Abstract
The past decade has seen an important revision of the traditional concept of the role and function of glial cells. From “passive support” for neurons, oligodendrocyte lineage cells are now recognized as metabolic exchangers with neurons, a cellular interface with blood vessels and responders to gut-derived metabolites or changes in the social environment. In the developing brain, the differentiation of neonatal oligodendrocyte progenitors (nOPCs) is required for normal brain function. In adulthood, the differentiation of adult OPCs (aOPCs) serves an important role in learning, behavioral adaptation and response to myelin injury. Here, we propose the concept of OPCs as environmental biosensors, which “sense” chemical and physical stimuli over time and adjust to the new challenges by modifying their epigenome and consequent transcriptome. Because epigenetics defines the ability of the cell to “adapt” gene expression to changes in the environment, we propose a model of OPC differentiation resulting from time-dependent changes of the epigenomic landscape in response to declining mitogens, raising hormone levels, neuronal activity, changes in space constraints or stiffness of the extracellular matrix. We propose that the intrinsically different functional properties of aOPCs compared to nOPCs result from the accrual of “epigenetic memories” of distinct events, which are “recorded” in the nuclei of OPCs as histone and DNA marks, defining a “unique epigenomic landscape” over time.
Keywords: histone, epigenetics, chromatin, DNA methylation, brain, aging
Graphical Abstract
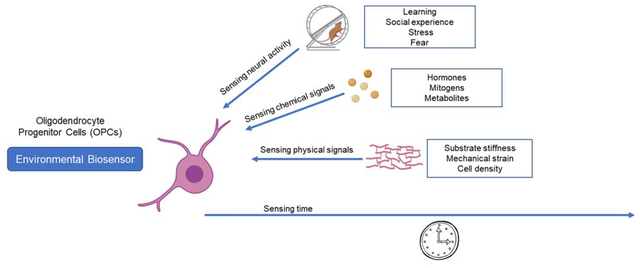
Oligodendrocyte progenitors as environmental biosensors. Oligodendrocyte progenitors (OPCs) adapt to the environment and respond to learning paradigms, social stimuli and stressors. At the cellular level, OPCs respond to chemical and physical cues. Finally, OPCs may also sense time and modify their intrinsic properties, so that OPCs in the adult brain are quite distinct from those in the neonatal brain.
1. Introduction
The past decade has seen an important revision of the traditional role and function of glial cells. The concept of myelinating oligodendrocytes (OLs) as simple “axonal insulators” and the textbook knowledge of either myelinated or unmyelinated fibers have been recently challenged by the discovery of discontinuous axonal myelination [1,2] and myelin plasticity in response to social interaction [3–5], mechano-stimulation [6], exercise [7,8], learning [9,10] and gut microbiota changes [11,12]. OLs derive from highly migratory and proliferative neonatal progenitors (OPCs), a proportion of which remains as adult undifferentiated OPCs in the central nervous system (CNS) [13,14]. New models accounting for the adaptability of myelinating glia to environmental conditions are needed. Because epigenetics defines the ability of the cell to “adapt” gene expression to changes in the environment, here we review a model of “time-dependent myelination” resulting from the unique epigenomic landscape and nuclear structure of myelinating glia in the developing or adult brain, due to the integration of chemical and physical stimuli over time. Epigenetics refers to changes in gene expression which are induced by post-translational modifications of DNA and histones, which are specific for each cell type and collectively define what is called the “epigenomic landscape”. An important feature of epigenetic marks is that they are stable, and thereby tend to accumulate over time in response to the exposure to distinct stimuli. The functional implications of histone marks depend on position of the lysine residues, whether they are acetylated or methylated, and the number of methyl groups added. For instance, trimethylation of K4 on histone H3 has been associated with transcriptionally active promoters, while trimethylation of K9 or K27 has been associated with transcriptional repression and chromatin compaction (i.e. repressive heterochromatin). Each of these marks is deposited (“writer”) or removed (“eraser”) by a specific enzyme and interpreted by nuclear proteins containing specialized recognition domains (“readers”) [15–17]. While new evidence is emerging on the distinct transcriptome of neonatal and adult OPCs, still very little is known about the unique epigenome at distinct ages and future studies will be needed to fill this critical gap of knowledge. In this review we first present evidence supporting the adaptability and responsiveness of OPCs to environmental stimuli affecting the whole organism (Chapter 2) and discuss the relationship between OPC differentiation and neuronal activity. We then address the definition of OPCs as responders to: chemical stimuli in Chapter 3, with an emphasis on mitogens, thyroid hormone, and systemic metabolites and to physical stimuli and mechanisms of mechano-transduction in Chapter 4. The concept of OPCs as time sensors will then be developed in Chapter 5 and concluding remarks provided at the end.
2. OPCs respond to neuronal activity in response to learning and social stimuli.
The concept that neuronal activity modulates the behavior of progenitor cells dates back to the ‘60’s [18] and revived in the ‘90s, when it was shown that disruption of neuronal activity, due to axonal transection or pharmacological block of axonal conduction, altered OPC proliferation and differentiation [19–21]. It was also reported that neurotransmitter receptors and ion channels directly affect the properties of cultured OPCs [22,23]. The later discovery of direct synapses between glutamatergic and GABAergic terminals on OPC cell bodies further supported the concept of a communication between neurons and oligodendroglial lineage cells [24–27]. More recently, a series of live imaging studies in zebrafish [28–30] and studies using optogenetics [31–33] or pharmacogenetics [34] in mice provided evidence of the relationship between neuronal activity and the behavior of OPCs. At an organism level, depriving mice of social contact during development (e.g. maternal deprivation, early weaning), or in adulthood (e.g. social isolation, chronic stress, social defeat), prevented new myelin formation in regions of the developing and adult brain related to social behavior, presumably due to decreased neuronal activation [3,4,35–38]. Conversely, exposing mice to motor, sensory or fearful learning paradigms, enhanced myelin plasticity by favoring new myelin formation and guaranteeing memory consolidation [1,7,39–42].
3. OPCs as chemosensors.
It is well established that the nuclei of proliferating and undifferentiated OPCs are mostly euchromatic, with relaxed and transcriptionally active chromatin structure, while differentiated OLs are characterized by heterochromatic electron-dense nuclei, indicative of transcriptional repression [43]. OPC proliferation is regulated by specific mitogens, such as PDGF-AA [44–46], while their differentiation into myelinating cells is favored by the presence of thyroid hormone [47]. More recently it has been shown that these and other chemical cues influence oligodendrocyte development by acting on the epigenome in OPCs [48,49]. Proliferating OPCs are characterized by relaxed euchromatin and global histone acetylation, due to mitogen-dependent stimulation of histone lysine acetyltransferases (KATs). We have previously reported the roles of the transcription factors c-Myc and E2F1 in maintaining OPC proliferation by recruiting KATs to cell cycle genes thereby regulating chromatin accessibility by influencing histone acetylation [48,49]. As mitogens were removed from the medium, the equilibrium between histone acetylation and deacetylation was skewed towards deacetylation as negative regulators of cell cycle, recruited HDAC1/2 to the same genes and to inhibitors of myelin gene transcription and cultured OPC spontaneously differentiated [50–52]. Silencing of E2F1 or c-Myc in OPCs induced decreased expression of cell cycle genes and of transcriptional inhibitors of differentiation while promoting histone reorganization and the initiation of the formation of heterochromatin, characteristic of the differentiated state [48,49]. The importance of the balance between histone acetylation and deacetylation as a critical step for differentiation was also suggested by a number of other studies. Treating OPCs with competing morphogenic signals either favoring (e.g. Sonic hedgehog, SHH) or antagonizing (e.g. Bone morphogenic protein, BMP) oligodendrogliogenesis, revealed a competitive regulation of histone acetylation. While BMP increased histone acetylation, SHH favored deacetylation and this led to specific effect on the transcriptome, resulting in opposing effects on OPC differentiation towards OL [53]. Besides the direct competition between KAT and HDAC activities, additional modalities by which chemical stimuli impact the epigenome and favor OPC differentiation is the promotion of post-translational modification of histone arginines. If arginine residues that are close to critical lysine residues (for instance R8 is close to K9 on histone H3; R3 is close to K5 on histone H4) are symmetrically methylated by specific enzymes (in this case PRMT5), the adjacent lysines can no longer be acetylated and this favors the OPC differentiation process, as shown by the defective myelination detected in mice with lineage specific genetic deletion of Prmt5 [54].
Finally, it is important to mention that while both deacetylation of lysine residues and repressive trimethylation of residues K9 and K27 of H3 are reversible histone marks, the recognition of trimethylated H3K9 and H3K27 by specialized readers and incorporation into facultative heterochromatin [55] or recruitment to the nuclear lamina [56], render these repressive marks more stable [57] during oligodendrocyte development. Treatment of cultured OPCs with thyroid hormone substantially increased the activity of selective histone methyltransferases (HMTs) and promoted the deposition of these repressive marks, characteristic of heterochromatin at genomic loci corresponding to tissue patterning, cell migration, and synaptic signaling pathways [58].
In addition to the influence of mitogens and hormonal signals, OPCs have been shown to be sensitive to chemical signals derived from the intestinal microbiota (reviewed in [59]). Studies performed in rodents conducted under germ-free (GF) animal housing have generated intriguing data highlighting a potential influence between the host microbiome and developmental myelination. Comparing specific-pathogen free and GF housed mice revealed structural alterations in white matter tract of the animals raised under GF conditions [60]. We also reported on the microbiota-derived metabolite p-cresol as negatively influencing OPC differentiation by blocking expression of myelin genes [11]. The production of this phenol-derivative was linked to the presence of certain microbial taxa commonly associated with gut dysbiosis [11], as often detected in demyelinating disorders (reviewed in [61]).
These studies highlight the contribution of chemical cues to the influence of OPC physiology and lineage development. Beyond local signals in the CNS, chemical cues originating from the gut microbiota appear to play a role in the healthy physiology of OPCs and may influence disease course in the case of demyelinating disorders. Further exploration may uncover novel mechanisms facilitating influence on the epigenome by chemical by-products of microbiota metabolism.
4. OPCs as mechanosensors.
OPCs can sense physical cues present in their environment such as spatial and geometric constraints, tensile strain and substrate stiffness. Spatial and geometric constraints influence OPC differentiation [62]. Building on older studies demonstrating that a critical density of cultured OPCs was needed to promote differentiation [63], the effect of spatial constraints or crowding on OPC differentiation, was demonstrated by culturing OPCs together with other cell types [62] or with inert polystyrene beads [6,62]. Differentiation was preceded by nuclear and epigenetic changes occurring in OPCs [6], thereby suggesting that OPCs were able to sense geometrical and spatial constraints and transduced this information in.
Novel culture methods have also been devised to test the influence of mechanical tensile strain on OPCs [64,65]. In these studies, the application of tensile strains within relevant physiological ranges inhibited proliferation of OPCs while enhancing their differentiation [64]. The applied mechanical strain caused changes to chromatin organization and decreased histone acetylation, with histone deacetylase 11 (HDAC11) acting as a key mediator of this physical cue [64]. Mechanical strain also altered the nuclear dynamics of OPCs as decreased nuclear fluctuations associated with differentiation (presumably linked to heterochromatin formation) were accelerated upon application of tensile strain to these cells [65].
Finally, the mechanosensitivity of OPCs was clearly demonstrated by in vitro studies where OPCs were cultured on polymer gels of varying stiffness, and found to modulate several functional properties of OPCs such as their survival, proliferation, migration and differentiation into mature OLs [66]. While polymer gel stiffness enhanced differentiation of OPCs [66], rigid lesion-like Matrigel substrates inhibited OPC differentiation [67]. Consistent with murine studies, the migration of OLs derived from human induced pluripotent stem cells (hiPSCs) was recently reported to increase in response to increased substrate stiffness [68]. However, the mechanosensitive response to stiffness in humans suggested a unique form of heterogeneity that was not seen in animal models [68].
Together, OPCs appear competent to sense mechanical forces specific to their microenvironment through a number of signaling pathways (reviewed in [15]). These forces modulate OPC physiology and influence their capacity to differentiate.
5. OPCs as time sensors.
During development, a majority of neonatal OPCs (nOPCs) differentiate into mature OLs. A proportion of nOPCs, however, maintain their progenitor state and transition into adult OPCs (aOPCs) [69,70]. Although aging has been associated with the progressive development of replicative senescence in distinct cell types [71], OPCs do not show these features [72] as they remain able to replicate and expand, unless exposed to chronic demyelinating conditions, such as primary progressive multiple sclerosis [73]. These aOPCs comprise 5–8% of the total cells in the adult rodent brain and are uniformly distributed in the brain’s grey and white matter [13]. Studies have shown that aOPCs respond to new learning task by proliferating and differentiating [7,74] and are responsible for remyelination following injury [75,76]. The persistence of OPCs in the adult CNS affords a unique opportunity to determine what biological changes occur over time (during the transition of nOPCs into aOPCs), a concept we describe here as sensing time. Since the identification of aOPCs, they have been shown to have intrinsically different properties from nOPCs. These differences were detected in cell culture studies where, despite being exposed to identical culture conditions, nOPCs and aOPCs exhibited disparate responsiveness in terms of proliferation, migration and differentiation [69,77,78]. Furthermore, isolated nOPCs maintained in culture for over a year displayed characteristics similar to isolated aOPCs suggesting a cell-intrinsic change in biological properties over time [79].
5.1. Decreased OPC proliferation with time.
Several studies have shown correlative links between the length of the cell cycle in OPCs to a factor of time. BrdU and EdU staining of OPCs in vivo reported significantly fewer numbers of proliferating cells in adult brains compared to neonatal brains both in resting conditions [78,80] or after white matter injury [81]. OPCs isolated from the optic nerves of neonatal and adult rats display differential cell cycle times in vitro, from less than 24 hours in nOPCs to 65 hours in aOPCs [69], data which has been mirrored in numerous other studies [78,82,83]. There are hypothetical explanations that could account for the lower proliferation rate observed in aOPCs, including intrinsic and extrinsic mechanisms. For instance, transcript analyses show higher expression levels of genes related to cell cycle (Ccnd1 and Cdk4) and proliferation (Pdgfrα and Mki67) in neonatal OPCs compared to aOPCs [83,84]. Extrinsic signals, such as an overall decrease in mitogen levels [85] or increase in cell contact among precursors [86] could explain the decrease in proliferation rate of nOPCs as they transition to aOPCs. The expression levels of voltage-gated ion channels and glutamate receptor subtypes differs over time suggesting that electrical activity could influence proliferation of OPCs in an age dependent manner [84]. The steady increase in AMPA receptor levels during aging [84] could be linked to the decrease in OPC proliferation over time since previous reports demonstrated that activation of AMPA receptors in OPCs inhibit OPC proliferation [87].
5.2. Decreased OPC migratory capacity over time.
Proliferating nOPCs migrate from their site of generation to other parts of the CNS during development [88], while aOPCs migrate to sites of injury in order to promote repair and remyelination [76]. In experimental approaches aimed at studying the rate of OPC migration in vivo, depletion of OPCs stimulates the migratory process. The rate of repopulation of OPC-depleted tissue by adjacent OPCs is significantly higher in young brains than in adult brains [14,89,90]. Intriguingly, both nOPCs and aOPCs maintain their original rates of repopulation into OPC-depleted tissue when transplanted into adult mice and young mice, respectively [89]. This suggests that the age-related decline in migration and repopulation rates of aOPCs are as a result of cell intrinsic properties. Consistent with the decline of OPC migration with time in vivo, the migratory rate of OPCs in culture declines as nOPCs transition to aOPCs [69]. In addition to cell-intrinsic differences, external factors such as age-related decline in the levels of mitogens such as PDGF and bFGF, which have previously been shown to enhance migration of OPCs, could account for the lower migratory rate of OPCs in the adult brain [91]. Transcriptional analyses of isolated nOPCs and aOPCs also help to explain the lower migratory rate of aOPCs. Bulk RNA sequencing revealed lower levels of genes relating to OPC migration (Ephb2 and Dcc) in aOPCs compared to nOPCs [84].
5.3. Changes in OPC differentiation capacity over time.
Myelination of axons by OLs is critical during development and this process continues into adulthood albeit at lower efficiency with age [92]. The age-related decrease in myelination is attributed to failure of OPC recruitment and differentiation [93]. The age-dependent decrease in differentiation capacity of OPCs and myelination in the adult CNS has been associated with inefficient epigenetic control of differentiation inhibitors [49], changes in immune response [94] or low levels of critical growth factors [95]. Several cell culture studies show that aOPCs take longer [69] or fail to differentiate [96] into myelinating OLs compared to nOPCs. In agreement to the observed phenotypic properties, aOPCs express lower levels of pro-differentiation genes (Myrf and Enpp6) compared to nOPCs [84]. Surprisingly, microarray analysis of fluorescence activated cell sorting of nOPCs and aOPCs discovered the transcriptome of aOPCs to be closer to that of the differentiated OL than to nOPCs [97]. However, when activated by demyelination, the transcriptome of aOPCs switched to resemble the transcriptome of nOPCs, although these activated aOPCs eventually fail to efficiently differentiate with aging [97]. Transcriptional changes leading to reduced expression of genes involved in cholesterol-biosynthesis and cell cycle and higher expression of genes involved in oxidative phosphorylation and inflammatory response may likely result from the reorganization of the epigenome at these specific genomic locations [98]. This study provides new avenues for further research into understanding remyelination failure in the adult brain [98].
5.4. Response of OPCs to metabolites over time
As proliferating cells, OPCs have significant demand for bioenergetic substrates to support replicatory biosynthesis and expansion. However, age related changes in sensitivity and responsiveness to metabolic signals have been identified between young and old OPCs. In studies comparing fetal versus adult stem-cell derived OPCs, oxygen glucose deprivation (OGD) was shown to be detrimental to fetal OPC survival, whereas adult OPCs were largely unaffected [99]. Sensitivity of neonatal OLs to metabolic regulation has also been supported by studies showing increased cell death in reduced glucose conditions in vitro [100,101]. Conversely, adult-derived OLs appeared more resilient to glucose stress by adapting towards mitochondrial oxidative phosphorylation and maintaining survival, albeit with lower ATP production [100]. These data suggest that during developmental myelination, neonatal OPC survival is strongly influenced by the availability of glucose, and differentiating OLs are particularly susceptible to disruptions in glycolysis and oxidative phosphorylation. While in adult tissues, OPCs and OLs display a greater capacity to adapt to metabolic alterations. Aging OPCs are characterized by impaired mitochondrial function [97], which has been in part associated with the reduced regenerative myelin capacity in old mice [97]. Recent studies aimed at restoring the declining differentiation capacity of aOPCs identified alternate day fasting as potential metabolic intervention for rejuvenating aOPCs [96]. Interestingly, the differentiation capacity of aOPCs significantly increased upon exposure of aOPCs to the fasting mimetic Metformin, due to improved mitochondrial function [96]. Given the importance of bioenergetics and the recent interest in metabolic influence on epigenetic regulation of gene expression, it will be important to study the intersection between these two variables and age-related metabolic intervention.
5.5. Intrinsic transcriptional differences suggest unique epigenomic landscapes.
Cell intrinsic differences observed between nOPCs and aOPCs as evidenced by their unique transcriptional signatures [84,102] suggested differential gene regulation in nOPCs and aOPCs may occur through epigenetic mechanisms such as histone post-translational modifications (PTMs) or DNA methylation. Our lab has previously provided evidence to support a critical role of epigenetics in the regulation of remyelination in aging [49]. We first showed that inefficient HDAC recruitment to promoter regions of differentiation inhibitors in aOPCs leads to failure of remyelination [49]. Recently we identified DNA hydroxymethylation, mediated by Ten-eleven translocation 1 (TET1), as a key regulator during adult myelination [103]. Inefficient myelination, reminiscent of myelination in aged mice, was observed upon both constitutive and conditional ablation of Tet1 in young mice [103]. Further supporting a role for DNA modifications in time sensing of OPCs, assessment of the Global DNA Methylation profile of cultured aOPCs indicated lower levels of DNA methylation in these cells with significant downregulation of DNA methyltransferase 1 (DNMT1) [104].
Taken together, both intrinsic and extrinsic cues regulate the unique properties of nOPCs and aOPCs in terms of proliferation, migration, differentiation and in response to metabolic regulation. A unique transcriptome in nOPCs and aOPCs underlie the cell intrinsic differences seen in these cells suggesting that epigenetic regulation modulates the differential gene expression in OPCs over time (Fig. 1)
Fig. 1. Distinct epigenetic changes are responsible for the intrinsic functional differences between nOPC and aOPC.
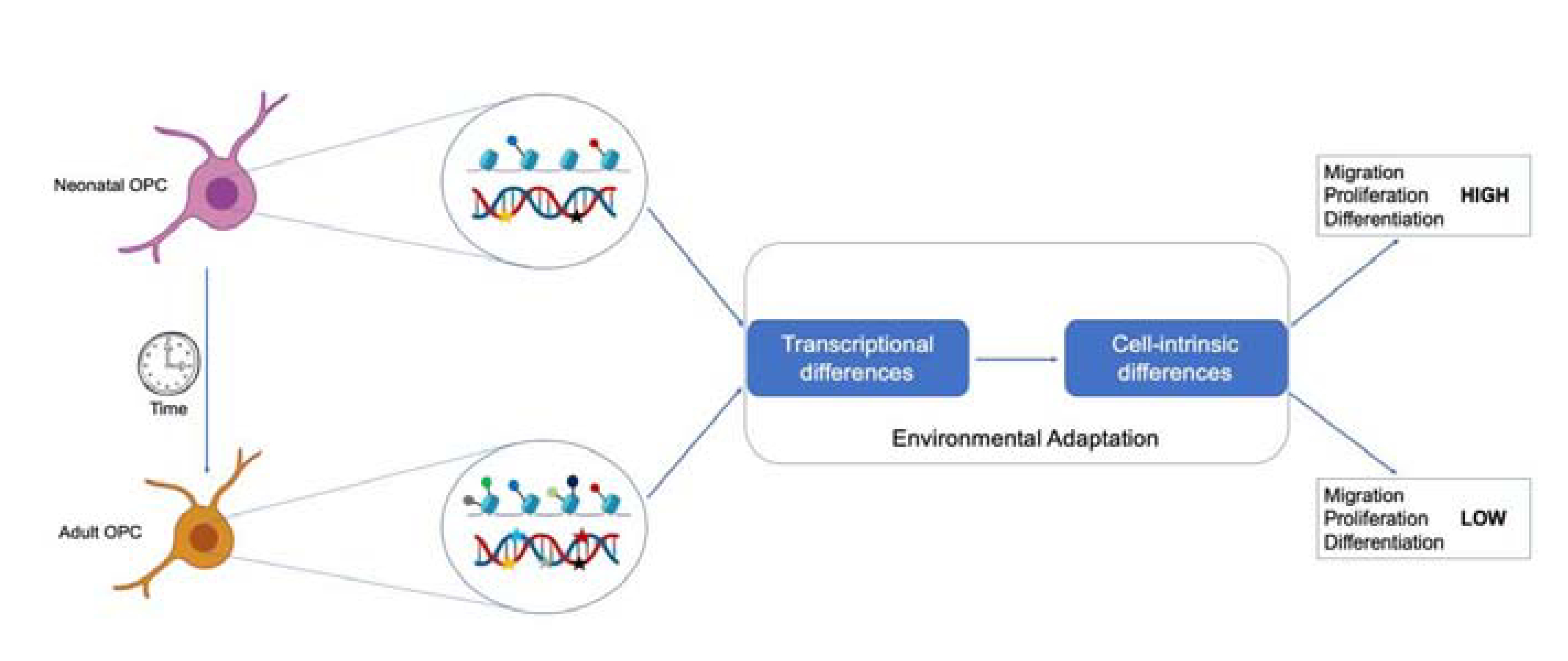
Functional differences exist between neonatal and adult OPC. The ability of progenitors to migrate, proliferate and differentiate gradually decreases as neonatal progenitors transition into adulthood. These functional difference result from progressive environmental adaptation leading to distinct epigenetic landscapes and transcriptional changes.
6. Conclusions.
Past research was based on the notion of “a myelination pathway” indistinguishable between neonatal and adult brain. This concept led to the direct translation of molecular mechanisms of developmental myelination into therapeutic approaches aimed at adult remyelination, which unfortunately proved to be only moderately effective [105]. This review provides a different perspective and highlights evidence supporting the concept of unique responsiveness of nOPCs and aOPCs to stimuli, due to their unique epigenome, with the goal to provide a conceptual framework for the potential development of novel myelin regenerative strategies [106,107]. Based on the widespread occurrence of myelination deficits in children, due to genetic or vascular causes [108,109] and in adults, due to myelin dysfunction consequent to autoimmunity [107,110], aging [106,111,112], neurodegenerative [113–117] and psychiatric disorders [118,119], we believe that a careful experimental design, which takes into account the age of intervention for repair, and the intrinsic differences between nOPC and aOPC, is critical for success. The existence of distinct epigenomic landscapes supporting transcriptional changes responsible for the intrinsic functional differences between neonatal and adult OPCs, implies that the responsiveness to pharmacological intervention would vary with age. Overall, future studies are needed to clarify these concepts and bear the promise of effective restoration of myelin.
Highlights.
Oligodendrocyte progenitors (OPCs) are functionally heterogeneous
The functional properties of OPCs change with time
OPCs respond to neuronal activity, chemical and physical stimuli
External stimuli modulate the epigenome of OPCs
Acknowledgments
This review summarizes concepts that were presented by Dr. Casaccia at the meeting “The Dark Side of the Brain” held at the Karolinska Institute October 11, 2019. The Casaccia Lab is supported by NIH-R35 NS111604 to PC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Hill RA, Li AM, Grutzendler J, Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain, Nat. Neurosci 21 (2018) 683–695. 10.1038/s41593-018-0120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, Arlotta P, Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex, Science. 344 (2014) 319–324. 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu J, Dietz K, Deloyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P, Impaired adult myelination in the prefrontal cortex of socially isolated mice, Nat. Neurosci 15 (2012) 1621–1623. 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu J, Dietz K, Hodes GE, Russo SJ, Casaccia P, Widespread transcription alternations in oligodendrocytes in the adult mouse brain following chronic stress, Dev. Neurobiol 78 (2018) 152–162. 10.1002/dneu.22533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu J, Dupree JL, Gacias M, Frawley R, Sikder T, Naik P, Casaccia P, Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice, J. Neurosci 36 (2016) 957–962. 10.1523/JNEUROSCI.3608-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hernandez M, Patzig J, Mayoral SR, Costa KD, Chan JR, Casaccia P, Mechanostimulation promotes nuclear and epigenetic changes in oligodendrocytes, J. Neurosci 36 (2016) 806–813. 10.1523/JNEUROSCI.2873-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McKenzie IA, Ohayon D, Li H, deFaria JP, Emery B, Tohyama K, Richardson WD, Motor skill learning requires active central myelination., Science. 346 (2014) 318–322. 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sampaio-Baptista C, Khrapitchev AA, Foxley S, Schlagheck T, Scholz J, Jbabdi S, DeLuca GC, Miller KL, Taylor A, Thomas N, Kleim J, Sibson NR, Bannerman D, Johansen-Berg H, Motor skill learning induces changes in white matter microstructure and myelination., J. Neurosci 33 (2013) 19499–19503. 10.1523/JNEUROSCI.3048-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F, Extensive piano practicing has regionally specific effects on white matter development, Nat. Neurosci 8 (2005) 1148–1150. 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- [10].Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H, Training induces changes in white-matter architecture, Nat. Neurosci 12 (2009) 1370–1371. 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gacias M, Gaspari S, Santos PMG, Tamburini S, Andrade M, Zhang F, Shen N, Tolstikov V, Kiebish MA, Dupree JL, Zachariou V, Clemente JC, Casaccia P, Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior, Elife. 5 (2016) e13442. 10.7554/eLife.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ntranos A, Casaccia P, The Microbiome-Gut-Behavior Axis: Crosstalk Between the Gut Microbiome and Oligodendrocytes Modulates Behavioral Responses, Neurotherapeutics. 15 (2018) 31–35. 10.1007/s13311-017-0597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dawson MR, Polito A, Levine JM, Reynolds R, NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS, Mol. Cell. Neurosci 24 (2003) 476–488. 10.1016/S1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- [14].Nishiyama A, Komitova M, Suzuki R, Zhu X, Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity, Nat. Rev. Neurosci 10 (2009) 9–22. 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- [15].Tsai E, Casaccia P, Mechano-modulation of nuclear events regulating oligodendrocyte progenitor gene expression, Glia. 67 (2019) 1229–1239. 10.1002/glia.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hernandez M, Casaccia P, Interplay between transcriptional control and chromatin regulation in the oligodendrocyte lineage, Glia. 63 (2015) 1357–1375. 10.1002/glia.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu J, Moyon S, Hernandez M, Casaccia P, Epigenetic control of oligodendrocyte development: adding new players to old keepers, Curr. Opin. Neurobiol 39 (2016) 133–138. 10.1016/J.CONB.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gyllensten L, Malmfors T, Myelinization of the optic nerve and its dependence on visual function-a quantitative investigation in mice., J. Embryol. Exp. Morphol 11 (1963) 255–266. https://dev.biologists.org/content/11/1/255. [PubMed] [Google Scholar]
- [19].Barres BA, Raff MC, Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons, Nature. 361 (1993) 258–260. 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- [20].Barres BA, Jacobson MD, Schmid R, Sendtner M, Raff MC, Does oligodendrocyte survival depend on axons?, Curr. Biol 3 (1993) 489–497. 10.1016/0960-9822(93)90039-Q. [DOI] [PubMed] [Google Scholar]
- [21].Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C, Induction of myelination in the central nervous system by electrical activity, Proc. Natl. Acad. Sci. U. S. A 93 (1996) 9887–9892. 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC, Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block, J. Neurosci 16 (1996) 2659–2670. 10.1523/jneurosci.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ghiani CA, Yuan X, Eisen AM, Knutson PL, DePinho RA, McBain CJ, Gallo V, Voltage-activated K+ channels and membrane depolarization regulate accumulation of the cyclin-dependent kinase inhibitors p27(Kip1) and p21(CIP1) in glial progenitor cells, J. Neurosci 19 (1999) 5380–5392. 10.1523/jneurosci.19-13-05380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bergles DE, Roberts JDB, Somogyl P, Jahr CE, Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus, Nature. 405 (2000) 187–191. 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- [25].Lin SC, Bergles DE, Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus, Nat. Neurosci 7 (2004) 24–32. 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- [26].Kukley M, Capetillo-Zarate E, Dietrich D, Vesicular glutamate release from axons in white matter, Nat. Neurosci 10 (2007) 311–320. 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- [27].Káradóttir R, Cavelier P, Bergersen LH, Attwell D, NMDA receptors are expressed in oligodendrocytes and activated in ischaemia, Nature. 438 (2005) 1162–1166. 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Czopka T, Ffrench-Constant C, Lyons DA, Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo, Dev. Cell 25 (2013) 599–609. 10.1016/j.devcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B, Neuronal activity biases axon selection for myelination in vivo, Nat. Neurosci 18 (2015) 683–689. 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA, Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo, Nat. Neurosci 18 (2015) 628–630. 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M, Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain, Science. 344 (2014). 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Geraghty AC, Gibson EM, Ghanem RA, Greene JJ, Ocampo A, Goldstein AK, Ni L, Yang T, Marton RM, Paşca SP, Greenberg ME, Longo FM, Monje M, Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment, Neuron. 103 (2019) 250–265. 10.1016/j.neuron.2019.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ortiz FC, Habermacher C, Graciarena M, Houry PY, Nishiyama A, Oumesmar BN, Angulo MC, Neuronal activity in vivo enhances functional myelin repair, JCI Insight. 4 (2019) e123434. 10.1172/jci.insight.123434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mitew S, Gobius I, Fenlon LR, McDougall SJ, Hawkes D, Xing YL, Bujalka H, Gundlach AL, Richards LJ, Kilpatrick TJ, Merson TD, Emery B, Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner, Nat. Commun 9 (2018) 1–16. 10.1038/s41467-017-02719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kodama Y, Kikusui T, Takeuchi Y, Mori Y, Effects of early weaning on anxiety and prefrontal cortical and hippocampal myelination in male and female Wistar rats, Dev. Psychobiol 50 (2008) 332–342. 10.1002/dev.20289. [DOI] [PubMed] [Google Scholar]
- [36].Makinodan M, Rosen KM, Ito S, Corfas G, A critical period for social experience-dependent oligodendrocyte maturation and myelination, Science. 337 (2012) 1357–1360. 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yang Y, Cheng Z, Tang H, Jiao H, Sun X, Cui Q, Luo F, Pan H, Ma C, Li B, Neonatal Maternal Separation Impairs Prefrontal Cortical Myelination and Cognitive Functions in Rats Through Activation of Wnt Signaling, Cereb. Cortex 27 (2016) 2871–2884. 10.1093/cercor/bhw121. [DOI] [PubMed] [Google Scholar]
- [38].Bonnefil V, Dietz K, Amatruda M, Wentling M, V Aubry A, Dupree JL, Temple G, Park H-J, Burghardt NS, Casaccia P, Liu J, Region-specific myelin differences define behavioral consequences of chronic social defeat stress in mice., Elife. 8 (2019) e40855. 10.7554/eLife.40855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE, Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex, Nat. Neurosci 21 (2018) 696–706. 10.1038/s41593-018-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pan S, Mayoral SR, Choi HS, Chan JR, Kheirbek MA, Preservation of a remote fear memory requires new myelin formation, Nat. Neurosci 23 (2020) 487–499. 10.1038/s41593-019-0582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Steadman PE, Xia F, Ahmed M, Mocle AJ, Penning ARA, Geraghty AC, Steenland HW, Monje M, Josselyn SA, Frankland PW, Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice, Neuron. 105 (2020) 150–164. 10.1016/j.neuron.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bacmeister CM, Barr HJ, McClain CR, Thornton MA, Nettles D, Welle CG, Hughes EG, Motor learning promotes remyelination via new and surviving oligodendrocytes, Nat. Neurosci 23 (2020) 819–831. 10.1038/s41593-020-0637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mori S, Leblond CP, Electron microscopic identification of three classes of oligodendrocytes and a preliminary study of their proliferative activity in the corpus callosum of young rats, J. Comp. Neurol 139 (1970) 1–28. 10.1002/cne.901390102. [DOI] [PubMed] [Google Scholar]
- [44].Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P, Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor ceil, Nature. 333 (1988) 560–562. 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- [45].Raff MC, Lillien LE, Richardson WD, Burne JF, Noble MD, Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture, Nature. 333 (1988) 562–565. 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- [46].Richardson WD, Pringle N, Mosley MJ, Westermark B, Dubois-Dalcg M, A role for platelet-derived growth factor in normal gliogenesis in the central nervous system, Cell. 53 (1988) 309–319. 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- [47].Barres BA, Lazar MA, Raff MC, A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development, Development. 120 (1994) 1097–1108. https://dev.biologists.org/content/120/5/1097. [DOI] [PubMed] [Google Scholar]
- [48].Shen S, Li J, Casaccia-Bonnefil P, Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain, J. Cell Biol 169 (2005) 577–589. 10.1083/jcb.200412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJM, Casaccia-Bonnefil P, Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency., Nat. Neurosci 11 (2008) 1024–1034. 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Magri L, Swiss VA, Jablonska B, Lei L, Pedre X, Walsh M, Zhang W, Gallo V, Canoll P, Casaccia P, E2F1 coregulates cell cycle genes and chromatin components during the transition of oligodendrocyte progenitors from proliferation to differentiation., J. Neurosci 34 (2014) 1481–1493. 10.1523/JNEUROSCI.2840-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Magri L, Gacias M, Wu M, Swiss VA, Janssen WG, Casaccia P, C-Myc-dependent transcriptional regulation of cell cycle and nucleosomal histones during oligodendrocyte differentiation, Neuroscience. 276 (2014) 72–86. 10.1016/j.neuroscience.2014.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Marin-Husstege M, Muggironi M, Liu A, Casaccia-Bonnefil P, Histone deacetylase activity is necessary for oligodendrocyte lineage progression., J. Neurosci 22 (2002) 10333–10345. 10.1523/JNEUROSCI.22-23-10333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wu M, Hernandez M, Shen S, Sabo JK, Kelkar D, Wang J, O’Leary R, Phillips GR, Cate HS, Casaccia P, Differential modulation of the oligodendrocyte transcriptome by sonic hedgehog and bone morphogenetic protein 4 via opposing effects on histone acetylation, J. Neurosci 32 (2012) 6651–6664. 10.1523/JNEUROSCI.4876-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Scaglione A, Patzig J, Liang J, Frawley R, Bok J, Mela A, Yattah C, Zhang J, Teo SX, Zhou T, Chen S, Bernstein E, Canoll P, Guccione E, Casaccia P, PRMT5-mediated regulation of developmental myelination, Nat. Commun 9 (2018) 1–14. 10.1038/s41467-018-04863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jamieson K, Wiles ET, McNaught KJ, Sidoli S, Leggett N, Shao Y, Garcia BA, Selker EU, Loss of HP1 causes depletion of H3K27me3 from facultative heterochromatin and gain of H3K27me2 at constitutive heterochromatin, Genome Res. 26 (2016) 97–107. 10.1101/gr.194555.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Black JC, Van Rechem C, Whetstine JR, Histone Lysine Methylation Dynamics: Establishment, Regulation, and Biological Impact, Mol. Cell 48 (2012) 491–507. 10.1016/j.molcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mews P, Zee BM, Liu S, Donahue G, Garcia BA, Berger SL, Histone Methylation Has Dynamics Distinct from Those of Histone Acetylation in Cell Cycle Reentry from Quiescence, Mol. Cell. Biol 34 (2014) 3968–3980. 10.1128/mcb.00763-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu J, Magri L, Zhang F, Marsh NO, Albrecht S, Huynh JL, Kaur J, Kuhlmann T, Zhang W, Slesinger PA, Casaccia P, Chromatin landscape defined by repressive histone methylation during oligodendrocyte differentiation., J. Neurosci 35 (2015) 352–365. 10.1523/JNEUROSCI.2606-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sauma S, Casaccia P, Does the gut microbiota contribute to the oligodendrocyte progenitor niche?, Neurosci. Lett 715 (2020) 134574. 10.1016/j.neulet.2019.134574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lu J, Synowiec S, Lu L, Yu Y, Bretherick T, Takada S, Yarnykh V, Caplan J, Caplan M, Claud EC, Drobyshevsky A, Microbiota influence the development of the brain and behaviors in C57BL/6J mice, PLoS One. 13 (2018) e0201829. 10.1371/journal.pone.0201829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sauma S, Casaccia P, Gut-brain communication in demyelinating disorders, Curr. Opin. Neurobiol 62 (2020) 92–101. 10.1016/j.conb.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rosenberg SS, Kelland EE, Tokar E, De La Torre AR, Chan JR, The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 14662–14667. 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Levi G, Agresti C, Cellular interactions and oligodendrocyte differentiation in vitro, Cytotechnology. 5 (1991) 158–161. 10.1007/BF00736837. [DOI] [PubMed] [Google Scholar]
- [64].Jagielska A, Lowe AL, Makhija E, Wroblewska L, Guck J, Franklin RJM, Shivashankar GV, Van Vliet KJ, Mechanical strain promotes oligodendrocyte differentiation by global changes of gene expression, Front. Cell. Neurosci 11 (2017) 93. 10.3389/fncel.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Makhija E, Jagielska A, Zhu L, Bost AC, Ong W, Chew SY, Shivashankar GV, Van Vliet KJ, Mechanical strain alters cellular and nuclear dynamics at early stages of oligodendrocyte differentiation, Front. Cell. Neurosci 12 (2018) 59. 10.3389/fncel.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Jagielska A, Norman AL, Whyte G, Vliet KJV, Guck J, Franklin RJM, Mechanical environment modulates biological properties of oligodendrocyte progenitor cells, Stem Cells Dev. 21 (2012) 2905–2914. 10.1089/scd.2012.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Urbanski MM, Kingsbury L, Moussouros D, Kassim I, Mehjabeen S, Paknejad N, Melendez-Vasquez CV, Myelinating glia differentiation is regulated by extracellular matrix elasticity, Sci. Rep 6 (2016) 1–12. 10.1038/srep33751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Espinosa-Hoyos D, Burstein SR, Cha J, Jain T, Nijsure M, Jagielska A, Fossati V, Van Vliet KJ, Mechanosensitivity of Human Oligodendrocytes, Front. Cell. Neurosci 14 (2020) 1–15. 10.3389/fncel.2020.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wolswijk G, Noble M, Identification of an adult-specific glial progenitor cell, Development. 105 (1989) 387–400. https://dev.biologists.org/content/105/2/387.long. [DOI] [PubMed] [Google Scholar]
- [70].Ffrench-Constant C, Raff MC, Proliferating bipotential glial progenitor cells in adult rat optic nerve, Nature. 319 (1986) 499–502. 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- [71].Sousa-Victor P, Gutarra S, García-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardí M, Ballestar E, González S, Serrano AL, Perdiguero E, Muñoz-Cánoves P, Geriatric muscle stem cells switch reversible quiescence into senescence, Nature. 506 (2014) 316–321. 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- [72].Tang DG, Tokumoto YM, Apperly JA, Lloyd AC, Raff MC, Lack of replicative senescence in cultured rat oligodendrocyte precursor cells, Science. 291 (2001) 868–871. 10.1126/science.1056780. [DOI] [PubMed] [Google Scholar]
- [73].Nicaise AM, Wagstaff LJ, Willis CM, Paisie C, Chandok H, Robson P, Fossati V, Williams A, Crocker SJ, Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis, Proc. Natl. Acad. Sci. U. S. A 116 (2019) 9030–9039. 10.1073/pnas.1818348116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, Emery B, Li H, Richardson WD, Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning, Nat. Neurosci 19 (2016) 1210–1217. 10.1038/nn.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gensert JM, Goldman JE, Endogenous progenitors remyelinate demyelinated axons in the adult CNS., Neuron. 19 (1997) 197–203. 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- [76].Franklin RJM, Ffrench-Constant C, Remyelination in the CNS: From biology to therapy, Nat. Rev. Neurosci 9 (2008) 839–855. 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- [77].Wolswijk G, Riddle PN, Noble M, Coexistence of perinatal and adult forms of a glial progenitor cell during development of the rat optic nerve, Development. 109 (1990) 691–698. https://dev.biologists.org/content/109/3/691. [DOI] [PubMed] [Google Scholar]
- [78].Shi J, Marinovich A, Barres BA, Purification and Characterization of Adult Oligodendrocyte Precursor Cells from the Rat Optic Nerve, J. Neurosci 18 (1998) 4627–4636. 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tang DG, Tokumoto YM, Raff MC, Long-term culture of purified postnatal oligodendrocyte precursor cells: Evidence for an intrinsic maturation program that plays out over months, J. Cell Biol 148 (2000) 971–984. 10.1083/jcb.148.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Segel M, Neumann B, Hill MFE, Weber IP, Viscomi C, Zhao C, Young A, Agley CC, Thompson AJ, Gonzalez GA, Sharma A, Holmqvist S, Rowitch DH, Franze K, Franklin RJM, Chalut KJ, Niche stiffness underlies the ageing of central nervous system progenitor cells, Nature. 573 (2019) 130–134. 10.1038/s41586-019-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Dingman AL, Rodgers KM, Dietz RM, Hickey SP, Frazier AP, Clevenger AC, Yonchek JC, Traystman RJ, Macklin WB, Herson PS, Oligodendrocyte Progenitor Cell Proliferation and Fate after White Matter Stroke in Juvenile and Adult Mice, in: Dev. Neurosci, 2019: pp. 601–616. 10.1159/000496200. [DOI] [PubMed] [Google Scholar]
- [82].Chan CLH, Wigley CB, Berry M, Oligodendrocyte-type 2 astrocyte (O-2A) progenitor cells from neonatal and adult rat optic nerve differ in their responsiveness to platelet-derived growth factor, Dev. Brain Res 55 (1990) 275–282. 10.1016/0165-3806(90)90209-H. [DOI] [PubMed] [Google Scholar]
- [83].Lin G, Mela A, Guilfoyle EM, Goldman JE, Neonatal and adult O4+ oligodendrocyte lineage cells display different growth factor responses and different gene expression patterns, J. Neurosci. Res 87 (2009) 3390–3402. 10.1002/jnr.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Spitzer SO, Sitnikov S, Kamen Y, Evans KA, Kronenberg-Versteeg D, Dietmann S, de Faria O, Agathou S, Káradóttir RT, Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age, Neuron. 101 (2019) 459–471. 10.1016/J.NEURON.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Van Heyningen P, Calver AR, Richardson WD, Control of progenitor cell number by mitogen supply and demand, Curr. Biol 11 (2001) 232–241. 10.1016/S0960-9822(01)00075-6. [DOI] [PubMed] [Google Scholar]
- [86].Zhang H, Miller RH, Density-dependent feedback inhibition of oligodendrocyte precursor expansion, J. Neurosci 16 (1996) 6886–6895. 10.1523/jneurosci.16-21-06886.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gudz TI, Komuro H, Macklin WB, Glutamate stimulates oligodendrocyte progenitor migration mediated via an av integrin/myelin proteolipid protein complex, J. Neurosci 26 (2006) 2458–2466. 10.1523/JNEUROSCI.4054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bergles DE, Richardson WD, Oligodendrocyte development and plasticity, Cold Spring Harb. Perspect. Biol 8 (2016) a020453. 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Chari DM, Crang AJ, Blakemore WF, Decline in rate of colonization of oligodendrocyte progenitor cell (OPC)-depleted tissue by adult OPCs with age, J. Neuropathol. Exp. Neurol 62 (2003) 908–916. 10.1093/jnen/62.9.908. [DOI] [PubMed] [Google Scholar]
- [90].Franklin RJM, Blakemore WF, To what extent is oligodendrocyte progenitor migration a limiting factor in the remyelination of multiple sclerosis lesions?, Mult. Scler 3 (1997) 84–87. 10.1177/135245859700300205. [DOI] [PubMed] [Google Scholar]
- [91].Milner R, Anderson HJ, Rippon RF, Mckay JS, Franklin RJM, Marchionni MA, Reynolds R, Ffrench-Constant C, Contrasting effects of mitogenic growth factors on oligodendrocyte precursor cell migration, Glia. 19 (1997) 85–90. . [DOI] [PubMed] [Google Scholar]
- [92].Shields SA, Gilson JM, Blakemore WF, Franklin RJM, Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination, Glia. 28 (1999) 77–83. . [DOI] [PubMed] [Google Scholar]
- [93].Sim FJ, Zhao C, Penderis J, Franklin RJM, The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation., J. Neurosci 22 (2002) 2451–2459. https://doi.org/20026217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhao C, Li WW, Franklin RJM, Differences in the early inflammatory responses to toxin-induced demyelination are associated with the age-related decline in CNS remyelination, Neurobiol. Aging 27 (2006) 1298–1307. 10.1016/j.neurobiolaging.2005.06.008. [DOI] [PubMed] [Google Scholar]
- [95].Hinks GL, Franklin RJM, Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats, Mol. Cell. Neurosci 16 (2000) 542–556. 10.1006/mcne.2000.0897. [DOI] [PubMed] [Google Scholar]
- [96].Neumann B, Baror R, Zhao C, Segel M, Dietmann S, Rawji KS, Foerster S, McClain CR, Chalut K, van Wijngaarden P, Franklin RJM, Metformin Restores CNS Remyelination Capacity by Rejuvenating Aged Stem Cells, Cell Stem Cell. 25 (2019) 473–485. 10.1016/j.stem.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Moyon S, Dubessy AL, Aigrot MS, Trotter M, Huang JK, Dauphinot L, Potier MC, Kerninon C, Parsadaniantz SM, Franklin RJM, Lubetzki C, Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration, J. Neurosci 35 (2015) 4–20. 10.1523/JNEUROSCI.0849-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].de la Fuente AG, Queiroz RML, Ghosh T, McMurran CE, Cubillos JF, Bergles DE, Fitzgerald DC, Jones CA, Lilley KS, Glover CP, Franklin RJM, Changes in the oligodendrocyte progenitor cell proteome with ageing, Mol. Cell. Proteomics (2020). 10.1074/mcp.ra120.002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Baldassarro VA, Marchesini A, Giardino L, Calzà L, Differential effects of glucose deprivation on the survival of fetal versus adult neural stem cells-derived oligodendrocyte precursor cells, Glia. 68 (2020) 898–917. 10.1002/glia.23750. [DOI] [PubMed] [Google Scholar]
- [100].Rao VTS, Khan D, Cui QL, Fuh SC, Hossain S, Almazan G, Multhaup G, Healy LM, Kennedy TE, Antel JP, Distinct age and differentiation-state dependent metabolic profiles of oligodendrocytes under optimal and stress conditions, PLoS One. 12 (2017). 10.1371/journal.pone.0182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Cui QL, Khan D, Rone M, Rao VTS, Johnson RM, Lin YH, Bilodeau PA, Hall JA, Rodriguez M, Kennedy TE, Ludwin SK, Antel JP, Sublethal oligodendrocyte injury: A reversible condition in multiple sclerosis?, Ann. Neurol 81 (2017) 811–824. 10.1002/ana.24944. [DOI] [PubMed] [Google Scholar]
- [102].Marques S, Zeisel A, Codeluppi S, Van Bruggen D, Falcão AM, Xiao L, Li H, Häring M, Hochgerner H, Romanov RA, Gyllborg D, Muñoz-Manchado AB, La Manno G, Lönnerberg P, Floriddia EM, Rezayee F, Ernfors P, Arenas E, Hjerling-Leffler J, Harkany T, Richardson WD, Linnarsson S, Castelo-Branco G, Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system, Science. 352 (2016) 1326–1329. 10.1126/science.aaf6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Moyon S, Frawley R, Marshall-Phelps KLH, Kegel L, Bøstrand SMK, Sadowski B, Huang D, Jiang Y-H, Lyons D, Möbius W, Casaccia P, TET1-mediated DNA hydroxy-methylation regulates adult remyelination, BioRxiv. (2020). 10.1101/819995. [DOI] [Google Scholar]
- [104].Zhou J, chao Wu Y, jun Xiao B, dong Guo X, xin Zheng Q, Wu B, Age-related Changes in the Global DNA Methylation Profile of Oligodendrocyte Progenitor Cells Derived from Rat Spinal Cords, Curr. Med. Sci 39 (2019) 67–74. 10.1007/s11596-019-2001-y. [DOI] [PubMed] [Google Scholar]
- [105].Green AJ, Gelfand JM, Cree BA, Bevan C, Boscardin WJ, Mei F, Inman J, Arnow S, Devereux M, Abounasr A, Nobuta H, Zhu A, Friessen M, Gerona R, von Büdingen HC, Henry RG, Hauser SL, Chan JR, Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial, Lancet. 390 (2017) 2481–2489. 10.1016/S0140-6736(17)32346-2. [DOI] [PubMed] [Google Scholar]
- [106].Franklin RJM, Ffrench-Constant C, Edgar JM, Smith KJ, Neuroprotection and repair in multiple sclerosis, Nat. Rev. Neurol 8 (2012) 624–634. 10.1038/nrneurol.2012.200. [DOI] [PubMed] [Google Scholar]
- [107].Franklin RJM, Ffrench-Constant C, Regenerating CNS myelin - From mechanisms to experimental medicines, Nat. Rev. Neurosci 18 (2017) 753–769. 10.1038/nrn.2017.136. [DOI] [PubMed] [Google Scholar]
- [108].Aylward GP, Neurodevelopmental outcomes of infants born prematurely, J. Dev. Behav. Pediatr 26 (2005) 394–407. 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- [109].The Merck Manual of Diagnosis and Therapy, 18th edition, Clin. Exp. Optom 90 (2007) 64–66. 10.1111/j.1444-0938.2007.00102.x. [DOI] [Google Scholar]
- [110].Love S, Demyelinating diseases, J. Clin. Pathol 59 (2006) 1151–1159. 10.1136/jcp.2005.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Copray S, Huynh JL, Sher F, Casaccia-Bonnefil P, Boddeke E, Epigenetic mechanisms facilitating oligodendrocyte development, maturation, and aging, Glia. 57 (2009) 1579–1587. 10.1002/glia.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Shen S, Liu A, Li J, Wolubah C, Casaccia-Bonnefil P, Epigenetic memory loss in aging oligodendrocytes in the corpus callosum, Neurobiol. Aging 29 (2008) 452–463. 10.1016/j.neurobiolaging.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Jin J, Peng Q, Hou Z, Jiang M, Wang X, Langseth AJ, Tao M, Barker PB, Mori S, Bergles DE, Ross CA, Detloff PJ, Zhang J, Duan W, Early white matter abnormalities, progressive brain pathology and motor deficits in a novel knock-in mouse model of Huntington’s disease, Hum. Mol. Genet 24 (2015) 2508–2527. 10.1093/hmg/ddv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, Rothstein JD, Bergles DE, Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis, Nat. Neurosci 16 (2013) 571–579. 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].McKenzie AT, Moyon S, Wang M, Katsyv I, Song WM, Zhou X, Dammer EB, Duong DM, Aaker J, Zhao Y, Beckmann N, Wang P, Zhu J, Lah JJ, Seyfried NT, Levey AI, Katsel P, Haroutunian V, Schadt EE, Popko B, Casaccia P, Zhang B, Multiscale network modeling of oligodendrocytes reveals molecular components of myelin dysregulation in Alzheimer’s disease, Mol. Neurodegener 12 (2017) 82. 10.1186/s13024-017-0219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Narayanan M, Huynh JL, Wang K, Yang X, Yoo S, McElwee J, Zhang B, Zhang C, Lamb JR, Xie T, Suver C, Molony C, Melquist S, Johnson AD, Fan G, Stone DJ, Schadt EE, Casaccia P, Emilsson V, Zhu J, Common dysregulation network in the human prefrontal cortex underlies two neurodegenerative diseases, Mol. Syst. Biol 10 (2014) 743. 10.15252/msb.20145304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wang M, Roussos P, McKenzie A, Zhou X, Kajiwara Y, Brennand KJ, De Luca GC, Crary JF, Casaccia P, Buxbaum JD, Ehrlich M, Gandy S, Goate A, Katsel P, Schadt E, Haroutunian V, Zhang B, Integrative network analysis of nineteen brain regions identifies molecular signatures and networks underlying selective regional vulnerability to Alzheimer’s disease, Genome Med. 8 (2016) 104. 10.1186/s13073-016-0355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Nave KA, Ehrenreich H, Myelination and oligodendrocyte functions in psychiatric diseases, JAMA Psychiatry. 71 (2014) 582–584. 10.1001/jamapsychiatry.2014.189. [DOI] [PubMed] [Google Scholar]
- [119].Ehrenreich H, Mitjans M, Van Der Auwera S, Centeno TP, Begemann M, Grabe HJ, Bonn S, Nave KA, OTTO: A new strategy to extract mental disease-relevant combinations of GWAS hits from individuals, Mol. Psychiatry 23 (2018) 476–486. 10.1038/mp.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]


