Abstract
Introduction:
Minimally invasive repair of pectus excavatum (Nuss procedure) is associated with significant pain, and efforts to control pain impact resource utilization. Bilateral thoracic intercostal nerve cryoablation has been proposed as a novel technique to improve post-operative pain control, though the impact on hospital cost is unknown.
Methods:
We conducted a retrospective study of patients undergoing a Nuss procedure from 2016 to 2019. Patients who received cryoablation were compared to those that received traditional pain control (patient-controlled analgesia or epidural). Outcome variables included postoperative opioid usage (milligram morphine equivalents, MME), length of stay (LOS), and hospital cost.
Results:
35 of 73 patients studied (48%) received intercostal nerve cryoablation. LOS (1.0 vs 4.0 days, p<0.01) and total hospital cost ($21,924 versus $23,694, p=0.04) were decreased in the cryoablation cohort, despite longer operative time (152 vs 74 minutes, p<0.01). Cryoablation was associated with decreased opioid usage (15.0 versus 148.6 MME, p<0.01) during the 24 hours following surgery and this persisted over the entire postoperative period, including discharge opioid prescription (112.5 vs 300.0 MME, p<0.01).
Conclusion:
Bilateral intercostal nerve cryoablation is associated with decreased postoperative opioid usage and decreased resource utilization in pediatric patients undergoing a minimally invasive Nuss procedure for pectus excavatum.
Keywords: minimally invasive Nuss procedure, pectus excavatum, cryoablation, hospital cost, post-op pain, pediatric
1. Introduction
Repair of pectus excavatum with a minimally invasive Nuss procedure is associated with significant postoperative pain despite improved perioperative morbidity compared to the traditional open procedure (Ravitch procedure) [1, 2]. Multiple approaches to perioperative pain control have been utilized, including thoracic epidurals, patient-controlled analgesia (PCA), and various peripheral nerve blocks using local anesthetics [3, 4]. All of these techniques are associated with significant opioid utilization and a typical length of stay (LOS) of 4 to 5 days [1, 5].
Thoracoscopic-assisted bilateral intercostal nerve cryoablation has been gaining popularity as a technique to control perioperative pain in pediatric patients undergoing repair of pectus excavatum and is associated with decreased opioid utilization and reduced length of stay [6–8]. Despite improved perioperative pain control, intercostal nerve cryoablation requires additional capital equipment, disposable supplies, and longer operative time to perform [8–10]. Given the complex impact of intraoperative bilateral intercostal nerve cryoablation on costs—reducing opioid use and hospital stay while increasing other components of cost (longer operative time, supplies)—the fiscal implications for hospital systems has yet to be determined.
Most studies of hospital cost utilize surrogate measures such as charges, cost-to-charge ratios, and reimbursements. To better capture the hospital cost of patients undergoing repair of pectus excavatum, we utilized an institutional accounting dataset that includes actual hospital financial data capturing the supplies, labor, equipment, and facilities associated with each admission and procedure. This better reflects the cost to the hospital system than most surrogate metrics such as reimbursements or charges, which may be influenced by patient or insurance factors [11].
This study sought to compare the index hospital cost of pediatric patients undergoing a minimally invasive Nuss procedure for pectus excavatum with bilateral thoracic intercostal nerve cryoablation to those receiving traditional pain control strategies (PCA or epidural) at an academic medical center. In addition, we analyzed other metrics that have been previously described in this patient population (LOS, pain scores, and opioid utilization) in order to provide a more complete assessment of resource utilization.
2. Methods
Pediatric patients that underwent a minimally invasive Nuss procedure for pectus excavatum at a single institution from July 2016 to September 2019 were identified by Current Procedural Terminology (CPT) code corresponding to repair of pectus excavatum within the electronic medical record (Epic Clarity, Epic Systems Corporation, Verona, WI). Inclusion criteria included all pediatric patients (<18 years of age) undergoing a minimally invasive Nuss procedure. Exclusion criteria included patients undergoing re-operative procedures. This study was approved by the University of Wisconsin Health Sciences IRB.
Cost data representing actual hospital costs were extracted from the institutional Legacy Data Warehouse (Epic Systems Corporation, Verona, WI). These data are obtained from hospital accounting algorithms and are regularly updated as information regarding quarterly cost data are incorporated (construction, facility costs, etc.). Data from these sources were merged using two patient identifiers to create the final dataset for analysis. Individual components of hospital cost (direct variable cost, direct fixed cost, indirect variable cost, and indirect fixed cost) were also obtained to provide more granular cost data [12]. Direct variable cost includes items immediately associated with patient care (consumables, medications). Direct fixed costs include capital equipment and labor associated directly with patients that do not change with hospital volume. Indirect variable cost includes overhead costs that change with hospital volume (electricity, laundry). Finally, indirect fixed costs include facility costs that do not depend on volume (maintenance). Costs were adjusted for medical price inflation using the Producer Price Index (PPI) for hospital inpatient care provided by the Bureau of Labor Statistics and are reported in 2018 dollars [13, 14].
Analgesic variables including postoperative inpatient opioid usage, uncontrolled postoperative pain, discharge opioid prescription, and opioid refills were collected by automated data abstraction and subsequently validated by manual chart review. Opioid usage was normalized to oral morphine milligram equivalents (MME) [15]. Severe postoperative pain was defined as reporting a 9 or a 10 on the Numeric Rating Scale (NRS), which has been validated for children 8 years and older [16]. Patients requiring a refill were defined as patients receiving a new opioid prescription within 30 days of discharge.
Patients receiving intraoperative bilateral thoracic intercostal nerve cryoablation were compared to patients that received a standardized pain control regimen without cryoablation. Prior to June 2018, all patients undergoing minimally invasive pectus repair were managed with a postoperative pain control strategy based upon either thoracic epidural or patient-controlled analgesia (PCA) in addition to a standardized medical regimen that was developed in collaboration with the Pediatric Acute Pain Service, consisting of scheduled acetaminophen and non-steroidal anti-inflammatory drugs (ketorolac or ibuprofen), rescue IV opioids as needed and diazepam as needed for muscle spasms. Intraoperative bilateral thoracic cryoablation was utilized starting in June 2018, and all subsequent pediatric patients underwent intraoperative bilateral intercostal nerve cryoablation as previously described [6, 9]. All procedures were performed by three fellowship-trained pediatric surgeons already experienced with minimal invasive pectus repair prior to the introduction of intercostal nerve cryoablation.
In our cohort, after placement of a double lumen endotracheal tube for sequential single lung isolation and thoracoscopic identification of intercostal nerves, patients underwent bilateral thoracic intercostal nerve cryoablation from T3-T7 consisting of a 2-minute freeze of each nerve to −60°C followed by a 10 second rewarming process. Since the cryoablation does not reach maximal analgesic effect until approximately 24 hours later, bilateral thoracoscopic assisted injection of 3–5 mL of 0.5% bupivacaine within the T3-T7 intercostal spaces was also performed. Pediatric patients who underwent bilateral thoracic intercostal nerve cryoablation followed a perioperative pain control protocol (Figure 1) developed in conjunction with our Pediatric Acute Pain Service, consisting of a single pre-operative dose of pregabalin, IV methadone prior to incision, IV or oral opiates, non-steroidal anti-inflammatory drugs, acetaminophen, and diazepam as needed for muscle spasms postoperatively.
Figure 1:
Multi-disciplinary perioperative pain management protocol.
Demographics, pre-operative clinical characteristics, and postoperative outcomes were compared between patients that received intercostal nerve cryoablation and those that did not. Mann-Whitney U test and chi-square test were used where appropriate for bivariate outcomes. All statistical analyses were performed in SAS 9.4 (SAS Institute Inc, Cary, NC).
3. Results
73 pediatric patients met eligibility criteria for this study, of which 35 underwent intraoperative bilateral intercostal nerve cryoablation during a minimally invasive Nuss procedure. The groups were similar in terms of patient age, gender, race, American Society of Anesthesiologist’s (ASA) class, or body mass index (BMI). In the pre-cryoablation cohort, 6 patients received thoracic epidural and 32 patients received PCA (Table 1).
Table 1:
Demographic factors in cryoablation and non-cryoablation cases
|
No Cryoablation (N=38) |
Cryoablation (N=35) |
|||
|---|---|---|---|---|
| n | Summary | n | Summary | |
| Age | 38 | 15.0 (14.0–16.0) | 35 | 15.0 (14.0–17.0) |
| Sex | 38 | 35 | ||
| . Male | 33 (86.8) | 31 (88.6) | ||
| . Female | 5 (13.2) | 4 (11.4) | ||
| Race | 38 | 35 | ||
| . Caucasian | 34 (89.5) | 30 (85.7) | ||
| . Hispanic/Latino | 4 (10.5) | 5 (14.3) | ||
| ASA Class | 38 | 35 | ||
| . Class 1 | 10 (26.3) | 13 (37.1) | ||
| . Class 2 | 27 (71.1) | 22 (62.9) | ||
| . Class 3 | 1 (2.6) | 0 (0.0) | ||
| . Class 4 | 0 (0.0) | 0 (0.0) | ||
| Body Mass Index | 38 | 19.6 (17.7–21.6) | 35 | 20.0 (18.2–21.0) |
| Epidural | 38 | 35 | ||
| . Yes | 6 (15.8) | 0 (0.0) | ||
| . No | 32 (84.2) | 35 (100.0) | ||
Values presented as Median (Interquartile Range) or N (column %).
ASA = American Society of Anesthesiologists
p-values: m= Mann-Whitney U test c=Pearson's chi-square test.
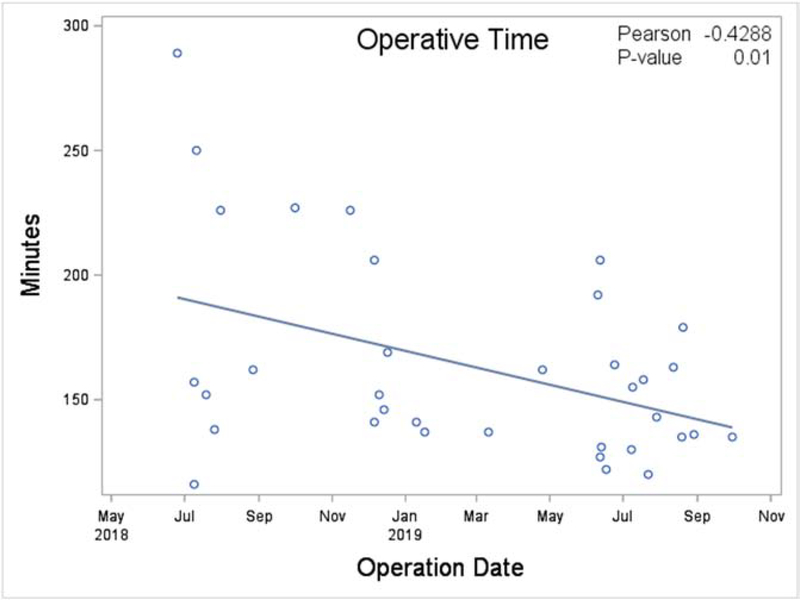
Bilateral intercostal nerve cryoablation was associated with lower total hospital cost ($21,924 versus $23,694, p=0.04, Table 2) despite an overall longer operative time (152 versus 74 minutes, p<0.01). Of note, operative time in the cryoablation group decreased over time as experience with the cryoablation technique was acquired (p=0.01, Figure 2). The length of stay was significantly lower in the cryoablation cohort (1.0 versus 4.0 days, p<0.01). Among the individual components of total cost, the largest differences in cost were observed in direct variable cost ($9,690 versus $10,711, p=0.06) and indirect fixed cost ($8,341 versus $9,775, p<0.01). There was no significant difference in readmission rates between cohorts (p=0.17).
Table 2:
Resource utilization and clinical outcomes in cryoablation and non-cryoablation cases
|
No Cryoablation (N=38) |
Cryoablation (N=35) |
||||
|---|---|---|---|---|---|
| n | Summary | n | Summary | p-value | |
| Cost Components ($) | 38 | 35 | |||
| . Direct Fixed Cost | 828 (734–946) | 572 (468–722) | <0.01m | ||
| . Indirect Fixed Cost | 9,775 (8,298–11,746) | 8,341 (6,844–9,374) | <0.01m | ||
| . Direct Variable Cost | 10,711 (9,368–13,701) | 9,690 (9,071–11,435) | 0.06m | ||
| . Indirect Variable Cost | 2,137 (1,940–2,939) | 2,414 (1,957–2,697) | 0.67m | ||
| . Total Cost | 23,694 (20,808–29,810) | 21,924 (18,374–24,554) | 0.04m | ||
| Operative Time (minutes) | 38 | 74 (65–95) | 35 | 152 (136–179) | <0.01m |
| Length of Stay (days) | 38 | 4 (3–5) | 35 | 1 (1–2) | <0.01m |
| Readmission | 38 | 2 (5.3) | 35 | 0 (0.0) | 0.17c |
Values presented as median, (interquartile range) or N (column %)
p-values:
= Mann-Whitney U test
=Pearson’s chi-square test.
Figure 2.
Operative length over time after adoption of intercostal nerve cryoablation at a pediatric tertiary care center.
Patients undergoing bilateral intercostal nerve cryoablation had significantly lower opioid usage during the first 24-hour (15.0 versus 148.6 MME, p<0.01, Table 3) and 24 to 48-hour (7.5 versus 115.6 MME, P<0.01) interval following surgery. Cryoablation was associated with lower total opioid consumption during the entire hospital stay (22.5 versus 410.8 MME, p<0.01). Despite lower opioid utilization, the fraction of patients reporting severe pain was lower in the cryoablation cohort for the first 24 hours (0.0% versus 29.0%, p<0.01) and total hospital stay (5.7% versus 42.1%, p<0.01). At discharge, patients undergoing cryoablation were given lower opioid prescriptions compared to patients not undergoing cryoablation (112.5 versus 300.0 MME, p<0.01). There was no difference in the fraction of patients requiring an opioid refill between groups (22.9% vs 29.0%, p=0.55). Two patients (5.7%) in the cryoablation cohort reported neuralgia at the time of discharge and required outpatient gabapentin use which was discontinued in both patients by 6 weeks.
Table 3:
Analgesic utilization for cryoablation and non-cryoablation cases
|
No Cryoablation (N=38) |
Cryoablation (N=35) |
||||
|---|---|---|---|---|---|
| n | Summary | n | Summary | p-value | |
| Opioid Use (MME) | 38 | 35 | |||
| . Perioperative | 57.5 (41.7–75.5) | 47.5 (29.2–68.3) | 0.10m | ||
| . 0–24 hr. | 148.6 (98.5–203.2) | 15.0 (8.4–37.5) | <0.01m | ||
| . 24–48 hr. | 115.6 (87.9–192.4) | 7.5 (0.0–30.0) | <0.01m | ||
| . Total Admission | 410.8 (331.8–605.3) | 22.5 (7.5–67.5) | <0.01m | ||
| Uncontrolled Pain Reported | |||||
| . 0–24 hr. | 38 | 11 (29.0) | 35 | 0 (0.0) | <0.01c |
| . 24–48 hr. | 38 | 3 (7.9) | 25 | 2 (8.0) | 0.99c |
| . Total | 38 | 16 (42.1) | 35 | 2 (5.7) | <0.01c |
| Opioid Prescription (MME) | 38 | 300.0 (187.5–375.0) | 31 | 112.5 (90.0–150.0) | <0.01m |
| Opioid Refill Required (N) | 38 | 11 (29.0) | 38 | 8 (22.9) | 0.55c |
Values presented as Median (Interquartile Range) or N (column %).
MME = morphine milligram equivalents
p-values:
= Mann-Whitney U test
=Pearson’s chi-square test.
4. Discussion
The findings in this study quantify the hospital cost savings associated with bilateral thoracic intercostal nerve cryoablation during a minimally invasive Nuss procedure to repair pectus excavatum. Bilateral intercostal nerve cryoablation was associated with reduced cost relative to non-cryoablation cases despite a longer operative time and additional surgical device cost. This can be attributed in part to a significant reduction in LOS (3 days) between groups, which has been demonstrated previously [7, 8, 10]. We also observed similar reductions in inpatient opioid requirement and discharge opioid prescriptions [6]. Overall, our findings demonstrate that the cost reductions associated with bilateral intercostal nerve cryoablation are greater than the additional costs incurred with this new technique. While the improvement in pain control and opioid use should be the primary factor driving the adoption of intercostal nerve cryoablation during a minimally invasive pectus repair, our data suggests that this improvement does not incur increased costs for the hospital system. This information has implications for both hospital-level decision making and for negotiations with insurance providers regarding coverage and reimbursement for intercostal nerve cryoablation.
The hospital cost reduction associated with intercostal thoracic nerve cryoablation is driven by multiple factors and the components of this difference provide useful information regarding the importance of each factor. Indirect fixed costs (commonly thought of as “hospital overhead”) were the largest contributor to the cost reduction. This accounting category reflects static facility costs (building maintenance, construction) which are largely driven by LOS, suggesting that this factor contributed the most to the observed cost reduction. Direct variable costs were the second largest contributor to the cost reduction, and these costs include intraoperative supplies (including the single use cryoablation probe), some labor costs, and postoperative analgesic medications. Our data suggest that the reduction in postoperative analgesic medications and labor related to patient care was more important than the cost associated with additional operative supplies. Direct fixed costs include the cost of capital equipment, and these costs were similar in both cohorts. This suggests that the increased cost of capital equipment needed to perform bilateral thoracic nerve intercostal cryoablation did not contribute significantly to the cost associated on a per-patient basis.
While a cost data analysis for the use of cryoablation during a minimally invasive Nuss procedure has not been previously reported in this pediatric patient population, prior published studies have reported information regarding opioid utilization. We observed a 90% reduction in opioid utilization during the first 24 hours and a 95% reduction in opioid use over the entire hospital stay. A similar recent study demonstrated a 52% reduction in opioid use during the first 24 hours and a 61% reduction over the entire stay [7]. Reductions in opioid use over the first 24 hours compared to previous studies are likely due in part to intercostal nerve blocks with bupivacaine, which can serve as a bridge to the onset of cryoanalgesia. Our observed reduction in discharge opioid prescription (63%) was higher compared to another recent study (32%) [6]. As part of a multimodal analgesic protocol (Figure 1), these interventions result in profound opioid sparing over the entire postoperative period in an adolescent population that is particularly vulnerable to negative consequences of medical opioid use [17, 18]. While our non-cryoablation cohort included patients receiving both thoracic epidural and PCA (unlike these previous studies, which only included epidural patients), these data add further evidence to the improved postoperative pain control provided by bilateral thoracic nerve intercostal cryoablation compared to traditional pain control methods.
There are important limitations to consider in the present study. It is a retrospective single institution study, which limits generalizability and introduces the potential for selection bias and cohort imbalance in both measured and unmeasured confounders. However, all consecutive patients received cryoablation once it was introduced, reducing the chance for selection bias. It is also possible that other aspects of the cryoablation protocol, such as pre-operative pregabalin and intercostal nerve blocks with bupivacaine, contributed to the overall decreased opioid use. However, the persistent opioid reduction beyond the first 24 hours suggests that these factors were not solely responsible for the overall opioid reduction. Finally, it is also possible that the introduction of cryoablation led to differences in opioid prescribing unrelated to the efficacy of the intervention (novelty bias) [19].
5. Conclusions
This study provides a detailed cost analysis of bilateral intercostal nerve cryoablation in pediatric patients undergoing a minimally invasive Nuss procedure for repair of pectus excavatum in our hospital system. We found that intercostal thoracic nerve cryoablation is associated with decreased costs relative to traditional pain control methods, in addition to confirming the reduction in opioid use and hospital length of stay that has been reported previously. This information should be used to support the continued adoption of thoracoscopic assisted bilateral intercostal nerve cryoablation as an effective and fiscally responsible method of pain control during a minimally invasive Nuss procedure for pectus excavatum.
6. Acknowledgements
Grant Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number T32 CA090217. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
7. Abbreviations
- ASA
American Society of Anesthesiologists
- BMI
body mass index
- CPT
current procedural terminology
- LOS
length of stay
- MME
milligram morphine equivalents
- NRS
numeric rating scale
- PCA
patient controlled anesthesia
- PPI
producer price index
Footnotes
Level of Evidence: retrospective comparative study, level III
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Papic JC, Finnell SME, Howenstein AM, Breckler F, Leys CM. Postoperative opioid analgesic use after Nuss versus Ravitch pectus excavatum repair. Journal of Pediatric Surgery 2014;49(6):919–23. [DOI] [PubMed] [Google Scholar]
- [2].Mao YZ, Tang ST, Li S. Comparison of the Nuss versus Ravitch procedure for pectus excavatum repair: an updated meta-analysis. Journal of Pediatric Surgery 2017;52(10):1545–52. [DOI] [PubMed] [Google Scholar]
- [3].St Peter SD, Weesner KA, Weissend EE, Sharp SW, Valusek PA, Sharp RJ, et al. Epidural vs patient-controlled analgesia for postoperative pain after pectus excavatum repair: a prospective, randomized trial. Journal of Pediatric Surgery 2012;47(1):148–53. [DOI] [PubMed] [Google Scholar]
- [4].Lukosiene L, Rugyte DC, Macas A, Kalibatiene L, Malcius D, Barauskas V. Postoperative pain management in pediatric patients undergoing minimally invasive repair of pectus excavatum: The role of intercostal block. Journal of Pediatric Surgery 2013;48(12):2425–30. [DOI] [PubMed] [Google Scholar]
- [5].Nuss D, Obermeyer RJ, Kelly RE. Nuss bar procedure: past, present and future. Annals of Cardiothoracic Surgery 2016;5(5):422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Harbaugh CM, Johnson KN, Kein CE, Jarboe MD, Hirschl RB, Geiger JD, et al. Comparing outcomes with thoracic epidural and intercostal nerve cryoablation after Nuss procedure. Journal of Surgical Research 2018;231:217–23. [DOI] [PubMed] [Google Scholar]
- [7].Graves CE, Moyer J, Zobel MJ, Mora R, Smith D, O’Day M, et al. Intraoperative intercostal nerve cryoablation During the Nuss procedure reduces length of stay and opioid requirement: A randomized clinical trial. Journal of Pediatric Surgery 2019;54(11):2250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dekonenko C, Dorman RM, Duran Y, Juang D, Aguayo P, Fraser JD, et al. Postoperative pain control modalities for pectus excavatum repair: A prospective observational study of cryoablation compared to results of a randomized trial of epidural vs patient-controlled analgesia. Journal of Pediatric Surgery 2020;55(8):1444–7. [DOI] [PubMed] [Google Scholar]
- [9].Keller BA, Kabagambe SK, Becker JC, Chen YJL, Goodman LF, Clark-Wronski JM, et al. Intercostal nerve cryoablation versus thoracic epidural catheters for postoperative analgesia following pectus excavatum repair: Preliminary outcomes in twenty-six cryoablation patients. Journal of Pediatric Surgery 2016;51(12):2033–8. [DOI] [PubMed] [Google Scholar]
- [10].Sujka J, Benedict LA, Fraser JD, Aguayo P, Millspaugh DL, St Peter SD. Outcomes Using Cryoablation for Postoperative Pain Control in Children Following Minimally Invasive Pectus Excavatum Repair. Journal of Laparoendoscopic & Advanced Surgical Techniques 2018;28(11):1383–6. [DOI] [PubMed] [Google Scholar]
- [11].Roudsari B, McWilliams J, Bresnahan B, Padia SA. Introduction to Cost Analysis in IR: Challenges and Opportunities. Journal of Vascular and Interventional Radiology 2016;27(4):539–45. [DOI] [PubMed] [Google Scholar]
- [12].Aiken T, Barrett J, Stahl CC, Schwartz PB, Udani S, Acher AW, et al. Operative Delay in Adults with Appendicitis: Time is Money. Journal of Surgical Research In Press. [DOI] [PubMed] [Google Scholar]
- [13].PPI Commodity data for Health care services-Inpatient care, seasonally adjusted. Bureau of Labor Statistics. [Google Scholar]
- [14].Dunn A, Grosse SD, Zuvekas SH. Adjusting Health Expenditures for Inflation: A Review of Measures for Health Services Research in the United States. Health Services Research 2018;53(1):175–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Von Korff M, Saunders K, Ray GT, Boudreau D, Campbell C, Merrill J, et al. De facto long-term opioid therapy for noncancer pain. Clinical Journal of Pain 2008;24(6):521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain 2009;143(3):223–7. [DOI] [PubMed] [Google Scholar]
- [17].Miech R, Johnston L, O’Malley PM, Keyes KM, Heard K. Prescription Opioids in Adolescence and Future Opioid Misuse. Pediatrics 2015;136(5):E1169–E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Levy S Youth and the Opioid Epidemic. Pediatrics 2019;143(2):7. [DOI] [PubMed] [Google Scholar]
- [19].Fossati R, Confalonieri C, Apolone G, Cavuto S, Garattini S. Does a drug do better when it is new? Annals of Oncology 2002;13(3):470–3. [DOI] [PubMed] [Google Scholar]