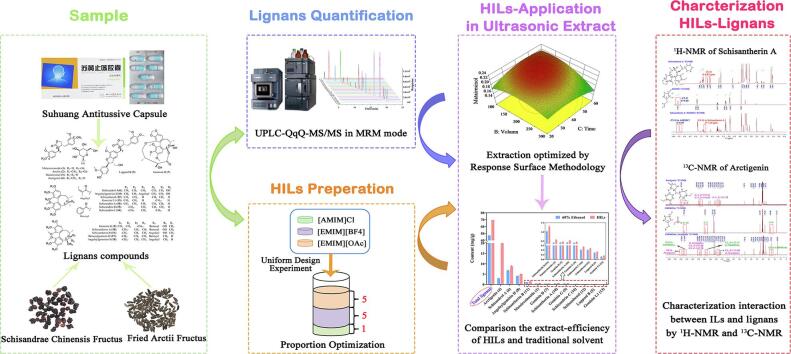
Graphical abstract
Abbreviations: TCMs, traditional Chinese medicines; HILs, hybrid ionic liquids; [EMIM][BF4], 1-ethyl-3-methylimidazolium tetrafluoroborate; [EMIM][OAc], 1-ethyl-3-methylimidazolium acetate; [AMIM]Cl, 1-allyl-3-methylimidazolium chloride; SH, Suhuang antitussive capsule; SCF, Schisandrae Chinensis Fructu; AF, fried Arctii Fructus; MRM, multiple reaction monitoring
Keywords: Suhuang antitussive capsule, Lignans, Ionic liquids, Ultrasonication, Response surface methodology
Highlights
-
•
A new UPLC-QqQ-MS/MS method in the MRM mode is established for qualitation and quantitation of natural lignans in complicated samples.
-
•
A novel ultrasonic-assisted extraction based on hybrid ionic liquids (HILs) was firstly proposed for simultaneous extraction of different polarities components from traditional Chinese medicines.
-
•
The HILs significantly improved the extract efficiency of lignans compared to conventional organic solvent and single ionic liquid, and the content of total lignans in the HILs system was 47% higher than that in ethanol system.
Abstract
Recently, efficient extraction of natural products from traditional Chinese medicines (TCMs) by green solvents is deemed an essential area of green technology and attracts extensive attentions. In this work, a green protocol for simultaneous ultrasonic-extraction of the native compounds with different polarities of TCMs by using a hybrid ionic liquids (HILs)-water system was reported for the first time. As a case study, three superior ILs (1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIM][BF4]), 1-ethyl-3-methylimidazolium acetate ([EMIM][OAc]), and 1-allyl-3-methylimidazolium chloride ([AMIM]Cl)) were chosen as the compositions of the HILs system, and the TCMs Suhuang antitussive capsule (SH) containing different-polarity lignans was selected. Primarily, an ultra-performance liquid chromatography coupled to triple quadrupole tandem mass spectrometry (UPLC-QqQ-MS/MS) method in the multiple reaction monitoring (MRM) mode was established for qualitative and quantitative analysis of 18 lignans. After majorization by uniform design experiment, the HILs prepared with [AMIM]Cl, [EMIM][BF4], and [EMIM][OAc] at a volume ratio of 1:5:5 could simultaneously extract multi-polarity lignans compared to single IL. Subsequently, the conditions of ultrasonic extraction employing with HILs and traditional organic solvent were optimized by the response surface methodology, respectively. The results indicated that the extract efficiency of the HILs system for target compounds was significantly improved compared with the traditional organic solvent-extraction, i.e. the content of total lignans in ethanol system was up to 47 mg/g, while that in the HILs system was up to 69 mg/g, with an increasing of 47%. Additionally, 1H-NMR and 13C-NMR spectra were used to characterize the hydrogen-bond interactions in the HILs-lignan mixtures. Extraction with the HILs in TCMs is a new application schema of ILs, which not only avoids the use of volatile toxic organic solvents, but also shows the potential to be comprehensively applied for the extraction of bioactive compounds from TCMs.
1. Introduction
Quality control of traditional Chinese medicines (TCMs) is an important subject that has attracted much attention and continuously explores with the rapid development of modern analytical techniques. How to simultaneously extract diverse active ingredients as comprehensively as possible for quality assessment of TCMs is what the scientists concern about. Suhuang antitussive capsule (SH), as one traditional Chinese formula agent, has been well known for the remarkable effect on the treatment of cough variant asthma (CVA) and post infectious cough (PIC) during the long-term clinical application, with the sales reaching about 2.0 billion RMB in 2019. SH is composed of nine Chinese medicinal herbs, including honey-fried Ephedrae Herba, Perillae Folium, Perillae Fructus, Schisandrae Chinensis Fructus (SCF), fried Arctii Fructus (AF), honey-fried Eriobotryae Folium, Peucedani Radix, Cicadae Periostracum, and Pheretima. And recent studies have further explored its mechanism of CVA and sputum obstruction in our laboratory respectively [1], [2]. Our previous investigation showed that 121 constituents have been identified by high-resolution mass spectrometry (HRMS), and bioactive lignans in SH were abundant and originated from SCF and AF [3]. SCF and AF as two famous TCMs were commonly used as edible medicinal plants around the world, and numerous studies have demonstrated diverse pharmacological activities, including anti-inflammation [4], anti-viral [5], anti-obesity [6], against drug-induced liver injury [7] and anti-cancer potential [8]. Further investigation exhibited that lignans with dibenzocyclooctadiene and dibenzylbutyrolactone skeletons played crucial roles as active components in SCF and AF, respectively [9], [10]. Thus, it is necessary to establish a qualitative and quantitative method, based on bioactive lignans, to control the quality of SH.
Given the diversity of chemical components and the complexity of production process in TCMs, it is urgent to establish a simultaneous determination of the multi-components analytical method, for comprehensive supervision of TCMs. Generally, determination for lignans was accomplished by various analytical strategies, mainly based on HPLC-UV and LC-MS [11], [12]. Currently, LC-MS has been gradually cut a striking figure due to its short-time running, high sensitivity, along with the superiority in determining the several ingredients with similar structure and polarity. With these attributions in mind, liquid chromatography-tandem triple quadrupole mass spectrometry (LC-QqQ-MS/MS), operating in multiple reaction monitoring (MRM) mode would be the preference for determination of lignans. This technique with the merits of higher sensitivity and less-time operation was appropriate for identification of the multi-components at low concentration without interference [13].
On extract methods, numerous safer, greener and targeted alternatives have been reported for extraction of botanical products to supersede the conventional organic solvents which could be hazardous to the environment [14], for example, supercritical antisolvent precipitation [15], the use of ionic liquid [16] and deep eutectic solvent [17] as the green reagent extraction. Noticeably, in the multi-components system of TCMs, the selection of extract-solvents should give consideration to compounds with different polarities; otherwise, it would result in inaccurate quantification. Thus, proposing an efficient and green approach that can simultaneously extract the multi-polarity components in TCMs is a critical subject in developing green chemistry. Ionic liquid (IL) was a complicated system that is composed of organic cations (imidazolium, pyridium, ammonium) and organic or inorganic anions (tetrafluoroborate, acetate, bromide anion, and chloride anion) [18]. Compared with traditional organic solvents, ILs possessed a series of unique properties due to the independent properties of ions and the multiplicity of existing interactions within the molecule [19], for instance, low flammability, nonvolatility, retrievability, excellent chemical thermal stability, and wide liquid range [20], [21], [22]. Recently, a significant number of studies have reported the effectiveness of ILs as solvents coupling with other techniques in the extraction of bioactive compounds such as carotenoids [23], furocoumarin [24], protein [25] and essential oil [26]. It is worth noting that IL-based extraction in previous studies usually used a single IL to extract lignans with similar polarity [27], while hardly prepared with two or more ILs to simultaneously extract different-polarity compounds.
Considering polarities of lignans in TCMs with large differences, it could not be thoroughly extracted by one IL. Therefore, we selected several ILs with better extract efficiency on some lignans in sample. Then the composition and proportion of ILs were optimized to obtain a hybrid ionic liquids (HILs) system. Finally, the HILs system was combined with ultrasonic extraction and expected that could improve the extract yields of lignans with different polarities in TCMs. Therefore, an eco-friendly and efficient method was proposed to extract 18 lignans in SH simultaneously by HILs-based ultrasonic-extraction. Meanwhile, a sensitive and specific analytical measure employing LC-MS/MS was established to identify and quantify 18 lignans in the MRM mode. Three superior ILs (1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIM][BF4]), 1-ethyl-3-methylimidazolium acetate ([EMIM][OAc]), and 1-allyl-3-methylimidazolium chloride ([AMIM]Cl)) were chosen as the extract system after optimizing proportions. Eventually, 1H-NMR and 13C-NMR spectra were used to characterize the hydrogen-bond interactions in the ILs-lignans mixtures.
2. Experimental
2.1. Chemicals and reagents
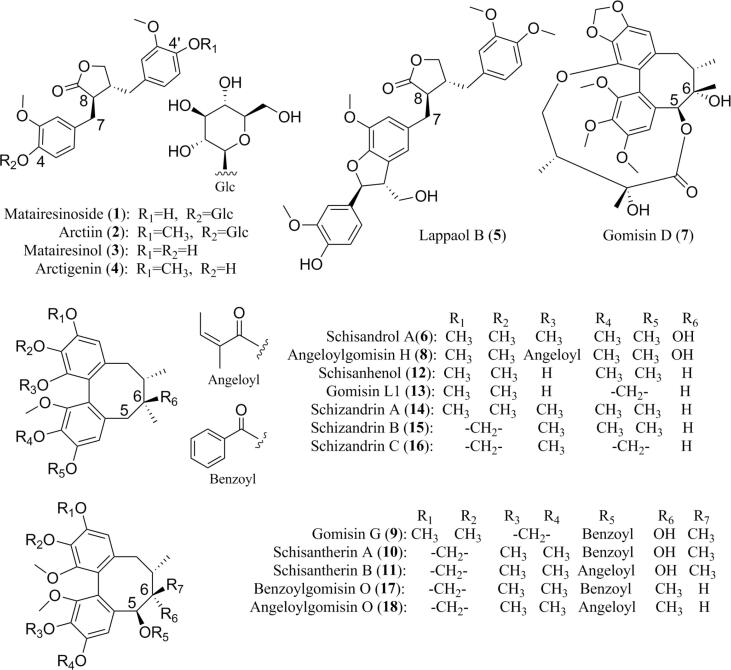
SH (18041311), SCF (304047–1711013) and AF (320278–1802002) were provided by Yangtze River Pharmaceutical Group Beijing Haiyan Pharmaceutical Co., Ltd. (Beijing, China). The collected information and chemical structures of 18 lignans were shown in Table S1 and Fig. 1, respectively. The purity of each compound was higher than 95% detected by HPLC-PDA. 1-Ethyl-3-methylimidazolium tetrafluoroborate ([EMIM][BF4], >99%) and 1-ethyl-3-methylimidazolium acetate ([EMIM][OAc], > 98%) were obtained from Lanzhou Institute of Chemical Physics (Lanzhou, China), and 1-allyl-3-methylimidazolium chloride ([AMIM]Cl, >99%) was bought from Shanghai Cheng Jie Chemical Co., Ltd.
Fig. 1.
Chemical structures of lignans (1–18).
Acetonitrile and methanol of chromatographic grade were purchased from Merck (Darmstadt, Germany). HPLC grade formic acid was supplied by Thermo Fisher Scientific (China) Co., Ltd. (Shanghai, China). Analytical grade methanol and ethanol were provided by Wuxi City Yasheng Chemical Co., Ltd and Shanghai Titan Scientific Co., Ltd, respectively. Deionized water was prepared by using a Milli-Q Integral Water Purification System (Millipore, USA). Other reagents were analytical grade.
2.2. UPLC-QqQ-MS analysis conditions
UPLC-QqQ-MS analysis system for determining lignans was performed using a Waters ACQUITY ® UPLC H Class system (Waters, USA) coupled to a Waters XEVO® TQD system triple quadrupole mass spectrometer which was equipped with Masslynx data processing system (Waters Corp., Milford, MA). Analyte separation was achieved on a Waters ACQUITY UPLC ® BEH C18 column (100 mm × 2.1 mm, 1.7 μm) at room temperature. The mobile phase was consisted of 0.1% formic acid water (A) and acetonitrile (B). Gradient elution was performed to investigate the optimal separation conditions, shown as follow: 0–7.1 min, 10%-34% B; 7.1–7.7 min, 34%-35.5% B; 7.7–12.0 min, 35.5%-58% B; 12.0–18.0 min, 58%-70% B; 18.0–18.6 min, 70%-70.2% B; 18.6–22.0 min, 70.2%-82% B; 22.0–24.0 min, 82%-100% B. The flow rate, injection volume and detection wavelength were 0.3 mL/min, 2 μL and 278 nm, respectively. The MS analyses were carried out utilizing electrospray ionization (ESI) source in positive mode of multiple reactions monitoring (MRM), with the mass spectra record ranged from 100 to 1000 m/z. The optimized parameters were as follows: cone gas rate, 50 L/h; desolvation temperature, 450 °C; capillary voltage, 2.5 kV; collision gas, argon. The data of cone voltage, collision energy, parent ion and characteristic product ion acquired with MRM for each analyte were listed in Table 1.
Table 1.
Optimized MRM transitions and mass spectrometer parameters for each lignan.
| NO. | Analytes | Formula | Parent ion (m/z) | Product ion (m/z) | Dwell (s) | Cone (V) | Collision (V) |
|---|---|---|---|---|---|---|---|
| 1 | Matairesinoside | C26H32O11 | 543.05 | 381.20 | 0.02 | 60 | 30 |
| 2 | Arcttin | C27H34O11 | 557.05 | 395.15 | 0.02 | 60 | 30 |
| 3 | Matairesinol | C20H22O6 | 359.10 | 137.20 | 0.02 | 20 | 30 |
| 4 | Arctigenin | C21H24O6 | 373.15 | 137.15 | 0.02 | 20 | 25 |
| 5 | Lappaol B | C31H34O9 | 573.10 | 543.15 | 0.02 | 60 | 25 |
| 6 | Schisandrol A | C24H32O7 | 455.20 | 409.20 | 0.02 | 40 | 25 |
| 7 | Gomisin D | C28H34O10 | 531.15 | 401.30 | 0.02 | 50 | 25 |
| 8 | Angeloylgomisin H | C28H36O8 | 523.20 | 315.20 | 0.02 | 50 | 40 |
| 9 | Gomisin G | C30H32O9 | 559.05 | 415.17 | 0.02 | 40 | 20 |
| 10 | Schisantherin A | C30H32O9 | 559.10 | 415.15 | 0.02 | 40 | 20 |
| 11 | Schisantherin B | C28H34O9 | 537.05 | 415.20 | 0.02 | 40 | 20 |
| 12 | Schisanhenol | C23H30O6 | 403.15 | 302.15 | 0.02 | 40 | 20 |
| 13 | Gomisin L1 | C22H26O6 | 387.25 | 227.15 | 0.02 | 40 | 30 |
| 14 | Schizandrin A | C24H32O6 | 417.15 | 316.05 | 0.02 | 40 | 20 |
| 15 | Schizandrin B | C23H28O6 | 401.10 | 300.15 | 0.02 | 40 | 25 |
| 16 | Schizandrin C | C22H24O6 | 385.20 | 285.10 | 0.02 | 40 | 20 |
| 17 | Benzoylgomisin O | C30H32O8 | 543.15 | 399.20 | 0.02 | 30 | 15 |
| 18 | Angeloylgomisin O | C28H34O8 | 521.25 | 399.15 | 0.02 | 30 | 15 |
2.3. Ultrasonic extraction of lignans
2.3.1. Sample preparation
SH was processed into powder by grinding and mixing equably after removing the capsules. A proper amount of powder was ultrasonically extracted by solvent of ethanol or ILs. Solvent was added to compensate for the lost weight when the extract was cooled to room temperature. After centrifugation for 10 min at 14000 rpm, the supernatant was filtered through a 0.22 μm nylon membrane filter for subsequent analysis. For details, the extraction conditions were optimized depend on the results in 2.3.2. SCF and AF powder were extracted according to the production process of SH.
2.3.2. Response surface methodology (RSM) for optimizing the extraction efficiency of lignans
RSM was applied to optimize the traditional organic solvent extract conditions by selecting several factors that had great influences on the extract efficiency based on previous single-factor experiments, to further investigate the interaction between the factors. Box-Benhnken design (BBD) was operated to establish the multiorder polynomial model to analyze experimental data by using Design-Expert.V.8.0.6 [28]. Three factors were examined at three levels, namely, ethanol concentration (X1, %), liquid to solid ratio (X2, mL/g) and frequency (X3). The yields of 18 lignans were taken as responses, which were solved by 12 independent combinations and 4 replicates at the center of the experimental domain. The factors and their levels, with both coded and actual values, were listed in Table S2.
[EMIM][BF4], [EMIM][OAc] and [AMIM]Cl were participated in the HILs-based ultrasonic extraction after preliminary screening, and a proper proportion of three ILs was confirmed by Uniform Design Experimentation [29]. Based on single-factors experiment, BBD combined with RSM was used to further investigate the three factors at three-level, namely, total ILs content (X1, VILs/VH2O), liquid to solid ratio (X2, mL/g) and extract time (X3, min), to determine the optimal extract conditions. The factors and their levels, with both coded and actual values, were listed in Table S8. Accomplished the extract optimization, to ulteriorly compare the extract efficiency of HILs with that of the traditional organic solvent and single IL, different solvents including [EMIM][BF4], [EMIM][OAc], [AMIM]Cl and ethanol were used, respectively.
2.4. Validation of quantitation method
2.4.1. Linearity, limit of detection (LOD), and limit of quantification (LOQ)
Calibration samples were diluted from a standard solution, and the calibration curves were constructed based on the peak area of each analyte versus the corresponding concentration. Limits of detection (LOD) and limits of quantification (LOQ) were defined as the quantities of analytes at a signal-to-noise ratio (S/N) of 3 and 10, respectively, where N was the standard deviation of response and S was the slope of the corresponding calibration curve.
2.4.2. Precision, stability and repeatability
Precision, defined as relative standard deviation (RSD, %), was evaluated by analyzing retention time (Rt) and signal intensity (peak area) of mixed standards solution within one day and over 3 consecutive days for six replicates (n = 6), which to establish intra- and inter-day precision, respectively. Stability was calculated RSD % of peak areas for each analyte by taking the sample solution for injection analysis at 0, 2, 4, 6, 8, 12, 24, and 48 h. Repeatability was assessed by analysis of six replicates with one sample solution.
2.4.3. Recovery and matrix effect
Recovery was assessed by analyzing sample that had been accurately spiked known quantities for the standard at three different concentration levels (high, middle and low). The spiked samples were analyzed in triplicate. Recovery was calculated with the following equation:
Matrix effect referred to significant interference of other components in sample during analysis process except for the analytes, which would enhance or weaken influence on the analytic results and extremely affect accuracy of analysis. Because of extensive source of crude drugs and complex coexistence of multi-components in SH, it was difficult to accurately quantify some trace lignans in matrix by common techniques. Therefore, the equation described by Zhang et al was used to evaluate matrix effect for lignans in sample [13]. A matrix-based calibration curve was prepared with extracts dissolving the mixed standards. To compare the slope of the solvent (60% methanol/water, v/v)-based curve with that of the matrix-based curve, the influence of rest components in the matrix on the ionization of analytes would be evaluated. The equation of matrix effect was as followed:
2.5. Characterization of the interaction between ILs and lignans
Noncovalent interactions greatly affected the physical properties of the mixtures of ILs-target compound, and the key to understand the solubility-promotion effect of ILs on compounds was to investigate the molecular interactions between them. Among various interactions, undoubtedly, hydrogen-bonding is one of important kinds of force interactions. Arctigenin (4) and schisantherin A (10), with significant improved-yields in the HILs system, were selected to determination of interaction between ILs, as representative components in AF and SCF respectively. 1H-NMR and 13C-NMR spectra were acquired on the Bruker 400 MHz spectrometer to characterize the hydrogen-bond interactions in the ILs-lignans system. Before measurement, 20 mg of each sample was weighed and dissolved in 0.5 mL DMSO‑d6.
3. Results and discussion
3.1. Optimization of UPLC-MS/MS conditions
3.1.1. Optimization of LC conditions
Efficient chromatographic separation of analytes is important to avoid ionization interference in the MS source, which is good for improving the sensitivity and accuracy of the entire analysis [30]. Initially, the Waters ACQUITY UPLC ® BEH C18 column (100 mm × 2.1 mm, 1.7 μm) exhibited excellent performance by comparing several UPLC-analysis columns in our laboratory. Subsequently, acetonitrile was selected as the organic phase in terms of better peak separation and lower instrumental pressure than methanol. Then, 0.1% acidic aqueous mobile phase was necessary to improve the peak shape and ionization efficiency of the analytes. Lastly, the optimized gradient elution described in 2.2 was adopted to separate 18 compounds with different polarities, which could achieve satisfactory separation of the target analytes.
3.1.2. Optimization of MS conditions
The correct ionization mode was beneficial to increase the signal intensity of analytes and improve sensitivity of MS analysis [31]. Injection of the individual standard of each analyte in both positive and negative modes and the positive mode was selected for monitoring of lignans, which showed better protonation and sensitivity. After the optimization of desolvation temperature (450 °C), capillary voltage (2.5 kV) and gas flow (50 L/h) by manual experiments, the molecular ions of lignans [M + H]+ and [M + Na]+ were adopted as precursor ions of MRM transitions under the positive mode. Cone voltage (CV) and collision energy (CE) for each analyte were optimized by gradually changing the voltage and energy range while monitoring the signal intensity of ion. The value that gave the best signal intensity for the ion was considered optimum.
The interest lignans were unambiguously segregated into two groups by the skeletons of dibenzocyclooctadiene and dibenzylbutyrolactone. Main product ions were observed in the MS/MS spectra of these lignans (Table 1). The lignans with dibenzylbutyrolactone skeleton have similar cleavage pathway, which generated fragments by loss of a glucose residue, and subsequently obtained product ion (m/z 137) by benzylic cleavage of the C7-C8 bond (Fig. S1A). According to the position of the oxygen-containing substituent, dibenzocyclooctadiene skeleton lignans were divided into unsubstituted in the eight-membered dibenzene ring, substituented in C-5, substituented in C-6 and double-substituted in C-5 and C-6 (Fig. S1B-S1E). Unsubstituted lignans tended to open the ring by losing of C4H8 fragment (56 Da) to obtain the product ion. Lignans with substituent at C-5 or C-6 were characterized by loss of substituents, and subsequently generated the predominant product ions by loss of H2O or carboxylic acid.
Based on the above fragmentation pathways and optimization of dwell time, cone voltages, and collision energy, the specific and stable MRM method for 18 lignans was established. All transitions for each analyte and their corresponding parameters were summarized in Table 1.
3.2. Validation of quantitation method
The validation of the proposed quantitation method was performed according to international guidelines in terms of specificity, linearity, sensitivity, stability, repeatability, accuracy and matrix effect [30].
3.2.1. Specificity and selectivity
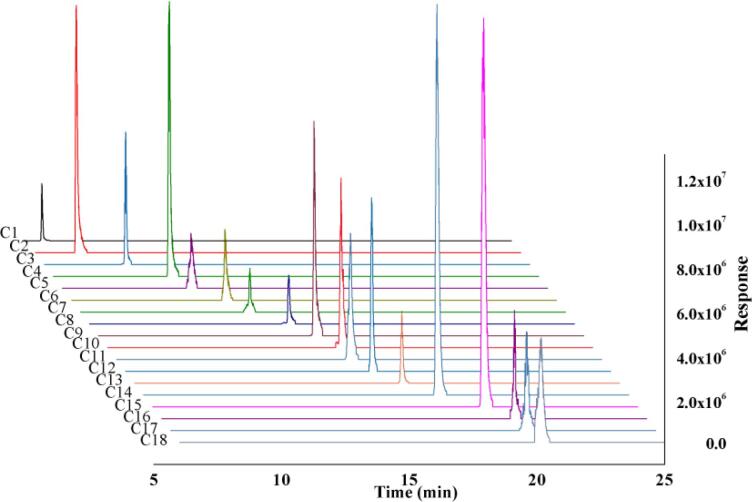
Fig. 2 displayed the typical LC-MS/MS MRM chromatograms of the 18 lignans standards mixture, in which no interference between 18 analytes at the retention time. Additionally, the signal intensity of the analytes in the MRM chromatograms was sufficient for quantitation, illustrating this method with high specificity and good sensitivity.
Fig. 2.
Typical LC-MS/MS MRM chromatograms of 18 lignans (C1-C18) standard mixtures, which are matairesinoside (C1), arcttin (C2), matairesinol (C3), arctigenin (C4), lappaol B (C5), schisandrol A (C6), gomisin D (C7), angeloylgomisin H (C8), gomisin G (C9), schisantherin A (C10), schisantherin B (C11), schisanhenol (C12), gomisin L1 (C13), schizandrin A (C14), schizandrin B (C15), schizandrin C (C16), benzoylgomisin O (C17) and angeloylgomisin O (C18), respectively.
3.2.2. Linear regression, LOD, and LOQ
The calibration curve of each analyte was established to investigate linearity by plotting the signal intensity (peak area, Y) as abscissa and the corresponding concentration as ordinate. As presented in Table 2, the calibration curves for 18 lignans presented satisfactory linearity (R2 > 0.99) within the calibration range of 0.01–24.20 μg/mL. The LOD and LOQ were deemed as minimum concentrations to confidently identify and quantify for analytes in method. The ranges of LOD and LOQ for all lignans were 0.26–70.60 pg and 0.52–141.20 pg, respectively (Table 2). The developed analysis significantly improved sensitivity when compared with the published UPLC-MS quantification, and demonstrated wider linear range of lignans [12].
Table 2.
Linear regression, LOD, LOQ, and precision data of lignans (1–18).
| NO. | Analytes | Range (μg/ml) | Regression equations | R2 | LOD (pg) | LOQ (pg) | Precision (RSD %) |
|
|---|---|---|---|---|---|---|---|---|
| Intra-day | Inter-day | |||||||
| (n = 6) | (n = 6) | |||||||
| 1 | Matairesinoside | 0.10–4.95 | y = 3.6808E-04x + 2.2906E-01 | 0.9961 | 19.80 | 99.00 | 2.38% | 3.11% |
| 2 | Arcttin | 0.45–22.50 | y = 1.5902E-04x − 2.5217E-01 | 0.9925 | 11.50 | 23.00 | 2.24% | 3.89% |
| 3 | Matairesinol | 0.01–2.00 | y = 1.5418E-05x − 9.3195E-04 | 0.9999 | 1.00 | 2.50 | 1.43% | 2.49% |
| 4 | Arctigenin | 0.48–24.20 | y = 1.3670E-05x − 7.1440E-01 | 0.9996 | 6.05 | 12.10 | 1.05% | 0.97% |
| 5 | Lappaol B | 0.14–7.06 | y = 8.8192E-04x + 6.5821E-02 | 0.9994 | 70.60 | 141.20 | 3.77% | 2.39% |
| 6 | Schisandrol A | 0.16–16.20 | y = 3.9203E-04x − 2.9038E-01 | 0.9988 | 9.95 | 19.90 | 4.29% | 0.98% |
| 7 | Gomisin D | 0.10–2.10 | y = 5.0992E-04x − 3.0819E-03 | 0.9999 | 42.00 | 105.00 | 1.29% | 7.12% |
| 8 | Angeloylgomisin H | 0.07–3.55 | y = 1.6117E-04x − 1.8634E-01 | 0.9957 | 35.50 | 71.00 | 3.51% | 1.06% |
| 9 | Gomisin G | 0.01–0.61 | y = 2.6153E-05x − 8.7045E-03 | 0.9985 | 1.53 | 3.05 | 2.48% | 2.52% |
| 10 | Schisantherin A | 0.02–1.05 | y = 1.8298E-05x − 3.2710E-02 | 0.9965 | 1.05 | 2.10 | 2.99% | 1.57% |
| 11 | Schisantherin B | 0.07–3.35 | y = 2.3898E-05x − 2.5098E-01 | 0.9936 | 1.68 | 3.35 | 2.26% | 1.55% |
| 12 | Schisanhenol | 0.01–0.60 | y = 9.8519E-06x + 1.6689E-03 | 1.0000 | 0.60 | 1.50 | 3.47% | 3.20% |
| 13 | Gomisin L1 | 0.01–0.95 | y = 1.8377E-05x − 4.6113E-04 | 0.9999 | 4.75 | 9.50 | 3.89% | 1.58% |
| 14 | Schizandrin A | 0.06–6.00 | y = 4.8897E-06x − 8.0361E-02 | 0.9997 | 1.50 | 3.00 | 2.21% | 0.29% |
| 15 | Schizandrin B | 0.12–11.50 | y = 5.6589E-06x − 3.1710E-01 | 0.9969 | 2.88 | 5.75 | 2.58% | 1.63% |
| 16 | Schizandrin C | 0.01–0.81 | y = 1.6225E-05x + 1.7799E-04 | 0.9999 | 0.81 | 8.10 | 3.89% | 2.98% |
| 17 | Benzoylgomisin O | 0.01–0.62 | y = 5.6597E-06x − 4.0086E-03 | 0.9999 | 0.31 | 0.63 | 2.23% | 1.40% |
| 18 | Angeloylgomisin O | 0.01–0.52 | y = 3.5111E-06x − 2.8068E-03 | 0.9999 | 0.26 | 0.52 | 2.36% | 2.92% |
3.2.3. Precision, stability, and repeatability
Intra-day precision of instrument was used to analyze the standard mixture for six replicates in one day, and the RSDs of peak area for the analytes were below 4.29%. Inter-day precision was calculated based on the data of six replicates in three consecutive days and the RSDs were entirely below 7.12% (Table 2). These results revealed that the method possessed good precision whether determination was conducted on an intra- or inter-day basis. To investigate stability of samples in different extract processes, the RSDs of peak areas for sample with ethanol-extraction were<10.45% (Table 3-1), while the RSDs for HILs-extraction were below 9.89% (Table 3-2). Repeatability of the quantitative approach was assessed by analyzing one sample for six continuous injections. The RSDs of repeatability were<7.48% in ethanol-sample (Table 3-1), while<5.88% in HILs-sample (Table 3-2). These results indicated that the sample possessed good stability and repeatability whether extracted with ethanol or HILs system.
Table 3.1.
48 h stability, repeatability, recovery data, and matrix effect of samples in the ethanol-extraction.
| NO. | Analytes | 48 h Stability (RSD%, n = 8) | Repeatability (RSD%, n = 6) | Recovery | (%, n = 3) | Matrix effect (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| high | middle | low | ||||||||
| Mean | RSD% | Mean | RSD% | Mean | RSD% | |||||
| 1 | Matairesinoside | 4.33% | 3.67% | 117.84% | 2.21% | 98.57% | 4.39% | 83.14% | 3.19% | 95.85% |
| 2 | Arcttin | 6.98% | 1.71% | 107.08% | 0.62% | 108.86% | 1.34% | 83.68% | 3.80% | 118.49% |
| 3 | Matairesinol | 2.83% | 2.97% | 86.30% | 1.91% | 90.17% | 1.69% | 110.81% | 5.57% | 118.82% |
| 4 | Arctigenin | 1.68% | 1.57% | 87.29% | 1.40% | 92.92% | 1.09% | 98.82% | 3.25% | 119.15% |
| 5 | Lappaol B | 6.17% | 7.37% | – | – | – | – | – | – | 80.20% |
| 6 | Schisandrol A | 4.75% | 3.55% | 103.02% | 0.84% | 110.85% | 1.94% | 86.67% | 4.48% | 89.13% |
| 7 | Gomisin D | 5.08% | 7.48% | 92.49% | 3.68% | 95.50% | 6.37% | 109.45% | 5.78% | 80.56% |
| 8 | Angeloylgomisin H | 6.71% | 2.83% | 99.85% | 3.17% | 103.28% | 6.04% | 104.38% | 8.69% | 103.19% |
| 9 | Gomisin G | 4.78% | 2.52% | 114.49% | 2.12% | 103.46% | 7.49% | 87.81% | 8.59% | 119.95% |
| 10 | Schisantherin A | 2.43% | 2.99% | 88.47% | 3.67% | 88.21% | 4.21% | 91.31% | 8.35% | 109.45% |
| 11 | Schisantherin B | 10.45% | 0.72% | 112.47% | 3.48% | 101.71% | 4.29% | 94.84% | 4.97% | 96.26% |
| 12 | Schisanhenol | 1.93% | 1.48% | 93.22% | 2.11% | 90.33% | 2.46% | 98.21% | 6.63% | 118.31% |
| 13 | Gomisin L1 | 2.74% | 1.54% | 93.87% | 2.47% | 80.49% | 1.88% | 90.33% | 3.94% | 95.00% |
| 14 | Schizandrin A | 2.52% | 0.87% | 88.27% | 1.02% | 92.28% | 3.85% | 96.40% | 4.43% | 81.27% |
| 15 | Schizandrin B | 2.36% | 1.22% | 108.75% | 2.15% | 105.09% | 2.98% | 102.71% | 4.43% | 117.50% |
| 16 | Schizandrin C | 3.08% | 1.45% | 112.41% | 3.41% | 105.52% | 6.12% | 92.42% | 4.09% | 87.79% |
| 17 | Benzoylgomisin O | 4.86% | 2.05% | 110.19% | 4.12% | 109.80% | 6.43% | 93.18% | 3.95% | 107.48% |
| 18 | Angeloylgomisin O | 8.21% | 3.95% | 120.11% | 1.89% | 105.34% | 2.63% | 89.44% | 5.27% | 87.95% |
Table 3.2.
48 h stability, repeatability, recovery data, and matrix effect of samples in the HILs-extraction.
| NO. | Analytes | 48 h Stability (RSD%, n = 8) | Repeatability (RSD%, n = 6) | Recovery | (%, n = 3) | Matrix effect (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| high | middle | low | ||||||||
| Mean | RSD% | Mean | RSD% | Mean | RSD% | |||||
| 1 | Matairesinoside | 4.60% | 2.25% | 119.28% | 0.90% | 114.91% | 5.39% | 84.57% | 6.06% | 96.66% |
| 2 | Arcttin | 3.81% | 3.03% | 102.11% | 5.14% | 101.47% | 5.57% | 87.08% | 9.51% | 92.29% |
| 3 | Matairesinol | 1.56% | 1.15% | 83.31% | 7.54% | 88.12% | 3.92% | 94.59% | 3.49% | 89.11% |
| 4 | Arctigenin | 1.76% | 0.51% | 82.63% | 4.52% | 95.75% | 2.87% | 90.98% | 10.40% | 88.04% |
| 5 | Lappaol B | 9.89% | 4.62% | 84.94% | 4.75% | 118.61% | 2.84% | 119.25% | 2.20% | 114.62% |
| 6 | Schisandrol A | 6.12% | 1.64% | 92.28% | 4.81% | 116.42% | 1.59% | 111.13% | 3.98% | 85.74% |
| 7 | Gomisin D | 5.41% | 2.38% | 102.05% | 2.32% | 99.45% | 3.58% | 115.05% | 1.96% | 97.76% |
| 8 | Angeloylgomisin H | 2.74% | 1.90% | 86.67% | 2.78% | 94.35% | 6.25% | 111.25% | 4.01% | 112.33% |
| 9 | Gomisin G | 4.23% | 1.55% | 89.10% | 4.53% | 105.32% | 2.39% | 83.81% | 2.68% | 81.65% |
| 10 | Schisantherin A | 5.42% | 4.30% | 83.35% | 5.95% | 106.05% | 3.81% | 83.12% | 1.20% | 83.14% |
| 11 | Schisantherin B | 2.31% | 2.23% | 89.03% | 4.39% | 89.42% | 5.82% | 114.34% | 2.18% | 81.33% |
| 12 | Schisanhenol | 2.64% | 1.89% | 81.09% | 1.01% | 86.30% | 5.44% | 87.34% | 8.41% | 82.35% |
| 13 | Gomisin L1 | 2.36% | 4.09% | 92.91% | 2.38% | 118.16% | 2.02% | 98.30% | 1.18% | 102.44% |
| 14 | Schizandrin A | 4.40% | 5.88% | 89.76% | 3.89% | 92.00% | 6.76% | 82.64% | 1.17% | 110.83% |
| 15 | Schizandrin B | 4.66% | 1.60% | 96.65% | 3.65% | 103.43% | 3.01% | 117.43% | 1.68% | 88.12% |
| 16 | Schizandrin C | 2.66% | 2.25% | 90.73% | 6.76% | 97.84% | 3.32% | 83.52% | 2.63% | 102.25% |
| 17 | Benzoylgomisin O | 4.06% | 4.79% | 98.34% | 4.81% | 96.15% | 5.61% | 115.32% | 0.38% | 101.36% |
| 18 | Angeloylgomisin O | 3.35% | 4.02% | 82.44% | 3.45% | 89.20% | 4.54% | 101.46% | 8.32% | 98.87% |
3.2.4. Recovery and matrix effect
Recovery experiment was carried out to evaluate the accuracy of the quantitation method by adding standards mixture at three different levels, equivalent to 50%, 100% and 150% of endogenous compounds concentrations, into samples [32]. In the samples processed by ethanol (Table 3-1), the ranges of recoveries were 83.14%-110.81% at 50% spiking level (RSDs < 8.69%), 80.49%-110.85% at 100% spiking level (RSDs < 7.49%), and 86.30%-120.11% at 150% level (RSDs < 4.12%). While in the sample with HILs-extraction (Table 3-2), the recoveries were in the range of 82.64%-119.25% (RSDs < 10.40%), 86.30%-118.61% (RSDs < 6.76%), 81.09%-119.28% (RSDs < 7.54%) at spiking levels of 50%, 100%, and 150%, respectively. It was failed to calculate the recovery of lappaol B (5) in ethanol-extraction due to low concentration. These results suggested that the proposed method has the acceptable accuracy. The matrix effects were evaluated by calculating the slope ratio of the calibrations curve that was obtained between matrix-free standard mixture and matrix-matched sample solutions. The values were accepted below a range of 20% in ethanol extracts (80.20%–119.95%) (Table 3-1) and HILs extracts (81.33%–114.62%) (Table 3-2) for 18 lignans, indicating that matrix has no significant effect on the quantitation for the interested analytes [13].
In summary, specificity, selectivity and precision of the quantitation with UPLC-QqQ-MS in the MRM mode and stability and accuracy of samples employing with ethanol or HILs for 18 lignans had been strongly demonstrated by the results of the methodological validation.
3.3. Extraction optimization of lignans
3.3.1. Optimization for extract conditions employing the conventional organic solvent
Contents of target compounds were conducted as the response values to investigate the influence of different extraction methods, extraction solvents, ultrasonic time, ultrasonic temperature, frequency, ethanol concentration, and liquid to solid ratio respectively on extraction efficiency. The results of the single-factor experimental analysis indicated that the target compounds obtained higher extract efficiency when it was performed under the ultrasonic extraction, ultrasonic time of 30 min, ultrasonic temperature of 40 °C, frequency of 2, ethanol concentration of 80% and liquid to solid ratio of 200 mL/g (Fig. S2). And the obvious influencing factors of ethanol concentration, liquid to solid ratio and extract frequency were selected for further optimization with RSM combined with BBD (Table S2). The ANOVA was performed to test the significance of models.
The results obtained from 17 experimental runs of BBD were shown in Table S3. The results of ANOVA for the regression models were shown in Tables S4. The significance of regression models was checked by F-test and p-values and the model behaved an excellent fitness if the p-value was below 0.05. The regression models of 17 lignans were extremely significant (p < 0.01), expect for lappaol B (5). The values of “lack of fit” were not significant (p > 0.05) for 17 lignans, except for schisandrol A (6). The results indicated that the significant models could suitable for parameter optimization with good predictability for variables.
The p-value was applied to verify the significance for each coefficient of the parameters and the F-value was used to evaluate the incidence of that on the experimental index, which would exert a greater influence with a larger value. The influence of various parameters on extract yields for lignans was shown in Table S4. Factors of X1, X2, and X3 collectively affected the extract yields of 10 compounds (1–4, 7, 11, 13–14 and 17–18). Noticeably, the liquid to solid ratio (X2) exerted no significant influence on the yields of compounds (6, 10, 12, 15 and 16), while the frequency (X3) had no impact on angeloylgomisin H (8). Besides, the extract efficiency of Gomisin (9) was independent of ethanol concentration (X1).
The predicted response of 18 lignans could be expressed as the following multiorder polynomial equations in Table S5 in terms of the coded levels and 3D response surface plots for that presented the visual graphics of the interaction effects between two independents variables. The interactions between ethanol concentration (X1) and liquid to solid ratio (X2) were presented in Fig. S3, with the frequency (X3) held at level of 0 (2). For yields of lignans (1–4, 7–8, 11, 13–14 and 17–18), ethanol concentration and liquid to solid ratio exhibited remarkable impacts on quadratic terms, and the correlative effects of them presented remarkable influences. As the ethanol concentration increased, the average yield of lignans in SCF was increased while that in AF was deceased relatively. This phenomenon was related to the polarity between solvent and constituents; because of lignans in SCF were small polarity while medium in AF [33]. Therefore, mostly lignans could achieve a good extract-efficiency when the concentration of ethanol was 60%. The 3D response surface for the interaction of liquid to solid ratio (X2) and frequency (X3) with fixed ethanol concentration (X1) (level 0) was displayed in Fig. S3, in which remarkable correlative effects were obtained in lignans (1–4, 7, 9, 11, 13–14 and 17–18). As the liquid to solid ratio and frequency increased, contents of lignans increased significantly and then tended to plateau or slightly reduced. The purpose of increasing liquid to solid ratio and frequency was to increase the contact area between solvent and sample, so that the liquid could fully extract target components; however, large solvent volumes could make the extraction difficult and lead to unnecessary waste [24]. A significant correlative effect between ethanol concentration (X1) and frequency (X3) was observed for contents of most lignans.
The optimization for conditions of conventional organic solvent-assisted ultrasonic extraction was fitted out using RSM and was predicted as follows: ethanol concentration of 60%, liquid to solid ratio of 300 mL/g and extract frequency of 2. And the predicted maximum contents of lignans were listed in Table 4. Three parallel experiments were conducted to verify the predicted optimal conditions, which gave the actual values of lignans shown in Table 4. The actual values were close to the predicted values, indicating that RSM was the available method for improving the extract yields of lignans in ultrasound-assisted extraction with the conventional organic solvent.
Table 4.
Comparison the predicted values of RSM with the actual values of verification experiment for lignans.
| NO. | Analytes | Extraction with ethanol |
Extraction with HILs |
||
|---|---|---|---|---|---|
| Predicted value (mg/g) | Measured value (mg/g) | Predicted value | Measured value | ||
| (mg/g) | (mg/g) | ||||
| 1 | Matairesinoside | 0.2654 | 0.2478 | 0.1885 | 0.1795 |
| 2 | Arcttin | 8.6021 | 7.4672 | 7.2159 | 7.1869 |
| 3 | Matairesinol | 0.2111 | 0.2029 | 0.2237 | 0.2363 |
| 4 | Arctigenin | 4.3236 | 3.9111 | 4.2100 | 4.3619 |
| 6 | Schisandrol A | 3.3475 | 2.7081 | 3.2770 | 3.6161 |
| 7 | Gomisin D | 0.1293 | 0.1183 | 0.1323 | 0.1361 |
| 8 | Angeloylgomisin H | 0.2589 | 0.2661 | 0.4976 | 0.5369 |
| 9 | Gomisin G | 0.1123 | 0.1071 | 0.1222 | 0.1214 |
| 10 | Schisantherin A | 0.0762 | 0.0767 | 0.1258 | 0.1131 |
| 11 | Schisantherin B | 0.1929 | 0.1779 | 0.3999 | 0.4217 |
| 12 | Schisanhenol | 0.0701 | 0.0706 | 0.0718 | 0.0724 |
| 13 | Gomisin L1 | 0.0345 | 0.0339 | 0.0334 | 0.0352 |
| 14 | Schizandrin A | 0.8363 | 0.9246 | 1.0071 | 1.0191 |
| 15 | Schizandrin B | 1.4602 | 1.4215 | 1.3260 | 1.4063 |
| 16 | Schizandrin C | 0.1410 | 0.1395 | 0.0855 | 0.0974 |
| 17 | Benzoylgomisin O | 0.0071 | 0.0072 | 0.0109 | 0.0115 |
| 18 | Angeloylgomisin O | 0.0021 | 0.0023 | 0.0052 | 0.0055 |
3.3.2. Optimization of extract conditions employing HILs
3.3.2.1. Optimization for the proportion of HILs using uniform design
According to the tentatively study, [EMIM][BF4], [EMIM][OAc] and [AMIM]Cl showed higher extractability for partial lignans respectively. Whereas, we found that use of a single IL could not increase the content of all target compounds due to their polarity differences. Therefore, we wondered whether extraction with a mixture of ILs could effectively improve the contents of 18 lignans simultaneously.
To further optimize the composition and proportion of HILs, three ILs mentioned above were chosen for uniform design from six levels, which ensured the experimental points distributed evenly under the premise of needing fewer trials. The software of the Data Processing System (DPS Version 7.01) was used to generate the experimental design. According to the uniform design table U6 (63), three ILs acted as the independent variables (X1, X2 and X3), and the contents of lignans (1–4 and 6–18) as the dependent variables except for lappaol B (5). Three parallel experiments were conducted by controlling the volume ratio of the total ILs to H2O to be 1:1, and the optimal proportion for HILs was obtained. The experimental design and results were listed in Table S6.
The SPSS 19.0 was applied for subsequent data analysis and regression model generation. The significant tests demonstrated the validity of models and multiple regression equation along with corresponding indexes, as listed in Table S7. The results indicated that the content of [AMIM]Cl (X1) showed a downside impact on yield for compounds (3, 7, 8, 10, 12 and 13), while a upside impact on matairesionside (1). Additionally, the content of [EMIM][OAc] (X2) exerted significant upside influences on yields of compounds (1–4, 6, 8, 9, 11 and 13), and [EMIM][BF4] (X3) positively affected compounds (1, 9, 14–18). Consequently, the extract yield of lignans could be improved by relatively raising the proportion of [EMIM][OAc] and [EMIM][BF4]. The optimized proportion for HILs obtained was: [AMIM]Cl:[EMIM][OAc]:[EMIM][BF4] of 1:5:5.
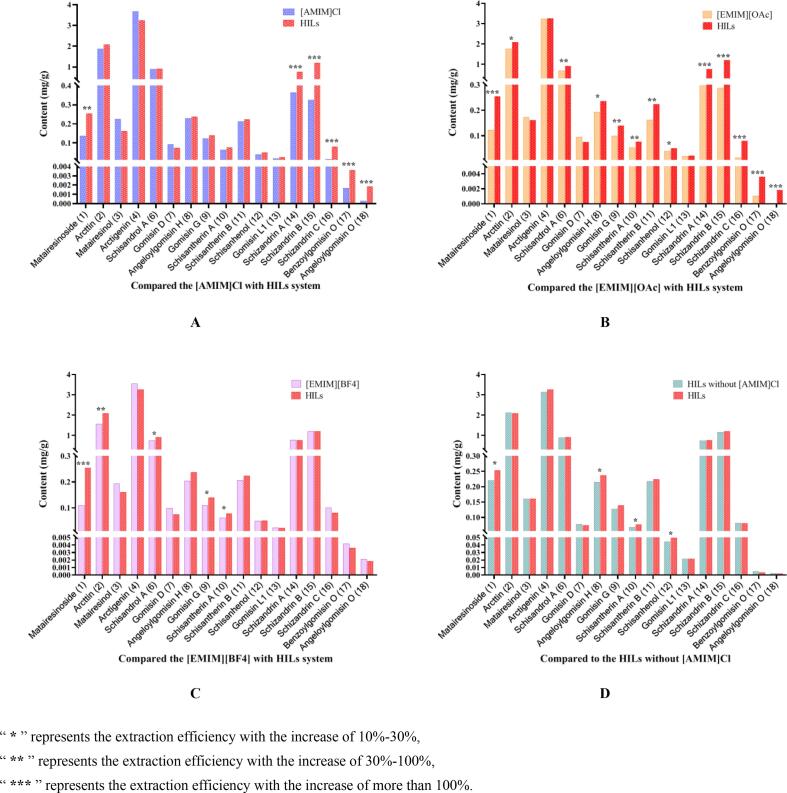
To verify the composition of HILs associated with the yield of target compounds, the verification experiment was conducted following the optimal proportion obtained previously and compared that with the single IL, as illustrated in Fig. 3. Comparison of extract abilities of the HILs with the [AMIM]Cl (Fig. 3A), the yields of the compounds (1, 14–18) increased greatly, in which, the yields of schizandrin A (14), schisandrin B (15), schizandrin C (16), benzoylgomisin O (17) and angeloylgomisin O (18) with an enhancement>100%. As for [EMIM][OAc] (Fig. 3B), the extract yields for compounds (6, 9–11) with an increased between 30% and 100%. Obviously, matairesinoside (1) and compounds (14–18) increased by over 100% after optimization. As to [EMIM][BF4] (Fig. 3C), the contents of matairesinoside (1) increased by 131%, while arcttin (2) increased by 32%. The uniform design results concluded the proportion of [AMIM]Cl was relatively low. Therefore, we compared the HILs with that without [AMIM]Cl (Fig. 3D), and found that the contents of matairesinoside (1), angeloylgomisin H (8), schisantherin A (10) and schisanhenol (12) were abundant in system containing [AMIM]Cl, but rare in that without [AMIM]Cl. Accordingly, the three ILs mixtures were determined as the extract system according to the above results.
Fig. 3.
Comparison of extraction yields of target compounds between the HILs and single IL: (A) comparison between HILs and [AMIM]Cl, (B) comparison between HILs and [EMIM][OAc], (C) comparison between HILs and [EMIM][BF4], (D) compared to the HILs without [AMIM]Cl.
3.3.2.2. Optimization for extract conditions using RSM
Based on the optimal proportion, we conducted the single-factor experiment and showed that the target compounds with higher extract yield by ultrasonic extraction, HILs content of 1:1 (VILs/VH2O), ultrasonic time of 30 min, ultrasonic temperature of 60 °C, ultrasonic power of 100 W, frequency of 1 and liquid to solid ratio of 200 mL/g (Fig. S4). Of which, the factors of HILs content, liquid to solid ratio and extract time were selected for further optimization with RSM combined with BBD (Table S8).
The results of BBD were shown in Table S9 and the ANOVA for the regression models was shown in Table S10. Considering the low response value of lappaol B (5), contents of 17 lignans were participated in fitting the regression models in RSM. The regression models for 17 lignans were extremely significant (p < 0.01), and the value of “lack of fit” was not significant (p > 0.05) for 16 lignans except for schisantherin A (10). The results indicated that the significant models could be applied for parameter optimization with a good prediction for variables.
The p-value and F-value for each coefficient of parameters were shown in Table S10. It was indicated that the ILs content (X1) played a critical role in the increasing of yields for 14 target compounds except for matairesinol (3), gomisin D (7) and schisanhenol (12). The contents of 13 target lignans were significantly affected by the liquid to solid ratio (X2) except for schisanhenol (12), schizandrin B (15), benzoylgomisin O (17) and angeloylgomisin O (18). And the extract time (X3) exerted a significant influence on the yields of lignans (1–3, 6, 8–10 and 14–16).
The multiorder polynomial equations for the responses and variables in terms of the coded levels were listed in Table S11, and the 3D response surface plots of interaction effects between two independents variables were shown in Fig. S5. The correlative effects between ILs content (X1) and liquid to solid ratio (X2) were presented remarkable influences on yields of 16 lignans except for schisanhenol (12), with the extract time (X3) held at level of 0 (40 min). The yields of lignans improved with ILs content increasing initially. After further increases, a significantly decrease in extract-yield was observed. We suggested that the high viscosity at high ILs concentration may result in poor solvent penetration into sample, leading to reduced extract-yield [24]. Moreover, as liquid to solid ratio increased, yields of lignans improved obviously and the reason for this phenomenon may be that concentration difference of target lignans between sample and solvent were increased with the increase of liquid to solid ratio. The increased concentration difference enhanced the mass transfer rate and the extract efficiency increased [16]. Another consideration was extract time (X3), it was demonstrated that remarkable correlative effects for the interaction of ILs content (X1) and extract time (X3) were obtained in mostly lignans, with fixed liquid to solid ratio (X2) (level 0). The extract-ability expanded within 40 min, and yields of lignans remained at a similar level during longer extract time for the extraction was complete after 40 min.
The optimization for extract conditions employing HILs were predicted as follows: total ILs content of 1.2:1 (VILs/VH2O), liquid to solid ratio of 210 mL/g and extract time of 40 min. The predicted maximum contents of lignans were listed in Table 4. And three parallel verification experiments were conducted under the optimized conditions, which gave the practical value for lignans were shown in Table 4. The actual values were close to the predicted values, indicating that RSM was a suitable method for improving the extract yields of lignans in ultrasound-assisted extraction with HILs.
3.3.3. Comparison of the efficiency employing HILs and traditional organic solvent in ultrasonic-assisted extraction
To more comprehensively investigate the difference of extract-abilities between HILs and organic solvent on the target compounds, a series of extract experiments were conducted under the ultrasound-assisted extraction.
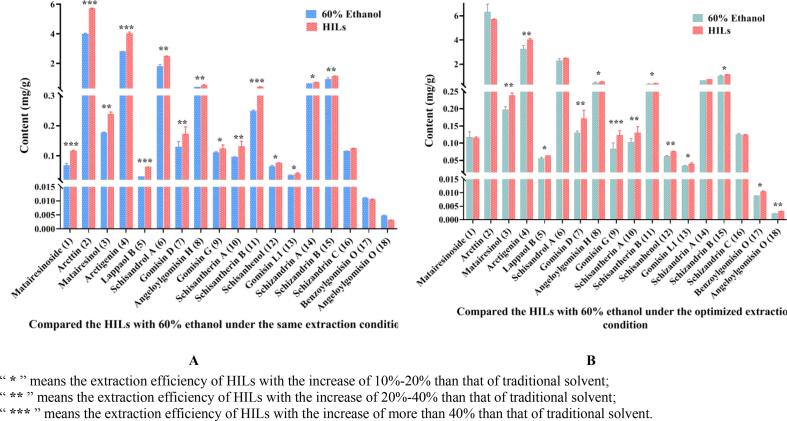
When compared with the ethanol-extraction under the same conditions, the proposed approach using HILs offered higher extract efficiency. It was noted that significant increases in the contents of 15 target compounds were shown in Fig. 4A and Table S12. Further analysis revealed that the contents on compounds (9 and 12–14) increased between 10% and 20%, and six compounds (3, 6–8, 10 and 15) with an increase between 20% and 40%. Besides, the contents of arcttin (2) and arctigenin (4) increased distinctly, reaching 43%. Moreover, the yields of matairesinoside (1), lappaol B (5), and schisantherin B (11) with an extremely significant enhancement, which were up to 69%, 105%, and 60%, respectively. It was concluded that the proposed HILs-based extraction was superior to traditional solvent under the same ultrasonic-extract conditions.
Fig. 4.
Comparison of the efficiency employing HILs and traditional organic solvent in ultrasonic-assisted extraction: (A) compared the HILs with 60% ethanol under the same extraction conditions, (B) compared the HILs with 60% ethanol under the optimized extraction conditions.
To better characterize the advantages of the HILs system, we compared it with the optimal ethanol-extract approach, as shown in Fig. 4B and Table S12. The experimental results manifested that 13 target lignans obtained by HILs possessed higher extract efficiency than optimal ethanol-extraction. Further data analysis discovered that the yields for six compounds in HILs (5, 8, 11, 13, 15 and 17) increased between 10% and 20%, and six lignans (3, 4, 7, 10, 12 and 18) with an increase of 20%-40%. Additionally, the extract yields of gomisin G (9) with obviously improvement reached 47%. These results indicated that even compared with the optimized ethanol-extraction, the higher extract efficiency of target compounds still could be achieved by using the HILs system. Briefly, it meant that the proposed HILs-based ultrasound-assisted extraction is a green and efficient extract protocol for lignans.
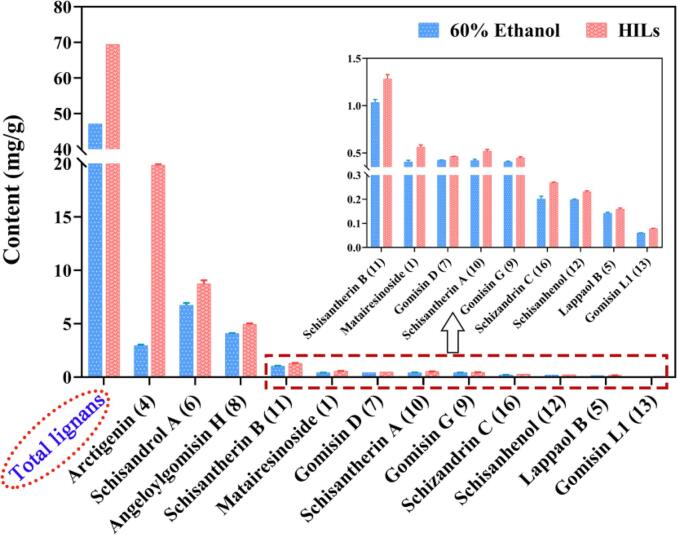
To demonstrate the feasibility of the constructing HILs system in the application of TCMs, according to the extraction process of SH, SCF and AF were extracted employing with 60% ethanol and HILs at the same conditions, respectively. The result indicated that the content of total lignans in the ethanol system was up to 47 mg/g, while that in HILs system was up to 69 mg/g, with an increasing of 47%. Remarkably, there were significant increases in the contents of 12 compounds in the TCMs, as shown in Fig. 5. Further data processing revealed that, in the HILs system, the yields of matairesinoside (1), arctigenin (4), schisandrol A (6), gomisin L1 (13) and schizandrin C (16) were increased more than 30%. Extraordinary, the concentration of bioactive arctigenin (4) in HILs was highly outstripped than that in ethanol, with an increase by nearly six times. Consequently, the HILs system could be widespread generalized in the simultaneous extraction of compounds with different polarities in TCMs.
Fig. 5.
Comparison of the efficiency in TCMs (SCF and AF) employing HILs and traditional organic solvent in ultrasonic-assisted extraction.
3.4. Characterization of the interaction between ILs and lignans
Arctigenin (4) as a representative lignan in AF, with obvious increased-yield in the HILs system, was selected by using 1H-NMR and 13C-NMR measurements of arctigenin, [EMIM][OAc], [AMIM]Cl, [EMIM][BF4], [EMIM][OAc]-arctigenin mixtures, [AMIM]Cl-arctigenin mixtures, and [EMIM][BF4]-arctigenin mixtures to determine interactions between ILs and arctigenin.
The analysis of 13C-NMR data (Fig. S6B) of [EMIM][OAc]-arctigenin mixtures, arctigenin, and [EMIM][OAc] showed an interesting phenomenon. Compared with their single substance, in the [EMIM][OAc]-arctigenin mixtures, the chemical shift of carbonyl (Δδ = 0.12 ppm) in [EMIM][OAc] moved to downfield at δC173.60, and the chemical shift of C-4 (Δδ = 2.19 ppm) in arctigenin moved to downfield at δC147.28 overlapped with C-4′, which were confirmed by HMBC correlations validly. Such a phenomenon indicated that the acetate anions of [EMIM][OAc] formed stronger hydrogen bond with arctigenin. The increase in chemical shift for carbonyl in the [EMIM][OAc] was an indication for the interaction between that with the phenolic hydroxyl proton in arctigenin, which led to a decrease of electron cloud density around C-3, C-4 and C-5 in arctigenin and causes a downfield shift [34]. Simultaneously, the carbonyl (Δδ = 0.14 ppm) in arctigenin may form weaker hydrogen bond with the ring protons (C-2, Δδ = -0.38 ppm) in the imidazolium cation and hydrogens in C-9 (Δδ = -0.25 ppm) of [EMIM][OAc]. Therefore the signals of the C-2 and C-9 in [EMIM][OAc] moved upfield. The chemical shift of C-2 in imidazolium cation was due to acidic and hydrogen bond donor properties in C2-H. And the upfield change of C9 was mainly attributed to the increase of electron density around the C-9 nucleus, owing to the stronger hydrogen bonds between the acetate anion and arctigenin [35]. Consequently, chemical shifts change of carbon atoms was ascribed to formation of new hydrogen bonds between arctigenin and acetate anion of [EMIM][OAc]. As shown in 1H-NMR (Fig. S6A), C2-H of imidazolium cation exhibited an obvious upfield shift (from 10.06 ppm to 9.76 ppm) against of pure [EMIM][OAc]. Owing to formation of the inter-molecular hydrogen bonds between arctigenin and [EMIM][OAc], the intra-molecular hydrogen bond between anion and cationic C2-H in the IL would be disturbed, resulting in the upfield shift of C2-H.
Similar phenomena could be observed in [AMIM]Cl-arctigenin mixtures (Fig. S7A). Compared to 1H-NMR spectrum of single substance, the signals of –OH in arctigenin moved downfield (Δδ = 0.40 ppm), while C2-H of [AMIM]Cl moved upfield (Δδ = -0.10 ppm). It was reported that the formation of hydrogen bond led to downfield chemical shift of the proton, while the weakening of hydrogen bond resulted in upfield chemical shift of them [36]. Considering the acidity of –OH of arctigenin was better than C2-H of [AMIM]Cl, the Cl- with strong hydrogen bond accepting ability in the IL would preferentially form hydrogen bond with –OH of arctigenin, which would weaken the hydrogen bond between anion and cationic C2-H in the IL [37]. Similarly, C-4 in arctigenin exhibited an obvious downfield shift (Δδ = 0.24 ppm) against of pure arctigenin in 13C-NMR, which confirmed the hydrogen-bond interaction with IL (Fig. S7B). The interaction of hydrogen bond between [EMIM][OAc] and arctigenin was superior to other ILs, and there was no significant chemical shifts observed in 1H-NMR and 13C-NMR of [EMIM][BF4]-arctigenin mixtures.
Schisantherin A (10), with a significant improved-yield in the HILs system, was typically chosen as small-polarity lignan in SCF to reveal the structural properties between that of IL.
It was noted from 1H-NMR spectra of [AMIM]Cl-schisantherin A mixtures (Fig S8A) and [EMIM]OAc-schisantherin A mixtures (Fig S9A) that signal of C4-H in schisantherin A moved toward downfield by 0.27 ppm and 0.10 ppm, respectively. While signals of C2-H in [AMIM]Cl and [EMIM][OAc] moved to upfield by 0.11 ppm and 0.50 ppm, respectively. The analysis of 13C-NMR data in [AMIM]Cl-schisantherin A mixtures (Fig S8B) and [EMIM][OAc]-schisantherin A mixtures (Fig S9B), we observed that C-4 and C-5 of schisantherin A were all shifted to downfield compared with pure schisantherin A. Particularly, shifted values of C-4 were 0.49 ppm and 0.22 ppm in [AMIM]Cl-schisantherin A mixtures and [EMIM][OAc]-schisantherin A mixtures, respectively. And the chemical shift of carbonyl (Δδ = 0.08 ppm) in [EMIM][OAc] moved to downfield at δC173.56 (Fig S9B). Combining the data of 1H-NMR, a possible explanation for such results were that hydrogen bonds were formed between C4-H of schisantherin A and anions of ILs, and the hydrogen bond formation ability of [AMIM]Cl surpassed other ILs in HILs system. There was no significant chemical shifts observed in 1H-NMR and 13C-NMR of [EMIM][BF4]-schisantherin A mixtures.
Consequently, the variation of chemical shifts in ILs-arctigenin mixtures and ILs-schisantherin A mixtures could be concluded that the ILs incorporated with the anions, which have strong hydrogen bond acceptor and can effectively dissolve target compounds by formation of hydrogen bonds between anions of ILs and active protons of lignans. Furthermore, superposition effect of hydrogen-bond formation with various ILs raises a rational interpretation why HILs system achieves a significant increase in extract efficiency than organic solvent and single IL.
4. Conclusions
In this study, firstly, a comprehensive identification and quantification of 18 lignans from SH was triumphantly developed based on UPLC-QqQ-MS/MS in the MRM mode method. It was concluded that the developed method has the advantages of linearity, sensitivity, precision, specificity and accuration. And the fragmentation patterns of 18lignans were proposed by MS/MS analysis. Moreover, this study provided a practical example by using lignans in SH illustrating the feasibility of constructing a HILs system for ultrasonic-extraction of natural compounds with different polarities. The results undoubtedly indicated that this HILs system, which was formed using [AMIM]Cl:[EMIM][OAc]:[EMIM][BF4] at the optimal proportion of 1:5:5, exhibited higher extract ability compared to that of traditional organic solvent and single IL. Compared with traditional organic solvent-extraction under the same conditions, the yields for 15 lignans increased obviously. Subsequently, compared with the optimized traditional organic solvent-extraction, there were still significant increases on extract efficiency for 13 lignans, among which 7 lignans with the enhancement of over 20%. Moreover, the total lignans were increased by 47% when the HILs system was generalized in the extraction of TCMs. Different from the use of single IL, a HILs system could be tailored for the maximal extractability of diverse polarity compounds. Finally, arctigenin (4) and schisantherin A (10) as representative lignans were selected by using 1H- NMR and 13C-NMR spectroscopy to characterize the interactions between ILs and compounds. It could be tentatively deduced that the phenolic hydroxyl in arctigenin and C4-H in schisantherin A as donors to form hydrogen-bond interactions with the anions of HILs, promoting its solubility in the HILs system. Consequently, the application of the HILs system in multi-compounds ultrasonic-extraction could avoid the employment of volatile toxic solvent, and provide a great protocol for the utilization of IL, which possesses a great application prospect. Although the method proposed in this paper has been proved to be effective and feasible for the extract of lignans from TCMs (SCF and AF), we have not applied it to extract other types of chemical ingredients from natural products. Therefore, further study on the application of HILs-extraction for more chemical compositions with different structure types should be developed, which will undoubtedly promote the large-scale application of ILs in various domains.
CRediT authorship contribution statement
Jiajia Wu: Investigation, Writing - original draft. Xingdong Wu: Conceptualization, Formal analysis, Validation. Rongrong Wu: Data curation. Zhen Wang: Project administration. Ninghua Tan: Supervision, Funding acquisition, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the “Double First-Class” University Project (CPU2018GF05) from China Pharmaceutical University and the Program for Innovative Research Team of Jiangsu Province.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105539.
Contributor Information
Zhen Wang, Email: drwang@cpu.edu.cn.
Ninghua Tan, Email: nhtan@cpu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Qin W.W., Wu X.D., Jia Y.N., Tong X.Y., Guo C., Chen D., Wang Z., Tan N.H. Suhuang antitussive capsule inhibits NLRP3 inflammasome activation and ameliorates pulmonary dysfunction via suppression of endoplasmic reticulum stress in cough variant asthma. Biomed. Pharmacother. 2019;118 doi: 10.1016/j.biopha.2019.109188. [DOI] [PubMed] [Google Scholar]
- 2.Tong X.Y., Liang R.Y., Jia Y.J., Qin W.W., Guo C., Wu X.D., Wang Z., Chen D., Tan N.H. Suhuang antitussive capsules-ameliorative effects on LPS-induced sputum obstruction in mice through promoting HGF secretion. Front. Pharmacol. 2019;10:1422. doi: 10.3389/fphar.2019.01422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X.D., Liu Q.Y., Chen D., Qin W.W., Lu B.Y., Bi Q.R., Wang Z., Jia Y.N., Tan N.H. Identification of quality control markers in Suhuang antitussive capsule based on HPLC-PDA fingerprint and anti-inflammatory screening. J. Pharm. Biomed. Anal. 2020;180 doi: 10.1016/j.jpba.2019.113053. [DOI] [PubMed] [Google Scholar]
- 4.Qin Z.B., Ding L.F., Wang X., Huang L.J., Liang M.J., Bin J., Luo N., Deng L., Guo Y.D. Lignans from the seeds of Arctium lappa L. (burdock) and their inhibitory effects on nitric oxide production. Phytochem. Lett. 2019;34:43–49. [Google Scholar]
- 5.Dias M.M., Zuza O., Riani L.R., Pinto P.D.F., Pinto P.L.S., Silva M.P., Moraes J.D., Ataíde A.C.Z., Silva F.D.O., Cecílio A.B., Filho A.A.D.S. In vitro schistosomicidal and antiviral activities of Arctium lappa L. (Asteraceae) against Schistosoma mansoni and Herpes simplex virus-1. Biomed. Pharmacother. 2017;94:489–498. doi: 10.1016/j.biopha.2017.07.116. [DOI] [PubMed] [Google Scholar]
- 6.Jang M.K., Yun Y.R., Kim J.H., Park M.H., Jung M.H. Gomisin N inhibits adipogenesis and prevents high-fat diet-induced obesity. Sci. Rep. 2017;7:40345. doi: 10.1038/srep40345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X.K., Yang H.J., Xiao J.C., Zhang J., Zhang J., Liu M., Zheng Y.C., Ma L. Network pharmacology based investigation into the bioactive compounds and molecular mechanisms of Schisandrae Chinensis Fructus against drug-induced liver injury. Bioorg. Chem. 2020;96 doi: 10.1016/j.bioorg.2019.103553. [DOI] [PubMed] [Google Scholar]
- 8.Don R.A.S.G., Yap M.K.K. Arctium lappa L. root extract induces cell death via mitochondrial-mediated caspase-dependent apoptosis in Jurkat human leukemic T cells. Biomed. Pharmacother. 2019;110:918–929. doi: 10.1016/j.biopha.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Kang T.G., Dou D.Q., Xu L. Establishment of a quality marker (Q-marker) system for Chinese herbal medicines using burdock as an example. Phytomedicine. 2019;54:339–346. doi: 10.1016/j.phymed.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Xiao W.L., Li R.T., Huang S.X., Pu J.X., Sun H.D. Triterpenoids from the Schisandraceae family. Nat. Prod. Rep. 2008;25:871–891. doi: 10.1039/b719905h. [DOI] [PubMed] [Google Scholar]
- 11.Zhao M.J., Bergaentzlé M., Flieller A., Marchioni E. Development and validation of an ultra-high performance liquid chromatography-high resolution mass spectrometry method for simultaneous quantification of cyanogenic glycosides and secoisolariciresinol diglucoside in flaxseed (Linum usitatissimum L.) J. Chromatogr. A. 2019;1601:214–223. doi: 10.1016/j.chroma.2019.04.072. [DOI] [PubMed] [Google Scholar]
- 12.Liu K.Y., Song Y.G., Liu Y.L., Peng M., Li H.Y., Li X.L., Feng B.W., Xu P.F., Su D. An integrated strategy using UPLC-QTOF-MSE and UPLC-QTOF-MRM (enhanced target) for pharmacokinetics study of wine processed Schisandra chinensis fructus in rats. J. Pharm. Biomed. Anal. 2017;139:165–178. doi: 10.1016/j.jpba.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X.J., Bi Q.R., Wu X.D., Wang Z., Miao Y.Y., Tan N.H. Systematic characterization and quantification of Rubiaceae-type cyclopeptides in 20 Rubia species by ultra performance liquid chromatography tandem mass spectrometry combined with chemometrics. J. Chromatogr. A. 2018;1581–1582:43–54. doi: 10.1016/j.chroma.2018.10.049. [DOI] [PubMed] [Google Scholar]
- 14.Guo Y.X., Han J., Zhang D.Y., Wang L.H., Zhou L.L. Aqueous two-phase system coupled with ultrasound for the extraction of lignans from seeds of Schisandra chinensis (turcz.) Baill. Ultrason. Sonochem. 2013;20:125–132. doi: 10.1016/j.ultsonch.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Huang T.L., Lin J.C.T., Chyau C.C., Lin K.L., Chang C.M.J. Purification of lignans from Schisandra chinensis fruit by using column fractionation and supercritical antisolvent precipitation. J. Chromatogr. A. 2013;1282:27–37. doi: 10.1016/j.chroma.2013.01.091. [DOI] [PubMed] [Google Scholar]
- 16.Wang T., Wang Q., Li P., Yang H. Temperature-responsive ionic liquids to set up a method for the simultaneous extraction and in situ preconcentration of hydrophilic and lipophilic compounds from medicinal plant matrices. Green Chem. 2019;21:4133–4142. [Google Scholar]
- 17.Hsieh Y.H., Li Y.B., Pan Z.C., Chen Z.J., Lu J.H., Yuan J.M., Zhu Z.Y., Zhang J.H. Ultrasonication-assisted synthesis of alcohol-based deep eutectic solvents for extraction of active compounds from ginger. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104915. [DOI] [PubMed] [Google Scholar]
- 18.Cao J.F., Xu W., Chen X.W., Shu Y. Improvement on the extraction efficiency of low density lipoprotein in an ionic liquid microemulsion. Talanta. 2019;195:720–727. doi: 10.1016/j.talanta.2018.11.111. [DOI] [PubMed] [Google Scholar]
- 19.Yavir K., Marcinkowski L., Marcinkowska R., Namieśnik J., Kloskowski A. Analytical applications and physicochemical properties of ionic liquid-based hybrid materials: A review. Anal. Chim. Acta. 2019;1054:1–16. doi: 10.1016/j.aca.2018.10.061. [DOI] [PubMed] [Google Scholar]
- 20.Benedéa J.L., Andersonb J.L., Chisvert A. Trace determination of volatile polycyclic aromatic hydrocarbons in natural waters by magnetic ionic liquid-based stir bar dispersive liquid microextraction. Talanta. 2018;176:253–261. doi: 10.1016/j.talanta.2017.07.091. [DOI] [PubMed] [Google Scholar]
- 21.Bediako B.B.A., Qian Q.L., Zhang J.J., Wang Y., Shen X.J., Shi J.B., Cui M., Yang G.Y., Wang Z., Tong S.R., Han B.X. Ru-Catalyzed methanol homologation with CO2 and H2 in an ionic liquid. Green Chem. 2019;21:4152–4158. [Google Scholar]
- 22.Jha R.R., Singh C., Pant A.B., Patel D.K. Ionic liquid based ultrasound assisted dispersive liquid-liquid micro-extraction for simultaneous determination of 15 neurotransmitters in rat brain, plasma and cell samples. Anal. Chim. Acta. 2018;1005:43–53. doi: 10.1016/j.aca.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Muradora D.C., Bragaa A.R.C., Martinsc P.L.G., Mercadanted A.Z., Rossoa V.V.D. Ionic liquid associated with ultrasonic-assisted extraction: A new approach to obtain carotenoids from orange peel. Food Res. Int. 2019;126 doi: 10.1016/j.foodres.2019.108653. [DOI] [PubMed] [Google Scholar]
- 24.Sui X.Y., Liu T.T., Liu J.C., Zhang J., Zhang H.L., Wang H.Y., Yang Y. Ultrasonic-enhanced surface-active ionic liquid-based extraction and defoaming for the extraction of psoralen and isopsoralen from Psoralea corylifolia seeds. Ultrason. Sonochem. 2020;69 doi: 10.1016/j.ultsonch.2020.105263. [DOI] [PubMed] [Google Scholar]
- 25.Lee S.Y., Show P.L., Ling T.C., Chang J.S. Single-step disruption and protein recovery from Chlorella vulgaris using ultrasonication and ionic liquid buffer aqueous solutions as extractive solvents. Biochem. Eng. J. 2017;124:26–35. [Google Scholar]
- 26.Hou K.X., Bao M.L., Wang L., Zhang H., Yang L., Zhao H.T., Wang Z.Y. Aqueous enzymatic pretreatment ionic liquid-lithium salt based microwave-assisted extraction of essential oil and procyanidins from pinecones of Pinus koraiensis. J. Clean. Prod. 2019;236 [Google Scholar]
- 27.Ma C.H., Liu T.T., Yang L., Zu Y.G., Wang S.Y., Zhang R.R. Study on ionic liquid-based ultrasonic-assisted extraction of biphenyl cyclooctene lignans from the fruit of Schisandra chinensis Baill. Anal. Chim. Acta. 2011;689:110–116. doi: 10.1016/j.aca.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Box G.E.P., Behnken D.W. Some new three level designs for the study of quantitative variables. Technometrics. 1960;2:455–475. [Google Scholar]
- 29.Tang Q., Feng M. China Agriculture Press; Beijing: 1997. Practical Statistics and DPS Data Processing System; p. 407. [Google Scholar]
- 30.Gai Q.Y., Jiao J., Wang X., Fu Y.J., Lu Y., Liu J., Wang Z.Y., Xu X.J. Simultaneous quantification of eleven bioactive phenolic compounds in pigeon pea natural resources and in vitro cultures by ultra-high performance liquid chromatography coupled with triple quadrupole mass spectrometry (UPLC-QqQ-MS/MS) Food Chem. 2021;335 doi: 10.1016/j.foodchem.2020.127602. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y., Sun H.Z., Zhou L., Luo F.J., Zhang X.Z., Chen Z.M. Quantitative determination and contamination pattern of perchlorate in tea by ultra performance liquid chromatography and tandem mass spectrometry. Food Chem. 2019;274:180–186. doi: 10.1016/j.foodchem.2018.07.113. [DOI] [PubMed] [Google Scholar]
- 32.Chinese Pharmacopoeia Commission . 11th ed. China Medical Science Press; Beijing, China: 2020. Chinese Pharmacopoeia. [Google Scholar]
- 33.Ramin T.B., Mohammad S.H., Morteza K., Pegah S., Iman N. Ultrasound-assisted packed-bed extraction of hypericin from Hypericum perforatum L. and optimization by response surface methodology. Ultrason. Sonochem. 2019;57:89–97. doi: 10.1016/j.ultsonch.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Chen Q.T., Xu A.R., Li Z.Y., Wang J.J., Zhang S.J. Influence of anionic structure on the dissolution of chitosan in 1-butyl-3-methylimidazolium-based ionic liquids. Green Chem. 2011;13:3446–3452. [Google Scholar]
- 35.Li Q.P., Sun J., Zhuang L.H., Xu X.Q., Sun Y., Wang G.W. Effect of urea addition on chitosan dissolution with [Emim]Ac-urea solution system. Carbohydr. Polym. 2018;195:288–297. doi: 10.1016/j.carbpol.2018.04.097. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y.Z., Wang N.N., Luo J.J., Zhou Y., Yu Z.W. Hydrogen-bonding interactions between [BMIM][BF4] and acetonitrile. Phys. Chem. Chem. Phys. 2013;15:18055–18064. doi: 10.1039/c3cp53356e. [DOI] [PubMed] [Google Scholar]
- 37.Zhu G.F., Gao X., Wang X.L., Wang J.J., Fan J. Influence of hydrogen bond accepting ability of anions on the adsorption performance of ionic liquid surface molecularly imprinted polymers. J. Chromatogr. A. 2018;1532:40–49. doi: 10.1016/j.chroma.2017.11.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.