Abstract
Background and purpose:
Randomised-controlled clinical trials (the ANCHOR and MARINA) examined the intravitreal anti-vascular endothelial growth factor (anti-VEGF) efficacy for eyes having fluorescein angiographic classic and occult (OCC) neovascular lesions. No significant difference in the treatment response between the lesion types was observed. Fundus fluorescein angiography and optical coherence tomography (OCT) are complementary devices that provide information about neovascular age-related macular degeneration (n-AMD). The aim of this retrospective study was to compare the clinical aspects of fluorescein angiographic characteristics in predominantly classic (PDC) and OCC subtypes of n-AMD treated with intravitreal ranibizumab.
Methods:
Treatment-naive fluorescein angiographic OCC-n-AMD and PDC-n-AMD patients, who received monthly intravitreal ranibizumab for 3 months after baseline, and were followed-up with pro re nata injections between March 2013 and February 2018, were included. Means of the visual acuity (VA), central macular thickness (CMT), and intravitreal injection and visit numbers of the groups were compared throughout 24 months.
Results:
We included 41 eyes of PDC-n-AMD patients and 36 eyes of OCC-n-AMD patients. The mean ages were 74.5 ± 10.6 and 71.9 ± 9.4, respectively. The baseline, and 3-, 6-, 12-, 18-, and 24-month VA results of the OCC group were significantly better than those in the PDC. However, VA gain in the PDC group at 3, 6, and 12 months was significantly higher than that in the OCC group. The mean of baseline CMT of the PDC (353 ± 118 µm) was significantly higher than that in the OCC group (293 ± 64 µm). No significant differences in terms of the number of visits or injections, or CMT change from the baseline values between groups were observed.
Conclusion:
The OCC-n-AMD patients had better baseline and follow-up VA and CMT means than the PDC-n-AMD patients. However, the PDC-n-AMD patients are expected to benefit more than the OCC-n-AMD patients in terms of VA gains.
Keywords: age-related macular degeneration, classical, fluorescein angiography, neovascular, occult, ranibizumab
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in individuals over the age of 50 years. It was predicted that by 2020, three million individuals would be affected in the United States.1 AMD is a progressive and degenerative disease that mostly affects the macula. Abnormal growth of choroidal vessels in AMD causes leakage, haemorrhage, fibrosis, and scarring, and is known as neovascular AMD (n-AMD) or wet AMD, as well as choroid neovascularisation.2,3
Despite the quality of configuration of n-AMD lesions in spectral-domain optical coherence tomography (OCT) scans, fundus fluorescein angiography is still a clinical routine for confirmation of an n-AMD diagnosis. Moreover, fundus fluorescein angiography is essential for exploration of the diagnostic accuracy of OCT scans and determination of the types of n-AMD.4
Commonly, n-AMD is classified according to the anatomical location of its origin, which is assessed by OCT scans in clinical practice. In type 1 neovascularisation, the new vessels originate from choriocapillaris and pass through a Bruch membrane defect into the subretinal pigment epithelium (RPE) layer. In type 2, the neovascularisation originates between the RPE and neurosensorial retina. Finally, in type 3, the neovascularisation develops from deep capillary plexus towards the RPE. Moreover, the fundus fluorescein angiography patterns of the n-AMD subtypes are classified as occult (OCC), minimally and predominantly classic (PDC), and also in some combination of both. The fluorescein angiographic appearance of classic n-AMD typically corresponds to type 2 neovascularisation, and OCC n-AMD is more consistent with type 1. Another type of n-AMD is intraretinal angiomatous proliferation over the RPE, which is classified as type 3 neovascularisation.5
Vascular endothelial growth factor-A (VEGF-A) has been found to be responsible for diabetic retinopathy with macular oedema and n-AMD-related choroid neovascularisations.6 Ranibizumab (Lucentis, Genentech) is an US Food and Drug Association (FDA)-approved, recombinant, humanised monoclonal antibody fragment (Fab) of active VEGF-A forms, and has been shown to be effective in the treatment of n-AMD.7,8
Fundus fluorescein angiography and OCT are complementary devices that provide information regarding n-AMD lesions. OCT images allow an improved understanding of the anatomical characteristics of the fundus fluorescein angiography defined n-AMD lesion subtypes.9 Fundus fluorescein angiography is still indispensable in order to determine the prognosis of n-AMD; however, the existing classifications are not thought to be predictive as required. Hence, recent classifications have been proposed in order to be guided more accurately in n-AMD management.10
The intravitreal anti-VEGF efficacy of ranibizumab treatment for eyes with classic and OCC neovascular lesions were examined in comprehensive randomised controlled clinical trials (the ANCHOR and MARINA); however, no significant differences were observed between the two lesion types (the MARINA trial).7,11 The aim of this study was to compare the clinical courses of fluorescein angiographic PDC and OCC n-AMD patients who received intravitreal ranibizumab therapy in a real-life setting over a period of 24 months.12
Methods
Ethics statement
All procedures performed in investigations involving human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards, and the study was approved by Ankara Numune Training and Research Hospital Ethics Committee (No: E-18-1810). The data were retrospectively collected from the patient records of participants who had provided a written, informed consent.
Selection of the patients
Patients above 50 years of age who had been referred to the Retina Service with an n-AMD diagnosis at Ankara Numune Training and Research Hospital, between March 2013 and February 2018, and received the three monthly loading doses of intravitreal ranibizumab treatment and had records of follow-up for 6 months after the first intravitreal injection were included. Subjects with a history of diabetic retinopathy, retinal vascular occlusion, moderate to severe hypertensive retinopathy, according to the Wong and Mitchell Hypertensive Retinopathy Classification,13 vitreous interface disorders, vitreous haemorrhage, degenerative myopia, anterior or posterior uveitis, malignancy, glaucoma, corneal ectatic disease, hereditary retinal dystrophy, previous history of trauma or retinal photocoagulation or any other cause of intravitreal injection were not included in the study.
Study design
Early Treatment Diabetic Retinopathy Study (ETDRS) letters for visual acuity (VA), anterior segment and fundus examination findings, and OCT scans using a Zeiss Cirrus HD-OCT 5000 (Carl Zeiss Meditec AG, Jena, Germany) for macular morphology and thickness (µm) of all of the patients were assessed at each scheduled visit. The OCT images were analysed using the Macular Cube database, and their reports were used to assess the retinal images. OCT scans with scan quality and central macular thickness (CMT) report values above 3 were included in the study.
The treatment decisions were given according to the pro re nata (as needed) treatment protocol after three monthly loading doses of ranibizumab therapy.14 An appointment system was used to perform all of the intravitreal injections within 1 to 3 days after the last visit, in an operating room. The patients were then recalled at 1-month intervals. Re-treatment with the intravitreal anti-VEGF was planned when the CMT was ⩾300 µm on the OCT scans with the remaining intraretinal and/or subretinal fluid or new retinal/subretinal haemorrhage associated with the n-AMD lesion at the fundus examination of the follow-up visit after completion of the loading doses. The n-AMD diagnosis was also confirmed with a digital fluorescein fundus angiography using a Visucam 500 (Carl Zeiss Meditec) in newly diagnosed eyes with intraretinal and/or subretinal fluid in OCT.
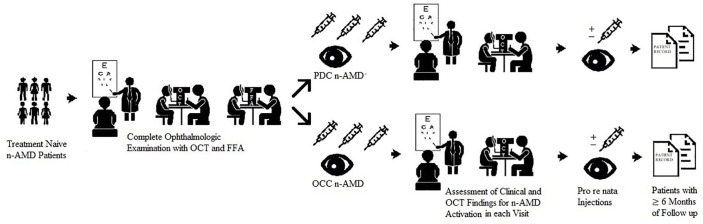
All of the patients underwent fluorescein angiography with 5 mL of 10% intravenous fluorescein solution at baseline. Each of the early, mid, and late phases of the angiography was analysed. The n-AMD subtypes were classified according to the fluorescein angiography images as classic or OCC. Classic lesions were defined as lacy, hyperfluorescent lesions that emerged in the early phase of the angiography and leaked progressively along with the late phases. These were also subclassified as PDC (classic component over 50% of the total lesion size) and minimally classic (classic component less than 50% of the total lesion size). OCC lesions are formed by late leakages from an undetermined source or fibrovascular or vascularised serous RPE detachment.2,5 A schematic description and representation of the fluorescein angiographic classification of the study are given in Figures 1 and 2, respectively.
Figure 1.
A descriptive scheme summarising the study design.
Figure 2.
Representative images of the fluorescein angiographic PDC and OCC n-AMDs, respectively. (a–d) Colour, and early, mid, and late phase representatives for the fundus fluorescein angiography of the PDC types, respectively. (e–h) Colour, and early, mid, and late phase representatives of the fundus fluorescein angiography of the OCC types, respectively.
The VA, CMT, mean number of visits, and number of intravitreal injections, in each period, were compared statistically for the PDC and OCC groups throughout the 24 months.
Statistical analysis
Statistical analyses were performed using SPSS Statistics 17.0 for Windows (SPSS Inc., Chicago, IL). The normality of the data was determined using the Shapiro–Wilk test. The independent samples t-test and Mann–Whitney U-test were used to compare the demographic data, VA, CMT, number of visits, and intravitreal injections between the two groups. After the intravitreal treatments, the related samples Wilcoxon signed rank test was used to determine the statistical significance of the changes within groups in terms of the VA and CMT. A chi-square test was used to analyse the statistical significance value of bilateral presentation of n-AMD in each group. The confidence interval level was set to 95%, where any output of p < 0.05 was accepted as statistically significant. The average number of visits and injections at 3, 6, 12, 18 and 24 months was given as the mean ± SD.
Results
Demographic data
The PDC group included 41 eyes of 40 (25 males, 15 female) patients, and 36 eyes of 32 patients (18 males, 14 females) were included in the OCC group. The mean age of the patients was 74.5 ± 10.6 years in the PDC group, and 71.9 ± 9.4 years in the OCC group. There was no significant difference in the age distribution between groups (p = 0.266, independent samples t-test). The OCC group was presented with frequent bilateral n-AMD cases than the PDC group, but there was no significant between groups (11 vs 2.4%, respectively, p = 0.141, chi-square test).
When the patients were asked about systemic diseases, 16 had systemic hypertension, 9 had comorbidity of diabetes and systemic hypertension without any diabetic retinopathy findings, and two had rheumatoid arthritis. Only 8 of the totally 25 systemic hypertension patients had mild hypertensive retinopathy changes according to the Wong and Mitchell Hypertensive Retinopathy Classification.13
Course of the VA
VA is the first quantitative parameter to assess the efficiency of the anti-VEGF treatment. The VA means of the PDC and OCC groups (with ETDRS letters) throughout the 24 months are given in Figure 3(a).
Figure 3.
(a) Diagram of the VA (ETDRS letters) of the PDC and OCC types of n-AMD after receiving anti-VEGF treatment throughout the 24 months. (b) Diagram of CMT of the PDC and OCC types of n-AMD after receiving anti-VEGF treatment throughout the 24 months.
The baseline, 3-, 6-, 12-, 18- and 24-month VA results of the groups were compared statistically, and the OCC group was observed to have significantly better VA results than the PDC group at all of the cut-off periods (p < 0.001, p = 0.005, p = 0.002, p = 0.001, p = 0.005, and p = 0.02, respectively). When compared to the baseline VA values, the PDC group had significant increases at 3, 6 and 12 months (p = 0.001, p = 0.002, p = 0.016, p = 0.107, and p = 0.259, respectively). Using the related samples Wilcoxon signed rank test, however, no significant changes were observed in the OCC group (P = 0.226, P = 0.733, P = 0.794, P = 0.327, and P = 0.392, respectively). The PDC and OCC groups were also compared in terms of the change in VA (VA gain) from the baseline values, and the change of VA of the PDC group at 3, 6, and 12 months was statistically significant (P = 0.001, P = 0.02, P = 0.016, P = 0.107, and P = 0.259, respectively). However, no significant change of the VA from baseline was observed in the OCC group (P = 0.226, P = 0.733, P = 0.794, P = 0.327 and P = 0. 392, respectively)
Course of the CMT in OCT
The other quantitative parameter was the measurement of the resolution of oedema, which was assessed as the CMT on the OCT, to assess the intravitreal anti-VEGF treatment efficiency. Figure 3(b) presents a diagram of the CMT means of the n-AMD groups throughout the 24 months. The time-varying CMT changes of the groups when compared to the baseline are presented in Table 1. All of the time-varying OCT results within the PDC and OCC groups were significantly lower than the baseline values. The CMT of the baseline, 3-, 6-, 12-, 18- and 24-month means were also compared between the groups (p = 0.025, p = 0.320, p = 0.674, p = 0.309, p = 0.838, and p = 0.423, respectively). The baseline CMT of the PDC group was significantly higher than that of the OCC group. Figure 4 is a representative for the lesion types (PDC or OCC) based on changes in the macular OCT images of the patients, which were presented in Figure 2. No significant differences were observed between groups in terms of change in the CMT from the baseline values throughout 18 months. However, the 24-month mean change of CMT from baseline was significantly higher than that of OCC (p = 0.153, p = 0.107, p = 0.262, p = 0.573, and p = 0.043 for 3-, 6-, 12, 18-, and 24 months, respectively).
Table 1.
CMT changes in the PDC and OCC n-AMD groups, and the statistical significance of the changes according to the baseline measurements after intravitreal anti-VEGF treatments at the follow-up visits.
| Month | n-AMD subtype | N | CMT Mean (µm) | SD | CMT Min | CMT Max | p * | p ** |
|---|---|---|---|---|---|---|---|---|
| Baseline | PDCa | 41 | 353 | 118 | 208 | 749 | − | − |
| OCCb | 36 | 293 | 64 | 142 | 420 | − | − | |
| 3 | PDC | 41 | 260 | 56 | 155 | 385 | <0.001 | − |
| OCC | 36 | 245 | 48 | 166 | 395 | − | 0.001 | |
| 6 | PDC | 39 | 264 | 75 | 147 | 567 | <0.001 | |
| OCC | 33 | 277 | 127 | 173 | 925 | − | 0.045 | |
| 12 | PDC | 35 | 280 | 80 | 172 | 523 | <0.001 | − |
| OCC | 32 | 255 | 46 | 190 | 366 | − | 0.004 | |
| 18 | PDC | 31 | 291 | 133 | 114 | 802 | 0.026 | − |
| OCC | 29 | 266 | 78 | 182 | 567 | − | 0.012 | |
| 24 | PDC | 28 | 243 | 71 | 161 | 411 | 0.002 | − |
| OCC | 27 | 254 | 57 | 164 | 351 | − | 0.016 |
CMT, central macular thickness; OCC, occult; n-AMD, neovascular age-related macular degeneration; PDC, predominantly classic; VEGF, vascular endothelial growth factor.
N: eyes meet the spectral-domain OCT-A scan quality requirements in the follow-up visits.
PDC n-AMD.
OCC n-AMD.
CMT changes when compared to baseline in the PDC n-AMD group using the Wilcoxon signed rank test for statistical significance, p < 0.05. **CMT change when compared to baseline in the OCC n-AMD group, using the Wilcoxon signed rank test, p < 0.05.
Figure 4.
Spectral-domain OCT scans and VA (ETDRS letters) changes throughout the 24 months in the PDC and OCC n-AMD patients, which were presented in Figure 2. (a–f) Baseline, and 3-, 6-, 12-, 18-, and 24-month macular OCT and VA values of the PDC n-AMD patients. (g–l) Baseline, and 3-, 6-, 12-, 18-, and 24-month macular OCT and VA values of the OCC n-AMD patients.
Assessment of the intravitreal injections
The treatment frequencies of the groups throughout the 24 months are given in Figure 5(a). No statistically significant differences were determined in terms of intravitreal ranibizumab treatment between the n-AMD types throughout 24 months (p = 0.625, p = 0.268, p = 0.761, p = 0.978, and p = 0.453 for 3-, 6-, 12, 18- and 24-month, respectively).
Figure 5.
(a) Mean number of injections in the PDC and OCC n-AMD groups at 3, 6, 12, 18 and 24 months, respectively. (b) Mean total number of visits in the PDC and OCC n-AMD groups at 3, 6, 12, 18 and 24 months, respectively.
All of the intravitreal treatment agents were tolerated well and none of these had procedure-related serious adverse events. Only three eyes in the OCC group and five in the PDC group had a temporary increase in intraocular pressure (range: 24–30 mmHg), which were all below 21 mmHg on day 3 postoperatively.
Assessment of the number of visits
The average of number of visits in each group throughout the 24 months is given in Figure 5(b). Moreover, there were no significant differences between the n-AMD groups regarding the number of visits throughout 24 months (p = 0.109, p = 0.562, p = 0.787, p = 0.658 and p = 0.672, respectively).
Discussion
Anti-VEGF therapy became the standard treatment after the discovery of the role of VEGF-A pathogenesis, and many randomised controlled studies with large sample sizes have indicated the efficiency of anti-VEGF agents in n-AMD.6,7,15 In this study, the role of the fluorescein angiographic pattern of PDC and OCC was explored via the assessment of baseline and follow-up clinical data. To the best of our knowledge, this is the first real-life study based on comparing the clinical courses of fluorescein angiographic PDC and OCC throughout a 24-month period.
According to the given VA results, it was obvious that anti-VEGF treatment in n-AMD treatment saved and prevented the worsening of the VA in the PDC and OCC groups. Moreover, VA gains were observed in both of the groups during the follow-up period. Although, there was statistical insignificance in the course of VA in OCC group, a significant increase in VA was observed in the PDC group throughout the 12 months.
Jonas and colleagues16 reported a case series about n-AMD subtypes in which the methodology was close to that of this study. They reported that improvement in VA did not differ markedly after intravitreal anti-VEGF treatment (bevacizumab) between various types of sub-foveal n-AMDs. However, in their 2-year study, the OCC n-AMD patients presented with significantly better baseline and follow-up VA means when compared to the PDC n-AMD patients. However, the VA gain in the PDC group, especially in the first year, was superior to that of the VA change in the OCC group after the ranibizumab therapy. Another close classification to this study was previously reported by Potter and colleagues;17 however, they investigated the role of photodynamic therapy and reported the loss of an average of 8.7 letters (1.9 lines) in the OCC n-AMD group and an average of 10 ETDRS letters (2 Snellen lines) in the PDC n-AMD group over 12 months. The VA gain in the PDC group and visual stabilisation in the OCC group showed that anti-VEGFs brought more successful outcomes than photodynamic therapy in OCC and PDC types according to the first-year results. Furthermore, 1- and 2-year results of the ANCHOR study (PDC patients were included) are comparable to the results of the PDC group of this study. Patients gained a mean of 11.3 and 10.7 versus 10.8 and 8.3 letters in the 12th and 24th months of the treatments, respectively.11,18
The initial and final VA means were better in the OCC group, suggesting that OCC n-AMD lesions have benign prognosis when compared to PDC n-AMD lesions. n-AMDs were first classified by Gass according to the location of the neovascular complex, based on the relationship of the RPE. In the type-1 classification, the neovascular complex is confined under the RPE, while the subretinal proliferated neovascular vessels of the complex exceed the RPE in type 2.19 Freund and colleagues made a modified classification based on multimodal imaging of fluorescein angiography, OCT and indocyanine green angiography. In addition to type 1, which was defined as OCC, and type 2, demonstrating classic n-AMDs, they asserted type 3, which represents the lesions of retinal angiomatous proliferation.5 Nearly 50% of the eyes enrolled in the Comparison of Age-related Macular Degeneration Treatments Trials study developed scars over a 2-year period. Eyes with a classic n-AMD pattern and a thicker retina, with more neovascular tissue or fluid under the central fovea, were more likely to present with a scar. Of the eyes, 20.6% had nonfibrotic scars and 24.7% had fibrotic scars. Moreover, VA of less than 20/40 and eyes with a larger n-AMD lesion size were the baseline risk factors to develop fibrotic scars.20,21 Furthermore, the percentages of subretinal fibrosis (92.7% vs 30%) and ellipsoid zone atrophy (29.3% vs 20%) were higher among the classic n-AMD patients.10 In addition, subjects with fibrotic scars presented with the lowest VA means and were the least likely to develop any type of scars in OCC n-AMD eyes.21 Thus, according to the given evidence from previous studies, the lesions of PDC n-AMD patients extend into the intra-retinal layers and affect the photoreceptors and/or neural retina and develop fibrosis, and these patients present with worse VA and CMT values.
Herein, the mean number of injections was 6 ± 2.3 and 6.1 ± 2.1 in the first year in the OCC and PDC groups, respectively. Vardarinos and colleagues22 reported 7.75 ± 1.3 injections in their treat- and extend-based study, and Ozkaya and colleagues23 reported 3.9 ± 1.0 pro re nata injections in their first-year results. The mean number of injections was higher in the treat and extend protocols than in the pro re nata protocols, as is expected in real-life circumstances as well. In this study, there was no significant difference between the groups in terms of the number of injections throughout the 2 years. Thus, the intravitreal anti-VEGF treatment was standardised across the groups to compare them in terms of the clinical findings. Moreover, both the PDC and OCC n-AMD groups required similar pro re nata anti-VEGF treatments.
The mean number of visits was 9.3 ± 1.8 and 9.4 ± 2 in the first year, and 15 ± 3.7 and 15.4 ± 3.6 at the end of the second year in PDC and OCC n-AMDs, respectively. When the mean number of visits was compared with the mean number of injections, each patient received 1 intravitreal injection per 1.52 and 1.56 visits in the first year, and 3.1 and 2.1 visits in the second year, respectively. The injection requirement of the n-AMD lesions decreased in both of the groups over time. Moreover, there was no significant difference in terms of the number of visits between the PDC and OCC n-AMD groups over time. Abedi and colleagues reported a mean of 8.6 ± 1.1 treat and extend injections (and visits) in the first year and 5.6 ± 2.0 in the second year. Thus, conducting a treat and extend injection regimen might provide predictability for intravitreal injections, especially in the second year of follow-up.24
The mean CMT changes in the OCT scans when compared to baseline were all statistically significant after initiation of the anti-VEGF therapy. According to the CMT changes in the study, the first 3 months of the intravitreal anti-VEGF treatment in n-AMD treatment was the most determinant period to monitor the anatomical morphology. The mean baseline CMT in the PDC group was significantly higher than that in the OCC group, and the following CMT means in the PDC group showed more fluctuation when compared to the OCC group over the 24 months. In addition, the 24-month change of CMT from baseline was significantly higher than the CMT change of OCC group. These results were also comparable with the study of Daniel and colleagues,21 who reported that patients with classic n-AMD presented with larger lesion diameters, foveal subretinal fluid, and increased risk of scar formation.
There is still a large proportion of n-AMD patients with ambiguous findings according to current fluorescein angiographic classification. After the daily practice of indocyanine green angiography, polypoidal choroidal vasculopathy and retinal angiomatous proliferation were added to the n-AMD sub-classification.25,26 Polypoidal choroidal vasculopathy has early subretinal focal indocyanine green angiography hyper-fluorescence with a definite polyp. Polypoidal choroidal vasculopathy and retinal angiomatous proliferation lesions mainly emerge with the OCC type of fundus fluorescein angiography characteristics (66% and 56%, respectively). However, they may also display classic angiographic features (34% and 13%, respectively).10 In addition, recently introduced micro-aneurysmal choroidal vasculopathies had focal hyperfluorescent areas in the early phase of indocyanine green angiography and mostly showed OCC fundus fluorescein angiography characteristics (83.5%), and they were also thought to be a variant of polypoidal choroidal vasculopathy.10 A recent fluorescein angiographic comprehensive study came up with a novel n-AMD classification model, and the authors investigated the anti-VEGF treatment efficiency according to the fundus fluorescein angiography classification of the lesions (OCC and classic) over 12 months. They reported compatible VA results to that of this study.10
The limitations of the study can be described as follows: the sample size of both groups was limited and minimally classic fluorescein angiographic n-AMD patients could not be evaluated due to their infrequency in the records, and there were only six eyes in which a statistical interpretation was unable to be made. Moreover, minimally classic fluorescein angiographic n-AMD was not included or classified under the title of classic n-AMD, so as to prevent any potential bias that originated from the lesion character. In addition, follow-up losses were observed in both of the groups throughout the 24 months.
In conclusion, with a standard anti-VEGF treatment (ranibizumab), the OCC n-AMD patients presented with better baseline and final VA means, and CMT measurements than the PDC n-AMD patients throughout the 24 months. It is foreseen that patients of n-AMD that at baseline showed better VA and smaller CMT will also have better VA and smaller CMT at the end of 24 months with anti-VEGF treatment. There were no significant differences in terms of the number of injections or visits between the groups. It could be also predicted that the PDC n-AMD patients responded better to the intravitreal ranibizumab therapy in terms changes in VA, even though the injection frequencies were similar. Despite recent advances in retinal imaging technology, fluorescein angiography remains not only a diagnostic tool, but also a prognostic marker in n-AMD management. Future long-term prospective studies with comprehensive participation, comprising similar clinical characteristics and including auxiliary imaging devices to define n-AMD lesion groups, would be useful for understanding the issue better.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Cemal Çavdarlı  https://orcid.org/0000-0001-8379-4384
https://orcid.org/0000-0001-8379-4384
Contributor Information
Cemal Çavdarlı, Department of Ophthalmology, Ankara City Hospital, University of Health Sciences, Bilkent, Çankaya, Ankara 06800, Turkey.
Sebile Çomçalı, Department of Ophthalmology, Ankara City Hospital, University of Health Sciences, Ankara, Turkey.
Pınar Topcu Yılmaz, Department of Ophthalmology, Ankara City Hospital, University of Health Sciences, Ankara, Turkey.
Mehmet Numan Alp, Department of Ophthalmology, Ankara City Hospital, University of Health Sciences, Ankara, Turkey.
References
- 1. Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA 2004; 291: 1900–1901. [DOI] [PubMed] [Google Scholar]
- 2. Novack GD. Pharmacotherapy for the treatment of choroidal neovascularization due to age related macular degeneration. Annu Rev Pharmacol Toxicol 2008; 48: 61–78. [DOI] [PubMed] [Google Scholar]
- 3. Nowak JZ. Age related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep 2006; 58: 353–363. [PubMed] [Google Scholar]
- 4. Mathew R, Pefkianaki M, Kopsachilis N, et al. Correlation of fundus fluorescein angiography and spectral-domain optical coherence tomography in identification of membrane subtypes in neovascular age-related macular degeneration. Ophthalmologica 2014; 231: 153–159. [DOI] [PubMed] [Google Scholar]
- 5. Freund KB, Zweifel SA, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina 2010; 30: 1333–1349. [DOI] [PubMed] [Google Scholar]
- 6. Witmer AN, Vrensen GF, Van Noorden CJ, et al. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 2003; 22: 1–29. [DOI] [PubMed] [Google Scholar]
- 7. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1419–1431. [DOI] [PubMed] [Google Scholar]
- 8. Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006; 26: 859–870. [DOI] [PubMed] [Google Scholar]
- 9. Liakopoulos S, Ongchin S, Bansal A, et al. Quantitative optical coherence tomography findings in various subtypes of neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 2008; 49: 5048–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bae K, Noh SR, Kang SW, et al. Angiographic subtypes of neovascular age-related macular degeneration in Korean: a new diagnostic challenge. Sci Rep 2019; 9: 9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1432–1444. [DOI] [PubMed] [Google Scholar]
- 12. Cebeci Z, Yilmaz YC, Kir N. Real-life experience of ranibizumab therapy for neovascular age-related macular degeneration from Turkey. Int J Ophthalmol 2018; 11: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med 2004; 351: 2310–2317. [DOI] [PubMed] [Google Scholar]
- 14. Augsburger M, Sarra GM, Imesch P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol 2019; 257: 1889–1895. [DOI] [PubMed] [Google Scholar]
- 15. Lotery A, Griner R, Ferreira A, et al. Real-world visual acuity outcomes between ranibizumab and aflibercept in treatment of neovascular AMD in a large US data set. Eye 2017; 31: 1697–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jonas JB, Libondi T, Ihloff AK, et al. Visual acuity change after intravitreal bevacizumab for exudative age-related macular degeneration in relation to subfoveal membrane type. Acta Ophthalmol Scand 2007; 85: 563–565. [DOI] [PubMed] [Google Scholar]
- 17. Potter MJ, Szabo SM, Li WW. Comparison of visual acuity outcomes in predominantly classic vs occult lesions in age-related macular degeneration treated with photodynamic therapy. Eye 2008; 22: 194–199. [DOI] [PubMed] [Google Scholar]
- 18. Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol 2007; 144: 850–857. [DOI] [PubMed] [Google Scholar]
- 19. Gass JD. Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranes. Am J Ophthalmol 1994; 118: 285–298. [PubMed] [Google Scholar]
- 20. CATT Research Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364: 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daniel E, Toth CA, Grunwald JE, et al. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014; 121: 656–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vardarinos A, Gupta N, Janjua R, et al. 24-month clinical outcomes of a treat-and-extend regimen with ranibizumab for wet age-related macular degeneration in a real-life setting. BMC Ophthalmol 2017; 17: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ozkaya A, Alkin Z, Togac M, et al. Five-year outcomes of ranibizumab in neovascular age-related macular degeneration: real-life clinical experience. Korean J Ophthalmol 2017; 31: 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abedi F, Wickremasinghe S, Islam AF, et al. Anti-VEGF treatment in neovascular age-related macular degeneration: a treat-and-extend protocol over 2 years. Retina 2014; 34: 1531–1538. [DOI] [PubMed] [Google Scholar]
- 25. Ciardella AP, Donsoff IM, Huang SJ, et al. Polypoidal choroidal vasculopathy. Surv Ophthalmol 2004; 49: 25–37. [DOI] [PubMed] [Google Scholar]
- 26. Slakter JS, Yannuzzi LA, Schneider U, et al. Retinal choroidal anastomoses and occult choroidal neovascularization in age-related macular degeneration. Ophthalmology 2000; 107: 742–753. [DOI] [PubMed] [Google Scholar]