Abstract
Background
Endocrine therapy (ET) plus cyclin-dependent-kinases 4/6 inhibitors (CDK4/6i) represents the standard treatment for luminal-metastatic breast cancer (MBC). However, prospective head-to-head comparisons are still lacking for 1st line (L) options, and it is still crucial to define the best strategy between 1st and 2nd L.
Materials and methods
717 consecutive luminal-MBC pts treated between 2008 and 2020 were analyzed at the Oncology Department of Aviano and Udine, Italy. Differences about survival outcomes (OS, PFS and PPS) were tested by log-rank test. The attrition rate (AR) between 1st and 2ndL was calculated.
Results
At 1stL, pts were treated with ET (49%), chemotherapy (CT) (31%) and ET-CDKi (20%) while, at 2ndL, 33% received ET, 33% CT and 8% ET-CDKi. Overall AR was 10%, 7% for CT, 8% for ET and 17% for ET-CDKi. By multivariate analysis, 1stL ET-CDK4/6i showed a better mPFS1 and OS. Moreover, 2ndL ET-CDK4/6i demonstrated better mPFS2 compared to ET and CT. Notably, 1stL ET-CDKi resulted in higher mPFS than 2ndL ET-CDKi. Intriguingly, 1stL ET-CDK4/6i was associated with worse mPPS compared to CT and ET. Secondarily, 1stL ET-CDK4/6i followed by CT had worse OS compared to 1stL ET-CDK4/6i followed by ET. Notably, none of baseline characteristics at 2ndL influenced 2ndL treatment choice (ET vs. CT) after ET-CDKi.
Conclusion
Our real-world data demonstrated that ET-CDKi represents the best option for 1stL luminal-MBC compared to ET and CT. Also, the present study pointed out that 2ndL ET, potentially combined with other molecules, could be a feasible option after CDK4/6i failure, postponing CT on later lines.
Keywords: Metastatic luminal breast cancer, Treatment strategies, CDK4/6 inhibitors, MBC
Highlights
-
•
To define the best treatment strategy for 1st and 2nd L is crucial for luminal mBC.
-
•
1st L CDK4/6i-based therapies represent the standard treatment in luminal mBC.
-
•
This study confirmed the key role of 1st L CDK4/6i on a real-world cohort.
-
•
This study suggested a preeminent role for ET after first-line CDK4/6i.
-
•
The 2nd line ET with other molecules could allow to further postpone CT.
1. Introduction
Over the last decade, thanks to a combination of new therapeutic agents and diagnostic strategies, the outcome and management of patients with metastatic breast cancer (mBC) gradually improved both on a survival and quality of life stand point. The combination CDK4/6 inhibitors (CDK4/6i) (i.e Palbociclib, Ribociclib and Abemaciclib) with endocrine therapy (ET, i.e. aromatase inhibitors (AI) or Fulvestrant) has significantly increased objective response rate (ORR) and progression-free survival (PFS) of first- and second-line treatments in patients with hormone receptor positive, HER2 negative (luminal) mBC. Although no head-to-head comparison has been tested in clinical trials, recent systematic reviews and meta-analyses have further supported the efficacy of the combinations in terms of PFS and of overall survival (OS) [[1], [2], [3], [4], [5], [6], [7], [8]].
The growing number of potentially active agents in different treatment lines is increasing the complexity of drug development making the choice of study endpoints even more critical [9]. Indeed, overall survival (OS) has been considered the most relevant outcome measure in mBC trials, being an objective measure of the clinical benefit. However, survival may be influenced by post-protocol treatments and some randomised trials might be underpowered to detect OS differences notwithstanding a benefit in PFS [10,11]. To overcome these limits, recent trials have been designed to detect a clinical benefit in terms of PFS and time-to-treatment progression (TTP), as competing causes of death and further-line treatments may have less impact [11].
A critical, still unmet, need is to improve resistance characterization to ET and CDK4/6i, such as ESR1 genetic and epigenetic alterations, FGFR1 amplification RB1 loss or CCNE1 amplification to anticipate clinical progression and guide subsequent treatment lines [[12], [13], [14], [15], [16]]. As a matter of fact, ET and CDK4/6i resistance might confer to MBC aggressive features such as epithelial to mesenchymal transition that eventually are clinically translated in rapid progression and ultimately in an unfavourable prognosis [15,17]. Furthermore, there is no evidence supporting post CDK4/6i progression decision-making, and different strategies such as CDK4/6i beyond progression, CDK4/6i or ET switch and the addition of other targeted agents, including PI3K inhibitors are being studied.
The aim of this study was to provide real-world treatment patterns data for luminal MBC, and to give hypothesis generation insights of their consequent impact on clinical outcome measures.
2. Materials and methods
2.1. Study design
This was an observational, multicentricer, retrospective study, that evaluated a consecutive series of 717 patients with luminal mBC. The study was conducted in accordance with the ethical principles deriving from the Helsinki Declaration and the current legislation on Observational Studies (Circular Min. Sal. Of September 6, 2002). The study was approved by the Departmental Review Board and by the Ethics Committee (Protocol number CRO-2019-85).
Data concerning clinico-pathological characteristics, treatments for metastatic disease, and blood tests performed within 30 days of the first- and second-line treatment start, were collected retrospectively. Primary objective of this study was to evaluate the impact first- and second-line of treatment strategies in terms of PFS, post-progression survival [PPS] after first-line, and OS) according to treatment type (chemotherapy, CT; ET alone; ET combined with CDK4/6i). Secondary objectives were to evaluate attrition rate according to first-line treatment, the probability of receiving chemotherapy after CDK4/6i, and factors determining the choice of second-line treatment after CDK4/6i.
PFS was defined as time from first-line therapy start until progression disease or death from any cause. According to the treatment line, PFS was defined as PFS1 and PFS2. PPS was defined as the interval between progression and death or last follow-up.
OS was defined as the time between first-line therapy start and death from any cause.
The attrition rate related to first-line treatment was defined as the proportion of patients who started therapy but who, at the time of disease progression were unable to receive further treatment due to disease progression, death, toxicity, or other clinical conditions.
2.2. Study population
All patients had histologically or cytologically confirmed luminal (HR positive/HER2 negative) mBC. They provided informed consent for the use of clinical data that were rendered anonymous for purposes of clinical research, epidemiology, training and disease study.
The study population included consecutive patients treated at the Department of Medical Oncology, National Cancer Institute of Aviano and at the Oncology Department of Udine, Italy, from January 2008 to Janury 2020. Data have been obtained from electronic and paper-chart review according to strict privacy standards.
2.3. Statistical analysis
Patients’ clinico-pathological characteristics were summarized through descriptive analysis. Categorical variables were reported as frequency distribution whereas continuous variables were reported as median value and range. Differences in terms of survival outcomes were tested by log-rank test and represented by Kaplan-Meier survival curves. Analyses were stratified by line of treatment and type of treatment received.
A Cox proportional-hazards regression model, including also potential confounders (e.g. age, ET naïve, ECOG PS) was used to calculate hazard ratios (HR) of death, with the corresponding 95% confidence intervals (CI), in the different subgroups of patients identified according treatment. To better evaluate if baseline features at second-line could influence the choice of CT or ET after fist-line CDK4/6i, a logistic analysis was performed.
A two-sided P < 0.05 was considered statistically significant. Statistical analyses will be performed with STATA (StataCorp. (2015) Stata Statistical Software: Release 14.2. College Station, TX: StataCorp LP).
2.4. Sample size calculation
The sample size was estimated in order to obtain a good performance of the statistical model for the association between patient and tumor characteristics with outcome measures in the multivariate analysis. The aim of the sampling was the achievement of a good “goodness of fit” from the regression model according to Peduzzi and Concato [18,19]. Therefore, considering 20–50 events per variable (EPV) and a final model with approximately 6–7 variables, more than 350 EPV are necessary to obtain an accurate estimation of the statistical model. In the present study, we observed 575 events for PFS1, 466 for PFS2, and 443 for OS. Therefore, we could define an accurate estimation for the multivariate model.
3. Results
3.1. Descriptive analysis
The study included 717 women with a diagnosis of luminal mBC who underwent first-line treatment (clinical, pathologic and treatment characteristics are displayed in Table 1). Overall, 29% of patients had a de novo mBC, 78% were post-menopausal and 15% had ECOG PS > 1. Moreover, 49%, 30% and 20% had respectively visceral, bone-only and non-visceral disease, while, 26% of cases had ≥3 metastatic sites and 66% had ≥5 metastatic lesions. Tumor burden was, moreover, classified in 3 groups by combining metastatic sites and number of lesions. In particular, group 1 (25%) had <3 metastatic sites and ≤5 lesions, group 2 (45%) <3 metastatic sites and >5 lesions or ≥3 metastatic sites and ≤5lesions, group 3 (23%) had ≥3 metastatic sites and >5 lesions.
Table 1.
Patient’s characteristics
| Variables | N (tot 717) | Frequencies |
|---|---|---|
| Age (years) | ||
| <45 | 106 | 14.78% |
| 45-65 | 314 | 43.79% |
| >65 | 297 | 41.42% |
| Histotype | ||
| Ductal | 513 | 71.55% |
| Lobular | 127 | 17.71% |
| Other | 14 | 1.95% |
| Missing | 63 | 8.79% |
| Ki-67 on primary tumor | ||
| <14 % | 181 | 25.24% |
| ≥14 % | 430 | 59.97% |
| Missing | 106 | 14.78% |
| ER | ||
| ≤10 % | 25 | 3.49% |
| >10 % | 594 | 82.85% |
| Missing | 98 | 13.67% |
| BMI (kg/m2) | ||
| < 25 | 263 | 36.68% |
| ≥ 25 | 293 | 40.86% |
| Missing | 161 | 22.45% |
| De novo metastatic disease | ||
| No | 503 | 70.15% |
| Yes | 208 | 29.01% |
| Missing | 6 | 0.84% |
| ET naïve | ||
| No | 271 | 37.80% |
| Yes | 445 | 62.06% |
| Missing | 1 | 0.14% |
| CT naïve | ||
| No | 311 | 43.38% |
| Yes | 405 | 56.49% |
| Missing | 1 | 0.14% |
| Endocrine responsiveness | ||
| No | 301 | 41.98% |
| Yes | 276 | 38.49% |
| Missing | 140 | 19.52% |
| Menopausal status | ||
| Premenopausal | 132 | 18.41% |
| Postmenopausal | 557 | 77.68% |
| Missing | 28 | 3.91% |
| Sites - first line | ||
| Bone only | 218 | 30.40% |
| No visceral | 141 | 19.67% |
| Visceral | 353 | 49.23% |
| Missing | 5 | 0.70% |
| Number of sites of metastasis | ||
| <3 | 532 | 74.20% |
| ≥3 | 184 | 25.66% |
| Missing | 1 | 0.14% |
| Number of metastatic lesions | ||
| ≤5 | 191 | 26.64% |
| >5 | 474 | 66.11% |
| Missing | 52 | 7.25% |
| Metastatic sites score | ||
| <3 sites and ≤5 lesions | 177 | 24.69% |
| ≥3 sites and ≤5 lesions/ < 3 sites and > 5 lesions | 321 | 44.77% |
| ≥ 3 sites and >5 lesions | 166 | 23.15% |
| Missing | 54 | 7.53% |
| ECOG PS – first line | ||
| ≤ 1 | 494 | 68.90% |
| > 1 | 108 | 15.06% |
| Missing | 115 | 16.04% |
| First line therapy | ||
| CT | 224 | 31.24% |
| ET | 352 | 49.09% |
| ET plus CDK 4/6 | 141 | 19.67% |
| Second line therapy | ||
| CT | 236 | 32.91% |
| ET | 239 | 33.33% |
| ET plus mTORi after ET or CT (17 pts) | ||
| ET plus mTORi after ET plus CDK4/6i (4 pts) | ||
| ET plus CDK 4/6i | 61 | 8.51% |
| None | 51 | 7.11% |
| Missing | 130 | 18.13% |
| Sites - second line | ||
| Bone only | 117 | 16.32% |
| No visceral | 103 | 14.37% |
| Visceral | 314 | 43.79% |
| None therapy | 51 | 7.11% |
| Missing | 132 | 18.41% |
| Number of lesions – second line | ||
| <3 sites | 343 | 47.84% |
| ≥3 | 199 | 27.75% |
| no therapy | 51 | 7.11% |
| Missing | 124 | 17.29% |
| Number of lesions - second line | ||
| ≤5 lesions | 70 | 9.76% |
| <5 lesions | 449 | 62.62% |
| No therapy | 51 | 7.11% |
| Missing | 147 | 20.50% |
| Metastatic sites score- second line | ||
| <3 sites and ≤5 lesions | 65 | 9.07% |
| ≥3 sites and ≤5 lesions/ < 3 sites and > 5 lesions | 260 | 36.26% |
| ≥ 3 sites and >5 lesions | 194 | 27.06% |
| No therapy | 51 | 7.11% |
| Missing | 147 | 20.50% |
| Change sites between first and second line | ||
| No visceral → no visceral | 106 | 14.78% |
| Visceral → visceral | 366 | 51.05% |
| No visceral → visceral | 62 | 8.65% |
| No therapy | 51 | 7.11% |
| Missing | 132 | 18.41% |
| ECOG PS- second line | ||
| ≤1 | 327 | 45.61% |
| >1 | 97 | 13.53% |
| No therapy | 51 | 7.11% |
| Missing | 242 | 33.75% |
| Treatment strategies | ||
| ET plus CDK 4/6 → ET | 19 | 2.65% |
| ET plus CDK 4/6 → CT | 29 | 4.04% |
| Capecitabine 24 pts | ||
| Nab-paclitaxel 2 pts | ||
| Paclitaxel 1 pt | ||
| Vinorelbine 2 pts | ||
| ET followed by ET plus CDK 4/6 | 40 | 5.58% |
| ET followed by ET or CT | 254 | 35.43% |
| CT followed by ET or CT | 194 | 27.06% |
| No therapy | 51 | 7.11% |
| Missing | 130 | 18.13% |
| Death events | ||
| Censored | 269 | 37.52% |
| Uncensored | 443 | 61.79% |
| Missing | 5 | 0.70% |
| PD 1 events | ||
| Censored | 140 | 19.53% |
| Uncensored | 575 | 80.20% |
| Missing | 2 | 0.28% |
| PD 2 events | ||
| Censored | 63 | 8.79% |
| Uncensored | 466 | 64.99% |
| Missing | 188 | 26.22% |
| PD 3 events | ||
| Censored | 22 | 3.07% |
| Uncensored | 135 | 18.83% |
| Missing | 560 | 78.10% |
Overall, 31% received first-line CT, 49% ET alone and 20% received ET plus CDK4/6i. In second-line, 33% of patients received CT, 33% ET alone and 8% ET plus CDK4/6i. In terms of treatment strategy, 27% of patients received CT followed by ET or CT, 35% received ET followed by CT or ET, 3% ET plus CDK4/6i followed by CT, 3% ET plus CDK4/6i followed by ET and 6% received ET followed by ET plus CDK4/6i (Table S1–S2).
With a median follow-up of 72.82 months (25th-75th percentile: 32.65–102.94 months), median PFS1 was 14.96 months (25th-75th percentile: 6.77–28.27), median PFS2 was 7.07 months (25th-75th percentile: 3.39–13.71) median PPS1 was 28.44 months (25th-75th percentile: 11.97–46.92), and median OS was 47.15 months (25th-75th percentile: 22.88–77.52).
3.2. Treatment strategies and tumor burden have a significant impact on survival outcomes
3.2.1. PFS1
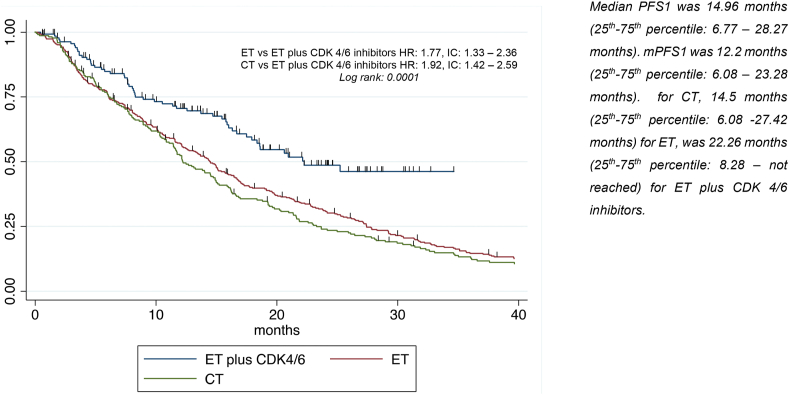
Univariate analysis for PFS1 showed that visceral disease, having ≥3 and >5 metastatic sites, ECOG PS > 1, Ki67 ≥ 14% and being previously exposed to ET were significantly associated with worse survival (Figure S1A-B-C). Conversely, de novo metastatic disease was associated with better PFS1. Patients treated with first-line ET (HR 1.77, p < 0.001, 95% C.I. 1.33–2.36) or CT (HR 1.92, p < 0.001, 95% C·I 1.42–2.59) experienced worse prognosis than patients treated with ET plus CDK4/6i. All variables retained their significance after multivariate analysis, in particular type of treatment received (HR 1.94, p < 0.001, 95%C.I. 1.37–2.73 for ET, HR 1.93, p < 0.001, 95%C.I. 1.36–2.75 for CT) (Table 2, Fig. 1).
Table 2.
Univariate and multivariate analysis for PFS1
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | P | 95 % CI | HR | P | 95 % CI | |
| Age (years) | ||||||
| <45 | 1.00 | |||||
| 45-65 | 1.10 | 0.459 | 0.86 -1.40 | |||
| >65 | 1.26 | 0.063 | 0.99 – 1.62 | |||
| BMI (kg/m2) | ||||||
| <25 | 1.00 | |||||
| ≥25 | 0.99 | 0.896 | 0.82 – 1.19 | |||
| ET naive | ||||||
| Yes | 1.00 | |||||
| No | 1.21 | 0.028 | 1.02 – 1.43 | 1.45 | 0.001 | 1.17-1.79 |
| De novo metastatic disease | ||||||
| No | 1.00 | |||||
| Yes | 0.82 | 0.038 | 0.69 – 0.99 | |||
| Sites | ||||||
| Bone only | 1.00 | |||||
| Not visceral | 1.17 | 0.188 | 0.93 – 1.48 | 1.26 | 0.132 | 0.93-1.71 |
| Visceral | 1.29 | 0.009 | 1.07 – 1.56 | 1.03 | 0.812 | 0.78-1.37 |
| Metastatic sites score | ||||||
| <3 sites and ≤5 lesions | 1.00 | |||||
| ≥3 sites and ≤5 lesions/ < 3 sites and > 5 lesions | 1.09 | 0.442 | 0.88 – 1.34 | 1.25 | 0.087 | 0.97-1.60 |
| ≥ 3 sites and >5 lesions | 1.48 | 0.001 | 1.16 – 1.87 | 1.64 | 0.003 | 1.18-2.28 |
| ECOG PS-first line | ||||||
| ≤1 | 1.00 | |||||
| >1 | 1.60 | <0.001 | 1.27 – 2.01 | 1.36 | 0.025 | 1.04-1.77 |
| ER | ||||||
| ≤10 % | 1.00 | |||||
| >10 % | 1.18 | 0.499 | 0.73 – 1.88 | |||
| Ki-67 on primary tumor: | ||||||
| <14 % | 1.00 | |||||
| ≥14 % | 1.37 | 0.002 | 1.13 -1.67 | 1.36 | 0.006 | 1.09-1.70 |
| Endocrine responsiveness | ||||||
| No | 1.00 | |||||
| Yes | 0.98 | 0.842 | 0.82 – 1.18 | |||
| First-line therapy | ||||||
| ET plus CDK 4/6 inhibitors | 1.00 | |||||
| ET | 1.77 | <0.001 | 1.33 – 2.36 | 1.94 | <0.001 | 1.37-2.73 |
| CT | 1.92 | <0.001 | 1.42 – 2.59 | 1.93 | <0.001 | 1.36-2.75 |
Fig. 1.
First line treatment-PFS1.
3.2.2. PFS2
Visceral involvement (HR 1.36, p0.005, 95%C.I. 1.10–1.68), ECOG PS > 1 at second-line (HR 1.81, p < 0.001, 95%C.I. 1.42–2.31), having ≥3sites and >5 lesions (HR 1.69, p = 0.001, 95%C.I. 1.24–2.30), Ki-67 ≥14% (HR1.41, p = 0.002, 95%C.I. 1.13–1.76) and treatments other than ET plus CDK4/6i (ET: HR1.65, p = 0.004, 95%C.I. 1.17–2.32; CT: HR 1.75, p = 0.001, 95%C.I. 1.24–2.47) were linked with worse prognosis after univariate analysis in terms of PFS2. After multivariate analysis, second-line treatment retained its significance, showing that ET or CT compared with ET/CDK4/6i combination were burdened by worse prognosis (respectively HR 1.67, p = 0.009, 95%C.I. 1.13–2.44 and HR 1.79, p = 0.003, 95% C.I. 1.22–2.61) (Table S3, Figure S2).
Median PFS1 for patients receiving first-line CDK4/6i was 22.26 months (25th-75th percentile: 8.28 – not reached). Conversely, median PFS2 for patients receiving second-line CDK4/6i was 12.26 months (25th-75th percentile: 5.16–22.32 months) (Figure S3).
3.2.3. PPS
Univariate analysis for PPS1, showed a trend for better PPS in women treated with first-line ET as compared to ET plus CDK4/6i (HR: 0.63, C·I.: 0.37–1.06, p = 0.08) (Figure S4).
3.2.4. OS
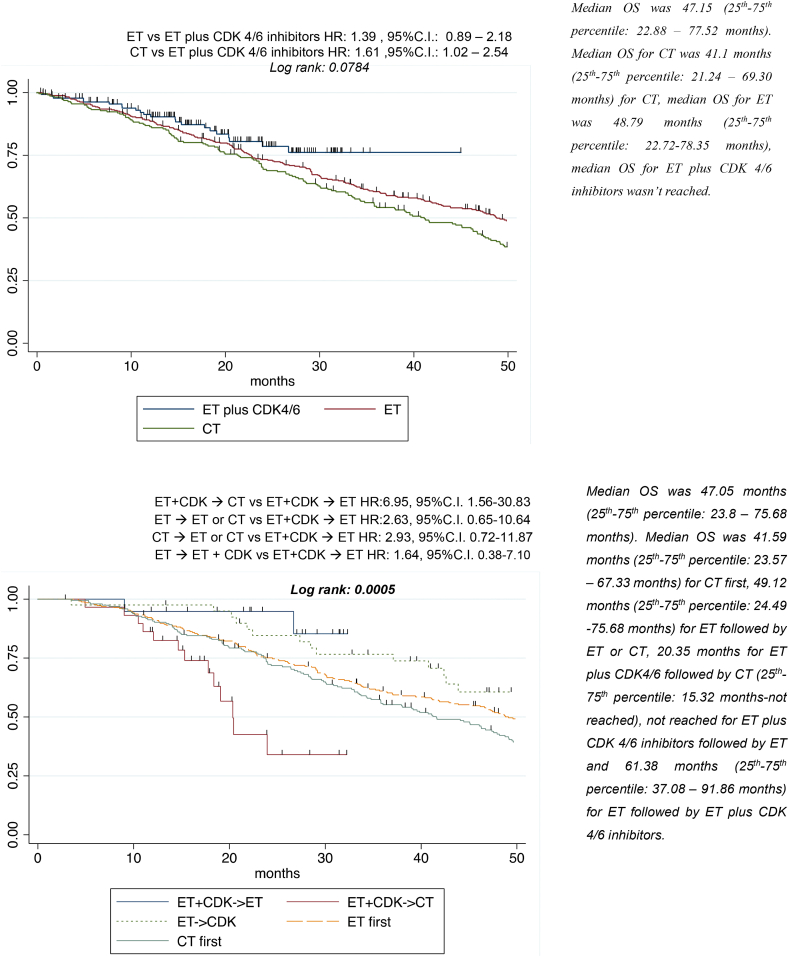
A trend towards better prognosis in terms of OS was observed in patients treated with first-line CDK4/6i (Table 3, Fig. 2A). In univariate analysis, ET followed by ET plus CDK4/6i, ET plus CDK4/6i followed by ET, ET followed by ET or CT and CT followed by CT or ET showed a better survival compared with ET plus CDK4/6i followed by CT. Results were confirmed in multivariate analysis (Table 3, Fig. 2B).
Table 3.
Univariate and Multivariate analysis-OS
| Univariate analysis |
Multivariate analysis (first-line therapy) |
Multivariate analysis (strategies) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | P | 95 % CI | HR | P | 95 % CI | HR | P | 95 % CI |
| Age (years) | |||||||||
| < 45 | 1.00 | ||||||||
| 45-65 | 0.97 | 0.812 | 0.73 – 1.28 | ||||||
| > 65 | 1.26 | 0.111 | 0.95 – 1.66 | ||||||
| BMI (kg/m2) | |||||||||
| < 25 | 1.00 | ||||||||
| ≥ 25 | 0.90 | 0.292 | 0.73 – 1.10 | ||||||
| ET naive | |||||||||
| Yes | 1.00 | ||||||||
| No | 1.18 | 0.097 | 0.97 – 1.43 | ||||||
| CT naive | |||||||||
| Yes | 1.00 | ||||||||
| No | 1.03 | 0.746 | 0.85-1.25 | ||||||
| De novo metastatic disease | |||||||||
| No | 1.00 | ||||||||
| Yes | 0.93 | 0.494 | 0.75 –1.15 | ||||||
| ER | |||||||||
| ≤10 % | 1.00 | ||||||||
| >10 % | 0.70 | 0.171 | 0.43 – 1.16 | ||||||
| Ki-67 on primary tumor: | |||||||||
| <14 % | 1.00 | ||||||||
| ≥14 % | 1.80 | <0.001 | 1.42 – 2.28 | 1.91 | <0.001 | 1.47-2.49 | 2.00 | <0.001 | 1.49-2.68 |
| Metastatic sites score | |||||||||
| No | 1.00 | ||||||||
| Yes | 1.10 | 0.369 | 0.89 – 1.37 | ||||||
| Sites | |||||||||
| Bone only | 1.00 | ||||||||
| No visceral | 1.25 | 0.107 | 0.95 – 1.62 | 1.30 | 0.119 | 0.93-1.82 | 1.23 | 0.263 | 0.86-1.76 |
| Visceral | 1.61 | <0.001 | 1.30– 2.01 | 1.20 | 0.262 | 0.87-1.66 | 0.99 | 0.960 | 0.70-1.41 |
| Sites score | |||||||||
| <3 sites and ≤5 lesions | 1.00 | ||||||||
| ≥3 sites and ≤5 lesions/ < 3 sites and > 5 lesions | 1.16 | 0.224 | 0.91 – 1.48 | 1.20 | 0.206 | 0.90-1.59 | 1.28 | 0.112 | 0.94-1.74 |
| ≥ 3 sites and >5 lesions | 1.97 | <0.001 | 1.51 – 2.56 | 1.60 | 0.009 | 1.13-2.28 | 1.83 | 0.002 | 1.24-2.68 |
| ECOG PS | |||||||||
| ≤ 1 | 1.00 | ||||||||
| > 1 | 2.39 | <0.001 | 1.89 – 3.02 | 2.07 | <0.001 | 1.59-2.70 | 1.71 | 0.001 | 1.25-2.33 |
| First-line therapy: | |||||||||
| ET plus CDK 4/6 inhibitors | 1.00 | ||||||||
| ET | 1.39 | 0.149 | 0.89 – 2.18 | 1.40 | 0.173 | 0.86-2.29 | |||
| CT | 1.61 | 0.042 | 1.02 – 2.54 | 1.24 | 0.404 | 0.75-2.03 | |||
| Treatment strategies | |||||||||
| ET +CDK → ET | 1.00 | ||||||||
| ET+ CDK →CT | 6.95 | 0.011 | 1.56-30.83 | 5.05 | 0.035 | 1.12-22.72 | |||
| ET → ET or CT | 2.63 | 0.176 | 0.65-10.64 | 2.35 | 0.235 | 0.57-9.61 | |||
| ET→ ET + CDK | 1.64 | 0.508 | 0.38-7.10 | 1.70 | 0.483 | 0.39-7.46 | |||
| CT → CT or ET | 2.93 | 0.133 | 0.72-11.87 | 2.05 | 0.318 | 0.50-8.39 | |||
Fig. 2.
AFirst-line treatment-OS. B. Treatment strategies-OS
3.2. Attrition rates across first-line treatment strategies
Attrition rate in the whole population was of 9.67% between first- and second-line treatment (PD1 observed in 575 patients, missing data in 130 patients and second-line treatment started in 536). Particularly, attrition rate was 7.31%, 8.25% and 17.24% for CT, ET alone and ET plus CDK4/6i, respectively. Moreover, probability to receive CT after ET plus CDK4/6i (treated in first- and second-line) was 52.80%. More in depth, the probability of receiving second- and third-line CT after ET plus CDK4/6i was 53.84% and 53.26%, respectively (Figure S5A, B).
Baseline characteristics at second-line that could influence the choice of CT or ET after CDK4/6i were analyzed. None of the variables considered, including rapidly progressive disease (defined as PFS ≤6 months at first-line) impacted on the choice of second-line treatment after CDK4/6i (Table S4).
4. Discussion
The addition of CDK4/6i in combination to ET has dramatically improved the clinical outcome of patients with luminal mBC, leading to rapid approval of these agents and recommendation of their use as the preferred first-line option by national and international guidelines [20,21].
The present study showed that first-line ET or CT alone were associated, in a real-world cohort, with worse PFS compared to ET plus CDK4/6i, with a 10 months difference in terms of mPFS1 for patients treated with combination therapy (mPFS1 12 months for CT, 14 months for ET and 22 months for ET plus CDK4/6i), consistently with what was observed in all main phase III studies [1,2,5,22].
Moreover, the present study showed a prolonged PFS2 in patients who received a CDK4/6i-based second-line as compared to ET or CT alone (12 months for ET plus CDK4/6i, 7 months for ET alone and 6 months for CT), consistently with PALOMA-3 trial which demonstrated an improvement in median PFS with the addition of Palbociclib to Fulvestrant of 9.5 months vs 4.6 months (HR 0.46, 95% CI 0.36–0.59, p < 0.0001)23.
Furthermore, MONALEESA-3 trials demonstrated a statistically significant benefit in OS with the combination of Ribociclib and ET. Consistently, in the phase III MONARCH-2 trial, Abemaciclib improved OS when combined with Fulvestrant in second-line (HR 0.75; 95% CI: 0.606–0.945; p = 0.0137) [6,[24], [25], [26], [27], [28], [29]]. Although the present study showed a trend towards better prognosis in terms of OS in patients treated with first-line CDK4/6i, the median follow-up was not adequate to generate conclusive results.
Notably, the study showed a greater improvement of CDK4/6i in first-line rather than in second-line second (median PFS1 and PFS2 respectively 22 vs 12 months) (Fig. 2). Moreover, the rate of patients that developed visceral metastases after CDK4/6i was similar in both second-line CT and ET cohorts. Conversely, the largest proportion of patients who switched from non-visceral to visceral involvement were those receiving ET followed by ET or CT (P < 0.001) (Table S1).
Luminal mBC is a clinically and molecularly heterogeneous disease, and the specific mechanisms though which it develops resistance to ET and CDK4/6i are still not fully elucidated, hindering the definition of biology-driven sequence strategies [12,14,16,30].
In the analyzed cohort, 60% of patients received CT and 40% received ET after a first-line CDK4/6i-based strategy. About CT, 24 pts received capecitabine, 2 nab-paclitaxel, 2 vinorelbine and 1 paclitaxel. Interestingly, after CDK4/6i failure, a worse prognosis was observed in patients who received CT over ET (HR 6.95, p = 0.01). Because of the potential bias that could have favored CT assignment in patients with an impending visceral crisis and therefore with an inherently worse prognosis, a logistic regression model was developed to explore potential factors that could have influenced clinical decision-making in second-line after CDK4/6i. None of the analyzed factors seemed to influence the choice of second-line CT or ET. Consistently, a recent study showed that ET after a combination of ET and Palbociclib is still effective, leading to a long median PFS2 after disease progression to first-line treatment [31].
Results from a logistic regression analysis conducted on 525 patients who had previously received CDK4/6i suggested that patients treated with CT after CDK4/6i were more likely to have a rapidly progressive disease compared to ET, everolimus or subsequent CDK4/6i (OR 0.46, 0.59, and 0.48)[32]. These observations might support the choice of ET after disease progression on a CDK4/6i in clinical practice.
The choice of second-line agents depends on previous lines of treatment and, to date, current options in progressive disease after CDK4/6i are represented by mTOR inhibitors (e.g everolimus plus exemestane), ET alone, CDK4/6i, PI3K inhibitors or CT.
The PI3K/Akt/mTOR pathway potentially plays a crucial role in secondary endocrine resistance in luminal mBC and there is a strong biological rationale supporting PI3K/AKT/mTOR axis as a therapeutic target [33]. The phase III SOLAR 1 trial showed the efficacy and safety of the PI3K inhibitor Alpelisib plus Fulvestrant in patients previously treated with ET (median PFS was 11 months in the Alpelisib arm vs 5.7 months in the placebo arm; HR:0.65). These results highlighted the potential need for an upfront evaluation of the PIK3CA mutational status and support the use of Alpelisib in mutated mBC with secondary resistance to ET [34]. However, this type of treatment is not yet available in all countries, except in clinical trials or specific programs.
Noteworthy, the efficacy of combinations of ET with mTOR, PI3K, and CDK4/6 inhibitors, the biological interplay between these pathways and the sensitization of cancer mutant cells led to the design of clinical studies investigating triplet combinations [[2], [3], [4], [5],22,23,[35], [36], [37], [38]].
Moreover, ESR1 mutations represent an established mechanism of acquired resistance to AI and, therefore, a combination of the same CDK4/6i with a more effective ET would overcome this resistance [39]. A recent study conducted on 16 mBC patients treated with Palbociclib and letrozole demonstrated that ESR1 mutations were significantly associated with worse PFS (3.3 vs 9.0 months; P = 0.038)39.
Consistently, the preliminary results of the PADA-1 study, suggested that the combination of Palbociclib with AIs could potentially overcome the initial impact of ESR1 mutations, leading to the clearance of mutated clones which eventually reoccurred at progression [40]. Of note, alternative resistance mechanisms could affect the ESR1 gene, such as epigenetic alterations, which can lead to early resistance and consequently to a dramatically shorter PFS1 under CDK4/6i [16].
Interestingly, the present study investigated attrition rate, a promising metric in evaluating the opportunity to receive a second-line treatment. Nuzzolese and Montemurro provided data on attrition rates in CDK4/6i trials. In the first-line setting, attrition rate ranged from 12% to 27% for ET and from 15% to 33% for CDK4/6i (PALOMA-2, PALOMA-3, MONARCH-2, MONARCH-3, MONALEESA-2, MONALEESA-3, MONALEESA-7 trials) [41]. In the present study, attrition rate was 7% for CT, 8% for ET and 17% for ET plus CDK4/6i after first-line therapy. Moreover, the probability to receive CT after CDK4/6i was 53%, regardless of the line of treatment, and approximately 54% and 53% after first- and second-line therapy, respectively.
This study, supports the hypothesis that second-line ET, combined with other targeted molecules, might potentially represent a feasible option after CDK4/6i failure, allowing to further postpone CT to later lines.
Noteworthy, this analysis contributes to the generation of real world data, which relevance has often been highlighted as an essential integration of randomized trials. Indeed, the use of data from the real-world scenario is garnering increased attention to provide information about clinical-relevant questions that cannot be answered using data from clinical trials [42].
Notwithstanding the intriguing insights, the present study has some limitations, such as the retrospective design and the small sample size. In particular, due to the paucity of patients receiving a CDK 4/6 Inhibitor (141 patients in the first line setting and 61 patients in the 2nd line setting) results need to interpreted with caution. Moreover, clinical decision-making is strongly influenced by patients’ and diseases’ characteristics, including age, PS, prior toxicities, potential adherence to treatment, previous treatment route of administration, and time to progression, with all of these characteristics being potentially sources of selection bias.
5. Conclusions
To date, first-line CDK4/6i-based therapies represent the gold standard in luminal mBC. The present study confirmed the significant impact of this strategy on a homogeneous real-world cohort. Moreover, the study suggested a preeminent role for ET after first-line CDK4/6i, supporting the concept of an ET switch to overcome potential resistance mechanisms and restore clinical response. Due to the retrospective nature of these results, further prospective studies will be needed to confirm these observations and to build the optimal treatment algorithms for luminal mBC.
6. Clinical practice points
-
•
First-line (1st L) CDK4/6i-based therapies represent the standard treatment in luminal mBC. However, prospective head-to-head comparisons are still lacking for many 1st line options, and it is still crucial to define the best treatment strategy for both 1st and 2nd L.
-
•
The present study confirmed the significant impact of 1st L CDK4/6i-based therapies on a homogeneous real-world cohort. Moreover, the study suggested a preeminent role for ET after first-line CDK4/6i, supporting the concept of an ET switch to overcome potential resistance mechanisms and restore clinical response.
-
•
The second-line ET, combined with other targeted molecules, might potentially represent a feasible option after CDK4/6i failure, allowing to further postpone CT to later lines.
Credit author statement
Debora Basile: Conceptualization, Methodology, Software, Formal analysis, Data curation, writing-review&editing, Visualization, Supervision, Project administration; Lorenzo Gerratana: Conceptualization, Methodology, Validation, Software, Visualization, Supervision; Carla Corvaja: Data curation, Writing – original draft, Visualization; Giacomo Pelizzari: writing-review&editing, Visualization, Supervision; Giorgia Franceschin: Data curation, Writing – original draft; Elisa Bertoli: Data curation, Visualization; Lorenza Palmero: Data curation, Visualization; Diego Zara: Data curation, Visualization; Martina Alberti: Data curation, Visualization; Silvia Buriolla: Data curation, Visualization; Lucia Da Ros: writing-review&editing, Visualization, Resources; Marta Bonotto: writing-review&editing, Visualization, Conceptualization; Mauro Mansutti: Visualization, Resources; Simon Spazzapan: Visualization, Resources; Marika Cinausero: writing-review&editing, Visualization, Resources; Alessandro Marco Minisini: writing-review&editing, Visualization, Resources, Supervision; Gianpiero Fasola: writing-review&editing, Visualization, Resources, Supervision; Fabio Puglisi: Methodology, writing-review&editing, Visualization, Resources, Supervision, Project administration
Funding
No specific funding was available for this work.
Declaration of competing interest
The authors declare no competing financial interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.02.015.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hortobagyi G.N., Stemmer S.M., Burris H.A. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018 doi: 10.1093/annonc/mdy155. Published online. [DOI] [PubMed] [Google Scholar]
- 2.Slamon D.J., Neven P., Chia S. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018 doi: 10.1200/JCO.2018.78.9909. Published online. [DOI] [PubMed] [Google Scholar]
- 3.Johnston S., Martin M., Di Leo A. npj Breast Cancer; 2019. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sledge G.W., Toi M., Neven P. The effect of Abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy - monarch 2: a randomized clinical trial. JAMA Oncology. 2019 doi: 10.1001/jamaoncol.2019.4782. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn R.S., Martin M., Rugo H.S. PALOMA-2: primary results from a phase III trial of palbociclib (P) with letrozole (L) compared with letrozole alone in postmenopausal women with ER+/HER2– advanced breast cancer (ABC) J Clin Oncol. 2016 doi: 10.1200/jco.2016.34.15_suppl.507. Published online. [DOI] [Google Scholar]
- 6.Schettini F., Giudici F., Giuliano M. Overall survival of CDK4/6-inhibitors-based treatments in clinically relevant subgroups of metastatic breast cancer: systematic review and meta-analysis. J Natl Cancer Inst. 2020 doi: 10.1093/jnci/djaa071. Published online May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frassoldati A., Biganzoli L., Bordonaro R. Endocrine therapy for hormone receptor-positive, HER2-negative metastatic breast cancer: extending endocrine sensitivity. Future Oncol. 2020;16(5):129–145. doi: 10.2217/fon-2018-0942. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano M., Schettini F., Rognoni C. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20(10):1360–1369. doi: 10.1016/S1470-2045(19)30420-6. [DOI] [PubMed] [Google Scholar]
- 9.Saad E.D. Endpoints in advanced breast cancer: methodological aspects & clinical implications. Indian J Med Res. 2011;134(4):413–418. [PMC free article] [PubMed] [Google Scholar]
- 10.Broglio K.R., Berry D.A. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst: J Natl Cancer Inst (Bethesda) 2009;101(23):1642–1649. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonotto M., Gerratana L., Poletto E. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncol. 2014;19(6):608–615. doi: 10.1634/theoncologist.2014-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Leary B., Cutts R.J., Liu Y. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Canc Discov. 2018;8(11):1390–1403. doi: 10.1158/2159-8290.CD-18-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrera-Abreu M.T., Palafox M., Asghar U. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor–positive breast cancer. Can Res. 2016;76(8):2301–2313. doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Condorelli R., Spring L., O’Shaughnessy J. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2018;29(3):640–645. doi: 10.1093/annonc/mdx784. [DOI] [PubMed] [Google Scholar]
- 15.Davis A.A., Zhang Q., Gerratana L. Association of a novel circulating tumor DNA next-generating sequencing platform with circulating tumor cells (CTCs) and CTC clusters in metastatic breast cancer. Breast Cancer Res. 2019;21(1):137. doi: 10.1186/s13058-019-1229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerratana L., Basile D., Franzoni A. Frontiers in Oncology. 2020. Plasma-based longitudinal evaluation of ESR1 epigenetic status in hormone receptor positive HER2 negative metastatic breast cancer. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulfoni M., Gerratana L., Del Ben F. In patients with metastatic breast cancer the identification of circulating tumor cells in epithelial-to-mesenchymal transition is associated with a poor prognosis. Breast Cancer Res. 2016;18(1):30. doi: 10.1186/s13058-016-0687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peduzzi P., Concato J., Kemper E., Holford T.R., Feinstein A.R. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 19.Steyerberg E.W., Eijkemans M.J., Harrell F.E., Habbema J.D. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Making. 2001;21(1):45–56. doi: 10.1177/0272989X0102100106. [DOI] [PubMed] [Google Scholar]
- 20.Linee A.I.O.M. guida-Neoplasie della mammella. 2019 [Google Scholar]
- 21.Cardoso, E. Senkus, A. Costa, E. Papadopoulos, M. Aapro, F. André, N. Harbeck, B. Aguilar Lopez, C. H. Barrios, J. Bergh, L. Biganzoli, C. B. Boers-Doets, M. J. Cardoso, L. A. Carey, J. Cortés, G. Curigliano, V. Diéras, N. S. El Saghir, A. Eniu, L. Fallowfield, P. A. Francis, K. Gelmon, S. R. D. Johnston, B. Kaufman, S. Koppikar, I. E. Krop, M. Mayer, G. Nakigudde, B. V. Offersen, S. Ohno, O. Pagani, S. Paluch-Shimon, F. Penault-Llorca, A. Prat, H. S. Rugo, G. W. Sledge, D. Spence, C. Thomssen, D. A. Vorobiof, B. Xu, L. Norton & E. P. Winer. 4th ESO–ESMO international Consensus guidelines for advanced breast cancer (ABC 4).
- 22.Finn R.S., Crown J.P., Lang I. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 23.Cristofanilli M., Turner N.C., Bondarenko I. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phas. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 24.Slamon D.J., Neven P., Chia S. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. New england Journal of medicine. 2019 doi: 10.1056/NEJMoa1911149. Published online December 11. [DOI] [PubMed] [Google Scholar]
- 25.Im S.-A., Lu Y.-S., Bardia A. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 26.Sledge G.W., Toi M., Neven P. The effect of Abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. Published online September. 2019;29 doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner N.C., Slamon D.J., Ro J. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 28.DeMichele A., Cristofanilli M., Brufsky A. Abstract P1-19-02: overall survival for first-line palbociclib plus letrozole vs letrozole alone for HR+/HER2- metastatic breast cancer patients in US real-world clinical practice. Can Res. 2020;80(4 Supplement) doi: 10.1158/1538-7445.SABCS19-P1-19-02. P1-19-02-P1-19-02. [DOI] [Google Scholar]
- 29.Finn R.S., Boer K., Bondarenko I. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2− advanced breast cancer (PALOMA-1, TRIO-18) Breast Canc Res Treat. 2020 doi: 10.1007/s10549-020-05755-7. Published online July 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xi J., Oza A., Thomas S. Retrospective analysis of treatment patterns and the effectiveness of palbociclib and subsequent regimens in metastatic breast cancer. J Natl Compr Canc Netw. 2019;17(2):141–147. doi: 10.6004/jnccn.2018.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi L., Biagioni C., McCartney A. Clinical outcomes after palbociclib with or without endocrine therapy in postmenopausal women with hormone receptor positive and HER2-negative metastatic breast cancer enrolled in the TREnd trial. Breast Cancer Res. 2019;21 doi: 10.1186/s13058-019-1149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.N.Princic, A. Aizer, D. H. Tang, D. M. Smith, W. Johnson ABardia. Predi ct ors of syst em i c t herapy sequences f ol l ow i ng a CD K 4/6 i nhi bi t based regi m en i n post m enopausal w om en w i t h horm one recept or posi t i ve , H EG FR- 2 negat i ve m et ast at i c breast cancer. Curr Med Res Opin. doi:10.1080/03007995.2018.1519500.
- 33.Miller T.W., Hennessy B.T., González-Angulo A.M. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010 doi: 10.1172/JCI41680. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.André F., Ciruelos E., Rubovszky G. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019 doi: 10.1056/NEJMoa1813904. Published online. [DOI] [PubMed] [Google Scholar]
- 35.Baselga J., Campone M., Piccart M. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012 doi: 10.1056/NEJMoa1109653. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baselga J., Im S.A., Iwata H. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017 doi: 10.1016/S1470-2045(17)30376-5. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vora S.R., Juric D., Kim N. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Canc Cell. 2014;26(1):136–149. doi: 10.1016/j.ccr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michaloglou C., Crafter C., Siersbaek R. Combined inhibition of mtor and cdk4/6 is required for optimal blockade of e2f function and long-term growth inhibition in estrogen receptor–positive breast cancer. Mol Canc Therapeut. 2018;17(5):908–920. doi: 10.1158/1535-7163.MCT-17-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gyanchandani R., Kota K.J., Jonnalagadda A.R. Detection of ESR1 mutations in circulating cell-free DNA from patients with metastatic breast cancer treated with palbociclib and letrozole. Oncotarget. Published online. 2017 doi: 10.18632/oncotarget.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bidard F.C., Callens C., Dalenc F. Prognostic impact of ESR1 mutations in ER+ HER2- MBC patients prior treated with first line AI and palbociclib: an exploratory analysis of the PADA-1 trial. J Clin Orthod. 2020;38(15_suppl) doi: 10.1200/JCO.2020.38.15_suppl.1010. 1010-1010. [DOI] [Google Scholar]
- 41.Nuzzolese I., Montemurro F. Attrition in metastatic breast cancer: a metric to be reported in randomised clinical trials? Lancet Oncol. 2020;21(1):21–24. doi: 10.1016/S1470-2045(19)30792-2. [DOI] [PubMed] [Google Scholar]
- 42.Booth C.M., Karim S., Mackillop W.J. Real-world data: towards achieving the achievable in cancer care. Nat Rev Clin Oncol. 2019;16(5):312–325. doi: 10.1038/s41571-019-0167-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.