Graphical abstract
Keywords: Aortic stenosis, Cardiac damage, Staging, Prognosis, Transcatheter aortic valve implantation
Highlights
-
•
Guideline recommendations improve the reproducibility of cardiac damage staging.
-
•
Guideline recommendations increase the sensitivity of cardiac damage staging.
-
•
The refined staging provides accurate prognostic value in patients undergoing TAVI.
-
•
The prognostic value was maintained after excluding cardiopulmonary comorbidities.
Abstract
Background
A new staging classification of aortic stenosis (AS) characterizing the extent of cardiac damage was established and validated in patients undergoing transcatheter aortic valve implantation (TAVI). The present study was aimed to refine the staging system by integrating a quantitative evaluation of right ventricular (RV) dysfunction defined by current echocardiographic guideline recommendations.
Methods and results
In a prospective TAVI registry, patients were categorized into the stages: no cardiac damage (Stage 0), left ventricular damage (Stage 1), left atrial or mitral valve damage (Stage 2), pulmonary vasculature or tricuspid valve damage (Stage 3), or RV damage (Stage 4) based on baseline echocardiography. Among 1133 eligible patients undergoing TAVI, 8 (3.4%) patients were categorized as Stage 0, 113 (10.0%) as Stage 1, 397 (35.0%) as Stage 2, 239 (21.1%) as Stage 3, and 346 (30.5%) as Stage 4. There was a stepwise increase in all-cause and cardiovascular mortality rates at 1 year according to increasing stages of secondary cardiac damage: 5.4% and 0% in Stage 0, 5.3% and 1.8% in Stage 1, 8.9% and 5.9% in Stage 2, 17.7% and 12.9% in Stage 3, and 25.8% and 19.9% in Stage 4, respectively. After multivariable adjustment, increasing stages of cardiac damage gradually correlated with all-cause and cardiovascular mortality.
Conclusion
A significant number of patients with AS underwent TAVI only once cardiac damage has already occurred. Integrating a guideline-based definition of RV dysfunction increased the sensitivity of the staging system to identify patients at increased risk of death after TAVI.
1. Introduction
Safety and efficacy of aortic valve replacement for the treatment of aortic stenosis (AS) is well established [1]. There is however a lack of evidence surrounding the optimal timing of intervention [2], [3], [4], [5]. Premature valvular replacement may expose patients to unnecessary peri-procedural risks while exchanging disease of the native aortic valve against the process of prosthetic valve degeneration and thrombosis. Conversely, delayed intervention may lead to irreversible myocardial damage that may increase peri-procedural risks and affect long-term clinical outcomes despite valvular replacement therapy. Recently, a staging system has been proposed to quantify the extent of cardiac damage associated with AS [6]. The classification was originally derived based on data from the PARTNER trial [6], and further validated in an independent cohort of 689 patients who underwent transcatheter aortic valve implantation (TAVI) at Pittsburg Medical Center [7]. In both studies, right ventricular (RV) damage was qualitatively estimated by visual assessment. This resulted in substantially lower rates of RV dysfunction (8.7% and 4%, respectively) as compared to the reported prevalence of RV dysfunction in a recent meta-analysis (37%) [8]. In another validation study conducted in 1189 symptomatic severe AS patients [9], [10], RV dysfunction was quantitatively assessed using tricuspid annular plane systolic excursion (TAPSE) < 1.6 cm, based on an old echocardiographic guideline [11]. In the study, RV dysfunction was identified in 12%, rather higher than the high-risk TAVI cohorts. Thus, we sought to refine and validate the proposed staging system by integrating a quantitative evaluation of RV dysfunction defined by latest echocardiographic guidelines [12].
2. Materials and methods
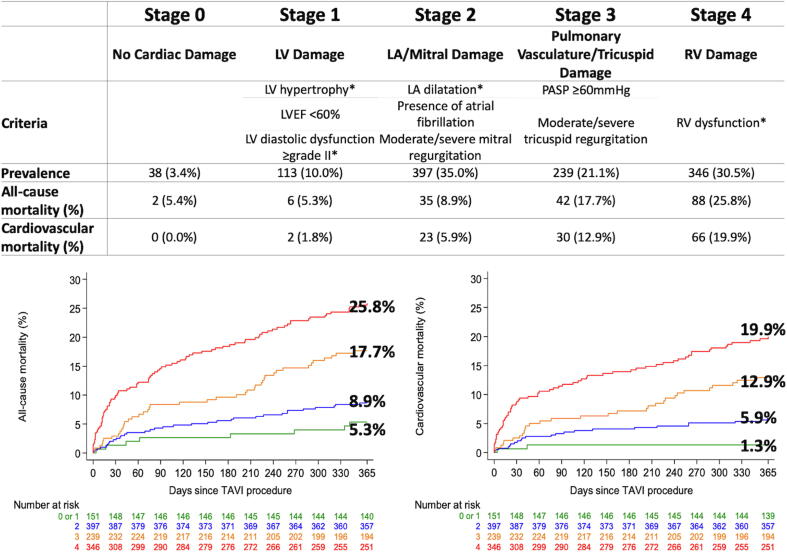
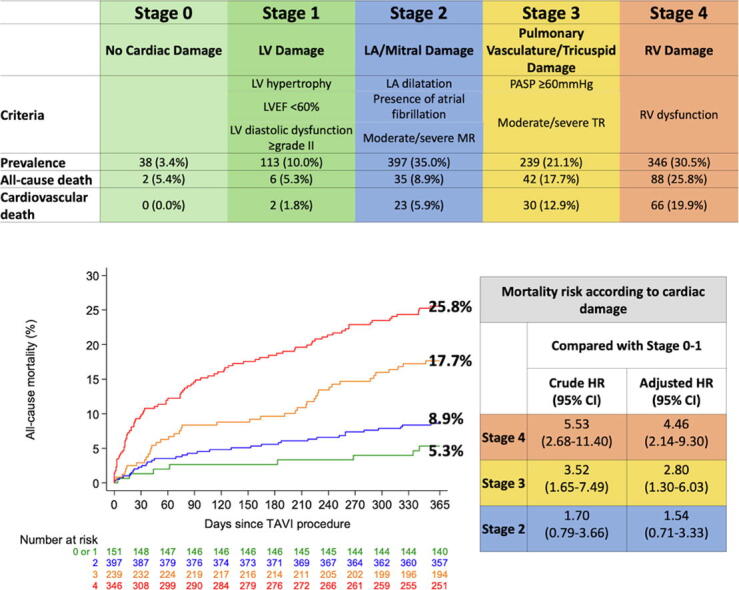
AS patients undergoing TAVI were consecutively enrolled into a prospective registry at Bern University Hospital, Bern, Switzerland (NCT01368250) [13]. The registry was approved by the local ethics committee, and patients provided written informed consent for participation. Based on transthoracic/transesophageal echocardiography performed within 3 months before TAVI, patients were retrospectively categorized into the following stages: Stage 0: no cardiac damage; Stage 1: left ventricular (LV) damage (LV ejection fraction < 60%, LV mass index > 95 g/m2 for women, >115 g/m2 for men, or LV diastolic dysfunction ≥ grade II [14]); Stage 2: left atrial (LA) or mitral valve damage (LA volume index > 34 ml/m2, mitral regurgitation ≥ moderate, or presence of atrial fibrillation); Stage 3: pulmonary vasculature or tricuspid valve damage (systolic pulmonary artery pressure ≥ 60 mmHg, or tricuspid regurgitation ≥ moderate); Stage 4: RV damage (RV dysfunction) (Fig. 1).
Fig. 1.
Prevalence of stages of cardiac damage and cardiovascular mortality. *LV hypertrophy, LV diastolic dysfunction, LA dilatation, and RV dysfunction were defined in accordance with the guideline recommendations [12], [14]. LV = left ventricular; LA = left atrial; RV = right ventricular; LVEF = left ventricular ejection fraction; PASP = pulmonary artery systolic pressure.
RV dysfunction was documented in the presence of at least two of the following parameters: TAPSE < 1.7 cm, S’ <9.5 cm/s, and fractional area change (FAC) < 35% [12]. If only two parameters were available and discrepant, RV dysfunction was defined by prioritizing in the order of TAPSE, S’, and FAC. All echocardiographic studies were re-evaluated by dedicated and experienced imaging specialists. Patients were hierarchically categorized into the most advanced stage if at least one of the criteria was met within that stage. Patients who could not be classified in any of the stages due to missing data were excluded from the present analysis.
Clinical follow-up was performed by standardized interviews, documentation from referring physicians, and hospital discharge summaries at 1 year. Mortality data were systematically collected and adjudicated by a dedicated clinical events committee according to the Valve Academic Research Consortium (VARC-2) recommendations [15].
Categorical variables are presented as frequencies and percentages, and the differences between groups were evaluated using the chi-square test or Fisher’s exact test. Continuous variables are presented as mean values ± standard deviation (SD), and were compared between groups using F test. Cumulative event curves of all-cause and cardiovascular mortality were constructed using the Kaplan-Meier method. Univariate and multivariate Cox proportional hazards models were used to calculate hazard ratios (HR) and 95% confidence intervals (95% CI). Multivariable adjustment was performed with baseline variables selected based on presumed association with mortality as well as those that may cause cardiac damage independently of AS: age, sex, body surface area, New York Heart Association (NYHA) functional class III or IV, Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM), hypertension, chronic kidney disease, coronary artery disease, moderate or severe mitral stenosis, chronic obstructive pulmonary disease (COPD), previous cardiac surgery, previous pacemaker implantation. A sensitivity analysis excluding patients with the comorbidities that may affect cardiac staging independently of AS were performed: coronary artery disease, moderate or severe mitral stenosis, COPD, previous cardiac surgery, and previous pacemaker implantation. All statistical analyses were performed using Stata 15.1 (StataCorp, College Station, TX, USA).
3. Results
Among 1133 eligible patients undergoing TAVI between 2007 and 2016, 38 (3.4%) patients were categorized as Stage 0, 113 (10.0%) as Stage 1, 397 (35.0%) as Stage 2, 239 (21.1%) as Stage 3, and 346 (30.5%) as Stage 4. Overall, the mean age in the cohort was 82.1 ± 6.3 years, mean STS PROM was 6.0 ± 4.2, and 50.6% of the patients were female. Patients in more advanced stages tended to have higher STS-PROM scores, more severe heart failure symptoms (NYHA III or IV), worse kidney function, and more frequently had a history of coronary artery disease, previous cardiac surgery, and permanent pacemaker implantation (Table 1).
Table 1.
Baseline characteristics according to cardiac damage.
| All patients (n = 1,133) |
Stage 0 or 1 (n = 151) |
Stage 2 (n = 397) |
Stage 3 (n = 239) |
Stage 4 (n = 346) |
omnibus p-value | |
|---|---|---|---|---|---|---|
| Age (years) | 82.1 ± 6.3 | 80.5 ± 6.6 | 82.7 ± 5.5 | 83.1 ± 5.7 | 81.4 ± 7.2 | <0.001 |
| Gender (female) | 576 (50.8%) | 82 (54.3%) | 190 (47.9%) | 155 (64.9%) | 149 (43.1%) | <0.001 |
| Body surface area (m2) | 1.82 ± 0.23 | 1.82 ± 0.24 | 1.85 ± 0.23 | 1.80 ± 0.23 | 1.81 ± 0.22 | 0.029 |
| STS-PROM | 6.07 ± 4.22 | 4.32 ± 2.70 | 5.37 ± 3.38 | 6.89 ± 5.05 | 7.08 ± 4.59 | <0.001 |
| NYHA III or IV | 775 (68.5%) | 88 (58.3%) | 258 (65.2%) | 167 (69.9%) | 262 (75.9%) | <0.001 |
| Hypertension | 946 (83.5%) | 123 (81.5%) | 338 (85.1%) | 202 (84.5%) | 283 (81.8%) | 0.543 |
| Chronic kidney disease (eGFR < 60) | 809 (71.5%) | 91 (60.3%) | 270 (68.0%) | 184 (77.0%) | 264 (76.5%) | <0.001 |
| Coronary artery disease | 715 (63.1%) | 89 (58.9%) | 243 (61.2%) | 142 (59.4%) | 241 (69.7%) | 0.023 |
| History of cerebrovascular accident | 135 (11.9%) | 12 (7.9%) | 57 (14.4%) | 29 (12.1%) | 37 (10.7%) | 0.170 |
| Peripheral artery disease | 170 (15.0%) | 20 (13.2%) | 56 (14.1%) | 37 (15.5%) | 57 (16.5%) | 0.742 |
| COPD | 162 (14.3%) | 18 (12.0%) | 55 (13.9%) | 38 (16.0%) | 51 (14.8%) | 0.717 |
| Previous Cardiac Surgery | 203 (17.9%) | 13 (8.6%) | 59 (14.9%) | 29 (12.1%) | 102 (29.5%) | <0.001 |
| Previous pacemaker implantation | 111 (9.8%) | 8 (5.3%) | 27 (6.8%) | 29 (12.1%) | 47 (13.6%) | 0.002 |
| Mitral stenosis (≥moderate) | 45 (4.4%) | 4 (2.8%) | 16 (4.4%) | 11 (5.3%) | 14 (4.7%) | 0.725 |
STS-PROM = Society of Thoracic Surgery Predicted Risk Of Mortality; NYHA = New York Heart Association; eGFR = estimated glomerular filtration rate; COPD = chronic obstructive pulmonary disease.
There was a stepwise increase in all-cause and cardiovascular mortality rates at 1 year according to increasing stages of secondary cardiac damage: 5.4% and 0% in Stage 0, 5.3% and 1.8% in Stage 1, 8.9% and 5.9% in Stage 2, 17.7% and 12.9% in Stage 3, and 25.8% and 19.9% in Stage 4, respectively (Fig. 1). After multivariable adjustment, increasing stages of cardiac damage gradually correlated with all-cause mortality (Stage 2 vs. Stage 0–1: HR 1.54, 95% CI 0.71–3.33, p = 0.273; Stage 3 vs. Stage 0–1: HR 2.80, 95% CI 1.30–6.03, p = 0.008; Stage 4 vs. Stage 0–1: HR 4.46, 95% CI 2.14–9.30, p < 0.001) and cardiovascular mortality (Stage 2 vs. Stage 0–1: HR 3.93, 95% CI 0.92–16.73, p = 0.064; Stage 3 vs. Stage 0–1: HR 7.67, 95% CI 1.82–32.35, p = 0.006; Stage 4 vs. Stage 0–1: HR 13.27, 95% CI 3.23–54.62, p < 0.001) at 1 year (Table 2). In a sensitivity analysis excluding patients with comorbidities that may cause cardiac damage independently of AS, the trends were consistent; all-cause and cardiovascular mortality rates gradually increased with advancing stages of secondary cardiac damage (all-cause mortality: 0% in Stage 0–1, 6.3% in Stage 2, 22.8% in Stage 3, 27.7% in Stage 4; cardiovascular mortality: 0% in Stage 0–1, 4.5% in Stage 2, 13.8% in Stage 3, 15.6% in Stage 4) (Table 3).
Table 2.
Risk of all-cause and cardiovascular mortality according to cardiac damage.
| Cardiac Stage |
Unadjusted |
Adjusted |
||
|---|---|---|---|---|
|
Hazard Ratio (95% CI) |
P Value |
Hazard Ratio (95% CI) |
P Value | |
| All-cause mortality | ||||
| Stage 0–1 | Reference | Reference | ||
| Stage 2 | 1.70 (0.79–3.66) |
0.176 | 1.54 (0.71–3.33) |
0.273 |
| Stage 3 | 3.52 (1.65–7.49) |
0.001 | 2.80 (1.30–6.03) |
0.008 |
| Stage 4 | 5.53 (2.68–11.40) |
<0.001 | 4.46 (2.14–9.30) |
<0.001 |
| Cardiovascular mortality | ||||
| Stage 0–1 | Reference | Reference | ||
| Stage 2 | 4.46 (1.05–18.91) |
0.043 | 3.93 (0.92–16.73) |
0.064 |
| Stage 3 | 10.00 (2.39–41.84) |
0.002 | 7.67 (1.82–32.35) |
0.006 |
| Stage 4 | 16.44 (4.03–67.14) |
<0.001 | 13.27 (3.23–54.62) |
<0.001 |
Table 3.
Sensitivity analysis excluding patients with other comorbidities potentially causing cardiac damage.
|
Stage 0 or 1 |
Stage 2 |
Stage 3 |
Stage 4 |
Crude risk ratios |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Stage 2 vs. Stage 0–1 |
Stage 3 vs. Stage 0–1 |
Stage 4 vs. Stage 0–1 |
||||||||
| n = 48 | n = 113 | n = 62 | n = 63 | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| At 1 year | ||||||||||
| All-cause death (n, %) | 0 (0.0) | 7 (6.3) | 14 (22.8) | 17 (27.7) | 6.41 (0.37–110.03) |
0.104 | 22.50 (1.38–367.84) |
<0.001 | 26.73 (1.65–433.53) |
<0.001 |
| Cardiovascular death (n, %) | 0 (0.0) | 5 (4.5) | 8 (13.8) | 9 (15.6) | 4.70 (0.27–83.35) |
0.323 | 13.19 (0.78–222.93) |
0.009 | 14.51 (0.87–243.22) |
0.005 |
Continuity corrected risk ratios (95% confidence intervals) with p-values from Fisher’s exact tests are provided.
4. Discussion
By integrating current guideline recommendations of RV dysfunction into the proposed staging system of pre-procedural cardiac damage [12], [14], we identified a substantially higher proportion of patients with advanced stages of disease as compared to the two previous studies (31% in Stage 4 as compared to 4–9% in the studies by Généreux and Fukui, respectively). The proportion was even higher than that of the study in symptomatic severe AS patients, in which RV dysfunction was assessed by TAPSE < 1.6 cm [9]. The difference may be attributed to the older age and higher risk nature of TAVI patients compared to the cohort, or a stricter cut-off of TAPSE than that of the current guideline recommendations [11], [12]. The refined staging system provided accurate prognostic value in patients undergoing TAVI. Stage 4, Stage 3, and Stage 2 conferred a 4.5-fold, 3-fold, and 1.5-fold increased risk of all-cause mortality and a 13-fold, 8-fold, and 4-fold increased risk of cardiovascular mortality, respectively, compared with Stage 0–1. An important limitation of previous studies was that the documented cardiac damage may have resulted from comorbidities rather than AS [6], [7]; in the present analysis, the prognostic value of the cardiac damage staging system was maintained even after exclusion of patients with relevant comorbidities potentially associated with cardiac injury.
A significant number of patients with AS may undergo valve replacement only once cardiac damage has already occurred. Integrating a refined definition of RV dysfunction increased the sensitivity of the staging system to identify patients at increased risk of death after TAVI. The implications of our findings are fourfold. First, quantitative assessment of cardiac damage in accordance with guideline recommendations [12], [14] improves reproducibility of the proposed staging system. Second, a more sensitive identification of patients prior to stage 4 cardiac damage may refine the timing of intervention for AS and improve outcomes. Third, recognition of advanced stages of cardiac damage may improve risk assessment of patients undergoing TAVI, and modulate subsequent follow-up and management strategies. Fourth, the staging system may be integrated into the decision-making process whether to perform TAVI or not in elderly patients with multiple comorbidities who are at risk of treatment futility of TAVI.
5. Study limitations
Several limitations should be acknowledged when interpreting the present analysis. First, about one third of patients were excluded from the present analysis due to incomplete echocardiographic assessment, which may have resulted in some degree of selection bias. Second, all patients were recruited at a single high-volume center. Finally, despite multivariate analyses, the possibility of residual confounding cannot be excluded.
6. Conclusions
A significant number of patients with AS underwent TAVI only once cardiac damage has already occurred. Integrating an echocardiographic guideline-based definition of RV dysfunction increased the sensitivity of the staging system to identify patients at increased risk of death after TAVI.
7. Disclosures
Dr. Windecker reports research and educational grants to the institution from Abbott, Amgen, BMS, Bayer, Boston Scientific, Biotronik, Cardinal Health, CardioValve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson&Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, Sinomed. Stephan Windecker serves as unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Sinomed, V-Wave and Xeltis, but has not received personal payments by pharmaceutical companies or device manufacturers. Dr. Windecker is also a member of the steering/excecutive committee group of several investigated-initiated trials that receive funding by industry without impact on his personal remuneration. Dr. Windecker is an unpaid member of the Pfizer Research Award selection committee in Switzerland. Dr. Pilgrim reports research grants to the institution from Edwards Lifesciences, Boston Scientifc and Biotronik, personal fees from Biotronik, Boston Scientific and HighLife SAS; and he is a proctor for Medtronic and Boston Scientific, speaker fees from Boston Scientific and consultant fees from BTG (former British Technology Group) and Teleflex. Dr. Praz has received travel expenses from Edwards Lifesciences, Abbott Medical, Polares Medical. Dr. Okuno reports speaker fees from Abbott. Dr. Heg reports and with CTU Bern, University of Bern, which has a staff policy of not accepting honoraria or consultancy fees. However, CTU Bern is involved in design, conduct, or analysis of clinical studies funded by not-for-profit and for-profit organizations. In particular, pharmaceutical and medical device companies provide direct funding to some of these studies. For an up-to-date list of CTU Bern’s conflicts of interest see http://www.ctu.unibe.ch/research/declaration_of_interest/index_eng.html. All other authors have no relationships relevant to the contents of this article to disclose.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Siontis G.C.M., Overtchouk P., Cahill T.J., Modine T., Prendergast B., Praz F., Pilgrim T., Petrinic T., Nikolakopoulou A., Salanti G., Sondergaard L., Verma S., Juni P., Windecker S. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. Eur. Heart J. 2019;40:3143–3153. doi: 10.1093/eurheartj/ehz275. [DOI] [PubMed] [Google Scholar]
- 2.Kang D.H., Park S.J., Lee S.A., Lee S., Kim D.H., Kim H.K., Yun S.C., Hong G.R., Song J.M., Chung C.H., Song J.K., Lee J.W., Park S.W. Early surgery or conservative care for asymptomatic aortic stenosis. N Engl. J. Med. 2020;382:111–119. doi: 10.1056/NEJMoa1912846. [DOI] [PubMed] [Google Scholar]
- 3.Spitzer E., Van Mieghem N.M., Pibarot P., Hahn R.T., Kodali S., Maurer M.S., Nazif T.M., Rodes-Cabau J., Paradis J.M., Kappetein A.P., Ben-Yehuda O., van Es G.A., Kallel F., Anderson W.N., Tijssen J., Leon M.B. Rationale and design of the transcatheter aortic valve replacement to UNload the Left ventricle in patients with ADvanced heart failure (TAVR UNLOAD) trial. Am. Heart J. 2016;182:80–88. doi: 10.1016/j.ahj.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Banovic M., Iung B., Bartunek J., Asanin M., Beleslin B., Biocina B., Casselman F., da Costa M., Deja M., Gasparovic H., Kala P., Labrousse L., Loncar Z., Marinkovic J., Nedeljkovic I., Nedeljkovic M., Nemec P., Nikolic S.D., Pencina M., Penicka M., Ristic A., Sharif F., Van Camp G., Vanderheyden M., Wojakowski W., Putnik S. Rationale and design of the Aortic Valve replAcemenT versus conservative treatment in Asymptomatic seveRe aortic stenosis (AVATAR trial): a randomized multicenter controlled event-driven trial. Am. Heart J. 2016;174:147–153. doi: 10.1016/j.ahj.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Bing R., Everett R.J., Tuck C., Semple S., Lewis S., Harkess R., Mills N.L., Treibel T.A., Prasad S., Greenwood J.P., McCann G.P., Newby D.E., Dweck M.R. Rationale and design of the randomized, controlled Early Valve Replacement Guided by Biomarkers of Left Ventricular Decompensation in Asymptomatic Patients with Severe Aortic Stenosis (EVOLVED) trial. Am Heart J. 2019;212:91–100. doi: 10.1016/j.ahj.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Genereux P., Pibarot P., Redfors B., Mack M.J., Makkar R.R., Jaber W.A., Svensson L.G., Kapadia S., Tuzcu E.M., Thourani V.H., Babaliaros V., Herrmann H.C., Szeto W.Y., Cohen D.J., Lindman B.R., McAndrew T., Alu M.C., Douglas P.S., Hahn R.T., Kodali S.K., Smith C.R., Miller D.C., Webb J.G., Leon M.B. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017;38:3351–3358. doi: 10.1093/eurheartj/ehx381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukui M., Gupta A., Abdelkarim I., Sharbaugh M.S., Althouse A.D., Elzomor H., Mulukutla S., Lee J.S., Schindler J.T., Gleason T.G., Cavalcante J.L. Association of structural and functional cardiac changes with transcatheter aortic valve replacement outcomes in patients with aortic stenosis. JAMA Cardiol. 2019;4:215–222. doi: 10.1001/jamacardio.2018.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.S.N. Grevious, M.F. Fernandes, A.K. Annor, M. Ibrahim, G.R. Saint Croix, E. de Marchena, G.C. M, C.E. Alfonso, Prognostic assessment of right ventricular systolic dysfunction on post-transcatheter aortic valve replacement short-term outcomes: systematic review and meta-analysis, J Am Heart Assoc, 2020;9:e014463. [DOI] [PMC free article] [PubMed]
- 9.Vollema E.M., Amanullah M.R., Ng A.C.T., van der Bijl P., Prevedello F., Sin Y.K., Prihadi E.A., Marsan N.A., Ding Z.P., Genereux P., Pibarot P., Leon M.B., Narula J., Ewe S.H., Delgado V., Bax J.J. Staging cardiac damage in patients with symptomatic aortic valve stenosis. J. Am. Coll Cardiol. 2019;74:538–549. doi: 10.1016/j.jacc.2019.05.048. [DOI] [PubMed] [Google Scholar]
- 10.Vollema E.M., Amanullah M.R., Prihadi E.A., Ng A.C.T., van der Bijl P., Sin Y.K., Ajmone Marsan N., Ding Z.P., Genereux P., Leon M.B., Ewe S.H., Delgado V., Bax J.J. Incremental value of left ventricular global longitudinal strain in a newly proposed staging classification based on cardiac damage in patients with severe aortic stenosis. Euro Heart J. Cardiovasc. Imaging. 2020;21:1248–1258. doi: 10.1093/ehjci/jeaa220. [DOI] [PubMed] [Google Scholar]
- 11.L.G. Rudski, W.W. Lai, J. Afilalo, L. Hua, M.D. Handschumacher, K. Chandrasekaran, S.D. Solomon, E.K. Louie, N.B. Schiller, Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography, J. Am. Soc. Echocardiogr. 2010;23:685-713; quiz 786-8. [DOI] [PubMed]
- 12.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., Lancellotti P., Muraru D., Picard M.H., Rietzschel E.R., Rudski L., Spencer K.T., Tsang W., Voigt J.U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28(1–39) doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Stortecky S., Franzone A., Heg D., Tueller D., Noble S., Pilgrim T., Jeger R., Toggweiler S., Ferrari E., Nietlispach F., Taramasso M., Maisano F., Grunenfelder J., Muller O., Huber C., Roffi M., Carrel T., Wenaweser P., Windecker S. Temporal trends in adoption and outcomes of transcatheter aortic valve implantation: A Swisstavi registry analysis. Eur. Heart J. Qual. Care Clin. Outfcomes. 2019;5:242–251. doi: 10.1093/ehjqcco/qcy048. [DOI] [PubMed] [Google Scholar]
- 14.Nagueh S.F., Smiseth O.A., Appleton C.P., Byrd B.F., 3rd, Dokainish H., Edvardsen T., Flachskampf F.A., Gillebert T.C., Klein A.L., Lancellotti P., Marino P., Oh J.K., Popescu B.A., Waggoner A.D. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Kappetein A.P., Head S.J., Genereux P., Piazza N., van Mieghem N.M., Blackstone E.H., Brott T.G., Cohen D.J., Cutlip D.E., van Es G.A., Hahn R.T., Kirtane A.J., Krucoff M.W., Kodali S., Mack M.J., Mehran R., Rodes-Cabau J., Vranckx P., Webb J.G., Windecker S., Serruys P.W. Leon MB and Valve Academic Research C. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2) Eur. J. Cardiothorac. Surg. 2012;42:S45–S60. doi: 10.1093/ejcts/ezs533. [DOI] [PubMed] [Google Scholar]