Abstract
Objective
Parkinson’s disease (PD) is a degenerative disorder characterized by steady motor function loss. PD pathogenesis remains inconclusive, but aberrant immune responses might play important roles. We hypothesized that imbalance between T helper (Th) 1 and regulatory T (Treg) cells was essential in experimental PD.
Methods
Th1 and Treg cells from the blood of patients with PD and healthy volunteer blood were measured by flow cytometry. Experimental PD was induced in mice by peritoneal injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Experimental PD severity was measured by open field test behavior assessments (distance moved, rearing, and grooming). Mice were administered neutralizing anti-tumor necrosis factor (TNF) α to inhibit Th1 effects. Treg cells were depleted by anti-CD25 neutralizing antibodies or isolated and transferred to experimental PD mice.
Results
Patients with PD had fewer Treg and more Th1 cells than healthy volunteers. Experimental PD mice exhibited fewer Treg and more Th1 cells. Treg cell depletion exacerbated experimental PD, whereas TNFα neutralization attenuated PD in mice. Treg transfer to experimental PD mice reduced PD severity. Mechanistically, anti-TNFα antibody administration and Treg transfer increased Treg and reduced Th1 cell abundance in the brain.
Conclusion
Th1 and Treg cell imbalance plays an essential role in mouse experimental PD pathogenesis.
Keywords: Parkinson’s disease, immune response, T helper 1 cell, regulatory T cell, tumor necrosis factor, CD25
Introduction
Parkinson’s disease (PD) is a progressive degenerative condition of the central nervous system.1 As a significant burden on society overall, PD affects a broad geriatric population and leads to the steady loss of motor functions. The hallmarks of PD are resting tremor, bradykinesia, rigidity, balance impairment, akinesia, and other symptoms and signs.2 One important pathogenesis of PD is related to the lack of dopamine-producing neurons in the substantia nigra pars compacta region (SNpc).3 The reduced number of dopaminergic neurons in the SNpc causes decreased dopamine levels in the striatum, which subsequently leads to motor impairment.
There are many pathological findings in patients with PD, including inflammatory immune cell infiltration of lesions of the central nervous system. For instance, microglia, a specific type of macrophage, are overactivated in the brain tissue of patients with PD.4 These overactivated microglia express human leukocyte antigen DR molecules, indicating their ability to present antigens to and activate cellular components in the adaptive immune system, likely T cells. Although tissue memory CD8+ T cells have been reported to exist in healthy human brain tissue,5,6 it is still unclear whether CD4+ T cells in the murine central nervous system exhibit a normal state.7,8 Notably, T cells of the central nervous system are sporadically present in the brain parenchyma of healthy human beings.9,10 However, our group and others11–13 have observed significant T cell infiltration in brain samples of patients with PD. These important results indicate that T cell activation and recruitment in the parenchyma of the central nervous system play a critical role in the pathogenesis of PD.
T helper (Th) cells, including Th1, Th2, and Th17, and regulatory T (Treg) cells as well as other subtypes participate in inflammatory processes.14 Th cells are particularly involved in the pathogenesis of PD. For instance, CD4+ T cells have been reported to differentiate toward a Th1 phenotype in patients with PD,15 indicating that Th1 cells might mediate PD pathogenesis. Moreover, regulatory immune cells including Treg cells have been observed to be decreased in the peripheral blood of patients with PD16 compared with healthy volunteers, suggesting that a lack of regulatory immune cells might also contribute to the development of PD.
Although both Th1 and Treg cells might be involved in the pathogenesis of PD, whether the imbalance between Th1 and Treg cells affects the pathogenesis of PD remains elusive. In this study, we tested our hypothesis that an imbalance involving increased Th1 and decreased Treg cell numbers is detrimental in experimental PD. Here, we demonstrate that the imbalance between Th1 and Treg cells exists in both patients with PD and mice with experimental PD. More importantly, restoration of the balance between Th1 and Treg cells attenuated the severity of experimental PD in mice. Therefore, the imbalance between Th1 and Treg cells is essential for the pathogenesis of experimental PD in mice. Strategies that restore this balance might be a therapeutic option for patients with PD in the near future.
Methods
Ethics approval and consent to participate
The ethics committee of Ningbo First Hospital in China approved and supervised the experimental proposal (approval number 2017-R044), which covered both human and animal studies. Medical proxies of patients with PD and healthy volunteers agreed to participation and signed written consent forms. The proxies of patients and healthy volunteers agreed to the publication of this research report.
Patients
Twenty patients diagnosed with PD were included in this study. Twenty age-matched healthy volunteers were also recruited. The sex ratio was 1:1 across the cohort (Table 1). The patients were admitted to Ningbo First Hospital in China between March and June of 2018 and were diagnosed according to the International Parkinson and Movement Disorder Society criteria.17,18 Healthy volunteers were recruited within the same timeframe and were determined to be free of PD and other neurological disorders.
Table 1.
Characteristics of patients with Parkinson’s disease and volunteers.
| Patients with Parkinson’s disease (n = 20) | Healthy volunteers (n = 20) | p value | |
|---|---|---|---|
| Sex (male, %) | 50 | 50 | 1 |
| Age (years; median, range) | 74.5, 50–79 | 72, 50–78 | 0.2069 |
Animals
Male C57/Bl6 mice, aged 10 to 12 weeks, were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Animals were kept in a specific pathogen-free environment with a controlled temperature (23°C) and humidity (40%–60%) and 12-hour light/dark cycles. Mice were allowed ad libitum access to food and autoclaved tap water. Experimental PD was induced in mice by intraperitoneal injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; Sigma-Aldrich, Shanghai, China) in accordance with a previous study.13 Briefly, 20 mg/kg of MPTP in 100 µL of normal saline was intraperitoneally injected four times at 2-hour intervals. Mice receiving normal saline served as controls. Mice were sacrificed by cervical dislocation under isoflurane anesthesia at 96 hours after the last injection.
Flow cytometry
Leukocytes were collected from the circulation of patients with PD and healthy volunteers and incubated with antibodies for flow cytometry analysis in accordance with a previously published protocol.19 Briefly, the peripheral blood was collected in K2-EDTA-anticoagulated tubes (BD Vacutainer™, Fisher Scientific Company, Ottawa, ON, Canada). A total of 200 μL of whole blood was transferred to a new tube, and 4 mL of RBC lysis buffer (BioLegend, San Diego, CA, USA) was added to the tube for 15 minutes. After washing with Cell Staining Buffer (BioLegend), the cells were incubated with anti-human CD16/CD32 antibody (1:200; eBioscience/Thermo Fisher, Waltham, MA, USA). Circulating leukocytes were incubated with antibodies against surface markers at 4°C for 30 minutes. Intracellular FoxP3 staining was performed after fixation/permeabilization using a commercial kit (eBioscience/Thermo Fisher). The antibodies used for staining are listed in Tables 2 and 3. Treg cells were defined as CD3+CD4+CD25+FoxP3+ cells, whereas Th1 cells were identified as CD3+CD4+T-bet+ cells. The percentages of Treg and Th1 cells among total CD4+ T cells were calculated in various organs.
Table 2.
Anti-mouse antibodies used for flow cytometry.
| Antibody | Clone | Manufacturer | Titration | Catalog Number |
|---|---|---|---|---|
| CD4-FITC | RM4-5 | eBioscience | 1:100 | 11-0042-85 |
| CD25-PE-Cy7 | PC61.5 | eBioscience | 1:100 | 25-0251-82 |
| FoxP3-AF700 | FJK-16s | eBioscience | 1:100 | 56-5773-82 |
| T-bet-PerCP-Cy5.5 | 4B10 | eBioscience | 1:100 | 45-5825-82 |
| CD3e-eF450 | 145-2C1 | eBioscience | 1:100 | 48-0031-82 |
Table 3.
Anti-human antibodies used for flow cytometry.
| Antibody | Clone | Manufacturer | Titration | Catalog Number |
|---|---|---|---|---|
| CD4-FITC | RPA-T4 | eBioscience | 1:100 | 11-0049-80 |
| CD25-PE-Cy7 | BC96 | eBioscience | 1:100 | 25-0259-42 |
| FoxP3-AF700 | PCH101 | eBioscience | 1:100 | 56-4776-41 |
| T-bet-PerCP-Cy5.5 | 4B10 | eBioscience | 1:100 | 45-5825-82 |
| CD3e-eF450 | OKT3 | eBioscience | 1:100 | 48-0037-42 |
Brain tissue from mice was digested using a commercial kit for brain tissue dissociation (Miltenyi Biotec, Bergisch Gladbach, Germany) to produce single-cell suspensions. After incubation with anti-mouse CD16/CD32 antibodies (1:200; eBioscience/Thermo Fisher), brain cells were incubated with antibodies against surface markers, and intracellular FoxP3 staining was performed similar to the procedure used for circulating leukocytes.
Cells were then analyzed with an Attune NxT flow cytometer (Applied Biosystems/Thermo Fisher). Data were analyzed using Kaluza software version 2.0 (Beckman Coulter, Indianapolis, IN, USA).
Open field tests
Behavioral assessments were conducted 96 hours after the last MPTP injection. The open field test was used to measure behavior because it is the most common behavioral assay of MPTP-treated mice.20,21 All field tests were conducted between 12:00 pm and 2:00 pm in normal lighting using a white plastic rectangular box (80 × 40×20 cm3). The bottom of the box contained a 5 × 5-cm2 grid of squares. The total distance moved by the mouse was determined by counting the number of squares. Rearing, a proxy of exploratory activity, and grooming, a surrogate of the displacement response, were recorded when a mouse was placed in the box for 5 minutes in accordance with a previously published article.22
Isolation and transfer of CD4+CD25+ cells
Single-cell suspensions from the mouse spleen were produced by mechanical isolation using 100-µm cell strainers. Circulating leukocytes from mice were obtained by drawing blood via cardiac puncture and applying RBC lysis buffer. CD25+ cells from the above tissues were isolated using a commercial CD4+CD25+ Treg cell isolation kit (STEMCELL, Vancouver, BC, Canada) following the manufacturer’s instructions. The purity of isolated CD4+CD25+ cells was between 90% and 95%. A total of 2 × 105 CD4+CD25+ Treg cells in 200 µL of phosphate buffered saline (PBS) were transferred to a recipient mouse by intraperitoneal injection 1 day before MPTP treatment, in accordance with an optimized protocol from a previously published paper.23
Neutralizing antibody treatment
To deplete Treg cells, mice were intraperitoneally injected with anti-CD25 neutralizing antibodies (0.5 mg/day, clone: PC-61.5.3; BioXcell, NH, USA) on days −5, −3, and −1 before MPTP treatment and days 1 and 3 after administration, in accordance with a previous publication.24 Control mice received the corresponding isotype control antibodies (rat IgG1, clone: HRPN; BioXCell) following the same regimen used for anti-CD25 antibodies. The depletion efficacy of CD25+ cells was confirmed in the blood and spleen by flow cytometry in a separate experiment.
To block the effects of tumor necrosis factor (TNF) α, 100 μg of an anti-TNFα antibody (clone: TN3-19.12; eBioscience/Thermo Fisher) was peritoneally injected on days −7 and −3 and the day of MPTP injection. Control mice received isotype control antibodies (Armenian Hamster IgG, clone: eBio299Arm; eBioscience/Thermo Fisher) according to the same schedule and dosage used for anti-TNFα antibodies.
Statistical analysis
Data are presented as mean ± standard deviation. Data were analyzed using either Student’s t tests for two groups or one-way analysis of variance (ANOVA) with Bonferroni’s post hoc test for more than two groups. A p value of less than 0.05 was considered to be statistically significant. Statistical analyses were conducted using GraphPad Prism 7 (GraphPad Software, Inc., San Diego, CA, USA).
Results
Patients with PD and mice with experimental PD exhibited lower Treg cell levels in the circulation
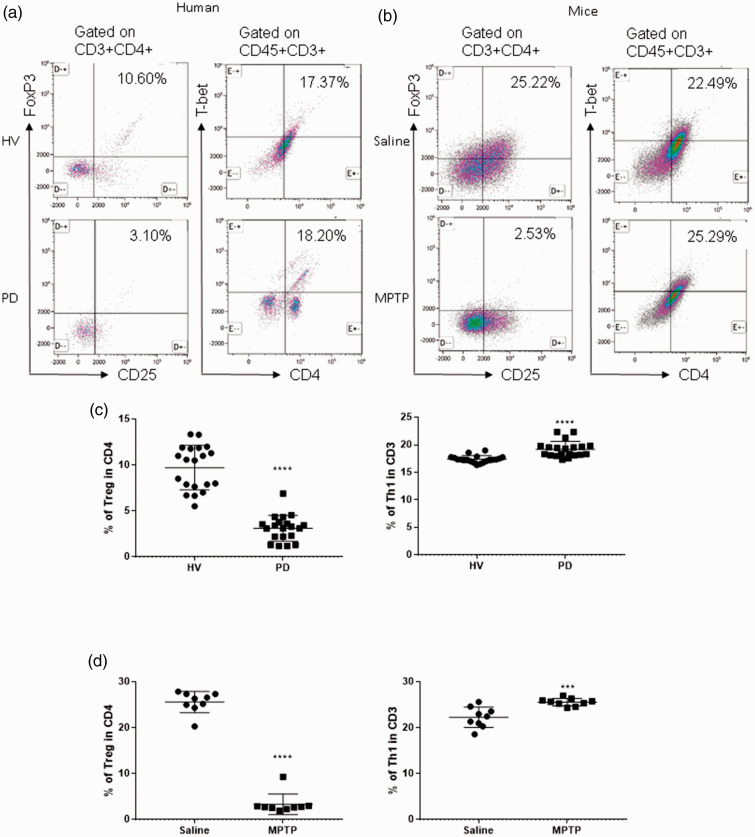
To determine the abundance of circulating Treg cells in patients with PD, we collected peripheral venous blood from patients with PD and age-matched healthy volunteers and measured Treg cell levels by flow cytometry. Treg cells were defined as CD3+CD4+CD25+FoxP3+ cells. As shown in Figure 1, patients with PD harbored fewer Treg cells in the circulation (healthy volunteers vs patients with PD, 9.723% ± 0.54% vs 3.114% ± 0.311%, p < 0.0001). It is worth noting that patients with PD also had more Th1 cells, which were defined as CD3+CD4+T-bet+ cells (healthy volunteers vs patients with PD, 17.4% ± 0.13% vs 19.19% ± 0.32%, p < 0.0001), indicating an imbalance between proinflammatory Th1 cells and the regulatory subset of T cells. In mice with experimental PD, the percentage of Treg cells in the blood was lower than that of control mice (saline vs MPTP, 25.6% ± 0.77% vs 3.344% ± 0.75%, p < 0.0001, Figure 1d). The level of Th1 cells in the blood was also higher in mice with experimental PD (saline vs MPTP, 22.29% ± 0.73% vs 25.59% ± 0.27%, p < 0.001, Figure 1d). Therefore, the level of circulating Treg cells was reduced and the number of circulating Th 1 cells was increased in patients with PD and mice with experimental PD, suggesting that the imbalance between the two cell types might be involved in the pathogenesis of PD.
Figure 1.
Patients with Parkinson’s disease (PD) and mice with experimental PD exhibit decreased regulatory T (Treg) and increased T helper 1 (Th1) cell numbers in the blood. Treg cells were defined as CD3+CD4+CD25+FoxP3+ cells, whereas Th1 cells were identified as CD3+CD4+T-bet+ cells. (a) Representative plots of Treg and Th1 cells in patients with PD and healthy volunteers (HVs). (b) Representative plots of Treg and Th1 cells in control mice (Saline) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. (c) Patients with PD exhibited lower Treg cell levels and greater Th1 cell levels in the blood than HVs. n = 20. ****, p < 0.0001 by a two-tailed Student’s t tests. (d) MPTP-induced experimental PD mice had fewer Treg and more Th1 cells in the circulation than saline-treated mice. n = 9/group in each experiment performed in triplicate. ***, p < 0.001; ****, p < 0.0001, according to Student’s t test.
Depletion of CD25+ T cells exacerbated experimental PD in mice
To explore the roles of Treg cells in experimental PD in mice, we applied anti-CD25 neutralizing antibodies to deplete Treg cells according to a previously described protocol.24 Administration of anti-CD25 neutralizing antibodies effectively depleted Treg cells in the blood (Figure 2a) and the spleen (data not shown). Anti-CD25 neutralizing antibody administration alone did not induce the symptoms of experimental PD in mice. More importantly, depletion of Treg cells exacerbated the severity of experimental PD in mice (Figure 2b–d). Upon analysis of CD4+ T cell subsets in brain tissues, we found that PD mice treated with anti-CD25 neutralizing antibodies harbored virtually no Treg cells and more Th1 cells than PD mice treated with isotype control antibodies (Figure 2e–f).
Figure 2.
Depletion of regulatory T (Treg) cells exacerbates the severity of experimental Parkinson’s disease (PD) in mice. Treg cells were depleted by administration of anti-CD25 antibodies. (a) Treg cells were effectively depleted in the blood of mice treated with anti-CD25 antibodies. Mice with PD induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine traveled a shorter distance (b) and exhibited decreased rearing (c) and grooming (d) in open field tests. n = 9/group in each experiment performed in triplicate. n.s., not significant; ****, p < 0.0001, by one-way analysis of variance (ANOVA) with Bonferroni’s post hoc test. Experimental PD mice administered anti-CD25 antibodies harbored fewer Treg cells (e) and more Th1 cells (f) in the brain. Mice in Panels E and F were from the same experiments as those in Panels B–D.***, p < 0.001; ****, p < 0.0001, according to Student’s t test.
Anti-CD25, anti-CD25 antibody treatment; CTRL, control group.
Transfer of CD25+ Treg cells attenuated the severity of experimental PD
Next, we sought to directly show the protective roles of CD25+ T cells in mice with experimental PD. We isolated CD4+CD25+ T cells from the blood and spleen of untreated mice and those with experimental PD. According to our preliminary experiments, Treg cells isolated from experimental PD mice seemed to have identical functions in relieving PD in mice to Treg cells collected from healthy mice (data not shown); therefore, we used Treg cells enriched from healthy mice for the remainder of our experiments. After receiving CD25+ Treg cells, experimental PD mice exhibited attenuated disease severity compared with PD mice treated with PBS (Figure 3a–c). At the time of analysis, PD mice receiving CD25+ cells harbored more Treg cells in the brain than those treated with PBS (Figure 3d). These data indicate that adoptively transferred Treg cells are protective against experimental PD in mice.
Figure 3.
Transfer of regulatory T (Treg) cells to mice with experimental Parkinson’s disease (PD) attenuates disease severity. The isolation and transfer of Treg cells were conducted as described. Experimental PD mice that received Treg cells showed attenuated disease severity, demonstrated as an increase in the distance traveled (a), increased rearing (b), and a greater degree of grooming (c). n=9/group in each experiment performed in triplicate. ****, p<0.0001, by one-way analysis of variance (ANOVA) with Bonferroni’s post hoc test. (d) 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced experimental PD mice that received Treg cells had more Treg cells in the brain. The same animals are included in Panels A–C. ****, p<0.0001, according to Student’s t test.
CTRL, control group; PBS, phosphate buffered saline.
Anti-TNFα antibodies attenuated experimental PD in mice and had a synergetic effect with CD25+ cell transfer in relieving experimental PD
Anti-TNFα antibodies have been reported to reduce the risk of PD in patients with inflammatory bowel disease (IBD) compared with patients with IBD who did not undergo anti-TNFα treatment;25 therefore, we examined whether the application of anti-TNFα antibodies would improve experimental PD in mice. Compared with isotype control antibodies, anti-TNFα antibodies indeed attenuated the severity of experimental PD. More importantly, co-administration of anti-TNFα and transfer of CD4+CD25+ Treg cells from healthy mice had a synergetic effect in alleviating MPTP-induced PD in mice (Figure 4). These results further indicate the importance of the imbalance between Th1 and Treg cells in the pathogenesis of PD.
Figure 4.
Tumor necrosis factor (TNF) α blocking and regulatory T (Treg) cell transfer had a synergic effect in attenuating experimental Parkinson’s disease (PD) in mice. The schedule of anti-TNFα antibody treatment and Treg cell transfer is indicated in the Methods section. Either anti-TNFα antibody treatment or Treg cell transfer decreased the severity of experimental PD, but co-administration of these treatments showed the most significant effect, characterized as a longer distance traveled (a), greater rearing (b), and increased degree of grooming (c). n=9/group in each experiment performed in triplicate. *, p<0.05; ****, p<0.0001, by one-way analysis of variance (ANOVA) with Bonferroni’s post hoc test.
Anti-TNFα, anti-TNFα antibodies; CTRL, control group; PBS, phosphate buffered saline.
Anti-TNFα antibodies and CD4+CD25+ Treg cell transfer reduced Th1 cells and increased Treg cells in the brains of experimental PD mice
To explore the mechanisms of the protection mediated by anti-TNFα antibodies and transfer of CD4+CD25+ Treg cells in experimental PD mice, we determined the immunophenotype of brain tissue of experimental animals. As shown in Figure 5, experimental PD mice treated with anti-TNFα antibodies and CD4+CD25+ Treg cell transfer had fewer Th1 cells in the brain (Figure 5a). Furthermore, PD mice receiving the combination therapy had more Treg cells in brain lesions (Figure 5b). These data indicate that anti-TNFα antibodies and CD4+CD25+ Treg cell transfer could at least partially restore the imbalance between Th1 and Treg cells to attenuate experimental PD.
Figure 5.
Combined treatment of anti-tumor necrosis factor (TNF) α antibodies and regulatory T (Treg) cell transfer decreased the levels of T helper 1 (Th1) cells and increased numbers of Treg cells in the brain of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease (PD) mice. (a) The combination of anti-TNFα antibodies and Treg cell transfer resulted in fewer Th1 cells in the brains of mice with experimental PD. (b) The combination therapy increased the level of Treg cells in the brains of mice with experimental PD. n=9/group in each experiment performed in triplicate. *, p<0.05; ****, p<0.0001, by one-way analysis of variance (ANOVA) with Bonferroni’s post hoc test.
PBS, phosphate buffered saline.
Discussion
In this study, we demonstrate the essential roles of the imbalance between Th1 and Treg cells in the pathogenesis of experimental PD in mice. We discovered that depletion of Treg cells exacerbated experimental PD, whereas transfer of Treg cells attenuated experimental PD. Blockade of TNFα, an important Th1 cytokine, reduced the severity of experimental PD. More importantly, neutralization of TNFα and transfer of Treg cells had a synergic effect in improving experimental PD in mice. Mechanistically, administration of anti-TNFα antibodies and Treg cell transfer increased Treg cells and decreased Th1 cells in the brains of PD mice.
Inflammation, especially Th1-driven inflammation, plays an important role in central nervous system disorders. The precise etiology of PD, a progressive degenerative disease of the central nervous system, has yet to be fully understood. The hallmark of PD is the loss of dopamine-producing neurons in the SNpc.13 It has been speculated that inflammation in the central nervous system might result in the development of PD. Our group and others have reported that abnormal T cell infiltration is observed in the brain tissue of patients with PD.11–13 In this study, we revealed that patients with PD had elevated and decreased numbers of Th1 and Treg cells in the circulation, respectively. Similarly, mice with experimental PD harbored increased numbers of Th1 cells and decreased numbers of Treg cells in the blood (Figure 1d). These data indicate that the imbalance between Th1 and Treg cells might participate in the pathogenesis of PD.
We applied two strategies in this study to investigate the roles of Treg cells in the pathogenesis of experimental PD. First, we depleted CD25+ cells using anti-CD25 neutralizing antibodies. After depletion of Treg cells, which was verified in the blood (Figure 2a) and spleen (Supplemental Figure 1), the severity of experimental PD worsened (Figure 2b–d). In agreement with reports in which some protective roles in the treatment of rodent experimental PD were found to be dependent on Treg cells,26 the results in this study indicate the direct effects of the loss of Treg cells in experimental PD, demonstrating the important roles of Treg cells in both the treatment and pathogenesis of PD. In line with a recent report,27 we subsequently demonstrated that transfer of Treg cells attenuated experimental PD (Figure 3). To date, this report provides the most supportive evidence that Treg cells are protective against neural destruction during experimental PD.
Patients with PD exhibit Th1-driven inflammation, including increased TNFα levels. This Th1-dominant inflammation was also observed by our group and others.11–13 Currently, we report that the abundance of Th1 cells was increased in the circulation of patients with PD as well as in mice with experimental PD, indicating the possible involvement of Th1-driven inflammation in the pathogenesis of PD. More importantly, inhibition of TNFα, a key Th1 cytokine, attenuated the severity of experimental PD in mice (Figure 4). These results provide evidence that Th1-driven inflammation might be a therapeutic target, in line with a previous clinical observation.25
Furthermore, when both anti-TNFα neutralizing antibodies and Treg transfer were applied, experimental PD mice exhibited the greatest attenuation of disease severity. Mice treated with the combination therapy harbored fewer Th1 cells and more Treg cells in the brain, indicating that the combination therapy might attenuate experimental PD by increasing the abundance of Treg cells and/or decreasing that of Th1 cells in the central nervous system.
This study has several unanswered questions. First, the precise effect of Th1 cells in mediating the pathogenesis of experimental PD has yet to be explored. Because blockade of TNFα signaling attenuates the severity of MPTP-induced PD, it is possible that Th1 cells infiltrate into the lesions in the central nervous system and produce TNFα. TNFα has been reported to be increased in patients with PD28 and mice with experimental PD;29,30 therefore, it is reasonable to speculate that overexpression of TNFα directly or indirectly acts on dopamine-producing neurons, which initiates cell death pathways in those neurons. Loss of dopamine-producing neurons leads to experimental PD. When TNFα is neutralized by antibodies, cell death in the brain is decreased. However, this theory requires further investigation. Second, we did not perform functional assays of Treg cells. Treg cells interact with effector T cells, including Th1 cells, to induce their suppression.31 Further investigation is required to determine whether Treg cells in experimental PD can suppress Th1 cells. Third, in this hypothesis-driven study, we only explored the functions of Th1 and Treg cells. Other immune cells, including microglia,4 also participate in the pathogenesis of PD. Therefore, it is important to study the role of other immune cells in the context of anti-TNFα and Treg transfer therapy. More importantly, the efficacy and safety of anti-TNFα antibodies in patients with PD are worthy of exploration.
In conclusion, the imbalance between Th1 and Treg cells plays an essential role in the pathogenesis of experimental PD. Restoration of this balance might be a therapeutic target for patients with PD in the near future.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060521998471 for Imbalance between T helper 1 and regulatory T cells plays a detrimental role in experimental Parkinson’s disease in mice by Wenjie Li, Yuan Luo, Hongyu Xu, Qianqian Ma and Qi Yao in Journal of International Medical Research
Footnotes
Author contributions: Dr. Wenjie Li conducted most of the experiments, including the animal work and flow cytometry. Drs. Yuan Luo, Hongyu Xu, and Qianqian Ma collected the clinical samples and performed flow cytometry. Dr. Qi Yao designed and supervised the study. Dr. Wenjie Li composed the first draft of this manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was supported by Zhejiang Province Chinese Medicine Program China (grant number: 2018ZA108). The funder had no role in the experimental design, data collection and analysis, manuscript composition, or publication decision.
ORCID iD: Qi Yao https://orcid.org/0000-0002-3786-2814
Supplemental material: Supplemental material for this article is available online.
References
- 1.The Lancet. Parkinson's disease: a complex disease revisited. Lancet 2017; 390: 430. DOI: 10.1016/S0140-6736(17)31997-9. [DOI] [PubMed] [Google Scholar]
- 2.Pringsheim T, Jette N, Frolkis A, et al. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2014; 29: 1583–1590. DOI: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 3.Fasano M, Lopiano L. Alpha-synuclein and Parkinson's disease: a proteomic view. Expert Rev Proteomics 2008; 5: 239–248. DOI: 10.1586/14789450.5.2.239. [DOI] [PubMed] [Google Scholar]
- 4.Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson's disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol 2007; 208: 1–25. DOI: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolders J, Heutinck KM, Fransen NL, et al. Tissue-resident memory T cells populate the human brain. Nat Commun 2018; 9: 4593. DOI: 10.1038/s41467-018-07053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 2012; 12: 623–635. DOI: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz M, Kipnis J, Rivest S, et al. How do immune cells support and shape the brain in health, disease, and aging? J Neurosci 2013; 33: 17587–17596. DOI: 10.1523/JNEUROSCI.3241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radjavi A, Smirnov I, Derecki N, et al. Dynamics of the meningeal CD4(+) T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol Psychiatry 2014; 19: 531–533. DOI: 10.1038/mp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellwardt E, Walsh JT, Kipnis J, et al. Understanding the role of T cells in CNS homeostasis. Trends Immunol 2016; 37: 154–165. DOI: 10.1016/j.it.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Smolders J, Remmerswaal EB, Schuurman KG, et al. Characteristics of differentiated CD8(+) and CD4 (+) T cells present in the human brain. Acta Neuropathol 2013; 126: 525–535. DOI: 10.1007/s00401-013-1155-0. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Chen S, Luo Y, et al. Interaction between ICAM1 in endothelial cells and LFA1 in T cells during the pathogenesis of experimental Parkinson's disease. Exp Ther Med 2020; 20: 1021–1029. DOI: 10.3892/etm.2020.8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders JA, Estes KA, Kosloski LM, et al. CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson's disease. J Neuroimmune Pharmacol 2012; 7: 927–938. DOI: 10.1007/s11481-012-9402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brochard V, Combadiere B, Prigent A, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 2009; 119: 182–192. DOI: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol 2010; 28: 445–489. DOI: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kustrimovic N, Comi C, Magistrelli L, et al. Parkinson's disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naive and drug-treated patients. J Neuroinflammation 2018; 15: 205. DOI: 10.1186/s12974-018-1248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez-Luquin DD, Arce-Sillas A, Leyva-Hernandez J, et al. Regulatory impairment in untreated Parkinson's disease is not restricted to Tregs: other regulatory populations are also involved. J Neuroinflammation 2019; 16: 212. DOI: 10.1186/s12974-019-1606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marras C, Lang A, Van De Warrenburg BP, et al. Nomenclature of genetic movement disorders: Recommendations of the International Parkinson and Movement Disorder Society Task Force. Mov Disord 2016; 31: 436–457. DOI: 10.1002/mds.26527. [DOI] [PubMed] [Google Scholar]
- 18.Postuma RB, Berg D. The new diagnostic criteria for Parkinson's disease. Int Rev Neurobiol 2017; 132: 55–78. DOI: 10.1016/bs.irn.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Draxler DF, Madondo MT, Hanafi G, et al. A flowcytometric analysis to efficiently quantify multiple innate immune cells and T cell subsets in human blood. Cytometry A 2017; 91: 336–350. DOI: 10.1002/cyto.a.23080. [DOI] [PubMed] [Google Scholar]
- 20.Meredith GE, Rademacher DJ. MPTP mouse models of Parkinson's disease: an update. J Parkinsons Dis 2011; 1: 19–33. DOI: 10.3233/JPD-2011-11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luchtman DW, Shao D, Song C. Behavior, neurotransmitters and inflammation in three regimens of the MPTP mouse model of Parkinson's disease. Physiol Behav 2009; 98: 130–138. DOI: 10.1016/j.physbeh.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Deacon RM, Koros E, Bornemann KD, et al. Aged Tg2576 mice are impaired on social memory and open field habituation tests. Behav Brain Res 2009; 197: 466–468. DOI: 10.1016/j.bbr.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 23.Liu D, Tu X, Huang C, et al. Adoptive transfers of CD4(+) CD25(+) Tregs partially alleviate mouse premature ovarian insufficiency. Mol Reprod Dev 2020; 87: 887–898. DOI: 10.1002/mrd.23404. [DOI] [PubMed] [Google Scholar]
- 24.Clemente-Casares X, Blanco J, Ambalavanan P, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 2016; 530: 434–440. DOI: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- 25.Peter I, Dubinsky M, Bressman S, et al. Anti-tumor necrosis factor therapy and incidence of Parkinson disease among patients with inflammatory bowel disease. JAMA Neurol 2018; 75: 939–946. DOI: 10.1001/jamaneurol.2018.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung ES, Kim H, Lee G, et al. Neuro-protective effects of bee venom by suppression of neuroinflammatory responses in a mouse model of Parkinson's disease: role of regulatory T cells. Brain Behav Immun 2012; 26: 1322–1330. DOI: 10.1016/j.bbi.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Liu Z, Cao BB, et al. Treg cells attenuate neuroinflammation and protect neurons in a mouse model of Parkinson's disease. J Neuroimmune Pharmacol 2020; 15: 224–237. DOI: 10.1007/s11481-019-09888-5. [DOI] [PubMed] [Google Scholar]
- 28.Dzamko N, Geczy CL, Halliday GM. Inflammation is genetically implicated in Parkinson's disease. Neuroscience 2015; 302: 89–102. DOI: 10.1016/j.neuroscience.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Sliter DA, Martinez J, Hao L, et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature 2018; 561: 258–262. DOI: 10.1038/s41586-018-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh N, Mitra S, Sinha P, et al. TNFR2 mediated TNF-alpha signaling and NF-kappaB activation in hippocampus of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice. Neurosci Res 2018; 137: 36–42. DOI: 10.1016/j.neures.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Piconese S, Barnaba V. Stability of regulatory T cells undermined or endorsed by different type-1 cytokines. Adv Exp Med Biol 2015; 850: 17–30. DOI: 10.1007/978-3-319-15774-0_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060521998471 for Imbalance between T helper 1 and regulatory T cells plays a detrimental role in experimental Parkinson’s disease in mice by Wenjie Li, Yuan Luo, Hongyu Xu, Qianqian Ma and Qi Yao in Journal of International Medical Research