Abstract
Objective:
This study was aimed at identifying Shigella and Salmonella infection, their antibiotic susceptibility pattern and associated risk factors among children with diarrhea who attended Alamura Health Center.
Method:
A facility-based cross-sectional study was conducted on 263 children aged below 14 years with diarrhea. A structured questionnaire was used to collect socio-demographic and clinical data after obtaining the necessary consent from their parents or caretakers. The culture and sensitivity tests were performed using the standard operating procedure of the microbiology laboratory.
Results:
Accordingly, 20/263 (7.6%), 95% confidence interval: 4.4%–11.4% Shigella and 1/263 (0.38%), 95% confidence interval: 0.0%–1.1% Salmonella were isolated. Shigella dysenteriae was dominant 11 (4.2%), followed by Shigella spp. 9 (3.42%) and Salmonella typ 1 (0.38%). The isolates showed 71.4% overall resistance to ampicillin and 61.9% for augmentin and tetracycline, whereas 95.2% of the isolates were sensitive to ciprofloxacin, 85.9% to ceftriaxone and ceftazidime, 81% to gentamycin, 76.2% to chloramphenicol, 66.7% to cefuroxime and 52.4% to cotrimoxazole. The habit of washing hands after toilet use for a while (adjusted odds ratio: 235.1, 95% confidence interval: 20.9–2643.3, p < 0.000) and storing cooked food in an open container for later use (adjusted odds ratio: 36.44, 95% confidence interval: 5.82–228.06, p < 0.000) showed a statistically significant association.
Conclusion:
High level of Shigella and single Salmonella was isolated. Ampicillin, augmentin and tetracycline were resistant and ciprofloxacin, ceftriaxone, ceftazidime, gentamycin, chloramphenicol, cefuroxime and cotrimoxazole were relatively sensitive. Hand-washing after defecation for some time and storing of foods for later use in an open container were statistically associated. Therefore, to alleviate this infection, the concerned body should focus on imparting health education for hand-wash after defecation and storing food in a closed container for later use is mandatory.
Keywords: Salmonella, Shigella, diarrhea, antibiotic resistance, children, Southern Ethiopia
Introduction
Diseases caused by enteric pathogens are of common public health concerns in many parts of the world including Ethiopia.1,2 Salmonella and Shigella are associated with a high burden of illness among children in the developing world.3 Children are one of the victims of these infections accounting for approximately 8% of all deaths among children under age 5 worldwide in 2017. This implies that over 1300 young children passed away each day, 480,000 children a year, regardless of the availability of humble active treatment. Most of these deaths are due to diarrhea in South Asia and sub-Saharan Africa.4 The rates of Shigella and Salmonella in Ethiopia reported from different studies are in the range of 4.3%–45%5–8 and 1%–12.6%,7,9,10 respectively.
They are species of particular concerns as they cause enteric fevers, food poisoning and gastroenteritis.9 They are Gram-negative rods that commonly inhabit the intestinal tracts of humans and many animals.10 It was estimated that 1.8 million cases of children died from diarrheal illness worldwide, a large proportion of which was attributed to infection by Shigella and Salmonella spp.11 Different studies have reported that Shigella spp. were associated with majority of cases of bacillary dysentery, which is prevalent mainly in developing nations;12,13 whereas, Salmonella spp. were the most common cause of food-borne infection outbreaks almost all over the world.14 In recent years, the emergence and global dissemination of Salmonella and Shigella species resistance to ampicillin, chloramphenicol, tetracycline and co-trimoxazole are increasingly documented in developing countries.15
Infections of Shigella and Salmonella can be asymptomatic and can be treated with rehydration solutions unless the infection is by invasive strains.16 Prescribing antibiotics might shorten the extent of diarrhea and control the organisms, which otherwise might continue to spread among people and in the environment, and furthermore, it would pose a public health concern.17 Children are at high risk of these infections due to their weakened immune status and ease of contamination.18 In developing countries, this infection increased due to poor sanitation, personal hygiene and lack of appropriate food supply that leads children to contaminate by themselves.19 Therefore, this study is aimed at identifying Shigella and Salmonella infections, antibiotic susceptibility and associated risk factors among children with diarrhea who visited Alamura Health Center in Southern Ethiopia.
Materials and methods
Study area and period
The study was conducted in the Southern Nations, Nationalities and Peoples Region (SNNPR) at Hawassa Alamura Health Center from 1 April 2019 to 30 August 2019. Hawassa is the capital city of the SNNPR, located in the Southern part of Ethiopia, on the shores of Lake Hawassa which is one of the Great Rift Valley lakes situated around 270 km from Addis Ababa, the capital city of Ethiopia. The mean annual rainfall is about 950 mm, temperature about 20°C and humidity 70%–80%. The rainy season generally extends from June to October. The human population of Hawassa for 2015 was estimated at 351,469, with an annual growth rate of just over 4%.20 Hawassa city has 7 sub-cities with 5 private, 1 general and 1 comprehensive specialized hospital and 10 health centers. Alamura Health Center is located in the Tabor sub-city and borderline between Fara and Hitata Kebele near Alamura Mountain.
Study design and population
A facility-based cross-sectional study design was conducted among children with diarrhea at Alamura Health Center. A convenient sampling technique was employed in which diarrheic pediatric patients below the age of 14 years were included. They were considered for the study only after obtaining the necessary consent from their parents or guardian and signing the document. The participants are excluded if their parents are not willing or refuse to sign. All diarrheic pediatric patients who visited Alamura Health Center for the diarrheal case of illness were the source of population.
Sample size determination
The sample size was calculated using a single population proportion formula: n = z2p (1−p)/d2, where n = sample size, z = confidence level at 95% (standard value of 1.96), M = margin of error at 5%, p = estimated prevalence of Shigella and Salmonella from the previous study 22.2%.21 Therefore, the calculated sample size for this study was 263.
Variable of study
The dependent variables were the presence of Salmonella and Shigella. The independent variables were socio-demographic factors, namely, age, sex, place of residence, educational status of the mothers, marital status, family size, monthly income, occupation of the family. Clinical variables collected include history and type of diarrhea, malnutrition and vaccination status of the children. Another variable considered for the study was behavioral factor, which includes a drinking water source, hand-wash after toilet use, food/drink consumption before illness, storage of cooked food for later use, the habit of hand-washing before and after a meal, washing habit of food containers and history of contact with domestic animals. These were assessed with a structured questionnaire.
Data collection
The socio-demographic and clinical data were collected after informing the parents/caregiver about the aim of the study. A face-to-face interview was conducted to collect the data with a structured questionnaire from parents or caretaker of the children who complained of diarrhea after they signed the consent and the child accepted the assent.
Laboratory diagnosis
The stool was collected using a screw cup container. The parents/caregiver was instructed to bring a fresh stool sample without any contamination before 30 min of collection. All stool specimens were placed into Carry Blair Transport Medium and transported to the Microbiology Laboratory of Hawassa University Comprehensive Specialized Hospital (HUCSH). The stool was inoculated on prepared culture media which is MacConkey, Xylose lysine deoxycholate (XLD) and selenite F-broth (Abtek, UK). The culture plates were incubated aerobically at 37°C for 24 h.
Bacterial identification
The colonies were examined morphologically for size, shape and ability to ferment lactose. Those bacterial colonies with non-lactose fermenting characteristics with H2S for Salmonella and without H2S for Shigella were picked up for biochemical identification. Indole test, urease production, mannitol fermentation, hydrogen sulfide, gas production test, citrate utilization test, motility test, carbohydrate fermentation test, lysine decarboxylase test (LDC) and oxidase test were used to identify the bacteria up to genus/species level.22
Antibiotics susceptibility testing
A pure colony of isolated bacteria was mixed with normal saline to make a 0.5 McFarland standard suspension for susceptibility testing and then swabbed on Mueller Hinton agar. The susceptibility pattern of the isolates was determined for ciprofloxacin (CIP; 5 μg), augmentin (AUG; 30 μg), gentamicin (GEN; 10 μg), chloramphenicol (CAF; 30 μg), co-cotrimoxazole (COT; 25 µg), tetracycline (TAT; 30 µg), ampicillin (AMP; 10 µg), ceftriaxone (CRO; 30 µg), cefuroxime (CRX; 30 µg) and ceftazidime (CAZ; 30 µg). After incubation for 24 h at 37°C, the diameter of each zone of inhibition was measured with a ruler in millimeters. The results were then interpreted according to Clinical and Laboratory Standards Institute (CLSI) guidelines antimicrobial susceptibility breaking points 2018 and recorded as sensitive (S), intermediate (I) or resistant (R).23
Quality control
A pre-test was conducted on 5% of the questionnaire before conducting the study. The validity and completeness of the data were verified daily. Sterility of culture media and biochemical tests were checked by overnight incubation of un-inoculated media from each batch of preparation. Standard strains of Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used for the culture and antibiotic susceptibility testing of internal quality assurance.
Data analysis
Data were entered into Statistical Package for the Social Sciences (SPSS) version 20 and were analyzed to make inferences on the frequency of occurrence of enteric pathogens associated with diarrhea and to show bacterial resistance pattern to locally prescribed antibiotic substances. Descriptive statistics were performed to get the frequency of dependent and independent variables. Binary logistic regression analysis was conducted to identify real predictor of Shigella and Salmonella. The strength of association was presented by odds ratio at 95% confidence interval (CI) and a p value of ⩽0.05 was considered as a statistically significant association.
Ethical consideration
The study was conducted after obtaining formal permission from the Southern Nations Nationality and People Regional Health Office, Hawassa City Administration Health Office, Alamura Health Center Manager and Laboratory Head. The patients were included in the study only when the parents or caretakers of the patients sign the consent letter. The culture and antimicrobial susceptibility results were communicated to the concerned bodies in the health center within 72 h.
Results
Socio-demographic characteristics of the study subjects
A total of 263 diarrheic pediatric patients from Alamura Health Center were enrolled for the study with a mean and standard deviation of age 6.8 ± 3.7 years. The frequency and percentage of pediatrics age range enrolled for the study were 0–4, 88 (33.5%); 5–9, 103 (39.2%); and 10–14, 72 (27.4%). An almost equal ratio of male to female was enrolled for the study (130:133). Regarding the residence, most of the study subjects 155 (58.9%) were from an urban area and 108 (41.1%) patients were from a rural area. Concerning the educational status of the mothers of pediatric patients, most of them were educated (81%) ranging from reading and writing to university graduate level and the rest 19% were illiterates. The marital status of their mothers: 178 (67.7%) were married, 43 (16.3%) divorced and 41 (15.6%) widowed. The mean and standard deviation of the family size was 5.6 ± 1.9 persons. The average income of the family was 3743.3 ± 2568.1 Ethiopian birr. Most of the study participants have a large family size with a relatively low income of <1500 birr per month and from this number, the diarrhea positive was 12 (57.1%; Table 2).
Table 2.
Bivariate analysis of socio-demographic characteristics and clinical data of diarrheic pediatric patients in the Alamura Health Center, Southern Ethiopia, 2019.
| Variables | Frequency (%) | Shigella/Salmonella | COR 95% CI | p value | AOR 95% CI | p value | |
|---|---|---|---|---|---|---|---|
| Yes (%) | No (%) | ||||||
| Age in group (years) | |||||||
| 0–4 | 88 (33.5) | 7 (33.3) | 81 (33.5) | 1.158 (0.35–3.82) | 0.809 | ||
| 5–9 | 103 (39.2) | 9 (42.9) | 94 (38.8) | 1.283 (0.41–4.00) | 0.668 | ||
| 10–14 | 72 (27.4) | 5 (23.8) | 67 (27.7) | 1 | |||
| Sex | |||||||
| Male | 130 (49.4) | 8 (38.1) | 122 (50.4) | 1 | |||
| Female | 133 (50.6) | 13 (61.9) | 120 (49.6) | 1.652 (0.66–4.13) | 0.283 | ||
| Residence | |||||||
| Rural | 108 (41.1) | 8 (38.1) | 100 (41.3) | 1 | |||
| Urban | 155 (58.9) | 13 (61.9) | 142 (58.7) | 0.874 (0.35–2.2) | 0.773 | ||
| Mother’s educational status | |||||||
| No formal education | 50 (19.0) | 2 (9.5) | 48 (19.8) | 1.125 (0.10–12.99) | 0.925 | ||
| Read and write | 56 (21.3) | 9 (42.9) | 47 (19.4) | 5.170 (0.62–43.05) | 0.129 | ||
| Elementary school | 76 (28.9) | 5 (23.8) | 71 (29.3) | 1.901 (0.21–17.03) | 0.566 | ||
| Secondary school | 53 (20.2) | 4 (19.0) | 49 (20.2) | 2.204 (0.23–20.7) | 0.489 | ||
| College/university | 28 (10.6) | 1 (4.8) | 27 (11.2) | 1 | |||
| Mother’s marital status | |||||||
| Married | 178 (67.7) | 14 (67.7) | 164 (67.8) | 1 | |||
| Divorced | 43 (16.3) | 5 (23.2) | 38 (15.7) | 1.057 (0.37–3.044) | 0.919 | ||
| Widowed | 41 (15.6) | 2 (9.5) | 39 (16.1) | 0.560 (0.070–4.468) | 0.584 | ||
| Family size (person) | |||||||
| 2–3 | 23 (8.8) | 2 (9.5) | 21 (8.7) | 1 | |||
| 4–5 | 129 (49.2) | 12 (57.1) | 117 (48.5) | 1.077 (0.23–5.163) | 0.926 | ||
| ⩾ 6 | 110 (42.0) | 7 (33.3) | 103 (42.7) | 0.714 (0.138–3.679) | 0.687 | ||
| Monthly income birr/ETB | |||||||
| 500–1500 | 51 (19.4) | 7 (33.3) | 44 (18.2) | 1 | |||
| > 1500 | 212 (80.6) | 14 (66.7) | 198 (81.8) | 0.444 (0.169–1.166) | 0.099 | ||
| Previous diarrhea | |||||||
| Yes | 162 (61.6) | 17 (81.0) | 145 (59.9) | 2.843 (0.928–8.706) | 0.067 | ||
| No | 101 (38.4) | 4 (19.0) | 97 (40.1) | 1 | |||
| Type of diarrhea | |||||||
| Bloody | 111 (42.2) | 1 (4.8) | 100 (43.1) | 1 | |||
| Watery | 49 (18.6) | 2 (9.5) | 47 (20.3) | 11.69 (0.99–138.44) | 0.051 | ||
| Mucoid | 103 (39.2) | 18 (85.7) | 85 (36.6) | 16.75 (2.13–131.67) | 0.007 | ||
| Frequency of diarrhea | |||||||
| Once | 170 (64.6) | 11 (52.4) | 159 (65.7) | 1.038 (0.125–8.598) | 0.973 | ||
| Twice | 77 (29.3) | 9 (42.9) | 68 (28.1) | 1.985 (0.234–16.88) | 0.530 | ||
| > 3 | 16 (6.1) | 1 (4.8) | 14 (5.8) | 1 | |||
| Malnutrition | |||||||
| Yes | 25 (9.5) | 3 (14.3) | 22 (9.1) | 1 | 0.441 | ||
| No | 238 (90.5) | 18 (85.7) | 220 (90.9) | 1.67 (0.46–6.121) | |||
| Vaccination | |||||||
| Yes | 202 (76.8) | 14 (66.7) | 188 (77.7) | 0.574 (0.221–1.495) | 0.256 | ||
| No | 61 (23.2) | 7 (33.3) | 54 (223) | 1 | |||
| Drinking H2O sources | |||||||
| Pipe | 159 (60.5) | 17 (81.0) | 142 (58.7) | 2.993 (0.978–9.162) | 0.055 | ||
| Other | 104 (39.5) | 4 (19.0) | 100 (41.3) | 1 | |||
| Child’s hand-wash after toilet | |||||||
| Always | 221 (84.0) | 1 (4.8) | 220 (90.9) | 1 | 0.000 | 235.1 (20.9–2643.3) | 0.000 |
| Sometimes | 42 (16.0) | 20 (95.20 | 22 (9.1) | 200 (25.6–1562.35) | |||
| Food taken before illness | |||||||
| Cooked food | 82 (31.2) | 5 (23.8) | 77 (31.8) | 0.801 (0.182–3.533) | 0.769 | ||
| Overnight food | 77 (29.3) | 8 (38.1) | 69 (28.5) | 1.430 (0.358–5.716) | 0.613 | ||
| Raw vegetable | 64 (24.3) | 5 (23.8) | 59 (24.4) | 1.045 (0.236–4.634) | 0.954 | ||
| Raw milk | 40 (15.2) | 3 (14.3) | 37 (15.3) | 1 | |||
| Storage of cooked food | |||||||
| Open containers | 40 (15.2) | 16 (76.2) | 24 (9.9) | 29.1 (9.78–86.372) | 0.000 | 36.44 | 0.000 |
| Closed containers | 223 (84.8) | 5 (23.8) | 218 (90.1) | 1 | |||
| Hand-washing before and after a meal | |||||||
| Yes | 85 (32.3) | 6 (28.6) | 79 (32.6) | 1 | |||
| No | 178 (67.7) | 15 (71.4) | 160 (66.1) | 1.212 (0.453–3.242) | 0.702 | ||
| Cleaning of cooking containers | |||||||
| Always | 157 (59.7) | 4 (19.0) | 153 (63.2) | 1 | |||
| Sometimes | 106 (40.3) | 17 (81.0) | 89 (36.8) | 7.306 (2.38–22.4) | 0.001 | 4.94 (0.795–30.74) | 0.087 |
| Contact with animals | |||||||
| Yes | 137 (52.1) | 13 (61.9) | 124 (51.2) | 1.546 (0.62–3.865) | 0.351 | ||
| No | 126 (47.9) | 8 (38.1) | 118 (48.8) | 1 | |||
COR: crude odds ratio; CI: confidence interval; AOR: adjusted odds ratio; ETB: Ethiopian Birr.
The magnitude of Shigella and Salmonella
The overall magnitude of Shigella and Salmonella among children with diarrhea in Alamura Health Center was 20/263 (7.6%), 95% CI: 4.4%–11.4% and 1/263 (0.38%), 95% CI: 0.0%–1.1%. Shigella dysenteriae was frequently isolated from 4.2% (11/263), 95% CI: 1.9–6.8, from patients followed by other Shigella spp. 3.42% (9/263), 95% CI: 1.5–5.7, and Salmonella spp. 0.38% (1/263), 95% CI: 0.0–1.1. In the rest, 92% (242/263) Shigella and Salmonella were not isolated from diarrheic pediatric patients (Figure 1).
Figure 1.
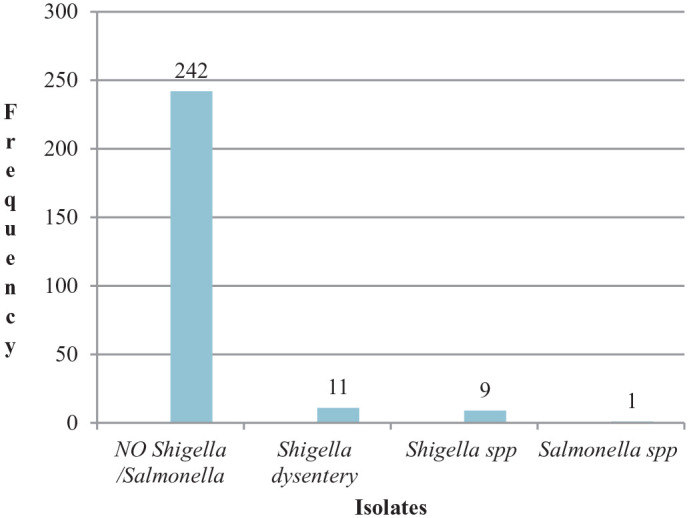
Magnitude of Shigella spp., Shigella dysenteriae and Salmonella typhi in diarrheic pediatric patients from Alamura Health Center, Southern Ethiopia, 2019.
Salmonella typhi
A single Salmonella typhi was isolated from the patient and it was sensitive to ciprofloxacin, gentamicin, ceftazidime, chloramphenicol, cefuroxime, ceftriaxone and co-trimoxazole and resistant to ampicillin and tetracycline.
Other Shigella species
Shigella spp. isolated were 100.0% sensitive to both ceftriaxone and ciprofloxacin, 77.8% to both ceftazidime and chloramphenicol, 66.7% to cefuroxime and 55.6% to gentamycin. Resistance was seen 81.8% for ampicillin, 72.7% for tetracycline and 55.6% for both co-trimoxazole and augmentin.
Shigella dysenteriae
Shigella dysenteriae isolate was 100% susceptible to gentamicin, 90.9% to ciprofloxacin, 90% to ceftazidime, 72% to both ceftriaxone and chloramphenicol. Resistance was seen for ampicillin (45.5%), 55% for co-trimoxazole, 72.7% for tetracycline and 91% for augmentin (Table 1).
Table 1.
Antimicrobial susceptibility profile of Salmonella typhi, Shigella spp. and Shigella dysenteriae isolated from diarrheic pediatric patients from Alamura Health Center, Southern Ethiopia, 2019.
| Antibiotics | Isolates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. typhi (1) | Shigella spp. (9) | S. dysentery (11) | Total (21) | ||||||||
| S | R | S | I | R | S | I | R | S (%) | I (%) | R (%) | |
| AMP | 0 | 1 | 0 | 4 | 5 | 0 | 2 | 9 | 0 (0.0) | 6 (28.6) | 15 (71.4) |
| COT | 1 | — | 5 | 0 | 4 | 5 | 3 | 3 | 11 (52.4) | 3 (14.3) | 7 (33.3) |
| CIP | 1 | — | 9 | 0 | 0 | 10 | 1 | 0 | 20 (95.2) | 1 (4.8) | 0 (0.0) |
| CRO | 1 | 0 | 9 | 0 | 0 | 8 | 0 | 3 | 18 (85.7) | 0 (0.0) | 3 (14.3) |
| CAZ | 1 | 0 | 7 | 1 | 1 | 10 | 1 | 0 | 18 (85.7) | 2 (9.5) | 1 (4.8) |
| GEN | 1 | 0 | 5 | 4 | 0 | 11 | 0 | 0 | 17 (81.0) | 4 (19.0) | 0 (0.0) |
| CAF | 1 | 0 | 7 | 1 | 1 | 8 | 2 | 1 | 16 (76.2) | 3 (14.3) | 2 (9.5) |
| CRX | 1 | 0 | 6 | 2 | 1 | 7 | 1 | 3 | 14 (66.7) | 3 (14.3) | 4 (19.0) |
| AUG | 0 | 1 | 1 | 4 | 4 | 0 | 3 | 8 | 1 (4.8) | 7 (33.7) | 13 (61.9) |
| TAT | 0 | 1 | 0 | 5 | 4 | 0 | 3 | 8 | 0 (0.0) | 8 (38.1) | 13 (61.9) |
S: sensitive; I: intermediate; R: resistant; AMP: ampicillin; COT: co-cotrimoxazole; CIP: ciprofloxacin; CRO: ceftriaxone; CAZ: ceftazidime; GEN: gentamicin; CAF: chloramphenicol; CRX: cefuroxime; AUG: augmentin; TAT: tetracycline.
Associated risk factors
Among the study participants, 162 (61.6%) patients showed a history of diarrhea; of these, 17 (81.0%) were positive for current infection. Of all diarrheic children, the type of diarrhea was watery for 111 (42.2%), mucoid for 103 (39.2%) and bloody for 49 (18.6%). Children with mucoid diarrhea were more 18 (85.7%) as compared to the rest of the patients. Most of the children, 170 (64.6%), had diarrhea once a day and most of the bacteria, 11 (52.4%), was isolated from these patients. Most of the study subjects used pipe water, 159 (60.5%), for drinking, and the children infected in these categories were more than 17 (81.0%).
Regarding hand-wash, 221 (84.0%) affected children practiced hand-washing after defecation and the rest 42 (16.0%) wash their hands only for some time. Among the 16% of patients who wash their hands only for some time, nearly 95.2% of the patients were affected. Most of the food taken by the children before the illness was cooked food, 82 (31.2%), even if the bacterial infection was dominantly isolated from children who ate food at night, 8 (38.1%). Most of the children enrolled in the study were those who stored their food in closed containers, 223 (84.8%); lack of hand-wash before and after meal, 178 (67.7%); wash food containers, 157 (59.7%); well-nourished, 238 (90.5%); vaccinated, 202 (76.8%); and had animal contact 137 (52.1%). Correspondingly, most of the bacteria were isolated from those who stored food in an open container, 16 (76.2%); lack of hand-wash after or before meal, 15 (71.4%); washing of food container for some time, 17 (81.0%); well-nourished, 18 (85.7%); vaccinated, 14 (66.7%); and had animal contacts, 13 (61.9%) (Table 2).
The bivariate analyses indicate that family with monthly income >1500 (crude odds ratio (COR) = 2.250, 95% CI: 0.86–5.902, p = 0.099), educational status of mother who can read and write (COR = 5.170, 95% CI: 0.62–43.05, p = 0.129), previous history of diarrhea (COR = 0.35, 95% CI: 0.115–0.078, p = 0.067), watery diarrheal type (COR = 11.69, 95% CI: 0.988–138.44, p = 0.051), mucoid (COR = 16.75, 95% CI: 2.130–131.67, p = 0.007) were the candidate variables for multivariable analysis.
Similarly, those who used pipe water source (COR = 2.993, 95% CI: 0.978–9.16, p = 0.055), wash hands of their child after toilet for some time (COR = 200, 95% CI: 25.602–1562.348, p = 0.000), store food in open containers (COR = 29.1, 95% CI: 9.78–86.37, p = 0.000) washing habit of food containers for some time (COR = 7.306, 95% CI: 2.38–22.4, p = 0.001) were candidate variables for multivariable analysis with p value of ⩽0.25 (Table 2).
However, in multivariate analysis, after adjustment, those who had a habit of washing the hands of children after toilet use for some time (adjusted odds ratio (AOR) = 235.1, 95% CI: 20.9–2643.3, p = 0.000) and store cooked food in an open container (AOR = 36.44, 95% CI: 5.82–228.06, p = 0.000) showed a statistically significant association of Shigella and Salmonella infection with p values ⩽0.05. However, factors like the type of diarrhea, history of contact with domestic animals, the habit of hand-washing before and after a meal and washing of food containers were not statistically significant (Table 2).
Discussion
The overall magnitude of Shigella and Salmonella isolated in this study was 8.0% (4.6%–11.4%), which is lower than the studies conducted in Tanzania 42.7%,24 Mozambique 27.2%,25 Ethiopia 22.3%,26 22.2%27 and 18.1%.28 It is comparable with a study reported in Ethiopia from Nekemte 9.2%28 and Southern Ethiopia 8.3%.21 The possible reason for such a difference may be the sample size, the method adopted and age variation.8,21
In this study, 7.6% (4.6%–11.0%) of Shigella spp. was isolated, which is comparable to the study conducted in Burkina Faso 5.8%,29 Kenya 7.4%,30 Nigeria 8%,31 Ethiopia 8.3%,32 9.1%.33 In contrast to our findings, a lower rate of Shigella infection was reported from China 1.4%,34 Nekemte 2.1%,28 Ambo 2.5%,35 Goba 4.3%,8 and a higher prevalence of Shigella was reported from Mekelle 13.3%,36 Botswana 21%.37 This study identified Shigella dysenteriae from another Shigella spp. with available biochemical tests and accordingly, 11 (4.2%), 95% CI: 1.9%–6.8%, were infected by Shigella dysenteriae. This rate is lower than the report from Nepal 14.5%.38 However, it is comparable with the findings from Central Africa 3%.39 The other nine (3.42%), 95% CI: 1.5–5.7, were other species of Shigella and this value is higher compared with the results reported from China 1.4%,34 Nigeria 1.4%,40 Addis Ababa 1.3%41 and Jimma 1.1%.42 Our findings are lower than the study reported from Jimma 20.1%,43 Bahir Dar 14.9%,44 9.5%,33 7.8%,33 Harar 14.6%,45 Addis Ababa 9.1%,32 South Africa 8.5%,46 Southwest Ethiopia 8.4%,17 Sudan 8%,47 Southern Ethiopia 7.0%,21 Eastern Ethiopia 6.9%48 and Northern Ethiopia 6.9%.49 This reported variation may be due to the geographical location, climatic change and age variation of the participant. A comparable result was reported from Gondar 4.6%,50 Nepal 4.6%,51 Butajira 4.5%,52 Kenya 4.0%,53 Turkey 3.2%54 and Ethiopia 2.3%.17
A single S. typhi 0.4%, 95% CI: 0%–1.1%, isolated in this study was in line with the findings reported from Addis Ababa, 0%,41 1.1%.50 In contrast to our finding, higher rates were reported from Sudan 4.0%,47 China 4.3%,34 Addis Ababa 3.95%,32 Kenya 3.4%,53 Turkey 3%,54 Gondar 1.6%55 and Hawassa 1.5%.21 This difference might be due to sampling size, climatic condition and age differences.8,21,41,56
Our study revealed that the highest rates of antibiotic resistance of Shigella spp. were against Ampicillin 81.8%, which is comparable with the studies from different areas of Ethiopia 70.1% from Jimma,43 79.9% from Gonder,57 86.7%28 and 88.9% from Mekelle.58 Our study also showed relatively low resistance compared to the findings from Nigeria 90.5%,59 Harar 100%,48 Jimma 100%,17 Hawassa 93%.60 This may be due to widespread resistant strains in the countries. Another antibiotic resistance of Shigella spp. was seen against tetracycline 71.4%, and this was comparable with the findings reported from Harar 70.6%,48 Jimma 63.6% 43 and Mekelle 77.8%.49 This result was slightly lower than the studies reported from Butajira 82.4%,52 Gondar 86%61 and 86%,57 Hawassa 90%.60 This may be due to the nature of the susceptibility of strains to tetracycline. Our results also indicated that 52.4% was resistant against co-trimoxazole and this was comparable with the studies done in Hawassa 56.0%,50 Addis Ababa 45.7%62 and Mekelle 55.6%.49 In contrast to our finding, higher results are reported from Gonder 73.4%.57 Several factors may contribute for the resistance, which may be related to the potency and quality of antimicrobials and the distribution of resistant strains.62
Studies have shown that Shigella is a global problem especially in developing countries.63,64 It is common in areas where living standards of people are very low and access to safe and adequate drinking water and proper waste disposal systems are often very limited or even absent.17,32,57,61,65 Deprived access to a good latrine, poor sanitation and hygienic status, hand-washing habit before and after a meal and/or latrine, absence of proper sewage disposal system were responsible for a typhoidal type of Salmonella infections.17,21,32 Our study assessed risk factors for acquiring Shigella/Salmonella infection. Accordingly, the socio-demographic factors like age group (5–9 years), sex, educational and marital status of mothers; family size (4–5 persons) and monthly income (>1500) were responsible for a higher percentage of infection. However, none of these variables were statistically associated. In agreement with our study, a statistically insignificant association with socio-demographic characteristic was reported from Ethiopia in Addis Ababa32 and Bahir Dar.33 In contrast to our study, age range1–3 from Bale,8 Burkina Faso66 and Mekelle Ethiopia;36 educational status of the family (illiterates) from Gonder;6 and family income in Thailand67 were statistically associated with p < 0.05.
The clinical variables showed that there are high rates of infection associated with mucoid diarrhea with a history of diarrhea, no malnutrition. However, none of these variables were statistically associated. Contrasting to our finding, the type of diarrhea with watery consistency from Bahir Dar was with high rates and statistically associated.33 Similarly, a study from Ambo showed that mucoid diarrhea was with higher rates of infection.35 Our study also showed that those who have taken vaccination were highly affected, which is in disagreement with the study reported from Ambo, which spells that children who were not vaccinated were at higher risk and significantly associated.35 Host factors associated with malnutrition, such as a compromised immune system, environmental enteric dysfunction and enteric microbiome may predispose malnourished children to be more severe to disease infection.68–71 Children with malnutrition may also be more likely to live in households of low socioeconomic status where poor access to clean water,72,73 sanitation and hygiene may expose them to greater fecal microbial loads and a higher risk of pathogens associated with mortality such as Shigella species.3 However, in our study, even if it is not statistically associated, high rates of infection were evident from malnourished children.
Behavioral factors such as the source of water from the pipeline, washing of hands after defecation for some time, consumption of food before illness, storing of food in open containers for later use, not washing hands before and after a meal and cleaning of cooking containers for some time constitute associate factors for the infection. However, the multivariate analysis showed that those who had a habit of washing hands for some time as compared to those who practice hand-washing always were at risk of infection. This can be justified that regular hand-washing using detergent is important for the prevention of Shigella and Salmonella transmission. Similarly, those who store cooked food in an open container for later use (34.44 times) are also at risk of infection as compared to those who practice closing the container, with a p value of ⩽0.05, which is in agreement with the studies conducted in Southern Ethiopia Arbaminch.7,74 This can be explained by the transmission of these infections by flies, cockroaches and rodents in the kitchen and, therefore, exposing food can lead to diarrhea in children through bacterial contamination.75–79
Limitation of study
Our study does not indicate the total magnitude of Salmonella and Shigella infection in Hawassa town.
It does not identify bacteria at the species level due to a lack of anti-sera in the local market.
Conclusion
Our study indicated that there was a high rate of Shigellosis and incidence of single Salmonella among children with diarrhea. Ampicillin, augmentin and tetracycline were resistant, while ciprofloxacin, ceftriaxone, ceftazidime, gentamycin, chloramphenicol, cefuroxime and cotrimoxazole were relatively sensitive. It was also found that those who practice hand-washing after defecation for some time, and store foods for later use in an open container were at risk of infection. Therefore, to alleviate this infection, the concerned body should be given health education for hand-washing after defecation and storing food in a closed container for later use.
Supplemental Material
Supplemental material, sj-pdf-1-smo-10.1177_20503121211009729 for Magnitude, risk factors and antimicrobial susceptibility pattern of Shigella and Salmonella, among children with diarrhea in Southern Ethiopia: A Cross-sectional Study by Manamo Hayamo, Tsegaye Alemayehu, Bereket Tadesse, Enkosilassie Mitiku and Zufan Bedawi in SAGE Open Medicine
Acknowledgments
We would like to acknowledge Hawassa University, SNNPR Health Bureau, Alamura Health Center and Hawassa University Comprehensive Specialized Hospital for their co-operation and study participants at large.
Footnotes
Author contributions: M.H., T.A., B.T., E.M., Z.B. equally conceived the idea, developed the proposal, collected the data, performed the analysis and prepared the manuscript; T.A. and Z.B. has made a final edition of the document. All authors have read and approved the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from *Hawassa University College of Medicine and Health Science Institutional Review Board (IRB/094/11)*.
Ethical clearance: The study was conducted after obtaining formal permission from the Southern Nation Nationality and People Regional Health Office, Hawassa City Administration Health Office, Alamura Health Center Manager and Laboratory Head. The patients were included in the study only when their parents or caretakers agree to the investigation and sign the consent. Culture results and antimicrobial susceptibility results were communicated to the concerned bodies in the health center within 72 h.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially supported for data collection by Hawassa University.
Informed consent: Written informed consent was obtained from all subjects before the study.
Availability of data and material: All the data supporting the findings can be obtained from the corresponding author.
ORCID iD: Tsegaye Alemayehu  https://orcid.org/0000-0001-7579-8991
https://orcid.org/0000-0001-7579-8991
Supplemental material: Supplemental material for this article is available online (https://www.researchsquare.com/article/rs-12803/v1).
References
- 1. Kefyalew S, Kebede G, Keneni A. Prevalence of Shigella related diarrhea in Ambo town and antibiotic susceptibility of the isolated strains. Greener J Epidemiol Public Health 2015; 3(1): 001–006. [Google Scholar]
- 2. Troeger C, Khalil IA, Reiner RC., Jr. Estimating health-loss due to enteric pathogens: importance and challenges. Lancet Glob Health 2019; 7(3): e284–e285. [DOI] [PubMed] [Google Scholar]
- 3. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrheal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382(9888): 209–222. [DOI] [PubMed] [Google Scholar]
- 4. UNICEF Data. Monitoring the situation of children and women, https://data.unicef.org/topic/childhealth/diarrhoeal-disease
- 5. Abebe W, Earsido A, Taye S, et al. Prevalence and antibiotic susceptibility patterns of Shigella and Salmonella among children aged below five years with diarrhea attending Nigist Eleni Mohammed memorial hospital, South Ethiopia. BMC Paediatr 2018; 18(1): 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alemu A, Geta M, Taye S, et al. Prevalence, associated risk factors and antimicrobial susceptibility patterns of Shigella infections among diarrheic pediatric population attending at Gondar town healthcare institutions, Northwest Ethiopia. Trop Dis Travel Med Vaccine 2019; 5(1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ameya G, Tsalla T, Getu F, et al. Antimicrobial susceptibility pattern, and associated factors of Salmonella and Shigella infections among under-five children in Arba Minch, South Ethiopia. Ann Clin Microbiol Antimicro 2018; 17(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Assefa A, Girma M. Prevalence and antimicrobial susceptibility patterns of Salmonella and Shigella isolate among children aged below five years with diarrhea attending Robe General Hospital and Goba Referral Hospital, South East Ethiopia. Trop Dis Travel Med Vaccine 2019; 5(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdullahi M. Incidence and antimicrobial susceptibility pattern of Salmonella species in children attending some hospitals in Kano metropolis, Kano state–Nigeria. Bayero J Pure Appl Sci 2010; 3(1): 58787. [Google Scholar]
- 10. Yildiz C, Öztürk C, Emekdas G. Research of the E. coli 0157: H7 strains cases of the gastro-enteritis. Infeksiyon Dergisi 2005; 19: 189–192. [Google Scholar]
- 11. Shao Y, Zhu S, Jin C, et al. Development of multiplex loop-mediated isothermal amplification-RFLP (mLAMP-RFLP) to detect Salmonella spp. and Shigella spp. in milk. Int J Food Microbiol 2011; 148(2): 75–79. [DOI] [PubMed] [Google Scholar]
- 12. Taneja N, Mewara A. Shigellosis: epidemiology in India. Indian J Med Res 2016; 143(5): 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huilan S, Zhen LG, Mathan M, et al. Etiology of acute diarrhea among children in developing countries: a multicentre study in five countries. Bull World Health Org 1991; 69(5): 549. [PMC free article] [PubMed] [Google Scholar]
- 14. Lauri A, Castiglioni B, Mariani P. Comprehensive analysis of Salmonella sequence polymorphisms and development of a LDR-UA assay for the detection and characterization of selected serotypes. Appl Microbiol Biotechnol 2011; 91(1): 189–210. [DOI] [PubMed] [Google Scholar]
- 15. Okeke IN, Klugman KP, Bhutta ZA, et al. Antimicrobial resistance in developing countries. Part II: strategies for containment. Lancet Infect Dis 2005; 5(9): 568–580. [DOI] [PubMed] [Google Scholar]
- 16. Pawlowski SW, Warren CA, Guerrant R. Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology 2009; 136(6): 1874–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beyene G, Tasew H. Prevalence of intestinal parasite, Shigella and Salmonella species among diarrheal children in Jimma health center, Jimma southwest Ethiopia: a cross-sectional study. Ann Clin Microbiol Antimicro 2014; 13(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pickering LK, Bartlett AV, Woodward WE. Acute infectious diarrhea among children in daycare: epidemiology and control. Clin Infect Dis 1986; 8(4): 539–547. [DOI] [PubMed] [Google Scholar]
- 19. Girma G. Prevalence, antibiogram and growth potential of Salmonella and Shigella in Ethiopia: implications for public health: a review. Res J Microbiol 2015; 10: 288–307. [Google Scholar]
- 20. Wikipedia. The Free Encyclopedia, https://en.wikipedia.org/wiki/Awasa_Zuria
- 21. Mulatu G, Beyene G, Zeynudin A. Prevalence of Shigella, Salmonella and Campylobacter species and their susceptibility patters among under-five children with diarrhea in Hawassa town, South Ethiopia. Ethiop J Health Sci 2014; 24(2): 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheesbrough M. Microbiological tests. District Laboratory Practice in Tropical Countries, Part 2, 2nd ed. Cambridge, Cambridge University Press, 2000. [Google Scholar]
- 23. M100:2018. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 24. Vargas M, Gascon J, Casals C, et al. Etiology of diarrhea in children less than five years of age in Ifakara, Tanzania. Am J Trop Med Hyg 2004; 70(5): 536–539. [PubMed] [Google Scholar]
- 25. Vandepitte J, Verhaegen J, Engbaek K, et al. Basic laboratory procedures in clinical bacteriology. Geneva: World Health Organization, 2003. [Google Scholar]
- 26. Beyene G, Haile-Amlak A. Antimicrobial sensitivity pattern of Campylobacter species among children in Jimma University Specialized Hospital, southwest Ethiopia. Ethiop J Health Develop 2004; 18(3): 185–189. [Google Scholar]
- 27. Teshome B, Teklemariam Z, Admassu Ayana D, et al. Salmonella and Shigella among patients with diarrhea at public health facilities in Adama, Ethiopia: prevalence, antimicrobial susceptibility pattern, and associated factors. SAGE Open Med 2019; 7: 2050312119846041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Terfassa A, Jida M. Prevalence and antibiotics susceptibility pattern of Salmonella and Shigella species among diarrheal patients attending Nekemte Referral Hospital, Oromia, Ethiopia. Int J Microbiol 2018; 2018: 9214689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nitiema LW, Nordgren J, Ouermi D, et al. Burden of rotavirus and other enteropathogens among children with diarrhea in Burkina Faso. Int J Infect Dis 2011; 15(9): e646–e652. [DOI] [PubMed] [Google Scholar]
- 30. Mbuthia OW, Mathenge SG, Oyaro MO, et al. Etiology and pathogenicity of bacterial isolates: a cross-sectional study among diarrheal children below five years in central regions of Kenya. Pan African Med J 2018; 31: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ngoshe IY, Denue BA, Bello HS, et al. Prevalence and antimicrobial susceptibility of Shigella species isolates from the diarrheal stool of patients in a tertiary health facility in northeastern Nigeria. Sub-Saharan African J Med 2017; 4(4): 96. [Google Scholar]
- 32. Mamuye Y, Metaferia G, Birhanu A, et al. Isolation and antibiotic susceptibility patterns of Shigella and Salmonella among under 5 children with acute diarrhoea: a cross-sectional study at selected public health facilities in Addis Ababa, Ethiopia. Clin Microbiol 2015; 4: 186. [Google Scholar]
- 33. Admassu M, Yemane G, Kibret M, et al. Prevalence and antibiogram of Shigella and Salmonella spp. from under-five children with acute diarrhea in Bahir Dar Town. Ethiop J Sci Tech 2015; 8(1): 27–35. [Google Scholar]
- 34. Qu M, Lv B, Zhang X, et al. Prevalence and antibiotic resistance of bacterial pathogens isolated from childhood diarrhea in Beijing, China (2010–2014). Gut Pathog 2016; 8: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tosisa W, Mihret A, Ararsa A, et al. Prevalence and antimicrobial susceptibility of Salmonella and Shigella species isolated from diarrheic children in Ambo town. BMC Paediatr 2020; 20(1): 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kahsay AG, Teklemariam Z. Prevalence of Shigella among diarrheic children under-5 years of age attending at Mekelle health center, north Ethiopia. BMC Res Notes 2015; 8(1): 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Urio E, Collison E, Gashe B, et al. Shigella and Salmonella strains isolated from children under 5 years in Gaborone, Botswana, and their antibiotic susceptibility patterns. Trop Med Int Health 2001; 6(1): 55–59. [DOI] [PubMed] [Google Scholar]
- 38. Wilson G, Easow JM, Mukhopadhyay C, et al. Isolation & antimicrobial susceptibility of Shigella from patients with acute gastroenteritis in western Nepal. Indian J Med Res 2006; 123(2): 145. [PubMed] [Google Scholar]
- 39. Lango-Yaya E, Djeintote M, Djimeli C. Contribution to the study of antibiotic resistance on Salmonella and Shigella strains isolated in the central African Republic. J Microbiol Exp 2017; 4(1): 00105. [Google Scholar]
- 40. Akinnibosun F, Nwafor F. Prevalence of diarrhea and antibiotic susceptibility test in children below 5 years at University of Benin Teaching Hospital, Nigeria. Int Res J Public Environ Health 2015; 2(4): 49–55. [Google Scholar]
- 41. Hawaz H, Girma S, Tezera Y, et al. Prevalence and drug susceptibility pattern of Shigella and Salmonella species in under ten diarrhoeic children admitted to Tirunesh-Beijing hospital. Int J Sci Rep 2017; 3(2): 637. [Google Scholar]
- 42. Lamboro T, Ketema T, Bacha K. Prevalence and antimicrobial resistance in Salmonella and Shigella species isolated from outpatients, Jimma University Specialized Hospital, Southwest Ethiopia. Can J Infect Dis Med Microbiol 2016; 2016: 4210760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mache A. Antibiotic resistance and serogroups of Shigella among paediatric out-patients in southern Ethiopia. East African Med J 2001; 78(6): 296–299. [DOI] [PubMed] [Google Scholar]
- 44. Debas G, Kibret M, Biadglegne F, et al. Prevalence and antimicrobial susceptibility patterns of Shigella species at Felege Hiwot Referral Hospital, Northwest Ethiopia. Ethiop Med J 2011; 49(3): 249–256. [PubMed] [Google Scholar]
- 45. Mekonnen H, Kebede A, Menkir S. Isolation rate and drug resistance patterns of Shigella species among diarrheal patients attending at Hiwot Fana hospital, Harar, Ethiopia. Ethiop J Sci Tech 2014; 7(1): 15–25. [Google Scholar]
- 46. Sarnie A, Guerrant R, Barrett L, et al. Prevalence of intestinal parasitic and bacterial pathogens in diarrheal and non-diarrheal human stools from Vhembe district, South Africa. J Health Popul Nutr 2009; 27(6): 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saeed A, Abd H, Sandstrom G. Microbial aetiology of acute diarrhea in children under five years of age in Khartoum, Sudan. J Med Microbiol 2015; 64(Pt. 4): 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reda AA, Seyoum B, Yimam J, et al. Antibiotic susceptibility patterns of Salmonella and Shigella isolates in Harar, Eastern Ethiopia. J Infect Dis Immun 2011; 3(8): 134–139. [Google Scholar]
- 49. Gebrekidan A, Dejene TA, Kahsay G, et al. Prevalence and antimicrobial susceptibility patterns of Shigella among acute diarrheal outpatients in Mekelle hospital, Northern Ethiopia. BMC Res Notes 2015; 8(1): 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Demissie A, Wubie T, Yehuala FM, et al. Prevalence and antimicrobial susceptibility patterns of Shigella and Salmonella species among patients with diarrhea attending Gondar Town Health Institutions, Northwest Ethiopia. Sci J Pub Health 2014; 2(5): 469–475. [Google Scholar]
- 51. Ansari S, Sherchand J, Parajuli K, et al. Bacterial aetiology of acute diarrhea in children under five years of age. J Nepal Health Res Council 2012; 10(22): 218–223. [PubMed] [Google Scholar]
- 52. Mengistu G, Mulugeta G, Lema T, et al. Prevalence and antimicrobial susceptibility patterns of Salmonella serovars and Shigella species. J Microb Biochem Technol 2014; 6(S2): S2006. [Google Scholar]
- 53. Shah M, Kathiiko C, Wada A, et al. Prevalence, seasonal variation, and antibiotic resistance pattern of enteric bacterial pathogens among hospitalized diarrheic children in suburban regions of central Kenya. Trop Med Health 2016; 44: 39–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kara TT, Özdemir H, Kurt F, et al. Prevalence of Salmonella and Shigella spp. and antibiotic resistance status in acute childhood gastroenteritis/Akut Çocukluk Çagi Gastroenteritlerindeki Salmonella-Shigella Sikligi ve Antibiyotik Direnç Durumlari. Cocuk Enfeksiyon Dergisi 2015; 9(3): 102–107. [Google Scholar]
- 55. Huruy K, Kassu A, Mulu A, et al. Intestinal parasitosis and Shigellosis among diarrheal patients in Gondar teaching hospital, northwest Ethiopia. BMC Res Notes 2011; 4(1): 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Soltan Dallal MM, Ehrampoush MH, Aminharati F, et al. Associations between climatic parameters and the human Salmonellosis in Yazd province, Iran. Environ Res 2020; 187: 109706. [DOI] [PubMed] [Google Scholar]
- 57. Yismaw GNC, Kassu A. A five-year antimicrobial resistance pattern observed in Shigella species isolated from stool samples in Gondar University hospital Northwest Ethiopia. Ethiop J Health Develop 2006; 20(3): 194–198. [Google Scholar]
- 58. Gebreegziabher G, Asrat D, W/Amanuel Y, et al. Isolation and antimicrobial susceptibility profile of Shigella and Salmonella species from children with acute diarrhoea in Mekelle Hospital and Semen Health Center, Ethiopia. Ethiop J Health Sci 2018; 28(2): 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Efuntoye MO, Adenuga A. Shigella serotypes among nursery and primary school children with diarrhea in Ago-Iwoye and Ijebu-Igbo, Southwestern Nigeria. J Phys: Conf Ser 2011; 2: 29–32. [Google Scholar]
- 60. Roma B, Worku S, Mariam ST, et al. Antimicrobial susceptibility pattern of Shigella isolates in Awassa. Ethiopian J Health Develop 2000; 14(2): 149–154. [Google Scholar]
- 61. Tiruneh M. Serodiversity and antimicrobial resistance pattern of Shigella isolates at Gondar University teaching hospital, Northwest Ethiopia. Jpn J Infect Dis 2009; 62(2): 93–97. [PubMed] [Google Scholar]
- 62. Asrat D. Shigella and Salmonella serogroups and their antibiotic susceptibility patterns in Ethiopia. East Mediterr Health J 2008; 14(4): 760–767. [PubMed] [Google Scholar]
- 63. Choi SYJY, Jeon YS, Lee JH, et al. Multilocus sequence typing analysis of Shigella flexneri isolates collected in Asian countries. J Med Microbiol 2007; 56(Pt. 11): 1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yan HLL, Alam MJ, Shinoda S, et al. Prevalence and antimicrobial resistance of Salmonella in retail foods in northern China. Int J Food Microbiol 2010; 143(3): 230–234. [DOI] [PubMed] [Google Scholar]
- 65. Chompook PTJ, Todd J, Wheeler JG, et al. Risk factors for Shigellosis in Thailand. Int J Infect Dis 2006; 10(6): 425–433. [DOI] [PubMed] [Google Scholar]
- 66. Nitiema LW, Nordgren J, Ouermi D, et al. Burden of rotavirus and other enteropathogens among children with diarrhea in Burkina Faso. Int J Infect Dis 2011; 15: e646–1e52. [DOI] [PubMed] [Google Scholar]
- 67. Pornthip Chompook JT, Jeremy G. Wheeler, Lorenz von Seidlein, John Clemens, Wanpen Chaicumpa. Risk factors for Shigellosis in Thailand. Int J Infect Dis 2006; 10: 425–433. [DOI] [PubMed] [Google Scholar]
- 68. Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol 2016; 37(6): 386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jones KD, Thitiri J, Ngari M, et al. Childhood malnutrition: toward an understanding of infections, inflammation, and antimicrobials. Food Nutr Bull 2014; 35(2Suppl): S64–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Prendergast AJ, Humphrey JH, Mutasa K, et al. Assessment of environmental enteric dysfunction in the SHINE trial: methods and challenges. Clin Infect Dis 2015; 61(suppl_7): S726–S732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rytter MJH, Kolte L, Briend A, et al. The immune system in children with malnutrition—a systematic review. PLoS ONE 2014; 9(8): e105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dangour AD, Watson L, Cumming O, et al. Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children. Cochrane Database Syst Rev 2013; 8: CD009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ngure FM, Reid BM, Humphrey JH, et al. Water, sanitation, and hygiene (WASH), environmental enteropathy, nutrition, and early child development: making the links. Ann N Y Acad Sci 2014; 1308: 118–128. [DOI] [PubMed] [Google Scholar]
- 74. Mama MAG. Prevalence, antimicrobial susceptibility patterns and associated risk factors of Shigella and Salmonella among food handlers in Arba Minch University, South Ethiopia. BMC Infect Dis 2016; 16(1): 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Heilmann M. Flies as vectors for Salmonella spp. and their control in pork butcheries in Kampala, Uganda–A contribution to improving public health. PhD Thesis, Freie Universitaet Berlin, Berlin, 2016
- 76. Cohen D, Green M, Block C, et al. Reduction of transmission of Shigellosis by control of houseflies (Musca domestica). Lancet 1991; 337(8748): 993–997. [DOI] [PubMed] [Google Scholar]
- 77. Tachbele E, Erku W, Gebre-Michael T, et al. Cockroach-associated food-borne bacterial pathogens from some hospitals and restaurants in Addis Ababa, Ethiopia: distribution and antibiograms. J Rural Tropical Public Health 2006; 5(1): 34–41. [Google Scholar]
- 78. Ribas A, Saijuntha W, Agatsuma T, et al. Rodents as a source of Salmonella contamination in wet markets in Thailand. Vector Borne Zoonotic Dis 2016; 16(8): 537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nashwa OK, Ebtesam MM. The possible role of rodent in transmission of salmonellosis to man. Suez Canal Vet Med J 2007; XII(2): 209–216. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-smo-10.1177_20503121211009729 for Magnitude, risk factors and antimicrobial susceptibility pattern of Shigella and Salmonella, among children with diarrhea in Southern Ethiopia: A Cross-sectional Study by Manamo Hayamo, Tsegaye Alemayehu, Bereket Tadesse, Enkosilassie Mitiku and Zufan Bedawi in SAGE Open Medicine


