Highlights
-
•
ACEs and PTSS did not significantly differ between patients and healthy controls.
-
•
Patients demonstrated greater corticolimbic connectivity compared to controls.
-
•
Greater PTSS and less corticolimbic connectivity increased headache frequency.
-
•
Less corticolimbic connectivity may indicate greater disease progression.
-
•
Patients may be more vulnerable to the effects of PTSS compared to controls.
Abbreviations: ACEs, adverse childhood experiences; CPSS-V, Child PTSD Symptom Scale; DTI, diffusion tensor imaging; ERQ-CA, Emotion Regulation Questionnaire for Children and Adolescents; FA, fractional anisotropy; MS-Q, Migraine Screen – Questionnaire; PTSS, post-traumatic stress symptoms; RMS, root mean square
Keywords: Brain, Headache, Pain, PTSD, Post-traumatic stress symptoms, Trauma
Abstract
Background/aims
Post-traumatic stress symptoms (PTSS) and chronic pain often co-occur at high rates in youth. PTSS may alter brain structure thereby contributing to headache chronicity. This study examined whether PTSS and altered limbic circuitry were associated with headache frequency in youth.
Methods
Thirty youth aged 10–18 years with chronic headaches and 30 age- and sex-matched controls underwent a 3T MRI scan. Volumes of the hippocampus and amygdala were obtained from T1-weighted images. Mean fractional anisotropy (FA, an index of white matter structure) axial and radial diffusivity values of the cingulum and uncinate fasciculus were extracted from diffusion-weighted images. Youth reported on their headaches daily, for one-month, and self-reported pubertal status, emotion regulation, adverse childhood experiences (ACEs) and PTSS using validated measures. Volumes of the hippocampus and amygdala and diffusivity values of the cingulum and uncinate were compared between patients and controls. Hierarchical linear regressions were used to examine the association between PTSS, subcortical volumes and/or diffusivity values and headache frequency.
Results
Mean FA values of the cingulum were higher in patients compared to controls (P = 0.02, Cohen’s d = 0.69). Greater PTSS (P = 0.04), smaller amygdala volumes (P = 0.01) and lower FA of the cingulum (P = 0.04) were associated with greater headache frequency, after accounting for age, puberty, pain duration, emotion regulation, and ACEs (Adjusted R2 ≥ 0.15). Headache frequency was associated with increases in radial diffusivity (P = 0.002, Adjusted R2 = 0.59), as opposed to axial diffusivity (n.s.).
Conclusions
PTSS, smaller amygdalar volume, and poorer cingulum structural connectivity were associated with headache frequency in youth, and may underlie headache chronicity.
1. Introduction
Across the lifespan, headaches are the second most prevalent medical disorder and the second leading cause of years lived with disability (G.B.D. Disease et al., 2018). Headaches affect approximately 8 to 83% of youth with increasing prevalence between preschool and mid-adolescence (King et al., 2011, Özge et al., 2011). Chronic headaches are more likely to persist into adulthood with earlier onset and greater lags between onset and diagnosis (Hernandez-Latorre and Roig, 2000, Kienbacher et al., 2006, Galinski et al., 2015, Brna et al., 2005). Therefore, to reduce the risk of headache chronicity, it is imperative to identify both behavioral and neurobiological factors associated with the development and maintenance of these pain conditions in order to help facilitate early diagnosis and the development of targeted interventions (Orr et al., 2018).
Trauma and chronic pain are intricately linked. Adverse childhood experiences (ACEs), including sexual and physical abuse, and household challenges, heighten risk for the development of chronic pain (Nelson et al., 2017), and post-traumatic stress symptoms (PTSS) are highly comorbid, and linked to worse pain outcomes (Asmundson et al., 2002, McWilliams et al., 2003, Noel et al., 2016). PTSS are defined by prolonged distress following exposure to a threat (Association, 2013, Association, 2013). PTSS include reexperiencing intrusive distressing recollections of the event, avoidance of trauma-related thoughts, feelings or reminders, and alterations in arousal and reactivity (Association, 2013, Association, 2013). Even subclinical levels of PTSS have been shown to place youth at risk of developing comorbid conditions (Copeland et al., 2007), including chronic pain (Pavlova et al., 2020). In adults, early life exposure to ACEs resulting in PTSS has been found to co-occur at high rates (10–80%) with chronic pain (Asmundson et al., 2002, McWilliams et al., 2003). The co-occurrence of ACEs and chronic pain in adolescence is also high (30%), and linked to impairment across behavioral, physical, social, and emotional domains (Noel et al., 2016). Relationships between ACEs, PTSS and chronic headaches has been well-established within the adult population (Peterlin et al., 2009, Beckham et al., 1997, Gerber et al., 2012, Zarei et al., 2016, Arcaya et al., 2017). Moreover, PTSS has been associated with greater headache-related disability (Peterlin et al., 2009, Gerber et al., 2012). Less is known about the relationships between ACEs, PTSS and chronic headaches in youth. However, in a cross-sectional study in 21 primary and secondary schools following an earthquake, 57.7% of youth with PTSS symptoms reported being bothered by headaches as compared to 33.4% without PTSS symptoms (Zhang et al., 2015). The high rate of comorbidity between PTSS and chronic headaches suggests that there are shared, mutually maintaining factors that lead to the development and/or maintenance of both conditions. Neurobiological factors are posited as critical; however, empirical research has not examined this to date (Vinall et al., 2016, Holley et al., 2016).
ACEs and subsequent PTSS might increase the risk for development or worsening of chronic pain via changes in shared brain circuitry that become altered following prolonged trauma or pain (Vinall et al., 2016, Holley et al., 2016). The amygdala, hippocampus, and medial prefrontal cortex are key brain regions in the corticolimbic system associated with stress responses and chronic pain (Timmers et al., 2019). Indeed, in both animal and adult pain literatures there is increasing evidence demonstrating that as pain transitions from an acute to a chronic state, neural activations within the brain move from primarily somatosensory regions to limbic regions (e.g. hippocampus, amygdala), indicating a shift from primarily sensory to emotional neural processing (Hubbard et al., 2015, Jensen et al., 2016, Mutso et al., 2014, Baliki et al., 2012, Jiang et al., 2016). Hippocampal and amygdala volumes are significantly reduced in individuals with post-traumatic stress disorder (PTSD) (O'Doherty et al., 2017), and trauma exposed individuals with and without a PTSD diagnosis demonstrate significant disruptions in white matter tracts connecting limbic structures, such as the amygdala to frontal brain regions (i.e., cingulum and uncinate fasciculus) (O'Doherty et al., 2018). Trauma-induced alterations to the hippocampus, amygdala, and associated connectivity may also predispose individuals to the development of chronic pain and/or contribute to the maintenance of chronic pain conditions. A recent study found that patients with PTSS and headaches had bilateral volume decreases in the caudate nucleus, putamen, hippocampus and lateral ventricles compared to PTSS patients without headaches (Filipovic et al., 2011). To date, no published studies have examined regional brain changes associated with PTSS and chronic headaches in youth.
The present study examined whether, structurally, limbic regions (i.e. hippocampus and amygdala) and their associated connections with the prefrontal cortex (i.e. cingulum and uncinate fasciculus) differed in youth with chronic headaches as compared to age- and sex-matched healthy controls. Moreover, we examined whether greater ACEs, PTSS symptoms, smaller hippocampal and amygdalar volumes, and reduced structural connectivity of these regions within the prefrontal cortex (i.e. cingulum and uncinate fasciculus) were associated with increased symptomology (i.e., headache frequency) in youth with chronic headaches and healthy controls. Given the lifelong impact of chronic headaches it is imperative that we identify the mechanisms associated with the development and maintenance of this condition.
2. Methods
This study was approved by the University of Calgary Conjoint Health Research Ethics Board (REB15-3100) and conducted in accordance with the Declaration of Helsinki. A parent of each participant provided informed and written consent. Youth under the age of 14 years provided informed and written assent, and youth over the age of 14 provided informed and written consent.
2.1. Participants
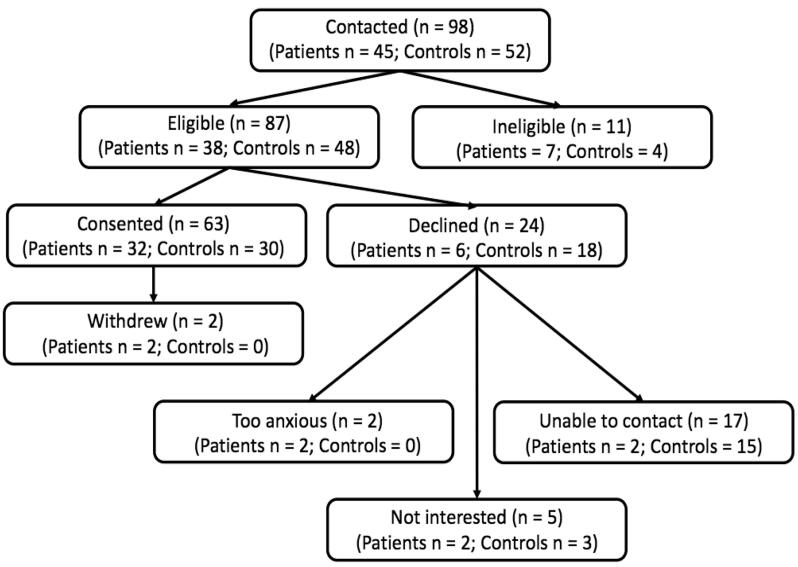
As part of a larger study examining the co-occurrence of pediatric pain and mental health issues, youth with chronic pain (e.g., headache, abdominal, musculoskeletal pain) between the ages of 10–18 years were recruited from outpatient multidisciplinary chronic pain programs at the Alberta Children’s Hospital (Pavlova et al., 2020, Neville et al., 2020, Neville et al., 2019, Soltani et al., 2020). This larger study included both new patients, as well as patients who had received care in the chronic pain clinics within the last two years. Following the completion of this three-month long study, families that had indicated they were interested in being contacted for future research and whose child had headache pain were invited to participate in this additional one-month long neuroimaging study. Patients were excluded if they had a neurodevelopmental or psychotic disorder, or contraindications to MRI. It was required that patients currently had headache pain for greater than three months that was not caused by an underlying disease process, and that they met a minimum of four out of the five criteria on the Migraine Screen – Questionnaire. Thirty youth between the ages of 10 and 18 years with chronic headaches, and 30 age- and sex-matched controls participated in the present study (see Fig. 1). The control group was primarily recruited using the Healthy Infants and Children Clinical Research Program at the University of Calgary, which is a large database of otherwise healthy youth and their families who have agreed to be contacted about participating in child health research as healthy controls. We also recruited healthy controls using community sources such as Kijiji.ca, Facebook advertisements, Alberta Children’s Hospital message boards, and community message boards. Healthy controls were excluded if they had a neurodevelopmental or psychotic disorder, contraindications to MRI, or a history of chronic pain (i.e., lasting longer than three months). To be included in the research study, healthy controls had to score less than four out of the five criteria on the Migraine Screen – Questionnaire.
Fig. 1.
Participant flow chart.
2.2. Measures
2.2.1. Demographics
Parents of youth with and without chronic headaches completed a demographic questionnaire, including but not limited to questions on the youth’s age, sex, ethnicity, duration of their pain problem and diagnosis (if applicable), and the family’s household annual income. Age was used as a covariate in the analyses. In addition, pain duration was added to the regression models to account for the fact that more pain-related brain changes may occur in youth that have had chronic headaches for a longer period.
2.2.2. Pubertal status
Youth reported on their pubertal status using A Self-Administered Rating Scale for Pubertal Development (Carskadon and Acebo, 1993). This is a 5-item scale scored on a 4-point Likert scale ranging from “not yet started” (i.e. 1) to “seems complete” (i.e. 4). Point values are averaged across items to derive pubertal status. A validation study comparing mean self-rating scores from the children to a pediatrician’s assessment of Tanner stage based upon pubic hair growth found the Spearman correlation coefficient between self-rated and pediatrician-rated physical development to be high (Carskadon and Acebo, 1993). Pubertal status, in addition to age, was used as a covariate in the analyses because research has shown that pubertal maturation has unique and additive influences on structural neurodevelopmental trajectories over and above age (Blakemore et al., 2010, Goddings et al., 2014, Herting and Sowell, 2017).
2.2.3. Headache screener
Both patients and controls were screened for the present study using the Migraine Screen – Questionnaire (MS-Q) (Láinez et al., 2005). The MS-Q was developed and validated in 2005, based on the International Headache Society criteria. It is a 5-item yes- or no-based questionnaire that addresses frequency/intensity, duration, and symptomology for the screening of headaches that is used in both clinical practice and research. A cut-off point of greater than or equal to four (i.e. four “yes” responses) showed a sensitivity of 0.93 (95% confidence interval = 0.87 to 0.99), specificity of 0.81 (95% confidence interval = 0.72 to 0.91), positive predictive value of 0.83 (95% confidence interval = 0.75 to 0.91), and negative predictive value of 0.92 (95% confidence interval = 0.85 to 0.99) (Láinez et al., 2005).
2.2.4. Headache tracking
Both patients and controls reported on the occurrence of their headaches and migraines and medication use daily for a one-month period, on a calendar that was provided. On days they experienced a headache or migraine, they were also asked to complete a supplementary questionnaire addressing the timing, location, symptomology and functional limitations (i.e. activity level, school functioning, chores and other tasks) associated with their headache. Both the headache calendar and supplementary questionnaire were developed and previously used in the Childhood and Adolescent Migraine Prevention study (trial registration: NCT01581281), which is a placebo-controlled, multicenter, comparative effectiveness study of amitriptyline and topiramate for the prevention of episodic and chronic migraine in children and adolescents (Hershey et al., 2013). Average number of self-reported headaches per day was calculated (number of headaches/the number of days reported) and included as the dependent variable.
2.2.5. PTSS
Youth PTSS was assessed using the Child PTSD Symptom Scale (CPSS-V) (Foa et al., 2001). The CPSS-V is a 20-item measure that maps on to the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition PTSD criteria (Association, 2013, Association, 2013), and assesses PTSS experienced by youth in the past month. Youth were asked to identify their most distressing or traumatic event that bothers them to think about. With that event in mind, they were asked to respond to 20 items assessing PTSS on a five-point Likert scale, ranging from “not at all” to “six or more times a week/almost always”. Total symptom severity scores were obtained by summing the 20 items (range: 0–80), with higher scores indicating higher PTSS. A score of 31 or above indicates clinically elevated PTSS. The CPSS-V has demonstrated excellent internal consistency, good test–retest reliability and good convergent validity (Foa et al., 2001).
2.2.6. Adverse childhood experiences (ACEs)
ACEs are stressful or traumatic events experienced before age 18. ACEs were assessed using the Center for Youth Wellness Adverse Childhood Experiences Questionnaire for Adolescents: Self Report (Burke Harris, 2015). This instrument is comprised of two sections: Section one consists of the traditional 10 ACEs for which we have population-level data for disease risk in adults (physical and sexual abuse, neglect, and household challenges [e.g. family member incarcerated, family member with mental illness, divorce]), and Section two includes nine items assessing for exposure to additional early life stressors identified by experts and community stakeholders (foster care, bullying/abuse from romantic partner, familial death, life-threatening procedure, trouble with the law). This instrument asks youth to identify how many of the 19 ACEs apply to them. The total number of ACEs (0–19) were used for analysis. An ACEs score of 1–3 with additional symptomology (e.g. sleep disturbance, anxiety, depression) or an ACEs score of 4 or more can be used to determine whether referral services are clinically indicated (Burke Harris, 2015). For the present study, we considered a score of 4 or more on the ACEs scale as reaching clinical cut-off. ACEs were included as a covariate in the regression models to account, in part, for the amount of traumatic experiences youth have been exposed to and the possible repeated effects of trauma on the brain (Kalmakis et al., 2015).
2.2.7. Emotion regulation
Youth self-reported on their ability to regulate their emotions using the Emotion Regulation Questionnaire for Children and Adolescents (ERQ-CA) (Gullone and Taffe, 2012). It is a 10-item scale designed to measure emotion regulation in two ways: Cognitive Reappraisal (6–items) and Expressive Suppression (4–items). Youth answered each item on a seven-point Likert-type scale ranging from “strongly disagree” to “strongly agree”. Internal consistencies for the subscales are high, and both demonstrate stability over a 12-month period (Gullone and Taffe, 2012). Higher Cognitive Reappraisal and lower Expressive Suppression scores are associated with more adaptive emotion regulation. Emotion regulation variables were included as covariates in the regression models as potential neuroprotective factors, given that more adaptive emotion regulation was previously found to be associated with changes in amygdala connectivity and PTSD symptom reduction in adolescent females with PTSD (Cisler et al., 2016).
2.3. Neuroimaging
Youth underwent MR scanning using a 32-channel head coil on a GE 3T Discovery MR750w (GE, Milwaukee, WI) system at the Alberta Children's Hospital. T1-weighted anatomical images (TI = 600 ms, TR/TE = 6.9 ms/3.0 ms, 0.8 mm × 0.8 mm × 0.8 mm resolution, scan duration 4:52 min:sec) and diffusion-weighted (spin-echo echo-planar imaging sequence with TR/TE = 12 s/88 ms, 2.2 mm × 2.2 mm × 2.2 mm resolution, 32 gradient encoding diffusion directions at b = 900 s/mm2, and 4 volumes at b = 0 s/mm2, scan duration = 7:24 min:sec) datasets were acquired. Using the same parameters as the diffusion imaging, 4 volumes at b = 0 s/mm2 were acquired in the opposite phase encoding direction (i.e., posterior to anterior). We failed to obtain this reversed phase encoding diffusion scan for one patient and two controls due to ending their scan early. Therefore, the diffusion data from these 3 participants was excluded from analysis. T1-weighted scans were reviewed for gross anatomical abnormalities. No abnormalities were identified requiring further review by a neuroradiologist.
2.3.1. Imaging processing
Freesurfer 5.3 was used for processing, editing, and segmenting structural brain images (Fischl, 2012). The automated recon-all pipeline was used to perform brain extraction, image registration, motion and intensity correction, and segmentation/parcellation (Dale et al., 1999). Each brain image was manually checked to ensure proper segmentation of volumes; no corrections or exclusions of volumes were required. After processing, volumes of the amygdala and hippocampus were extracted in each hemisphere. Left and right volumes of the amygdala and hippocampus were highly correlated (patients amygdala: r(28) = 0.80, P < 0.001; patients hippocampus: r(28) = 0.88, P < 0.001; controls amygdala: r(28) = 0.71, P < 0.001; controls hippocampus: r(28) = 0.85, P < 0.001), which is why left and right values were summed for each structure, for each participant, as has been done in previous studies (Perosa et al., 2020, Beauchet et al., 2016, Bitter et al., 2011, Wrase et al., 2008).
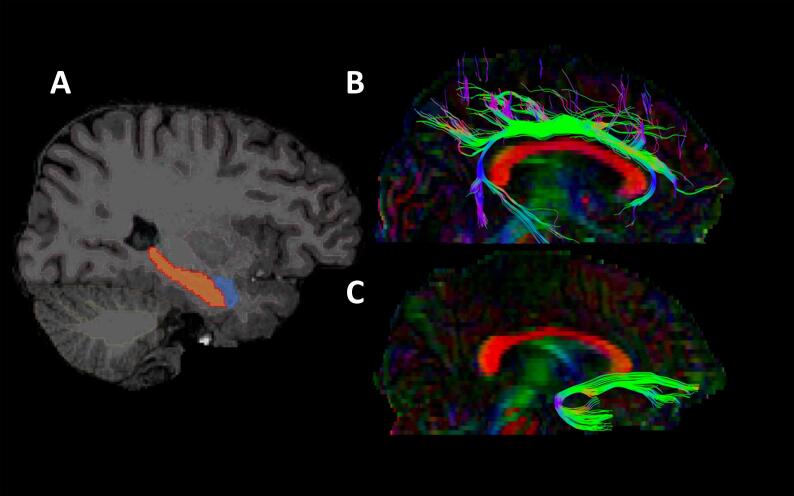
Preprocessing of the DWI was initiated with estimation of susceptibility-induced distortions making use of the two image sets acquired with opposing phase encoding directions (using FSL’s topup) (Andersson et al., 2003, Smith et al., 2004). Subsequently, the calculated susceptibility-induced off-resonance field was fed into FSL’s eddy (Andersson and Sotiropoulos, 2016). Here, susceptibility-induced distortions, eddy current-induced distortions and subject motion were corrected simultaneously, outlier (i.e., drop-out) slices were identified and replaced by Gaussian Process predictions (Andersson et al., 2016), and gradient directions were rotated in accordance with motion. As quality checks, motion reports (root mean square/RMS) were inspected (patients: M = 0.90, SD = 0.58; controls: M = 0.92, SD = 0.56) and potential outliers were identified (defined as equal or greater to mean + 3*SD). No outliers were identified and no significant differences were found in RMS between the 29 patients and 28 controls (t (Smith et al., 2004) = −0.16, P = 0.88). In ExploreDTI, constrained spherical deconvolution (CSD) was used to compute a whole brain tractogram (Farquharson et al., 2013), then semi-automated tractography was performed to extract the cingulum and uncinate fasciculus (Lebel et al., 2008). Similar to previous research, a 16-year old healthy female with typical data quality (see Fig. 2) was selected as the exemplar participant for this process (Geeraert et al., 2019). All participants’ FA maps were registered to this template and inverse normalization parameters computed. Regions of interest (ROIs) for each tract were drawn on this template based on a priori knowledge of tract location (Abdul-Rahman et al., 2011, Wakana et al., 2004, Plaisier et al., 2014, Larroza et al., 2014); detailed information about ROI placement is available online (Reynolds and Lebel, 2019). ROIs were then registered to each participant’s data for fiber tracking in native space using the inverse normalization parameters described above. Parameter maps and fiber tracts were manually inspected and data with motion corruption or poor tracking quality was removed (patients: uncinate fasciculus n = 2; controls: cingulum n = 2, uncinate fasciculus n = 2). There was cingulum diffusion data available for all 29 patients and 26/28 controls, and uncinate fasciculus data was available for 27/29 patients and 26/28 controls. Mean fractional anisotropy (FA), axial diffusivity, and radial diffusivity were extracted. Left and right cingulum FA values were moderately correlated for patients (cingulum: r(27) = 0.62, P < 0.001; uncinate fasciculus: r(25) = 0.53, P = 0.004) and controls (cingulum: r(24) = 0.63, P = 0.001; uncinate fasciculus: r(24) = 0.56, P = 0.003), so left and right FA values were averaged for each tract to reduce the number of comparisons, as we and others have done in the past (Lebel et al., 2008, Adams et al., 2010, Lebel and Beaulieu, 2011, Vinall et al., 2014). Similarly, left and right mean axial and radial diffusivity values were also averaged for patients and controls.
Fig. 2.
Brain regions of interest. A. Circled are the amygdala (blue) and hippocampus (orange) Freesurfer segmentations. B. FA color map and cingulum tract. C. FA color map and uncinate fasciculus tract. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.4. Analyses
Statistical analyses were performed using IBM SPSS Statistics Version 26.0 (Cooporation, 2019). Assumptions of normality (i.e., skewness and kurtosis) were evaluated. PTSS was skewed, therefore, this variable was base-10 log transformed for analysis (McDonald, 2014). Days of medication use and migraines were highly skewed, therefore non-parametric tests were used to compare patients to healthy controls. Days of medication use was transformed into a dichotomous variable for analysis. Chi-square and t-tests were performed to examine the differences between patients who filled out the PTSS questionnaire vs. those who did not to ensure that those who responded represented the overall sample. Chi-square and t-tests were also used to compare the characteristics of patients versus healthy controls. ANCOVA was used to compare the hippocampal and amygdala volumes between groups adjusting for age, pubertal status and total brain volume. ANCOVA was also used to compare cingulum and uncinate mean FA values, and radial and axial diffusivity between groups adjusting for age and pubertal status. Linear regressions were used to examine the relationship between the brain regions of interest (amygdala and hippocampal volumes, and cingulum and uncinate mean FA values), PTSS and average number of headaches, after accounting for group (patient or controls), age, pubertal status, pain duration, cognitive reappraisal, expressive suppression and ACEs and total brain volume (volume models only). Hierarchical linear regressions were used to determine whether: step 1) age, puberty and pain duration; step 2) cognitive reappraisal, expressive suppression, ACEs and PTSS; and step 3) brain structure; were associated with average number of headaches reported by youth. Four hierarchical linear regressions were performed, substituting variables in step 3 with: model 1) amygdala volume and total brain volume; model 2) hippocampal volume and total brain volume; model 3) mean FA of the cingulum; and model 4) mean FA of the uncinate fasciculus. These models were repeated in the healthy controls, excluding the pain duration variable. Significant models were also re-run with the addition of dichotomous variable, medication use. Moreover, if mean FA values in either hierarchical regression model 3 (mean FA of the cingulum) or model 4 (mean FA of the uncinate fasciculus) were significant, FA values were replaced with either the axial or radial diffusivity values (i.e., indices that provide additional information on white matter microstructure), to examine the diffusion direction associated with headache frequency.
3. Results
Participant characteristics are reported in Table 1. Fifteen youth (50%) had received a diagnosis of migraines, 1 (3%) had received a diagnosis of chronic headaches, 7 (23%) youth had not received a diagnosis, 2 (7%) youth had post-concussive headaches and 5 (17%) had received other diagnoses (i.e. anxiety disorder, complex regional pain syndrome, postural orthostatic tachycardia syndrome, tendonitis and vertigo), which may or may not have been related to their headaches (to be assessed, and followed by the clinic). Thirty-three percent (n = 10) of patients as compared to 73% of controls (n = 22) did not take medication for their pain one-month prior to their MRI. Only 2/30 patients (6.7%) took medication daily for their headaches. The types of medications utilized by patients to manage their pain were highly variable including: analgesics, anticonvulsants, antiemetics, nonsteroidal anti-inflammatory drugs, prokinetics, selective serotonin reuptake agonists, triptans (see Table 1). Patients and healthy controls were largely similar in their symptomology, with the exception of annual income and pain. One week prior to their MRI, patients and controls indicated that in the last week they had experienced aches and pains in their head (patients: 25/30, 83%; controls 5/30, 17%; P <= 0.001), muscles and joints (patients: 7/30, 23%; controls: 7/30, 23%; P > 0.99), stomach (patients: 6/30, 20%; controls: 2/30, 7%; P <= 0.001), legs (patients: 3/30, 10%; controls: 2/30, 7%; P = 0.45), chest (patients: 1/30, 3%; controls: 1/30, 3%; P > 0.99) and other regions (patients: 2/30, 7%; controls: 4/30, 13%; P = 0.16). Therefore, patients experienced significantly more head and stomache pain, but similar rates of muscle, joint, leg, chest and other pains compared to controls. Patients who did not complete the PTSS questionnaire (n = 5) did not significantly differ from the other patients in age, sex, pubertal status, pain duration, cognitive reappraisal or ACEs (all ns., P > 0.18). Patients who did not respond to the PTSS questionnaire (n = 5) did however have fewer headaches on average per month (M = 5.16, SD = 7.12) as compared to the patients who did respond to the PTSS questionnaire (M = 16.28, SD = 11.73, t(28) = -2.81, P = 0.02). Although the healthy controls reported more traumatic experiences compared to patients (see Table 2), their reports of PTSS symptomology did not significantly differ from patients with chronic headaches (t(52) = -1.63, P = 0.11).
Table 1.
Characteristics of the cohort.
| Characteristics | Patients n = 30 |
Controls n = 30 |
P value |
|---|---|---|---|
| Age | 14.4 (2.6) | 14.4 (2.6) | – |
| Sex (female), n (%) | 20.0 (67.0) | 20.0 (67.0) | – |
| Ethnicity (Caucasian) | 29.0 (96.7) | 26.0 (92.9) | 0.19 |
| Annual income (>$90,000), n (%) | 10.0 (37.0) | 21.0 (77.8) | <0.001 |
| Pubertal status (range: 5–20) | 14.7 (5.2) | 14.6 (4.6) | 0.97 |
| Duration of pain condition (months) | 26.8 (39.6) | – | – |
| Number of headaches per month | 16.2 (13.2) | 1.4 (1.7) | <0.001 |
| Number of migraines per month† | 7.5 (10.5) | 0.2 (0.6) | <0.001 |
| Number of days medication was used per month† | 7.5 (10.3) | 0.4 (0.8) | <0.001 |
| PTSS (range: 0–80)* | 6.8 (11.9) | 10.8 (13.4) | 0.11 |
| ACEs (range: 0–19) | 2.4 (2.9) | 1.6 (2.3) | 0.24 |
| Cognitive reappraisal (range: 6–30) | 16.1 (5.2) | 18.2 (5.6) | 0.15 |
| Expressive suppression (range: 4–20) | 10.6 (4.4) | 9.53 (2.7) | 0.26 |
| Left hippocampal volume | 4,213.3 (398.9) | 4,273.6 (343.6) | 0.53 |
| Right hippocampal volume | 4,301.3 (339.4) | 4,314.3 (397.5) | 0.89 |
| Total hippocampal volume | 8,514.6 (715.7) | 8,587.9 (712.3) | 0.69 |
| Left amygdala volume | 1,636.6 (184.4) | 1,617.5 (161.0) | 0.67 |
| Right amygdala volume | 1,752.1 (218.6) | 1,772.0 (189.2) | 0.71 |
| Total amygdala volume | 3,388.7 (382.1) | 3,389.5 (324.0) | 0.99 |
| Total brain volume | 1,209,171.5 (123,066.4) | 1,201,264.5 (123,696.0) | 0.81 |
| Left cingulum mean FA | 0.43 (0.03) | 0.40 (0.04) | 0.003 |
| Right cingulum mean FA | 0.39 (0.04) | 0.38 (0.03) | 0.14 |
| Cingulum mean FA | 0.41 (0.03) | 0.39 (0.03) | 0.01 |
| Right cingulum mean axial diffusivity | 1.22 (0.03) | 1.22 (0.03) | 0.75 |
| Left cingulum mean axial diffusivity | 1.28 (0.04) | 1.28 (0.06) | 0.88 |
| Cingulum mean axial diffusivity | 1.25 (0.03) | 1.25 (0.04) | 0.97 |
| Right cingulum mean radial diffusivity | 0.65 (0.04) | 0.67 (0.04) | 0.25 |
| Left cingulum mean radial diffusivity | 0.65 (0.05) | 0.69 (0.07) | 0.01 |
| Cingulum mean radial diffusivity | 0.65 (0.04) | 0.68 (0.05) | 0.03 |
| Right uncinate mean FA | 0.41 (0.02) | 0.40 (0.02) | 0.31 |
| Left uncinate mean FA | 0.41 (0.03) | 0.40 (0.03) | 0.20 |
| Uncinate mean FA | 0.41 (0.02) | 0.40 (0.02) | 0.15 |
| Right uncinate mean axial diffusivity | 1.31 (0.20) | 1.28 (0.05) | 0.57 |
| Left uncinate mean axial diffusivity | 1.27 (0.03) | 1.28 (0.05) | 0.80 |
| Uncinate mean axial diffusivity | 1.29 (0.12) | 1.28 (0.04) | 0.65 |
| Right uncinate mean radial diffusivity | 0.70 (0.20) | 0.68 (0.05) | 0.63 |
| Left uncinate mean radial diffusivity | 0.66 (0.03) | 0.68 (0.04) | 0.14 |
| Uncinate mean radial diffusivity | 0.68 (0.11) | 0.68 (0.04) | 0.92 |
Mean and standard deviation shown, unless otherwise indicated
*Raw data shown. Calculations conducted on transformed data.
Missing data: ACEs (Patients = 5, Controls 5); Annual income (Patients = 3, Controls = 3); Cingulum (Patients = 1, Controls = 4); Cognitive reappraisal (Patients = 2); Expressive suppression (Patients = 2); Left cingulum (Patients = 1, Controls = 4); Left uncinate (Patients = 3, Controls = 4); Pubertal status (Patients = 2, Controls = 1); PTSS (Patients = 5, Controls = 1); Right Cingulum (Patients = 1, Controls = 4) Right Uncinate (Patients = 1, Controls = 4); Uncinate (Patients = 3, Controls = 4).
Non-parametric tests were conducted.
Table 2.
Types of trauma reported by patients and controls.
| Types of Trauma | Patients (n = 25) | Controls (n = 29) |
|---|---|---|
| Direct exposure to threatened or actual 1) death, 2) serious injury and/or 3) sexual violence | 2 (8) | 5 (17) |
| Witnessing threatened or actual 1) death or 2) serious injury | 2 (8) | 0 (0) |
| Learning of threatened or actual 1) death or 2) serious injury of a close friend or family member | 3 (12) | 10 (34) |
| Intrusive thoughts about threatened or actual 1) death or 2) serious injury | 1 (4) | 2 (6) |
| Harassment/bullying | 1 (4) | 2 (6) |
| Parent’s divorce | 1 (4) | 0 (0) |
| Moving | 0 (0) | 2 (6) |
| Trauma not disclosed | 3 (12) | 0 (0) |
| None | 13 (52) | 8 (28) |
Number (Percent)
Three out of 25 patients (12%) versus 4/29 (14%) controls met the clinical cut-off on the CPSS-V questionnaire. Seven out of 25 (28%) patients versus 4/25 (16%) controls met the clinical cut-off on the ACEs questionnaire.
3.1. Differences in brain structures between groups
Median and interquartile ranges for the brain regions of interest are reported in Table 1. Hippocampal (F(4,56) = 0.29, P = 0.60) and amygdala (F(4,56) = 0.12, P = 0.73) volumes were similar between patients and healthy controls, after accounting for age, pubertal status and total brain volume. FA values of the cingulum (F(3,51) = 6.14, P = 0.02), but not the uncinate fasciculus (F(1,49) = 1.30, P = 0.26), were significantly higher in patients as compared to healthy controls, after accounting for age and pubertal status. Mean axial diffusivity of cingulum (F(3,51) = 0.03, P = 0.86), and uncinate fasciculus (F(3,49) = 0.22, P = 0.64) did not differ between patients and controls, after accounting for age and pubertal status. Mean radial diffusivity of the cingulum (F(3,51) = 4.76, P = 0.03), but not the uncinate fasciculus (F(3,49) = 0.05, P = 0.83), was significantly lower in patients as compared to healthy controls, after accounting for age and pubertal status.
3.2. Brain changes associated with PTSS and average headache frequency
Linear regression revealed that higher PTSS (ß = 0.30, t(45) = 2.15, P = 0.04), fewer average headaches per day (ß = −0.38, t(45) = -2.41, P = 0.02), greater total brain volume (ß = 0.66, t(45) = 5.56, P < 0.001) and belonging to the patient group (ß = −0.43, t(45) = -2.13, P = 0.04) was associated with larger amygdalar volumes (Adjusted R2 = 0.49), after accounting for age, pubertal status, pain duration, ACEs, cognitive reappraisal and expressive suppression. Only shorter pain duration (ß = −0.34, t(45) = −2.17, P = 0.04) and greater total brain volume (ß = 0.83, t(45) = 7.00, P < 0.001) was associated with larger hippocampal volumes (Adjusted R2 = 0.50), after accounting for the other factors. Youth with chronic headaches had higher mean FA values of the cingulum (ß = −0.59, t(21) = −2.18, P = 0.04, Adjusted R2 = 0.19) and uncinate (ß = −0.65, t(21) = −2.17, P = 0.04), after accounting for age, pubertal status, pain duration, PTSS, ACEs, cognitive reappraisal, expressive suppression and average headaches per day.
3.3. PTSS, brain volumes and average headache frequency
Hierarchical linear regression models revealed that over and above covariates (i.e. age, pubertal status, pain duration, ACEs, cognitive reappraisal, expressive suppression and total brain volume), greater PTSS (ß = 0.51, t(21) = 2.38, P = 0.04), and smaller volumes of the amygdala (ß = −0.96, t(21) = -2.92, P = 0.01), were associated with greater average headache frequency in youth with chronic headaches. PTSS explained 15% of the variance in average headache frequency, and amygdala volume explained another 29% of the variance in average headache frequency (see Table 3). These relationships remained unchanged by the addition of dichotomous variable medication use, which was non-significant. Hippocampal volumes were not associated with average headache frequency after accounting for age, pubertal status, pain duration, PTSS, ACEs, cognitive reappraisal, expressive suppression and total brain volume. No significant relationships were found with average headache frequency in healthy controls.
Table 3.
PTSS, amygdala volume and average headache frequency.
| Average Number of Headaches Per Day N = 22 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Step 1 |
Step 2 |
Step 3 |
|||||||
| ß | SE | P | ß | SE | P | ß | SE | P | |
| Age | −0.38 | 0.07 | 0.37 | −0.49 | 0.06 | 0.22 | −0.48 | 0.05 | 0.15 |
| Pubertal status | 0.62 | 0.04 | 0.15 | 0.74 | 0.03 | 0.09 | 0.17 | 0.03 | 0.67 |
| Duration of pain condition | −0.09 | 0.002 | 0.71 | −0.17 | 0.002 | 0.44 | −0.08 | 0.002 | 0.70 |
| PTSS | – | 0.51 | 0.18 | 0.07 | 0.51 | 0.15 | 0.04 | ||
| ACEs | – | −0.24 | 0.04 | 0.42 | −0.21 | 0.03 | 0.39 | ||
| Cognitive reappraisal | – | 0.24 | 0.02 | 0.28 | 0.27 | 0.01 | 0.15 | ||
| Expressive suppression | – | 0.10 | 0.02 | 0.70 | 0.44 | 0.02 | 0.07 | ||
| Total brain volume | – | – | – | – | 0.47 | 0.000 | 0.15 | ||
| Amygdala volume | – | – | – | – | −0.96 | 0.000 | 0.01 | ||
| Adjusted R2 = −0.02 | Adjusted R2 = 0.17 | Adjusted R2 = 0.46 | |||||||
3.4. PTSS, white matter tracts and average headache frequency
Hierarchical linear regression models revealed that over and above covariates (i.e. age, pubertal status, pain duration, ACEs, cognitive reappraisal, expressive suppression), greater PTSS (ß = 0.49, t(21) = 2.13P = 0.05), and lower mean FA values of the cingulum (ß = −0.47, t(21) = -2.30P = 0.04) were associated with greater average headache frequency. PTSS explained 15% of the variance and mean FA values of the cingulum explained another 19% of the variance in average headache frequency (see Table 4). With the addition of dichotomous variable medication use (n.s.), PTSS (ß = 0.49, t(21) = 2.07P = 0.06) and FA of the cingulum (ß = −0.45, t(21) = -2.12P = 0.06) remained moderately related to headache frequency. The relationship between mean FA values of the cingulum and average headache frequency were driven by alterations in radial diffusivity (ß = 0.74, t(21) = 3.93, P = 0.002, Adjusted R2 = 0.59; see Table 5) as opposed to axial diffusivity (n.s.). Mean FA values of the uncinate fasciculus were not associated with average headache frequency after accounting for age, pubertal status, pain duration, PTSS, ACEs, cognitive reappraisal and expressive suppression. No significant relationships were found with average headache frequency in healthy controls.
Table 4.
PTSS, cingulum FA and average headache frequency.
| Average Number of Headaches Per Day N = 22 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Step 1 |
Step 2 |
Step 3 |
|||||||
| ß | SE | P | ß | SE | P | ß | SE | P | |
| Age | −0.38 | 0.07 | 0.37 | −0.49 | 0.06 | 0.22 | −0.33 | 0.06 | 0.35 |
| Pubertal status | 0.62 | 0.04 | 0.15 | 0.74 | 0.03 | 0.09 | 0.79 | 0.03 | 0.04 |
| Duration of pain condition | −0.09 | 0.002 | 0.71 | −0.17 | 0.002 | 0.44 | −0.19 | 0.002 | 0.32 |
| PTSS | – | 0.51 | 0.18 | 0.07 | 0.49 | 0.16 | 0.05 | ||
| ACEs | – | −0.24 | 0.04 | 0.42 | −0.30 | 0.03 | 0.25 | ||
| Cognitive reappraisal | – | 0.24 | 0.02 | 0.28 | 0.35 | 0.01 | 0.09 | ||
| Expressive suppression | – | 0.10 | 0.02 | 0.70 | 0.10 | 0.02 | 0.64 | ||
| Cingulum FA | – | – | – | – | −0.47 | 2.92 | 0.04 | ||
| Adjusted R2 = −0.02 | Adjusted R2 = 0.17 | Adjusted R2 = 0.36 | |||||||
Table 5.
PTSS, cingulum radial diffusivity and average headache frequency.
| Average Number of Headaches Per Day N = 22 |
|||
|---|---|---|---|
| ß | SE | P | |
| Age | −0.17 | 0.05 | 0.55 |
| Pubertal status | 0.82 | 0.02 | 0.01 |
| Duration of pain condition | −0.31 | 0.001 | 0.07 |
| PTSS | 0.37 | 0.13 | 0.07 |
| ACEs | −0.36 | 0.03 | 0.10 |
| Cognitive reappraisal | 0.42 | 0.01 | 0.02 |
| Expressive suppression | 0.23 | 0.01 | 0.21 |
| Cingulum radial diffusivity | 0.74 | 1.82 | 0.002 |
| Adjusted R2 = 0.59 | |||
4. Discussion
The present study examined regional changes in subcortical volumes (hippocampus, amygdala) and white matter tracts (cingulum, uncinate fasciculus) in youth with chronic headaches as compared to healthy controls. Effects of PTSS, average headache frequency and brain volumes and connectivity were explored. It also examined whether greater PTSS symptoms, smaller hippocampal and amygdalar volumes, and reduced structural connectivity of these regions within the prefrontal cortex were associated with increased headache frequency in youth with chronic headaches and healthy controls. When examining differences between patients with chronic headache and healthy peers, only FA values in the cingulum (bundle of fibers connecting limbic structures to the prefrontal cortex, referred to hereafter as corticolimbic connections) were observed to be higher in patients. Although there were no differences in emotion regulation, number of ACEs, or degree of PTSS in patients versus healthy controls, patients appeared to be more sensitive to the effects of PTSS as compared to controls. Greater PTSS, smaller amygdala volumes and lower FA values in the cingulum were independently associated with greater headache frequency in youth with chronic headaches, after accounting for pubertal status, pain duration, ACEs, cognitive reappraisal and total brain volume.
Holley et al. (Holley et al., 2016) were the first to propose a conceptual pediatric model of the co-occurrence of PTSS and chronic pain, which posits that the mechanisms underlying PTSS and chronic pain in youth (i.e. pain characteristics, avoidance and cognitive bias, anxiety sensitivity, catastrophizing, activity restriction, depression, hyperarousal and fear encompassed in interpersonal and neurobiological factors) are likely bidirectional and mutually maintaining. We expanded on this model to further emphasize possible neurobiological underpinnings of both chronic pain and PTSS in youth (e.g. neural reorganization) (Vinall et al., 2016). To the best of our knowledge, this is the first study to quantitatively demonstrate the independent contributions of PTSS, altered amygdala volumes and cingulum connectivity to headache frequency in youth with chronic headaches, thus providing evidence for the involvement of trauma symptomology and altered corticolimbic connections in the maintenance of chronic headache pain.
Previously, in a US sample it had been shown that more youth with chronic pain (32%) reported clinically significant elevations in PTSD symptoms compared to youth without chronic pain (8%) (Noel et al., 2016). Although in the present study, self-reported ACEs and PTSS were similar between patients and healthy controls, greater PTSS was still associated with altered amygdala volumes, and greater headache frequency in patients, but not in healthy controls. Thus, patients may be more susceptible to the effects of PTSS as compared to controls. Using animal models, it has been shown that neonatal exposure to pain or trauma leads to the formation of an implicit traumatic memory, which primes the central nervous system, such that subsequent exposure to stress during adolescence and/or adulthood leads to the sensitization to pain and greater pain responses compared to animals without exposure to neonatal pain or trauma, exposed to the same stressors during adolescence or adulthood (Salberg et al., 2020, Beggs et al., 2012). Therefore, even though patients reported a similar number of adverse events, and subsequently reported similar levels of PTSS compared to controls, patients may be neurobiologically predisposed to be more sensitive to PTSS, thereby exacerbating their pain problem.
Indeed, we found differences in the corticolimbic circuity in patients as compared to controls. Patients had higher FA and lower radial diffusivity values of the cingulum relative to healthy controls. A recent study by Nahman-Averbuch et al. (Nahman-Averbuch, 2020) followed 20 youth aged 10–17 years with chronic headaches before and after 8 weeks of cognitive behavioral therapy, and found that greater amygdala-prefrontal functional connectivity at baseline was associated with greater headache reduction (Nahman-Averbuch, 2020). Greater corticolimbic connectivity may be associated with an earlier disease process. The authors suggested that cognitive behavioral therapy may have little to no effect in patients that already have weakened corticolimbic connectivity (Nahman-Averbuch, 2020). Previous studies involving animal models and adults have shown that as an individual transitions from an episodic to chronic pain state, there is less engagement of the somatosensory regions and greater engagement of the limbic regions of the brain (Hubbard et al., 2015, Jensen et al., 2016, Mutso et al., 2014, Baliki et al., 2012, Jiang et al., 2016), reflecting activity-dependent changes in connectivity. The continued presence of pain reinforces this signaling within the corticolimbic circuitry (Mansour et al., 2014). Moreover, factors such as PTSS can enhance these corticolimbic activations and (Suarez-Jimenez et al., 2020), thereby contributing to both the development and maintenance of chronic pain conditions. Recurring headaches and subclinical PTSS may explain why we found relative higher FA values in the cingulum in patients compared to healthy controls. However, a paradoxical effect of continuous excitation of cells due to ongoing exposure to pain and stress is excitoxicity (Rosso et al., 2017, Averill et al., 2017, Harnett et al., 2020, Tripathi et al., 2018, Kirkland et al., 2018).
Glutamate is the primary neurotransmitter involved in neuronal excitation, which contributes to the strengthening of signaling between neurons over time. However, continued excitation of glutamate receptors N-methyl-D-aspartate receptor and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid can lead to excessive calcium entry into neurons, triggering cell-damaging processes that ultimately lead to cell-death (Mark, 2001, Mody and MacDonald, 1995). Indeed, a recent study using high-field proton magnetic resonance spectroscopy found that relative to trauma-exposed controls, patients with PTSD had lower levels of N-acetyl aspartate, a biomarker of neuronal integrity in right and left hippocampus, and significantly higher levels of glutamate, a biomarker for neurotoxicity, in the right hippocampus (Rosso et al., 2017). Therefore, greater PTSS was associated with a higher concentration of glutamate and decreased cell survival. This is fitting with the extant adult PTSD diffusion-weighted imaging studies, which have reported lower FA in PTSD patients relative to healthy controls, most notably in the corticolimbic tracts (Daniels et al., 2013). Glutamate-mediated excitotoxity may explain why we found that greater PTSS, smaller amygdala volumes and lower FA values in the cingulum were associated with greater headache frequency in youth with chronic headaches. For youth with greater PTSS, glutamate levels may have no longer been facilitating neuronal connections, but rather were compromising neuronal integrity and enhancing headache frequency. Previous studies in both adults and youth have shown decreased corticolimbic connectivity in the chronification of pain (Mutso et al., 2014, Erpelding et al., 2016, Geha et al., 2008, Lieberman et al., 2014). One study also found increased left amygdala functional connectivity in individuals with episodic migraines compared to healthy controls, and decreased right functional connectivity in individuals with chronic migraines compared to controls, again reflecting neuronal changes with increasing symptomology (Chen et al., 2017). Future work focusing on excitatory neurotransmission (e.g., using positron emission tomography/PET) is needed to give us more insights into this potential mechanism.
Few studies to date, however, have applied quantitative brain imaging techniques to explore structural differences in the brains of youth with chronic headaches as compared to healthy controls. One study using voxel-based morphometry found that compared to healthy age- and sex-matched controls, pediatric headache patients had significantly smaller gray matter volumes in several frontal and temporal regions (Rocca et al., 2014). Similar to the present study, however, Santoro et al. (Santoro et al., 2018) found no significant differences in regional brain volumes between pediatric patients with either tension-type or migraine-type headaches compared to healthy controls (Santoro et al., 2018). Using atlas-based diffusion weighted imaging they found significantly greater mean diffusivity (a diffusion parameter with higher values possibly indicative of cell loss) in the hippocampus and brain stem of pediatric patients with either tension-type or migraine-type headaches as compared to healthy controls (Santoro et al., 2018). Further, patients with migraine-type headaches had increased mean diffusivity values in the thalamus, and were trending towards greater mean diffusivity values within the amygdala (Santoro et al., 2018). Santoro et al. (Santoro et al., 2018) suggested that early diffusion changes in children with chronic headaches may precede volumetric abnormalities reported later in adolescence and adulthood (Santoro et al., 2018). In examining the white matter tracts, Messina et al. (Messina et al., 2015) found that pediatric migraine patients, compared to age- and sex-matched controls, had lower mean diffusivity of the white matter tracts in the brainstem, thalamus and fronto-temporo-occipital lobes, bilaterally (Messina et al., 2015). Mean diffusivity has very low specificity, and is fairly homogenous across brain tissues with slightly lower values in the white matter as compared to the gray matter (Schilling et al., 2017). In contrast, mean FA values can vary considerably between white matter tracts (Schilling et al., 2017). Thus, this study builds on existing literature by examining the mean FA, radial and axial diffusivity of two corticolimbic white matter tracts.
In the present study, lower FA values of the cingulum were associated with greater average number of headaches in youth with chronic headaches. The relationship between FA of the cingulum and headache frequency was driven by changes in radial diffusivity as opposed to axial diffusivity. Higher radial diffusivity may represent decreased myelination, increased axonal membrane permeability, decreased axonal bundling and/or coherence of specific connections between the subcortical gray matter and prefrontal cortex (Beaulieu, 2002, Geeraert et al., 2018, Lebel et al., 2019). Therefore, while FA values may remain relatively higher in youth with chronic headaches as compared to healthy controls, lower values within the chronic headache group may belong to youth that are further along in their disease process. We were unable to explore the ameliorative or exacerbative effects of individual pain medications on the corticolimbic circuitry. Medication use lessened the relationship between corticolimbic circuitry and headache frequency in youth with chronic headaches. However, it is not clear whether this is demonstrative of brain protection, or a limitation of our sample size. This represents an important avenue of exploration for future research. Due to our sample size, we also limited our analyses to a priori identified brain regions white matter tracts. However, future studies should consider other brain regions or white matter microstructural pathways that may be impacted by chronic pain exposure during development. The present study adds to the growing body of literature demonstrating alterations to corticolimbic circuitry with the chronification of pain (Mutso et al., 2014, Erpelding et al., 2016, Geha et al., 2008, Lieberman et al., 2014).
5. Conclusions
Chronic headaches are highly prevalent in youth and often persist into adulthood. The mechanisms underlying the development and maintenance of chronic headaches in youth remain unclear. Although the relationship between PTSS and chronic headaches in adults has been well-established (Peterlin et al., 2009, Beckham et al., 1997, Gerber et al., 2012, Zarei et al., 2016, Arcaya et al., 2017), this association in youth has been relatively unexplored. Moreover, the mechanisms underlying these relationships are not known. This is the first study to link PTSS and alterations in corticolimbic circuity to headache frequency in youth.
Importantly, despite the known comorbidity of PTSS and chronic pain, most treatments to address chronic pain in youth take on a “one-size-fits-all-approach” and fail to address comorbid mental health issues, such as PTSS, resulting in small treatment effect sizes (Eccleston, 2014). By addressing PTSS, clinicians may be able to reduce the frequency of headaches in their pediatric patients. Future research is needed to explore whether PTSS management can: 1) prevent the transition from episodic to chronic headache pain, 2) normalize corticolimbic connectivity in youth with chronic headache pain, and 3) improve outcomes in youth with chronic headaches.
CRediT authorship contribution statement
Jillian Vinall Miller: Conceptualization, Project administration, Investigation, Methodology, Software, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Quinn Andre: Methodology, Software, Validation, Writing - original draft, Writing - review & editing. Inge Timmers: Methodology, Software, Investigation, Validation, Writing - original draft, Writing - review & editing. Laura Simons: Methodology, Resources, Writing - review & editing. Nivez Rasic: Supervision, Resources, Writing - review & editing. Catherine Lebel: Methodology, Resources, Writing - review & editing. Melanie Noel: Conceptualization, Funding acquisition, Supervision, Methodology, Resources, Writing - review & editing.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research Strategy for Patient Oriented Research (1041605), the Shaikh Family Research Award (1042861) and the Alberta Children’s Hospital Research Institute awarded to Dr. Noel. Dr. Miller was supported by Louise and Alan Edwards Foundation and Canadian Institutes of Health Research Fellowships. Currently, Dr. Miller is supported by the Alberta Children’s Hospital Research Institute & Vi Riddell Children’s Pain & Rehabilitation Program.
References
- Abdul-Rahman M.F., Qiu A., Sim K. Regionally specific white matter disruptions of fornix and cingulum in schizophrenia. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams E. Tractography-based quantitation of corticospinal tract development in premature newborns. J. Pediatr. 2010;156(6):882–888.e1. doi: 10.1016/j.jpeds.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Graham M.S., Zsoldos E., Sotiropoulos S.N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage. 2016;141:556–572. doi: 10.1016/j.neuroimage.2016.06.058. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Skare S., Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Arcaya M.C., Lowe S.R., Asad A.L., Subramanian S.V., Waters M.C., Rhodes J. Association of posttraumatic stress disorder symptoms with migraine and headache after a natural disaster. Health Psychol. 2017;36(5):411–418. doi: 10.1037/hea0000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson G.JG., Coons M.J., Taylor S., Katz J. PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. Can. J. Psychiatry. 2002;47(10):930–937. doi: 10.1177/070674370204701004. [DOI] [PubMed] [Google Scholar]
- A.P. Association, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (American Psychiatric Association, Arlington, VA, 2013).
- A.P. Association, Diagnostic and Statistical Manual of Mental Disorders: DSM-V (American Psychiatric Association, Washington, DC, 2013).
- Averill L.A. Glutamate dysregulation and glutamatergic therapeutics for PTSD: evidence from human studies. Neurosci. Lett. 2017;649:147–155. doi: 10.1016/j.neulet.2016.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Petre B., Torbey S., Herrmann K.M., Huang L., Schnitzer T.J., Fields H.L., Apkarian A.V. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012;15(8):1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O., Barden J., Liu-Ambrose T., Chester V.L., Szturm T., Allali G. The relationship between hippocampal volume and static postural sway: results from the GAIT study. Age (Dordr) 2016;38(1) doi: 10.1007/s11357-016-9883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15(7-8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beckham J.C., Crawford A.L., Feldman M.E., Kirby A.C., Hertzberg M.A., Davidson J.R.T., Moore S.D. Chronic posttraumatic stress disorder and chronic pain in Vietnam combat veterans. J. Psychosom. Res. 1997;43(4):379–389. doi: 10.1016/s0022-3999(97)00129-3. [DOI] [PubMed] [Google Scholar]
- Beggs S., Currie G., Salter M.W., Fitzgerald M., Walker S.M. Priming of adult pain responses by neonatal pain experience: maintenance by central neuroimmune activity. Brain. 2012;135(2):404–417. doi: 10.1093/brain/awr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter S.M., Mills N.P., Adler C.M., Strakowski S.M., DelBello M.P. Progression of amygdala volumetric abnormalities in adolescents after their first manic episode. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50(10):1017–1026. doi: 10.1016/j.jaac.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brna P., Dooley J., Gordon K., Dewan T. The prognosis of childhood headache: a 20-year follow-up. Arch. Pediatr. Adolesc. Med. 2005;159(12):1157. doi: 10.1001/archpedi.159.12.1157. [DOI] [PubMed] [Google Scholar]
- N. R. Burke Harris, T.;, Center for Youth Wellness ACE-Questionnaire (CYW ACE-Q Child, Teen, Teen SR) (Center for Youth Wellness, San Francisco, CA, ed. 7, 2015).
- Carskadon M.A., Acebo C. A self-administered rating scale for pubertal development. J. Adolesc. Health. 1993;14(3):190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Chen Z. Altered functional connectivity of amygdala underlying the neuromechanism of migraine pathogenesis. J. Headache Pain. 2017;18(1) doi: 10.1186/s10194-017-0722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Sigel B.A., Steele J.S., Smitherman S., Vanderzee K., Pemberton J., Kramer T.L., Kilts C.D. Changes in functional connectivity of the amygdala during cognitive reappraisal predict symptom reduction during trauma-focused cognitive-behavioral therapy among adolescent girls with post-traumatic stress disorder. Psychol. Med. 2016;46(14):3013–3023. doi: 10.1017/S0033291716001847. [DOI] [PubMed] [Google Scholar]
- I. Cooporation, IBM SPSS Statistics for Macintosh, Version 26.0 (IBM Corp., Armonk, NY, 2019).
- Copeland W.E., Keeler G., Angold A., Costello E.J. Traumatic events and posttraumatic stress in childhood. Arch. Gen. Psychiatry. 2007;64(5):577. doi: 10.1001/archpsyc.64.5.577. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Daniels J.K., Lamke J.P., Gaebler M., Walter H., Scheel M. White matter integrity and its relationship to PTSD and childhood trauma–a systematic review and meta-analysis. Depress Anxiety. 2013;30(3):207–216. doi: 10.1002/da.22044. [DOI] [PubMed] [Google Scholar]
- Disease G.B.D., Injury I., Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston, C., et al., Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst. Rev., 10.1002/14651858.CD003968.pub4, CD003968 (2014). [DOI] [PMC free article] [PubMed]
- Erpelding N. Rapid treatment-induced brain changes in pediatric CRPS. Brain Struct. Funct. 2016;221(2):1095–1111. doi: 10.1007/s00429-014-0957-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquharson S., Tournier J.-D., Calamante F., Fabinyi G., Schneider-Kolsky M., Jackson G.D., Connelly A. White matter fiber tractography: why we need to move beyond DTI. J. Neurosurg. 2013;118(6):1367–1377. doi: 10.3171/2013.2.JNS121294. [DOI] [PubMed] [Google Scholar]
- Filipovic B.R., Djurovic B., Marinkovic S., Stijak L., Aksic M., Nikolic V., Starcevic A., Radonjic V. Volume changes of corpus striatum, thalamus, hippocampus and lateral ventricles in posttraumatic stress disorder (PTSD) patients suffering from headaches and without therapy. Cent. Eur. Neurosurg. 2011;72(03):133–137. doi: 10.1055/s-0030-1253349. [DOI] [PubMed] [Google Scholar]
- Fischl, B., FreeSurfer. Neuroimage 62, 774-781 (2012). [DOI] [PMC free article] [PubMed]
- Foa E.B., Johnson K.M., Feeny N.C., Treadwell K.R.H. The child PTSD Symptom Scale: a preliminary examination of its psychometric properties. J. Clin. Child. Psychol. 2001;30(3):376–384. doi: 10.1207/S15374424JCCP3003_9. [DOI] [PubMed] [Google Scholar]
- Galinski M., Sidhoum S., Cimerman P., Perrin O., Annequin D., Tourniaire B. Early diagnosis of migraine necessary in children: 10-year follow-up. Pediatr. Neurol. 2015;53(4):319–323. doi: 10.1016/j.pediatrneurol.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Geeraert B.L. A comparison of inhomogeneous magnetization transfer, myelin volume fraction, and diffusion tensor imaging measures in healthy children. Neuroimage. 2018;182:343–350. doi: 10.1016/j.neuroimage.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Geeraert B.L., Lebel R.M., Lebel C. A multiparametric analysis of white matter maturation during late childhood and adolescence. Hum. Brain Mapp. 2019;40(15):4345–4356. doi: 10.1002/hbm.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha P.Y. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60(4):570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber M.R., Fried L.E., Pineles S.L., Shipherd J.C., Bernstein C.A. Posttraumatic stress disorder and intimate partner violence in a women's headache center. Women Health. 2012;52(5):454–471. doi: 10.1080/03630242.2012.684088. [DOI] [PubMed] [Google Scholar]
- Goddings A.-L., Mills K.L., Clasen L.S., Giedd J.N., Viner R.M., Blakemore S.-J. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullone E., Taffe J. The Emotion Regulation Questionnaire for Children and Adolescents (ERQ-CA): a psychometric evaluation. Psychol. Assess. 2012;24(2):409–417. doi: 10.1037/a0025777. [DOI] [PubMed] [Google Scholar]
- Harnett N.G., Goodman A.M., Knight D.C. PTSD-related neuroimaging abnormalities in brain function, structure, and biochemistry. Exp. Neurol. 2020;330:113331. doi: 10.1016/j.expneurol.2020.113331. [DOI] [PubMed] [Google Scholar]
- Hernandez-Latorre M.A., Roig M. Natural history of migraine in childhood. Cephalalgia. 2000;20(6):573–579. doi: 10.1046/j.1468-2982.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- Hershey A.D., Powers S.W., Coffey C.S., Eklund D.D., Chamberlin L.A., Korbee L.L. Childhood and Adolescent Migraine Prevention (CHAMP) study: a double-blinded, placebo-controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo in the prevention of childhood and adolescent migraine. Headache. 2013;53(5):799–816. doi: 10.1111/head.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Sowell E.R. Puberty and structural brain development in humans. Front. Neuroendocrinol. 2017;44:122–137. doi: 10.1016/j.yfrne.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley A.L., Wilson A.C., Noel M., Palermo T.M. Post-traumatic stress symptoms in children and adolescents with chronic pain: a topical review of the literature and a proposed framework for future research. Eur. J. Pain. 2016;20(9):1371–1383. doi: 10.1002/ejp.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard C.S., Khan S.A., Xu S.u., Cha M., Masri R., Seminowicz D.A. Behavioral, metabolic and functional brain changes in a rat model of chronic neuropathic pain: a longitudinal MRI study. Neuroimage. 2015;107:333–344. doi: 10.1016/j.neuroimage.2014.12.024. [DOI] [PubMed] [Google Scholar]
- Jensen K.B., Regenbogen C., Ohse M.C., Frasnelli J., Freiherr J., Lundström J.N. Brain activations during pain: a neuroimaging meta-analysis of patients with pain and healthy controls. Pain. 2016;157(6):1279–1286. doi: 10.1097/j.pain.0000000000000517. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Oathes D., Hush J., Darnall B., Charvat M., Mackey S., Etkin A. Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain. Pain. 2016;157(9):1970–1978. doi: 10.1097/j.pain.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmakis K.A., Meyer J.S., Chiodo L., Leung K. Adverse childhood experiences and chronic hypothalamic-pituitary-adrenal activity. Stress. 2015;18(4):446–450. doi: 10.3109/10253890.2015.1023791. [DOI] [PubMed] [Google Scholar]
- Kienbacher C., Wöber C., Zesch H.E., Hafferl-Gattermayer A., Posch M., Karwautz A., Zormann A., Berger G., Zebenholzer K., Konrad A., Wöber-Bingöl Ç. Clinical features, classification and prognosis of migraine and tension-type headache in children and adolescents: a long-term follow-up study. Cephalalgia. 2006;26(7):820–830. doi: 10.1111/j.1468-2982.2006.01108.x. [DOI] [PubMed] [Google Scholar]
- King S., Chambers C.T., Huguet A., MacNevin R.C., McGrath P.J., Parker L., MacDonald A.J. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152(12):2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Kirkland A.E., Sarlo G.L., Holton K.F. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730. doi: 10.3390/nu10060730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Láinez M.J.A., Domínguez M., Rejas J., Palacios G., Arriaza E., Garcia-Garcia M., Madrigal M. Development and validation of the Migraine Screen Questionnaire (MS-Q) Headache. 2005;45(10):1328–1338. doi: 10.1111/j.1526-4610.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- Larroza A., Moratal D., D'Ocon Alcaniz V., Arana E. I. por la Alzheimer's Disease Neuroimaging, [Tractography of the uncinate fasciculus and the posterior cingulate fasciculus in patients with mild cognitive impairment and Alzheimer disease] Neurologia. 2014;29:11–20. doi: 10.1016/j.nrl.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lebel C., Treit S., Beaulieu C. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. 2019;32(4):e3778. doi: 10.1002/nbm.v32.410.1002/nbm.3778. [DOI] [PubMed] [Google Scholar]
- Lieberman G. White matter involvement in chronic musculoskeletal pain. J. Pain. 2014;15(11):1110–1119. doi: 10.1016/j.jpain.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A.R., Farmer M.A., Baliki M.N., Apkarian A.V. Chronic pain: the role of learning and brain plasticity. Restor. Neurol. Neurosci. 2014;32:129–139. doi: 10.3233/RNN-139003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark L.P. Pictorial review of glutamate excitotoxicity: fundamental concepts for neuroimaging. AJNR Am. J. Neuroradiol. 2001;22:1813–1824. [PMC free article] [PubMed] [Google Scholar]
- McDonald, J.H., Handbook of Biological Statistics (Sparky House Publishing, Baltimore, Maryland, ed. 3rd Edition, 2014).
- McWilliams L.A., Cox B.J., Enns M.W. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- Messina R. White matter microstructure abnormalities in pediatric migraine patients. Cephalalgia. 2015;35(14):1278–1286. doi: 10.1177/0333102415578428. [DOI] [PubMed] [Google Scholar]
- Mody I., MacDonald J.F. NMDA receptor-dependent excitotoxicity: the role of intracellular Ca2+ release. Trends Pharmacol. Sci. 1995;16:356–359. doi: 10.1016/s0165-6147(00)89070-7. [DOI] [PubMed] [Google Scholar]
- Mutso A.A., Petre B., Huang L., Baliki M.N., Torbey S., Herrmann K.M., Schnitzer T.J., Apkarian A.V. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J. Neurophysiol. 2014;111(5):1065–1076. doi: 10.1152/jn.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahman-Averbuch H. Identification of neural and psychophysical predictors of headache reduction following cognitive behavioral therapy in adolescents with migraine. Pain. 2020 doi: 10.1097/j.pain.0000000000002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S.M., Cunningham N.R., Kashikar-Zuck S. A conceptual framework for understanding the role of adverse childhood experiences in pediatric chronic pain. Clin. J. Pain. 2017;33:264–270. doi: 10.1097/AJP.0000000000000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville A., Jordan A., Beveridge J.K., Pincus T., Noel M. Diagnostic uncertainty in youth with chronic pain and their parents. J. Pain. 2019;20(9):1080–1090. doi: 10.1016/j.jpain.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Neville A., Griep Y., Palermo T.M., Vervoort T., Schulte F., Yeates K.O., Sumpton J.E., Mychasiuk R., Noel M. A “dyadic dance”: pain catastrophizing moderates the daily relationships between parent mood and protective responses and child chronic pain. Pain. 2020;161(5):1072–1082. doi: 10.1097/j.pain.0000000000001799. [DOI] [PubMed] [Google Scholar]
- Noel M., Wilson A.C., Holley A.L., Durkin L., Patton M., Palermo T.M. Posttraumatic stress disorder symptoms in youth with vs without chronic pain. Pain. 2016;157(10):2277–2284. doi: 10.1097/j.pain.0000000000000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty D.C.M., Tickell A., Ryder W., Chan C., Hermens D.F., Bennett M.R., Lagopoulos J. Frontal and subcortical grey matter reductions in PTSD. Psychiatry Res. Neuroimaging. 2017;266:1–9. doi: 10.1016/j.pscychresns.2017.05.008. [DOI] [PubMed] [Google Scholar]
- O'Doherty D.C.M., Ryder W., Paquola C., Tickell A., Chan C., Hermens D.F., Bennett M.R., Lagopoulos J. White matter integrity alterations in post-traumatic stress disorder. Hum. Brain Mapp. 2018;39(3):1327–1338. doi: 10.1002/hbm.23920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr S.L., Kabbouche M.A., O’Brien H.L., Kacperski J., Powers S.W., Hershey A.D. Paediatric migraine: evidence-based management and future directions. Nat. Rev. Neurol. 2018;14(9):515–527. doi: 10.1038/s41582-018-0042-7. [DOI] [PubMed] [Google Scholar]
- Özge A., Termine C., Antonaci F., Natriashvili S., Guidetti V., Wöber-Bingöl Ç. Overview of diagnosis and management of paediatric headache. Part I: diagnosis. J. Headache Pain. 2011;12(1):13–23. doi: 10.1007/s10194-011-0297-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova M., Kopala-Sibley D.C., Nania C., Mychasiuk R., Christensen J., McPeak A., Tomfohr-Madsen L., Katz J., Palermo T.M., Noel M. Sleep disturbance underlies the co-occurrence of trauma and pediatric chronic pain: a longitudinal examination. Pain. 2020;161(4):821–830. doi: 10.1097/j.pain.0000000000001769. [DOI] [PubMed] [Google Scholar]
- Perosa V., Priester A., Ziegler G., Cardenas-Blanco A., Dobisch L., Spallazzi M., Assmann A., Maass A., Speck O., Oltmer J., Heinze H.-J., Schreiber S., Düzel E. Hippocampal vascular reserve associated with cognitive performance and hippocampal volume. Brain. 2020;143(2):622–634. doi: 10.1093/brain/awz383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- B. L. Peterlin et al., Posttraumatic stress disorder in migraine. Headache 49, 541-551 (2009). [DOI] [PubMed]
- Plaisier A., Pieterman K., Lequin M.H., Govaert P., Heemskerk A.M., Reiss I.K.M., Krestin G.P., Leemans A., Dudink J. Choice of diffusion tensor estimation approach affects fiber tractography of the fornix in preterm brain. AJNR Am. J. Neuroradiol. 2014;35(6):1219–1225. doi: 10.3174/ajnr.A3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, J.G., M.; Lebel, C., White Matter Tractography Guides. doi: 10.6084/m9.figshare.7603271.v1 (2019).
- Rocca M.A. Structural brain MRI abnormalities in pediatric patients with migraine. J. Neurol. 2014;261(2):350–357. doi: 10.1007/s00415-013-7201-y. [DOI] [PubMed] [Google Scholar]
- Rosso I.M., Crowley D.J., Silveri M.M., Rauch S.L., Jensen J.E. Hippocampus glutamate and N-acetyl aspartate markers of excitotoxic neuronal compromise in posttraumatic stress disorder. Neuropsychopharmacology. 2017;42(8):1698–1705. doi: 10.1038/npp.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salberg S., Noel M., Burke N.N., Vinall J., Mychasiuk R. Utilization of a rodent model to examine the neurological effects of early life adversity on adolescent pain sensitivity. Dev. Psychobiol. 2020;62(3):386–399. doi: 10.1002/dev.v62.310.1002/dev.21922. [DOI] [PubMed] [Google Scholar]
- Santoro J.D. Brain diffusion abnormalities in children with tension-type and migraine-type headaches. AJNR Am. J. Neuroradiol. 2018;39(5):935–941. doi: 10.3174/ajnr.A5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling K. Reproducibility and variation of diffusion measures in the squirrel monkey brain, in vivo and ex vivo. Magn. Reson. Imaging. 2017;35:29–38. doi: 10.1016/j.mri.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Soltani S., van Ryckeghem D.M.L., Vervoort T., Heathcote L.C., Yeates K., Sears C., Noel M. Attentional biases in pediatric chronic pain: an eye-tracking study assessing the nature of the bias and its relation to attentional control. Pain. 2020;161(10):2263–2273. doi: 10.1097/j.pain.0000000000001916. [DOI] [PubMed] [Google Scholar]
- Suarez-Jimenez B. Neural signatures of conditioning, extinction learning, and extinction recall in posttraumatic stress disorder: a meta-analysis of functional magnetic resonance imaging studies. Psychol. Med. 2020;50(9):1442–1451. doi: 10.1017/S0033291719001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers I., Quaedflieg C.W.E.M., Hsu C., Heathcote L.C., Rovnaghi C.R., Simons L.E. The interaction between stress and chronic pain through the lens of threat learning. Neurosci. Biobehav. Rev. 2019;107:641–655. doi: 10.1016/j.neubiorev.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi G.M., Kalita J., Misra U.K. Role of glutamate and its receptors in migraine with reference to amitriptyline and transcranial magnetic stimulation therapy. Brain Res. 2018;1696:31–37. doi: 10.1016/j.brainres.2018.05.046. [DOI] [PubMed] [Google Scholar]
- Vinall J., Miller S.P., Bjornson B.H., Fitzpatrick K.P.V., Poskitt K.J., Brant R., Synnes A.R., Cepeda I.L., Grunau R.E. Invasive procedures in preterm children: brain and cognitive development at school age. Pediatrics. 2014;133(3):412–421. doi: 10.1542/peds.2013-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinall J., Pavlova M., Asmundson G.J., Rasic N., Noel M. Mental health comorbidities in pediatric chronic pain: a narrative review of epidemiology, models, neurobiological mechanisms and treatment. Children (Basel) 2016;3 doi: 10.3390/children3040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S., Jiang H., Nagae-Poetscher L.M., van Zijl P.C.M., Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wrase J., Makris N., Braus D.F., Mann K., Smolka M.N., Kennedy D.N., Caviness V.S., Hodge S.M., Tang L., Albaugh M., Ziegler D.A., Davis O.C., Kissling C., Schumann G., Breiter H.C., Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. Am. J. Psychiatry. 2008;165(9):1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Zarei M.R., Shabani M., Chamani G., Abareghi F., Razavinasab M., Nazeri M. Migraine patients have a higher prevalence of PTSD symptoms in comparison to chronic tension-type headache and healthy subjects: a case-control study. Acta Odontol. Scand. 2016;74(8):633–635. doi: 10.1080/00016357.2016.1232435. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang J., Zhu S., Du C., Zhang W. Prevalence and predictors of somatic symptoms among child and adolescents with probable posttraumatic stress disorder: a cross-sectional study conducted in 21 primary and secondary schools after an earthquake. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137101. [DOI] [PMC free article] [PubMed] [Google Scholar]