Abstract
Identification and prevention of drug-related problems have become the central role of patient-centered pharmacy practitioners. After the initiation of patient-oriented pharmacy service, many studies evaluating magnitude of drug-related problems at facility level in Ethiopia have been conducted, though the extent of the problem at a national level remains unknown. Hence, this systematic review and meta-analysis is undertaken with the aim of quantifying the prevalence of drug-related problems in Ethiopian public healthcare settings using Cipolle/Strand classification system. Electronic databases were searched including PubMed, EMBASE, Web of Science, MEDLINE and HINARI, Google Scholar and ResearchGate for both published and unpublished works. Data on study characteristics and outcomes were extracted using the format developed on Microsoft Excel. The primary measure was the pooled prevalence of drug-related problems. The meta-analysis was conducted using OpenMeta[Analyst].A total of 17 studies were included in this systematic review and meta-analysis. The pooled prevalence of drug-related problems of patients who experienced at least one drug-related problem during their therapy was found to be 69.4% (95% confidence interval: 61.5–77.4). The most frequently reported types of drug-related problems were “need for additional drug and “noncompliance,” together accounting for more than half of the drug-related problems. The most frequently reported factors associated with drug-related problems were patients’ age, polypharmacy, comorbidities and the number days of hospital stay.The prevalence of drug-related problems in Ethiopian public healthcare settings was found to be high. Inconsistent reporting of drug-related problems was observed across the studies. It is imperative to design and implement interventions aimed at reducing drug-related problems. Responsible stakeholders should adopt uniform drug-related problem classification approach to ensure uniform reporting of drug-related problems in Ethiopian healthcare settings
Keywords: Ethiopia, review, drug-related problem, drug therapy
Introduction
The introduction of the concept of pharmaceutical care resulted in an ambition to apply a new philosophy of pharmacy practice.1 According to its creators Hepler and Strand, “Pharmacists must abandon factionalism and adopt patient-centered pharmaceutical care as their philosophy of practice,” as they phrased it and continued “Pharmacy’s re-professionalization will be completed only when all pharmacists accept their social mandate to ensure the safe and effective drug therapy of the individual patient.”2 Pharmaceutical care requires pharmacists to work with other healthcare professionals to assess, initiate, monitor and modify medication use to ensure the safety and effectiveness of drug therapy. Identifying, resolving and preventing drug-related problems (DRPs) are, therefore, considered to be cornerstones in pharmaceutical care.3
DRP refers to any undesirable event experienced by a patient that involves drug therapy and hinders achieving the desired therapeutic goals.4 Many classification systems have been developed to categorize DRPs based on the nature of errors and/or outcomes of the events.5 The ABC classification,6 the American Society of Hospital Pharmacists classification, 7 the Cipolle/Morley/Strand classification,4 the Granada Consensus,8 the Hanlon Approach,9 the Pharmaceutical Care Network Europe (PCNE) classification10 and the Hepler–Strand classification2 are some of the notable DRP classification systems that exist to this day.
The Cipolle/Morley/Strand classification of DRPs has been widely used. According to this system, DRPs are classified into seven categories based on the nature of discrepancies that led to their occurrence: unnecessary drug therapy, need for additional drug therapy, ineffective drug therapy, dose too high, dose too low, adverse drug reaction (ADR) and noncompliance.4 If not resolved, DRPs can result in harmful clinical outcomes ranging from temporary minor symptom exacerbations to permanent disability or death.11 In addition to unwanted clinical outcomes, the economic impact of DRPs is excessive. Additional hospitalizations, law suit costs, infections acquired during hospital visits, lost income, medical and disability expenses cost some countries between US$6 billion and US$29 billion each year.12,13
It is estimated that in the developed countries, 1 in 10 patients is harmed while receiving hospital care and the major causes of the harm are DRPs. In developing countries, the probability of patients being harmed because of DRPs is higher than that in the industrialized nations.13 Countless occurrences of DRPs are encountered every day because of the rapidly expanding array of drug products available, the growing number of diseases being identified and diagnosed, as well as the increasing number of patients entering the healthcare system.14 Furthermore, especially in developing countries, poorly developed healthcare facilities, shortage of well-trained professionals, unavailability and unaffordability of essential drugs as well as a high prevalence of infectious diseases contribute to the higher prevalence of DRPs and associated harmful outcomes.15,16
Patient-related factors such as polypharmacy, comorbidities, prolonged hospital stay and extremes of age have been identified as risk factors for the occurrence of DRPs.14,17,18 Hence, identifying risk factors, timely medication therapy review and taking correction measures for identified DRPs are important to reduce the harmful outcomes of DRPs.19–22 Data about the prevalence of DRPs in a healthcare system would be useful to plan and implement strategies to reduce the incidence and resolve a DRP before it harms the patient. Hence, this systematic review and meta-analysis was undertaken with the aim of quantifying the prevalence of DRPs and identifying commonly encountered types of DRPs in Ethiopian healthcare settings. This is expected to provide insight for the healthcare providers on the extent of DRPs and types of DRPs that need caution during their practice.
Methods
Review protocol
The protocol for the study was developed before the commencement of the review and it is available on reasonable request from the principal author. The Preferred Reporting Items for Systematic review and Meta-analysis (PRISMA) checklist was used for conducting and reporting this review. In addition, we used PRISMA flow diagram to depict the process of identification, screening for eligibility and final inclusion.23
Eligibility criteria
Original research articles that evaluated the magnitude of DRPs were included in the study. There were no restrictions on publication year, but only studies that were written in English and conducted in Ethiopian public healthcare settings were considered for inclusion without time limitation. Studies that identified DRPs using Cipolle/Morley/Strand DRPs classification algorism4 were included to ensure comparability. Studies with insufficient outcome measures or studies with outcomes of interest are missing or vague were excluded.
Data source and search strategy
The search was conducted in January 2020 (while the look for newly published articles continued until the review process was completed) on PubMed, EMBASE, Web of Science, MEDLINE and HINARI using the following keywords and indexing terms: “Drug related problems,” “Drug therapy problems,” “Medication related problems” and “Ethiopia.” Google Scholar and ResearchGate search was also conducted to identify other relevant published and unpublished works including dissertations, institutional repositories and organizational manuals, among others. Boolean operators (AND, OR) and truncation were used when appropriate to increase the number of relevant findings. In addition, we searched for the articles listed in the references of the retrieved articles to identify further relevant studies.
Study selection
The records retrieved from different databases were exported to EndNote version 9. The original articles identified using the search strategy were subject to screening by two reviewers, Y.A. and Z.T., independently after removing duplicate files. Initially, the articles were screened based on the title and abstract. Then the full text of each included based on the title and abstract article was screened for eligibility based on the established criteria.
Data extraction and outcome measurement
A data extraction format was developed on Microsoft Excel to extract the study characteristics and outcomes. Study characteristics such as study setting, study design, year of publication, study subjects, sample size, DRP classification and data source were extracted. The primary outcome measure was the magnitude of DRP prevalence. Moreover, the types of DRPs, the mean number of DRPs and factors associated with DRPs were accessed.
Study quality assessment
The quality of the studies, which fulfilled the inclusion criteria, was checked prior to data extraction using the Joanna Briggs Institute Prevalence Critical Appraisal Tool for prevalence studies.24 The instrument has 10 criteria with “Yes,” “No,” “Unclear” and “Not applicable” options. The mean score of the two authors was used for decision on the inclusion of each article, and studies with less than 50% score were excluded. Furthermore, the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) is used to grade the quality of each article. GRADE method has four levels of quality of evidence: very low, low, moderate and high.25
Data processing and statistical analysis
The extracted data were exported from Microsoft Excel to OpenMeta[Analyst] software for the analysis of outcome measures and sub-grouping. The pooled prevalence of outcomes was calculated assuming DerSimonian-Laird random-effects model at 95% CI. The significance of heterogeneity of the studies was assessed using I2statistics based on I2 percent variation across the studies. The presence of publication bias was assessed using funnel plots. A statistical test with a p value of less than 0.05 was considered significant.
Results
Study selection
To assess the magnitude of DRPs in Ethiopian public healthcare settings, our search identified 488 records, out of which 48 were duplicate titles. After evaluating the articles based on the titles and abstracts, 420 articles were excluded. Twenty-one articles were considered relevant based on titles and abstracts. These were further screened based on the established inclusion criteria and quality assessment. Finally, 17 articles were considered to be of good quality and included in the systematic review (Figure 1).
Figure 1.
PRISMA flow chart of literature search and study inclusion criteria.
Summary of study characteristics
Table 1 depicts the characteristics of the 17 studies included in the present systematic review and meta-analysis. All the studies included were published since 2015 and conducted using cross-sectional study design through medical record review and/or face-to-face patient interview. Regarding the study areas, two studies were conducted in Northern part of Ethiopia,31,36 six were conducted in the capital city, Addis Ababa,14,18,27,34,38,39 and four were from Western Ethiopia, Jimma.26,32,33,35 The remaining were from Southern Ethiopia and Eastern Ethiopia. The lion’s share of studies were conducted on a single disease such as diabetes mellitus30,33,37,39,40 and hypertension.29,36 The sample size in the included studies ranged from 10335 to 418.39 The prevalence of DRPs ranged from 42.3%39 to 88.0%.37 The mean number of DRPs per patient varied from 0.6814 to 2.6.32 The risk of bias was assessed using Joanna Briggs Institute Prevalence Critical Appraisal Tool (JBI) for prevalence studies. Majority of articles included scored at least 70%. In addition, the quality of evidence of each article was evaluated using GRADE approach and most of the articles was rated as low quality (the result for both JBI and GRADE evaluation is provided as supplementary file).
Table 1.
Characteristics of studies conducted on drug-related problems in Ethiopia.
| Studies | Year of publication | Study area | Study setting | Study design | Study subject | Sample size | Source of data | Prevalence of DRPs (%) | Total DRPs | Mean DRPS per patient |
|---|---|---|---|---|---|---|---|---|---|---|
| Yadesa et al.26 | 2015 | Jimma, Western Ethiopia | Medical ward of a single hospital | CS | Patients prescribed with antibiotics | 152 | Record review and patient interview | 75.7 | 206 | 1.4 |
| Sisay et al.a,27 | 2015 | Addis Ababa, Central Ethiopia | Oncology unit of a single hospital | CS | Cancer patients | 367 | Record review | 74.7 | 474 | 1.3 |
| Ayalew et al.a,14 | 2015 | Addis Ababa, Central Ethiopia | Medical ward of a single hospital | CS | All patients | 225 | Record review | 52.0 | 152 | 0.68 |
| Kaleab Gizaw28 | 2017 | Bonga, Southern Ethiopia | Ambulatory clinic of a single hospital | CS | CVS patients | 130 | Record review and patient interview | 72.0 | 163 | 1.5 ± 0.8 |
| Abadir and Daba29 | 2017 | Dire Dawa, Eastern Ethiopia | Ambulatory clinic of a single hospital | CS | HTN patients | 271 | Record review and patient interview | 71.2 | 378 | 1.4 ± 1.3 |
| Hailu et al.30 | 2017 | Wolaita, Southern Ethiopia | Ambulatory clinic of a single hospital | CS | T2DM patients | 243 | Record review and patient interview | 83.1 | 378 | 1.8 ± 0.7 |
| Belayneh et al.31 | 2018 | Dessie, Northern Ethiopia | Medical ward of a single hospital | CS | All patients | 147 | Record review and patient interview | 75.5 | 159 | 1.1 |
| Niriayo et al.32 | 2018 | Jimma, Western Ethiopia | Ambulatory clinic of a single hospital | CS | HF patients aged >18 years | 340 | Record review and patient interview | 83.5 | 880 | 2.6 ± 1.8 |
| Mohammed et al.33 | 2018 | Jimma, Western Ethiopia | Ambulatory clinic of a single hospital | CS | T2DM with HTN | 300 | Record review and patient interview | 82.0 | 494 | 1.6 ± 1.1 |
| Birarra et al.a,34 | 2018 | Addis Ababa, Central Ethiopia | Pediatric ward of a single hospital | CS | All patients | 285 | Record review | 31.6 | 106 | 0.37 |
| Aster et al.35 | 2019 | Jimma, Western Ethiopia | Medical ward of a single hospital | CS | CKD patients | 103 | Record review and patient interview | 78.6 | 200 | 1.9 ± 0.9 |
| Asgedom et al.36 | 2019 | Mekelle, Northern Ethiopia | Ambulatory clinic of a single hospital | CS | HTN patients | 241 | Record review and patient interview | 55.6 | 357 | 1.5 |
| Argaw et al.37 | 2019 | Bale, Southern Ethiopia | Ambulatory clinic of a single hospital | CS | T2DM patients | 216 | Record review and patient interview | 88.0 | 446 | 2.1 ± 0.9 |
| Malede et al.38 | 2019 | Addis Ababa, Central Ethiopia | Pediatric Oncology ward of a single hospital | CS | All patients | 156 | Record review and patient interview | 68.6 | 257 | 1.6 |
| Demoz and Berhaa,39 | 2019 | Addis Ababa, Central Ethiopia | Ambulatory clinic of a single hospital | CS | T2DM patients | 418 | Record review and patient interview | 42.3 | 207 | 1.16 ± 0.4 |
| Abdulmalik et al.a,40 | 2019 | Harar, Eastern Ethiopia | Ambulatory clinic of a single hospital | CS | T2DM patients | 148 | Record review | 64.2 | 127 | 0.9 |
| Nasir et al.a,18 | 2020 | Addis Ababa, Central Ethiopia | Ambulatory clinic of a single hospital | CS | Epilepsy patients | 291 | Record review and patient interview | 70.4 | 352 | 1.2 ± 0.4 |
DRP: drug-related problem; CS: cross-sectional; CKD: chronic kidney disease; HTN: hypertension; T2DM: type 2 diabetes mellitus; CVS: cardiovascular disease.
Adherence was not included as a part of DRP.
Prevalence of drug-related problems
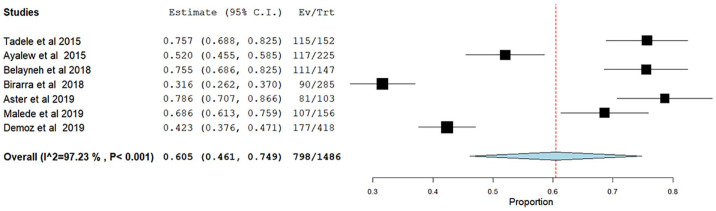
A meta-analysis of 17 studies was conducted to estimate the pooled prevalence of DRPs. Accordingly, it was found that 69.4% (95% CI: 61.5–77.4) of patients experienced at least one DRP during their therapy. There was a significant heterogeneity across the studies, as it was indicated by the I2 value of 97.22%, p < 0.001 (Figure 2).
Figure 2.
Forest plot showing the pooled prevalence of 17 studies reporting drug-related problems in Ethiopia.
Subgroup analysis
We conducted subgroup analysis based on DRP classification. Studies lacking adherence as a part of DRP, as recommended by Cipolle/Morley/Strand DRPs classification algorism, and those studies that reported all seven types of DRP were analyzed separately. Not surprisingly, studies that reported all types of DRP had significant higher pooled prevalence of DRP with 77% (95% CI: 72–82; Figure 3) compared to the studies that excluded adherence 55.9% (95% CI: 41.3–70.4; Figure 4).
Figure 3.
Forest plot showing the pooled prevalence of studies included adherence in their DRP reporting.
Figure 4.
Forest plot showing the pooled prevalence of studies excluded adherence in their DRP reporting.
We also conducted subgroup analysis to explore sources of heterogeneity and to evaluate whether there was significant difference in magnitude of DRP between ambulatory and inpatient settings. Pooled prevalence of DRP in ambulatory setting was higher than that of inpatient wards’ with 75.8% and 60.5%, respectively. There was significant heterogeneity in both settings (Figures 5 and 6). Only few studies were included in this meta-analysis; the heterogeneity observed might have been caused by random variation.
Figure 5.
Subgroup analysis of studies conducted in inpatient setting, Ethiopia.
Figure 6.
Subgroup analysis of studies conducted in ambulatory setting, Ethiopia.
Pooled prevalence of each drug-related problems category
In this section, we only included studies that reported DRP adhering strictly to Cipolle/Morley/Strand DRPs classification algorism. Eleven studies were analyzed for this section. Accordingly, “need for additional drugs” was the most frequently reported type of DRP, accounting for 33% (95% CI: 26%–39%), followed by “noncompliance,” 21% (95% CI: 15%–27%). “Dosage too high” and “adverse drug reaction” were the least frequently reported types of DRP, accounting for 4% each with CIs of (95% CI: 3%–6%) and (95% CI: 2%–5%), respectively (Table 2).
Table 2.
Pooled prevalence of drug therapy problems presented in seven categories.
| Types of DRPs | Ev/Trt | Pooled estimate 95% CI | I 2 |
|---|---|---|---|
| Needs additional drug | 1281/3918 | 0.33 (0.26–0.39) | 94.46 |
| Noncompliance | 791/3918 | 0.21 (0.15–0.27) | 96.67 |
| Dosage too low | 675/3918 | 0.15 (0.09–0.22) | 98.24 |
| Ineffective drug therapy | 533/3918 | 0.10 (0.05–0.15) | 97.64 |
| Unnecessary drug therapy | 296/3918 | 0.09 (0.06–0.11) | 93.81 |
| Dosage too high | 156/3918 | 0.04 (0.03–0.06) | 90.94 |
| Adverse drug reaction | 146/3918 | 0.04 (0.02–0.05) | 91.28 |
DRP: drug-related problem; CI: confidence interval.
Factors associated with drug therapy problems
The literatures included reported a wide variety of factors as risk factors for the occurrence of DRPs. The most frequently reported factors were patients’ age,31,33,34,38 number of medications (polypharmacy)30,29,32,37 and polymorbidity.29,30,32,33,36,37,39 In addition, the number of days of hospital stay38,33 a prior history of hospitalization,30 the level of patients involvement in the therapeutic decision and their belief about medication,32 as well as the stage of the disease (severity),37 were reported to have association with the occurrence of DRPs.
Publication bias
Analysis for publication bias using funnel plot (logit event rate vs standard error) revealed that there was no publication bias (Figure 7).
Figure 7.
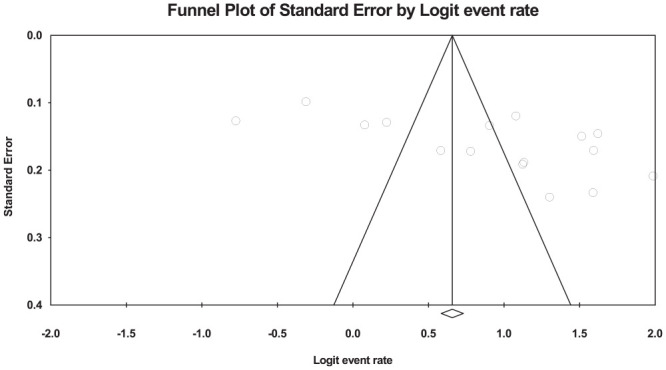
Funnel plot analysis for publication bias in the studies conducted on drug-related problem in Ethiopia.
Discussion
Although drugs are the cornerstone of modern medicine, they could sometimes be the causes of unwanted and harmful patient outcomes or DRPs.41 Hence, looking out for potential DRPs, minimizing their effect and taking corrective measures are some of the essential activities which involve the interactive work of physicians and pharmacists.42
In this systematic review, 69.4% (95% CI: 72%–82%) of patients were reported to have at least one DRP. In the subgroup analysis, the prevalence of DRP was high among studies conducted in ambulatory unit compared to inpatient. This might be due to lack of strict follow-up and monitoring for ambulatory patients. This review shows not only an alarmingly high number of DRPs, but also a wide variation from studies conducted elsewhere. For instance, a study conducted in Hong Kong reported only 21.0% incidence of DRPs.43 Attention should be given on reducing the occurrence of DRPs considering its impact on patient treatment outcomes and associated financial costs.
Understanding types of DRP gives added value in the effort of preventing their occurrence as well as dealing with their effects. Cipolle/Morley/Strand classify DRPs into seven categories, namely, needs additional drug, unnecessary drug use, ineffective drug therapy, dosage too high, dosage too low, ADR and noncompliance.4 In the current review, the most commonly reported types of DRP were found to be “Need for additional drug,” followed by “Noncompliance,” accounting for more than half of the DRPs. According to the authors, “need for additional drug” can occur in the face of diagnosis without an indication, or need for additive drug or preventive drug. Similarly, “noncompliance” could be an indicator of lack of access to prescribed drugs due to unaffordability and unavailability, failure to understand instructions and difficulty in administration. Thus, practitioners should consider such factors cautiously during their daily encounters.
On the contrary, ADR was reported as the least frequent type of DRP, with an incidence of 4% (95% CI: 2%–5%). This figure might not show the real picture of the burden of ADRs. This is because detecting ADR is often challenging as sign and symptoms of a disease may overlap with the manifestations of an ADR. Furthermore, all studies included in this review were conducted through document review and patient interview. These approaches have inherent downside when it comes to detecting ADRs, as there might be unrecorded ADR in patient charts and the potential for patient recall bias. In certain cases, laboratory tests might be needed to detect ADR. Therefore, this finding should be interpreted cautiously as there is a potential for underestimation.
Although factors associated with the occurrence of DRPs vary depending on the likes of the setting where research was conducted and study population, a variety of factors have been reported as a risk for DRPs. Examples of risk for DRPs indicated in the literatures included patient age, polypharmacy, polymorbidity, patient cognitive condition and the types of medication used (the risk is high with drugs such as antiepileptics, anticoagulants).44,45 In this review, most of the studies included reported age of patients as a risk factor for DRPs. This can be explained by the likely increased number of comorbidities in elderly population; prescribers’ tendency of overlooking drug selection and dosing guidelines for geriatric patients; and socio-economic and behavioral changes that emerge with aging, such as financial constraints and forgetfulness.
Similarly, patients diagnosed with more than one disease were reported to have higher risk of developing DRPs. This could be due to the fact that patients with multiple comorbidities are more likely to receive larger number of medications. This brings us to the issue of polypharmacy, which is also reported to have a significant association with DRPs as patients on multiple drugs were at high risk of experiencing DRPs. However, it should be noted that the cutoff number of drugs used to declare polypharmacy was varied across the studies reviewed. Thoroughly assessing patients’ medical history as well as undertaking medication reconciliation can significantly reduce DRPs related to multiple comorbidities and polypharmacy.
Looking at the study characteristics, nearly all studies conducted on DRP were published 2015 onward, converging with the initiation of patient-oriented pharmacy service in Ethiopia. Such an exploding number of research outputs are expected to play a vital role in the full implementation of clinical pharmacy services in Ethiopia and improve the overall quality of the service. The majority of the studies used Cipolle/Morley/Strand classification of DRPs with the exception of few studies which used PCNE classification approach.46,47 While there are several types of DRP classification, there is no single most effective DRP categorization approach based on applicability, comprehensiveness and internal consistency.48 Although different classification approaches are used across countries, it is very important to adopt a single classification to ensure the consistency of practice and reporting. In addition, modification of the adopted classification system should be considered for special groups of patients such as the elderly.49
Furthermore, we witnessed incomplete reporting and deviation from the selected DRP classification system. For instance, rather than reporting “adherence” as one type of DRP, the authors reported it separately.18 Other studies reported drug–drug interaction as a separate DRP.14,18 In fact, according to Cipolle/Morley/Strand classification, drug–drug interaction is a cause of DRPs such as ineffectiveness, high or low dosage. Future studies should take this into consideration as it will not only create inconsistency across studies, but also make it difficult to interpret and use the generated information.
Moreover, a considerable number of studies were conducted in the ambulatory ward assessing DRPs in chronic care patients such as diabetes mellitus and hypertension. While these types of studies are expected to improve the pharmacotherapy of chronic diseases that are becoming a common healthcare problem in the country, equal attention should be given to DRPs occurring in inpatient wards to gain insight into the whole picture of the problem.
Limitation
We were not able to find pooled magnitude of each factor associated with DRPs because of wide variation in statistics used to report the findings. Furthermore, this systematic review included observational studies with low to moderate quality of evidence and should be interpreted accordingly. Drugs commonly involved in DRPs were not assessed in this review because only few of the studies included in this review reported drugs involved in the DRPs. Despite the limitations, the findings of this review provide a new set of information on the extent of the problem at national level.
Conclusion
The review shows a high prevalence of DRPs in Ethiopian public healthcare settings. Need for additional drug and noncompliance were most frequently reported DRPs. It is imperative to design and implement interventions aimed at reducing DRPs. One possible solution is to actively engage clinical pharmacy practitioners in the patient care process. In addition, responsible stakeholders should adopt a uniform DRP classification approach to ensure uniform reporting of DRPs. Future studies should consider the suitability of selected DRP classification for the study settings.
Supplemental Material
Supplemental material, sj-doc-1-smo-10.1177_20503121211009728 for Drug-related problems in Ethiopian public healthcare settings: Systematic review and meta-analysis by Yohanes Ayele and Zelalem Tilahun Tesfaye in SAGE Open Medicine
Supplemental material, sj-pdf-2-smo-10.1177_20503121211009728 for Drug-related problems in Ethiopian public healthcare settings: Systematic review and meta-analysis by Yohanes Ayele and Zelalem Tilahun Tesfaye in SAGE Open Medicine
Supplemental material, sj-pdf-3-smo-10.1177_20503121211009728 for Drug-related problems in Ethiopian public healthcare settings: Systematic review and meta-analysis by Yohanes Ayele and Zelalem Tilahun Tesfaye in SAGE Open Medicine
Supplemental material, sj-pdf-4-smo-10.1177_20503121211009728 for Drug-related problems in Ethiopian public healthcare settings: Systematic review and meta-analysis by Yohanes Ayele and Zelalem Tilahun Tesfaye in SAGE Open Medicine
Acknowledgments
We would like to extend our appreciation to the authors who cooperated with us through giving additional information regarding their articles.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yohanes Ayele  https://orcid.org/0000-0002-6473-7752
https://orcid.org/0000-0002-6473-7752
Supplemental material: Supplemental material for this article is available online.
References
- 1. Berenguer B, la Casa C, de la Mata MJ, et al. Pharmaceutical care: past, present and future. Current Pharmaceutical Design 2004; 10: 3931. [DOI] [PubMed] [Google Scholar]
- 2. Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm 1990; 47(3): 533–543. [PubMed] [Google Scholar]
- 3. Morak S, Vogler S, Walser S, et al. Understanding the pharmaceutical care concept and applying it in practice. Vienna: Gesundheit Ӧsterreich, 2010. [Google Scholar]
- 4. Cipolle RJ, Strand LM, Morley PC. Pharmaceutical care practice: the clinician’s guide. Int J Toxicol 2004; 23: 379–380. [Google Scholar]
- 5. van Mil JF, Westerlund LT, Hersberger KE, et al. Drug-related problem classification systems. Ann Pharmacother 2004; 38(5): 859–867. [DOI] [PubMed] [Google Scholar]
- 6. Meyboom RH, Lindquist M, Egberts AC. An ABC of drug-related problems. Drug Saf 2000; 22(6): 415–423. [DOI] [PubMed] [Google Scholar]
- 7. ASHP guidelines on a standardized method for pharmaceutical care. In: Deffenbaugh J. (ed.) Best Practices for Health-system Pharmacy. Bethesda, MD: American Society of Health-system Pharmacists, 1996, pp. 109–111. [DOI] [PubMed] [Google Scholar]
- 8. Grupo de Investigacíon en Aténcion Farmacéutica. Ars Pharmaceutica 2002; 43: 175–184. [Google Scholar]
- 9. Hanlon J, Schmader K, Samsa G, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol 1992; 45(10): 1045–1051. [DOI] [PubMed] [Google Scholar]
- 10. (PCNE) PCNE. PCNE DRP-Classification V9.0 2020, https://www.pcne.org/working-groups/2/drug-related-problem-classification
- 11. Viktil KK, Blix HS. The impact of clinical pharmacists on drug-related problems and clinical outcomes. Basic Clin Pharmacol Toxicol 2008; 102(3): 275–280. [DOI] [PubMed] [Google Scholar]
- 12. Rashed AN, Neubert A, Tomlin S, et al. Epidemiology and potential associated risk factors of drug-related problems in hospitalised children in the United Kingdom and Saudi Arabia. Eur J Clin Pharmacol 2012; 68(12): 1657–1666. [DOI] [PubMed] [Google Scholar]
- 13. Lenander C, Elfsson B, Danielsson B, et al. Effects of a pharmacist-led structured medication review in primary care on drug-related problems and hospital admission rates: a randomized controlled trial. Scand J Prim Health Care 2014; 32(4): 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ayalew MB, Megersa TN, Mengistu YT. Drug-related problems in medical wards of Tikur Anbessa specialized hospital, Ethiopia. J Res Pharm Pract 2015; 4: 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orach CG. Health equity: challenges in low income countries. Afri Health Sci 2009; 9: S49–S51. [PMC free article] [PubMed] [Google Scholar]
- 16. Chudi I. Healthcare problems in developing countries. Med Pract Rev 2010; 1: 9–11. [Google Scholar]
- 17. Fattinger K, Roos M, Vergères P, et al. Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol 2000; 49(2): 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nasir BB, Berha AB, Gebrewold MA, et al. Drug therapy problems and treatment satisfaction among ambulatory patients with epilepsy in a specialized hospital in Ethiopia. PLoS ONE 2020; 15(1): e0227359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greeshma M, Lincy S, Maheswari E, et al. Identification of drug related problems by clinical pharmacists in prescriptions with polypharmacy: a prospective interventional study. J Young Pharm 2018; 10(4): 460–465. [Google Scholar]
- 20. Khdour MR, Jarab AS, Adas HO, et al. Identification of drug-related problems: a prospective study in two general hospitals. Curr Clin Pharmacol 2012; 7(4): 276–281. [DOI] [PubMed] [Google Scholar]
- 21. MacDonald DA, Chang H, Wei Y, et al. Drug therapy problem identification and resolution by clinical pharmacists in a family medicine residency clinic. Pharm Pract 2018; 9(2): 2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ojeh VB, Naima N, Abah IO, et al. Pattern of drug therapy problems and interventions in ambulatory patients receiving antiretroviral therapy in Nigeria. Pharm Pract 2015; 13(2): 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Munn Z, Moola S, Riitano D, et al. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Polic Manag 2014; 3(3): 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. David A, Dana B, Peter AB, et al. Grading quality of evidence and strength of recommendations. BMJ 2014; 328(7454): 1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yadesa TM, Gudina EK, Angamo MT. Antimicrobial use-related problems and predictors among hospitalized medical in-patients in Southwest Ethiopia: prospective observational study. PLoS ONE 2015; 10(12): e0138385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sisay EA, Engidawork E, Yesuf TA, et al. Drug related problems in chemotherapy of cancer patients. J Cancer Sci Ther 2015; 7(2): 055–059. [Google Scholar]
- 28. Kaleab Gizaw. Drug related problems and contributing factors among adult ambulatory patients with cardiovascular diseases at Gebretsadik Shawo General Hospital, Bonga, South west Ethiopia. J Nat Sci Res 2017; 7(1): 2224. [Google Scholar]
- 29. Abadir H, Daba FB. Drug therapy problems and their predictors among hypertensive patients on follow up In Dil-Chora Referral Hospital, Dire-Dawa, Ethiopia. Int J Pharmaceut Sci Res 2017; 8(6): 2712–2719. [Google Scholar]
- 30. Hailu CK, Seble BT, Tufa EG. Epidemiology and predictors of drug therapy problems among type 2 diabetic patients at Wolaita Soddo University Teaching Hospital, Southern Ethiopia. Am J Pharmacol Sci 2017; 5(2): 40–48. [Google Scholar]
- 31. Belayneh YM, Amberbir G, Agalu A. A prospective observational study of drug therapy problems in medical ward of a referral hospital in northeast Ethiopia. BMC Health Serv Res 2018; 18(1): 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niriayo YL, Kumela K, Kassa TD, et al. Drug therapy problems and contributing factors in the management of heart failure patients in Jimma University Specialized Hospital, Southwest Ethiopia. PLoS ONE 2018; 13(10): e0206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mohammed YH, Habtem J, Tigestu AD. Determinants of drug-related problems among ambulatory type 2 diabetes patients with hypertension comorbidity in Southwest Ethiopia: a prospective cross sectional study. BMC Research Notes 2018; 11: 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Birarra MK, Heye TB, Shibeshi W. Assessment of drug-related problems in pediatric ward of Zewditu Memorial Referral Hospital, Addis Ababa, Ethiopia. Int J Clin Pharm 2017; 39(5): 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aster WG, Eshetu MB, Amare DW, et al. Drug-related problems and associated factors among patients admitted with chronic kidney Disease at Jimma University Medical Center, Jimma Zone, Jimma, Southwest Ethiopia: a hospital-based prospective observational study. Int J Nephrol 2019; 371: 1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Asgedom SW, Fekadu T, Atey TM, et al. Assessment of drug therapy problem and associated factors among adult hypertensive patients at Ayder comprehensive specialized hospital, Northern Ethiopia. Afri Health Sci 2019; 19(3): 2571–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Argaw AM, Hiwet TT, Derse BB. Drug therapy problems and determinants among ambulatory type 2 diabetes mellitus patients: pharmacists’ intervention in South-East Ethiopia. Endocrinol Metab Syndr 2019(8): 303. [Google Scholar]
- 38. Malede B, Ephrem E, Adam H. Identification and resolution of drug related problems in pediatric hematology/oncology ward of Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, 2019, http://213.55.95.56/handle/123456789/17843
- 39. Demoz GT, Berha AB. Drug therapy problems, medication adherence and treatment satisfaction among diabetic patients on follow-up care at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. PLoS ONE 2019; 14(10): e0222985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abdulmalik H, Tadiwos Y, Legese N. Assessment of drug-related problems among type 2 diabetic patients on follow up at Hiwot Fana Specialized University Hospital, Harar, Eastern Ethiopia. BMC Res Notes 2019; 12(1): 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roughead EE, Semple SJ. Medication safety in acute care in Australia: where are we now? Part 1: a review of the extent and causes of medication problems 2002-2008. Australia New Zealand Health Policy 2009; 6: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guignard B, Bonnabry P, Perrier A, et al. Drug-related problems identification in general internal medicine: the impact and role of the clinical pharmacist and pharmacologist. Eur J Intern Med 2015; 26(6): 399–406. [DOI] [PubMed] [Google Scholar]
- 43. Rashed AN, Wilton L, Lo CCH, et al. Epidemiology and potential risk factors of drug-related problems in Hong Kong paediatric wards. Br J Clin Pharmacol 2014; 77(5): 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaufmann CP, Stämpfli D, Hersberger KE, et al. Determination of risk factors for drug-related problems: a multidisciplinary triangulation process. BMJ Open 2015; 5(3): e006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peterson C, Gustafsson M. Characterisation of drug-related problems and associated factors at a clinical pharmacist service-naïve hospital in Northern Sweden. Drugs Real World Outcomes 2017; 4(2): 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abdela OA, Bhagavathula AS, Getachew H, et al. Risk factors for developing drug-related problems in patients with cardiovascular diseases attending Gondar University Hospital, Ethiopia. J Pharm Bioallied Sci 2016; 8(4): 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ayele Y, Melaku K, Dechasa M, et al. Assessment of drug related problems among type 2 diabetes mellitus patients with hypertension in Hiwot Fana Specialized University Hospital, Harar, Eastern Ethiopia. BMC Research Notes 2018; 11(1): 728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Basger BJ, Moles RJ, Chen TF. Application of drug-related problem (DRP) classification systems: a review of the literature. Eur J Clin Pharmacol 2014; 70(7): 799–815. [DOI] [PubMed] [Google Scholar]
- 49. Lim XY, Yeo QQ, Kng GLL, et al. Validation of a drug-related problem classification system for the intermediate and long-term care setting in Singapore. Pharmacy 2018; 6(4): 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-smo-10.1177_20503121211009728 for Drug-related problems in Ethiopian public healthcare settings: Systematic review and meta-analysis by Yohanes Ayele and Zelalem Tilahun Tesfaye in SAGE Open Medicine
Supplemental material, sj-pdf-2-smo-10.1177_20503121211009728 for Drug-related problems in Ethiopian public healthcare settings: Systematic review and meta-analysis by Yohanes Ayele and Zelalem Tilahun Tesfaye in SAGE Open Medicine
Supplemental material, sj-pdf-3-smo-10.1177_20503121211009728 for Drug-related problems in Ethiopian public healthcare settings: Systematic review and meta-analysis by Yohanes Ayele and Zelalem Tilahun Tesfaye in SAGE Open Medicine
Supplemental material, sj-pdf-4-smo-10.1177_20503121211009728 for Drug-related problems in Ethiopian public healthcare settings: Systematic review and meta-analysis by Yohanes Ayele and Zelalem Tilahun Tesfaye in SAGE Open Medicine