problems across sibling type and birth order (see Supplemental Table S3 for full results from the PCAs). As expected, internalizing and externalizing both loaded strongly and significantly on the severity factor (loadings ranged from .57–.65 across sibling types and birth order). In all cases externalizing problems loaded strongly and positively on the directionality factor, and internalizing problems loaded equally and negatively on the directionality factor (ranging from +/− .79 – 1.02).
Analytic Strategy
We conducted two separate univariate ACE decompositions in order to understand the extent to which genetic and environmental influences explain the variance in severity and directionality. ACE decompositions leverage the differences between twins/siblings who vary in degree of genetic relatedness in order to parse the variance in the phenotype into additive genetic (A), shared environmental (C), and nonshared environmental (E) components. According to quantitative genetic theory, by definition, twins and siblings who live together share 100% of their shared environment – that is, their non-genetic influences that account for family member similarity. Sibling types vary in average degree of genetic relatedness in a systematic and consistent way due to inheritance patterns. MZ twins share 100% of their segregating genes, DZ twins and full siblings share 50% of their segregating genes on average, half siblings share 25% of their segregating genes on average, and stepsiblings who are genetically unrelated share 0% of their segregating genes on average. Comparisons of the similarity of sibling 1 with sibling 2 in a given pair across different types thus inform about genetic and environmental influences. Specifically, if MZ adolescent twins are twice as similar for their severity of symptoms as DZ/Full siblings are (on average), then genetic influences must be operating. If MZ twins are just as similar in terms of severity as DZ/full siblings are, or if genetically unrelated siblings are similar at all, then shared environmental influences are contributing to severity of symptoms. The extent to which MZ twins are not perfectly correlated indicates nonshared environmental influences (i.e., any environmental influence that account for differences among family members). We first present and examine the sibling correlations separately by type in order to predict the relative contributions of genetic and environmental influences and to corroborate findings from the ACE decomposition.
We then conducted a univariate ACE decomposition using R(openMx) (Boker, 2010; code available in the appendix) separately for symptom severity and directionality in order to test the hypotheses that 1) symptom severity would be predominately genetically-influenced with potentially some small shared and nonshared environmental influences, whereas 2) symptom directionality would be predominately nonshared environmentally influenced, with a small role for genetic influence. First the full ACE model was fit, and then A and C parameters were dropped from the model (e.g., set to 0) independently and together to test for a decrement in model fit (via chi-square difference tests) when excluded. Results from the best-fitting, most parsimonious model are preferred. Standardized variance component estimates are presented.
Results
Severity.
Twin/sibling correlations (Supplemental Table S4) showed a cascade where MZ twins were nearly double the correlation for DZ, FI, and FS pairs, and US pairs showed the lowest correlation (MZ = .79, DZ = .43, FI = .36, FS = .42, HS = .40, US = .33) indicating clear genetic influence2. The US correlation indicates some likely shared environmental influence. The high MZ twin correlation indicates a relatively limited role of nonshared environmental influences.
The ACE decomposition (Figure 2) yielded a heritability estimate of 57.7% (95% Confidence Interval [CI] = 41%–72%). In other words, 58% of the variance in symptom severity was attributable to additive genetic influences. There was also a meaningful shared environmental component of 19.6% (95% CI = 8%–30%), and a nonshared environmental component of 22.7% (95% CI = 17%–32%). None of these influences could be dropped without a decrement in model fit (see Supplemental Table S5).
Figure 2.

Results for the preferred ACE models. Percent variance explained by each type of influence in the preferred mode is presented.
Directionality.
For directionality, twin/sibling correlations (Supplemental Table S4) also showed a cascade where MZ twins were nearly double the correlation for DZ, FI, and FS pairs, and US pairs showed the lowest correlation (MZ = .65, DZ = .45, FI = .22, FS = .22, HS = .32, US = .07) indicating clear genetic influence. The US correlation was very low, indicating that there is not likely meaningful shared environmental influence. The moderate MZ twin correlation indicated a somewhat greater role of nonshared environmental influences as compared with the severity score.
The full ACE decomposition yielded a heritability estimate of 58.9% (95% Confidence Interval [CI] = 38%–73%). In other words, 59% of the variance in symptom directionality was attributable to additive genetic influences. There was not a meaningful shared environmental component – the shared environment explained 4.4% of the variance (95% CI = 0%–17%). There was a moderate nonshared environmental component of 36.7% (27%–49%). Shared environmental influences could be dropped without a decrement in model fit, χ2change(1) = 0.49, p = .49 (see also Supplemental Table S6). Thus, the ACE decomposition including only genetic and nonshared environmental components was preferred (Figure 2), with estimates of 64.7% genetic influence (95% CI = 54%–73%) and 35.3% nonshared environmental influence (95% CI = 27%–46%).
Discussion
The present study sought to test the generalist genes specialist environments model for the comorbidity of internalizing and externalizing problems during adolescence by leveraging the severity-directionality model of psychopathology. There was some support for the generalist genes hypothesis in that severity was under considerable genetic influence. However, we also found support for generalist environments (e.g., both shared and non-shared) contributing to symptom severity. There was some support for a larger role for nonshared environmental influences and negligible shared environmental influence on directionality, as would be expected by the generalist genes specialist environments model, although this was quite weak evidence (the difference in nonshared environmental contributions to directionality relative to severity was 12%, and there was some overlap in the confidence intervals). Further, the large genetic influence on directionality (e.g., specialist genes) was unexpected and not predicted by the generalist genes specialist environments model.
Severity and Directionality
Figure 3 presents the findings in terms of the Plinko board metaphor for severity and directionality. Severity was conceptualized as the density of the ball, leading to its penetrance down the board. These circles, when represented as pie charts, depict findings that about three quarters of the variance in density is explained by familial influence, with over half of the variance attributable to genetics. This primarily familial influence on comorbidity is consistent with twin studies examining covariation of internalizing and externalizing scores (Chen et al., 2016; Pesenti-Gritti et al., 2008). Using the same NEAD data in a bivariate framework consistent with the approach to comorbidity typically taken in the twin literature, O’Connor et al., (1998a) showed that the internalizing and externalizing composites were correlated for genetic (45%), shared environmental (30%) and nonshared environmental influences (25%) reasons. The present findings – that all three of these factors are important for the etiology of symptom severity – are consistent with O’Connor et al., (1998a) and findings from other multivariate variance decompositions (e.g., common pathways model; Pettersson et al., 2018; Waldman et al., 2016). Not all multivariate twin studies have found shared environmental influences to be important, however; sometimes adolescent internalizing and externalizing factors are correlated for genetic and nonshared environmental reasons, with little or no shared environment (Cosgrove et al., 2011; Lahey et al., 2011; Pettersson et al., 2013). In the literature predicting severity, the types of influences identified – early life stress, parent-adolescent conflict resolution, marital hostility – are familial in nature, and based on the current findings, most likely represent a combination of genetic and shared environmental influences.
Figure 3. Results depicted in the context of the Plinko board representing the severity-directionality model.
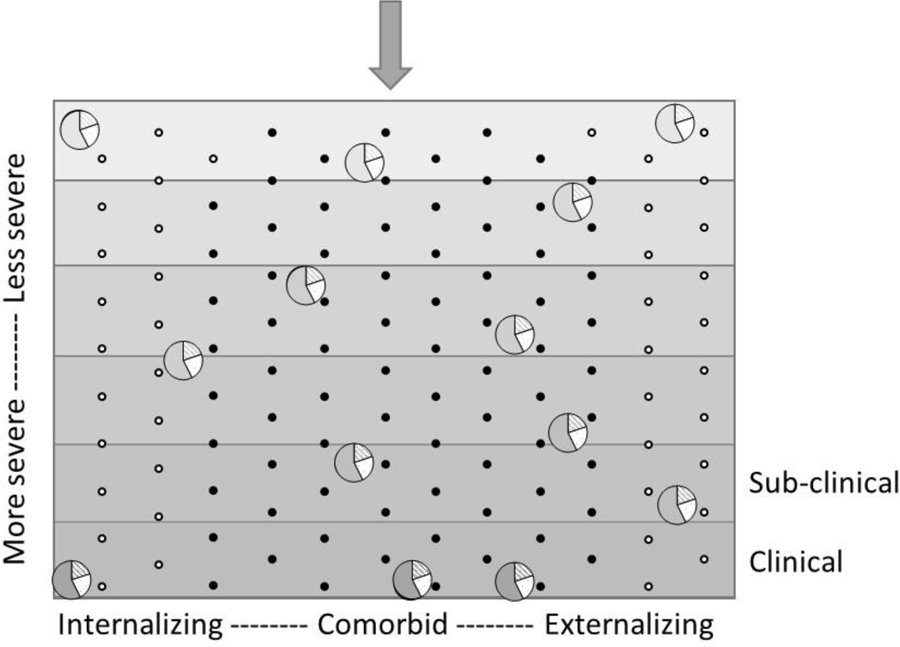
Circles represent individuals. The y-axis represents symptom severity, with gravity pulling balls downward to more severe symptomatology. The shaded gradations of the background correspond to the density of the liquid filling that part of the plinko board. The shade of the circle also represents density, which is key for determining the penetrance into symptom severity. Here, the pie-chart pieces of the circle depicts the percentage of variance each influence exerts on that ‘density’ – solid color indicates genetic influence (58%), diagonal lines indicate shared environmental influence (20%), and white background dots indicate nonshared environmental influence (23%). The x-axis represents symptom directionality. The dots (e.g., the pegs) represent various ‘specialist’ influences that push individuals towards internalizing or externalizing or comorbid problems, regardless of how deep into the board they sink. Black dots represent specialist genetic influences (65%), whereas white dots represent the hypothesized ‘specialist environments’ that are nonshared environmental in nature (35%).
Figure 3 also demonstrates that the pegs, which were conceptualized as specialist environments (nonshared environmental influences: white pegs) contributing to differentiation of internalizing and externalizing problems actually take up little of the board – a much larger proportion of the variance is explained by genetic influences (e.g., black pegs in Figure 3). Although symptom severity maps conceptually onto a common factor fairly well, symptom directionality conceptualizes specialist genes and environments quite differently than previous studies. Differentiation of internalizing and externalizing has previously been conceptualized as unique or residual genetic and environmental influences after accounting for the common genetic and environmental influences (i.e., through a correlation or common factor). In this way, tests of specialist genes and environments have been phenotype-specific. Although the terms generalist genes and specialist environments were initially developed to describe the results of multivariate models (e.g., common pathways models), they have become a conceptual framework that have then been tested via other formulations of multivariate models (e.g., independent pathways models). In this analysis, we take the next step in testing whether it is a generalizable concept by applying a novel way of testing for the role of specialist environments and genetics. Here, genetic and environmental influences on directionality are actually a direct measure of genetic and environmental influences on differentiation that is not specific to one problem type or another. That is, directionality rests on a conceptualization of comorbidity that puts ‘pure’ internalizing and externalizing-type problems on opposite ends of a single psychopathology dimension, and thus is a single score that differentiates problem types.
The first step in placing these findings on directionality in the literature is a comparison to findings using the typical approach in the same NEAD data. O’Connor et al., (1998a) found unique genetic, shared, and nonshared environmental influences on externalizing problems and unique genetic and nonshared environmental influences on internalizing problems. Interestingly, these data showed a distinct pattern for internalizing and externalizing in terms of the residual variance: externalizing problems were influenced far more by specialist genes than environments, whereas internalizing problems were influence much more by specialist environments than genes. These findings underscore that previous studies yield phenotype-specific information on specialist genes and environments that are qualitatively different from the examination of directionality in this study. That is, the differences between our findings and those of O’Connor et al. (1998a) may be explained by the fact that the directionality score assesses a measure of within-person comorbidity placed on a spectrum of pure internalizing to mixed/comorbid to pure externalizing problems, which is quite different than phenotype-specific influences at the between-person level.
Some twin studies have found specific genetic, shared, and nonshared environmental influences on some problem types after accounting for the comorbidity (Waldman et al., 2016). However, in other studies, there were primarily genetic and nonshared environmental (and no or very little shared environmental) influences on internalizing and externalizing problems (Chen et al., 2016; Lahey et al., 2011; O’Connor, McGuire, et al., 1998; O’Connor, Neiderhiser, Reiss, Hetherington, & Plomin, 1998; Pettersson et al., 2013). Although on the surface this pattern of findings looks consistent with findings of directionality in the current analysis, these studies varied widely in terms of the strength of support for specialist genes vs. environments. For example, Chen et al., (2016) showed primarily specialist genes (consistent with the current findings), Pettersson et al., (2013) showed both specialist genes and environments without lending more weight to either, and Lahey et al., (2011) showed mostly specialist environments and much weaker influences of specialist genes. Further, one study found little evidence of genetic or shared environmental influences specific to problem type (which were measured as specific disorders), with only nonshared environmental influence playing a systematic role (Cosgrove et al., 2011), the clearest support of specialist environments. In other words, the most consistent finding across these studies is that there is a lack of consistency, which may be due, at least in part, to differences in the ages and assessment of the samples examined. In addition, twin only studies (e.g., Chen et al., 2016; Lahey et al., 2011; Pettersson et al., 2013) are typically unable to clearly distinguish between heritability and shared environmental influences (e.g., Burt, 2009).
The current findings showed more support for specialist genes than specialist environments. By using a different conceptual lens, these findings may sway the balance of the literature away from solid support for the supposed predominant role of specialist environments differentiating internalizing and externalizing problems. Instead, it is likely that specialist genes and environments both contribute to the differentiation of problem type, to different extents depending on the measurement of internalizing and externalizing problems and whether differentiation is conceptualized as explicitly differentiation or as phenotype-specific residual variation. Possibly, the severity-directionality model is better suited to uncover real effects of specialist genes on the tendency of youth towards acting out vs. closing in, in a way that the predominantly used factor structures are not. That is, there may be a unique set of genes that serve as differentiators of somewhat normative adolescent problem types (picked up in this analysis), separate from genes driving mental illness (picked up by traditional multivariate approaches). Thus, the conceptualization of these emotional and behavioral styles as a continuum separate from severity of problem behavior may be particularly useful for understanding normative and sub-clinical variations in these symptoms.
Strengths and Limitations
The present study had several strengths, including the inclusion of non-twin siblings and reliable multi-measure multi-rater assessments of internalizing and externalizing problems that include observer ratings. However, the sample was predominately White, middle class, and came from an unselected sample, and thus findings may not generalize to diverse or high-risk populations, particularly as heritability estimates are known to be sample-dependent and vary across those dimensions. Second, the measurement error is contained by non-shared environmental influences on directionality (as opposed to severity, since unsystematic error cannot contribute to variance that is common across internalizing and externalizing scores). However, this limitation should upwardly bias the likelihood of finding evidence for specialist environments, which we found less evidence of than expected. Third, although in this first-pass test of generalist genes and specialist environments using severity and directionality we controlled for age and sex, findings may vary meaningfully by age (given the wide age-range included in this study) or by sex (given sex differences in antisocial and depressive sypmtoms during adolescence; Zahn-Waxler, Shirtcliff, & Marceau, 2008).
Finally, the internalizing and externalizing composites primarily reflected depression and antisocial behavior, respectively. Thus, the present findings about severity and directionality may be more applicable to comorbidity of antisocial phenotypes (a more disruptive phenotype than would be measured if attention/hyperactivity measures were included) and depression (more low mood, sad and blue focused and less worry focused than if anxiety measures were included), than an analysis of broader internalizing and externalizing measures. In the Colorado Twin Registry, a bivariate model of depression and conduct disorder and a broader common factors analysis of the same sample of internalizing and externalizing both found genetic and nonshared environmental correlations, indicating that conduct/antisocial and depressive phenotypes are somewhat consistent with the broader externalizing and internalizing phenotypes (Cosgrove et al., 2011; Subbarao et al., 2008). Findings regarding the generalist genes specialist environments model may differ with inclusion of other features of internalizing and externalizing problems, for example a more equal weighting of anxiety and depression in internalizing scores, and a better representation of attentional and impulsivity items in externalizing scores. Thus, replication of these results are needed in studies that include broader measures of internalizing and externalizing problems.
Conclusions
In all, the present study largely conformed to the prior literature examining genetic and environmental influences on the comorbidity of internalizing and externalizing problems. There was substantial genetic influence on severity, and a (slightly) larger role of nonshared environmental influence on directionality as compared with severity. However, by leveraging the severity-directionality model of psychopathology in adolescence, a greater influence of specialist genes on problem type differentiation was found than was previously reported in the literature. This finding potentially weakens the argument for specialist environments being primarily responsible for what differentiates internalizing and externalizing problems. Further, the strong contribution of shared environmental influence on severity, while not precisely novel (e.g., see Burt, 2009), is evidence of generalist environments and adds to a growing body of findings highlighting the important role of shared environmental influences on comorbidity of internalizing and externalizing problems during adolescence. Overall, the generalist genes specialist environments model (although originally developed for the intersection of anxiety and depression symptoms) is a nice heuristic for thinking about comorbid internalizing and externalizing problems. However, the present data generally show that comorbidity is more nuanced, and that specialist genes and generalist environments are also important for understanding the commonalities and differentiation of internalizing and externalizing problems during adolescence.
Supplementary Material
Table 1.
Sample demographic characteristics
| Characteristic | Sibling 1 | Sibling 2 |
|---|---|---|
| Age | 13.5 (2.0) | 12.1 (1.3) |
| Age Range | 10–18 | 9–18 |
| Age Difference | 1.6 (1.3) | |
| % Female | 48.4% | |
| Mother | Father | |
|
|
||
| Parent Age | 38.1 (5.2) | 41.0 (6.5) |
| Parent Education (years) | 13.8 (2.3) | 13.9 (2.7) |
| Median family income | $25,000–$35,000 | |
Acknowledgements.
We thank the principal investigators and investigator team not listed as coauthors: David Reiss, E. Mavis Hetherington, and Robert Plomin, as well as families of the Nonshared Environment in Adolescent Development project. The Nonshared Environment in Adolescent Development project was supported by National Institute of Mental Health Grant R01MH43373, R01MH48825, and by the William T. Grant Foundation (David Reiss, Principal Investigator [PI]). Data analysis and manuscript preparation were supported in part by the National Institute on Drug Abuse Grant K01DA039288 (Marceau, PI).
Footnotes
Conflicts of Interest: None.
Findings were highly consistent when severity and directionality were computed within sibling 1 and 2 separately, but combining across sibling types, data available upon author request.
An imperfect cascade is commonly found in twin and sibling samples, including this one (see Reiss et al., 2000). The ACE model weights each group by sample size and pools the groups based on genetic relatedness, so the DZ, FI, and FS pairs are considered together with the relative value of covariance weighted by sample size. Thus, the overall pattern suggests a general cascade.
References
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick TR, et al. (2010). OpenMx: Multipurpose Software for Statistical Modeling. R package version.
- Brikell I, Larsson H, Lu Y, Pettersson E, Chen Q, Kuja-Halkola R, … Martin J (2018). The contribution of common genetic risk variants for ADHD to a general factor of childhood psychopathology. Molecular Psychiatry, [ePub ahead of print]. 10.1038/s41380-018-0109-2 [DOI] [PMC free article] [PubMed]
- Burt SA (2009). Rethinking environmental contributions to child and adolescent psychopathology: A meta-analysis of shared environmental influences. Psychological Bulletin, 135(4), 608–637. 10.1037/a0015702 [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, … Moffitt TE (2014). The p Factor: One General Psychopathology Factor in the Structure of Psychiatric Disorders? Clinical psychological science : a journal of the Association for Psychological Science, 2(2), 119–137. 10.1177/2167702613497473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-J, Ji C-Y, Wang S-S, Lichtenstein P, Larsson H, & Chang Z (2016). Genetic and environmental influences on the relationship between ADHD symptoms and internalizing problems: A Chinese twin study. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 171(7), 931–937. 10.1002/ajmg.b.32411 [DOI] [PubMed] [Google Scholar]
- Cosgrove VE, Rhee SH, Gelhorn HL, Boeldt D, Corley RC, Ehringer MA, … Hewitt JK (2011). Structure and etiology of co-occurring internalizing and externalizing disorders in adolescents. Journal of Abnormal Child Psychology, 39(1), 109–123. 10.1007/s10802-010-9444-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley TC (1997). General genes: A new theme in developmental psychopathology. Current Directions in Psychological Science, 6, 90–95. 10.1111/1467-8721.ep11512831 [DOI] [Google Scholar]
- Essex MJ, Klein MH, Cho E, & Kraemer HC (2003). Exposure to Maternal Depression and Marital Conflict: Gender Differences in Children’s Later Mental Health Symptoms. Journal of the American Academy of Child & Adolescent Psychiatry, 42(6), 728–737. 10.1097/01.CHI.0000046849.56865.1D [DOI] [PubMed] [Google Scholar]
- Essex MJ, Kraemer HC, Armstrong JM, Boyce T, Goldsmith HH, Klein MH, … Kupfer DJ (2006). Exploring risk factors for the emergence of children’s mental health problems. Archoves of General Psychiatry, 63, 1246–1256. 10.1001/archpsyc.63.11.1246 [DOI] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, … Armstrong JM (2011). Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Development and Psychopathol, 23(4), 1039–1058. 10.1017/s0954579411000484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH (1991). A zygosity questionnaire for young twins: A research note. Behavior Genetics, 21, 257–269. 10.1007/BF01065819 [DOI] [PubMed] [Google Scholar]
- Hetherington EM, & Clingempeel WG (1992). Coping with marital transitions: A family systems perspective. Monographs of the Society for Research in Child Development, 57(2–3). 10.2307/1166050 [DOI] [Google Scholar]
- Jones HJ, Heron J, Hammerton G, Stochl J, Jones PB, Cannon M, … Linden DE (2018). Investigating the genetic architecture of general and specific psychopathology in adolescence. Translational Psychiatry, 8(1), 145. 10.1038/s41398-018-0204-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M (1985). The Children’s Depression Inventory. Psychopharmacology Bulletin, 21(4), 995–998. [PubMed] [Google Scholar]
- Kovas Y, & Plomin R (2007). Learning Abilities and Disabilities: Generalist Genes, Specialist Environments. Current Directions in Psychological Science, 16(5), 284–288. 10.1111/j.1467-8721.2007.00521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, & Zald DH (2017). A hierarchical causal taxonomy of psychopathology across the life span. Psychological Bulletin, 143(2), 142. 10.1037/bul0000069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Van Hulle CA, Singh AL, Waldman ID, & Rathouz PJ (2011). Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Archives of General Psychiatry, 68(2), 181–189. 10.1001/archgenpsychiatry.2010.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebbe AM, Elledge LC, Kiel EJ, & Stoppelbein L (2012). Cortisol Predicts Behavioral Dysregulation and Length of Stay Among Children Admitted for Psychiatric Inpatient Treatment. Journal of Clinical Child & Adolescent Psychology, 41(2), 227–238. 10.1080/15374416.2012.652000 [DOI] [PubMed] [Google Scholar]
- Marceau K, Ruttle PL, Shirtcliff EA, Hastings PD, Klimes-Dougan B, & Zahn-Waxler C (2015). Within-person coupling of changes in cortisol, testosterone, and DHEA across the day in adolescents. Developmental Psychobiology, 57(6), 654–669. 10.1002/dev.21173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Zahn-Waxler C, Shirtcliff EA, Schreiber JE, Hastings P, & Klimes-Dougan B (2015). Adolescents’, mothers’, and fathers’ gendered coping strategies during conflict: Youth and parent influences on conflict resolution and psychopathology. Development and Psychopathology, 27(4pt1), 1025–1044. 10.1017/S0954579415000668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiderhiser JM, Marceau K, De Araujo-Greecher M, Ganiban JM, Mayes LC, Shaw DS, … Leve LD (2016). Estimating the Roles of Genetic Risk, Perinatal Risk, and Marital Hostility on Early Childhood Adjustment: Medical Records and Self-Reports. Behavior Genetics, 46(3), 334–352. 10.1007/s10519-016-9788-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiderhiser JM, Reiss D, & Hetherington EM (2007). The Nonshared Environment in Adolescent Development (NEAD) Project: A longitudinal family study of twins and siblings from adolescence to young adulthood. Twin Research and Human Genetics, 10(1), 74–83. 10.1375/twin.10.1.74 [DOI] [PubMed] [Google Scholar]
- Nichols RC, & Bilbro WC (1966). The diagnosis of twin zygosity. Acta Genetica, 16, 265–275. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, McGuire S, Reiss D, Hetherington EM, & Plomin R (1998). Co-occurrence of depressive symptoms and antisocial behavior in adolescence: A common genetic liability. Journal of Abnormal Psychology, 107, 27–37. 10.1037/0021-843X.107.1.27 [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Neiderhiser JM, Reiss D, Hetherington EM, & Plomin R (1998). Genetic contributions to continuity, change, and co-occurrence of antisocial and depressive symptoms in adolescence. Journal of Child Psychology and Psychiatry, 39(3), 323–336. 10.1111/1469-7610.00328 [DOI] [PubMed] [Google Scholar]
- Pesenti-Gritti P, Spatola CA, Fagnani C, Ogliari A, Patriarca V, Stazi MA, & Battaglia M (2008). The co-occurrence between internalizing and externalizing behaviors. A general population twin study. European Child & Adolescent Psychiatry, 17(2), 82–92. 10.1007/s00787-007-0639-7 [DOI] [PubMed] [Google Scholar]
- Pettersson E, Anckarsater H, Gillberg C, & Lichtenstein P (2013). Different neurodevelopmental symptoms have a common genetic etiology. Journal of Child Psychology and Psychiatry, 54(12), 1356–1365. 10.1111/jcpp.12113 [DOI] [PubMed] [Google Scholar]
- Pettersson E, Lahey BB, Larsson H, & Lichtenstein P (2018). Criterion validity and utility of the general factor of psychopathology in childhood: predictive associations with independently measured severe adverse mental health outcomes in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry, 57(6), 372–383. 10.1016/j.jaac.2017.12.016 [DOI] [PubMed] [Google Scholar]
- Reiss D, Neiderhiser JM, Hetherington EM, & Plomin R (2000). The relationship code: Deciphering genetic and social influences on adolescent development. Cambridge, MA: Harvard University Press. [Google Scholar]
- Rhee SH, Lahey BB, & Waldman ID (2015). Comorbidity Among Dimensions of Childhood Psychopathology: Converging Evidence From Behavior Genetics. Child Development Perspectives, 9(1), 26–31. 10.1111/cdep.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, & Essex MJ (2015). Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Developmental Psychobiology, 57(6), 688–704. 10.1002/dev.21138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzam S, Coleman JR, Caspi A, Moffitt TE, & Plomin R (2018). A polygenic p factor for major psychiatric disorders. Translational Psychiatry, 8(1), 205. 10.1038/s41398-018-0217-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, & Essex MJ (2008). Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology, 50(7), 690–703. 10.1002/dev.20336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatola CAM, Fagnani C, Pesenti-Gritti P, Ogliari A, Stazi MA, & Battaglia M (2007). A general population twin study of the CBCL/6–18 DSM-oriented scales. Journal of the American Academy of Child and Adolescent Psychiatry, 46(5), 619–627. 10.1097/CHI.0b013e3180335b12 [DOI] [PubMed] [Google Scholar]
- Subbarao A, Rhee SH, Young SE, Ehringer MA, Corley RP, & Hewitt JK (2008). Common genetic and environmental influences on major depressive disorder and conduct disorder. Journal of Abnormal Child Psychology, 36(3), 433–444. 10.1007/s10802-007-9189-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman ID, Poore HE, van Hulle C, Rathouz PJ, & Lahey BB (2016). External validity of a hierarchical dimensional model of child and adolescent psychopathology: Tests using confirmatory factor analyses and multivariate behavior genetic analyses. Journal of Abnormal Psychology, 125(8), 1053–1066. 10.1037/abn0000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, & Marceau K (2008). Disorders of childhood and adolescence: gender and psychopathology. Annual Review of Clinical Psychology, 4, 275–303. 10.1146/annurev.clinpsy.3.022806.091358 [DOI] [PubMed] [Google Scholar]
- Zill N (1985). Behavior problems scales developed from the 1981 Child Health Supplement to the National Health Interview Survey. Washington, D.C.: Child Trends, Inc. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


