Abstract
Renal cell carcinoma (RCC) is a type of urinary tumor with a high incidence and is often associated with tumor metastasis. Long non-coding RNA (lncRNA) regulates tumorigenesis, progression, and metastasis. However, the role and the predictive value of lncRNA in RCC progression and metastasis have not been elucidated. The purpose of this study was to evaluate the effect of a newly discovered lncRNA LOC648987 on RCC proliferation and metastasis. LOC648987 was identified by RT-PCR for high expression in human RCC tissues as well as in metastatic RCC tissues. In the cell experiments, we infected the RCC cell lines ACHN and 786-O cells with LOC648987-shRNA and its negative control (shNC). The results showed that the knockdown of LOC648987 inhibited the proliferation of ACHN and 786-O cells and colony formation. The cell cycle and the apoptosis progression of ACHN and 786-O cells were assessed using flow cytometry. The knockdown of LOC648987 significantly inhibited the progression of ACHN and 786-O cells from G0/G1 to S phase and promoted cell apoptosis. The metastasis promoting effects of LOC648987 on ACHN and 786-O cells were verified by transwell migration assays, which depended on vimentin and MMP-9 to regulate the epithelial–mesenchymal transition. Finally, the promotion of LOC648987 on RCC tumorigenesis was evaluated in BALb/c nude mice. These data confirmed that lncRNA LOC648987 promoted RCC cell proliferation and tumor metastasis and regulated the expression of EMT-related proteins in RCC cells.
Keywords: renal cell carcinoma, lncRNA LOC648987, EMT, proliferation, metastasis
Introduction
Renal cell carcinoma (RCC) is one of the most common genitourinary tumors in the world, accounting for approximately 3% of all global malignancies, with more than 300,000 new patients worldwide each year.1 RCC accounts for 5% and 3% of all the malignant tumors in men and women, respectively. The incidence rate ranks seventh among male cancer patients and tenth among female cancer patients. Currently, the most effective way to treat RCC is surgical resection.2 RCC is divided into multiple pathological subtypes. Clear-cell RCC is the most common subtype, accounting for approximately 75% of the total cases of renal cancers.3 Early RCC is often difficult to detect, 30% of patients with RCC have metastasized at the time of detection, and patients with metastatic RCC have a poor prognosis.4 Once RCC metastasizes, the patient loses the opportunity to undergo surgical resection for radical treatment. Therefore, it is important to inhibit tumor metastasis in patients with distant metastases. Currently, a variety of targeted drugs have showed exciting therapeutic effects in treating RCC, however, because of tumor heterogeneity and multi-drug resistance, patients are likely to develop resistance to a single targeted therapy, which may decrease the therapeutic effect. Therefore, combination of different targeted drugs or combination of targeted drugs with chemoradiotherapy, can make full use of their advantages and play synergistic effects in treating RCC.2
Long non-coding RNAs (lncRNAs) are a class of non-coding RNAs between 200 nt and 100 kb in length, localized in the nucleus and cytoplasm.5 Studies have confirmed that lncRNA plays an important role in many life activities such as epigenetic regulation and post-transcriptional regulation. The abnormal expression of lncRNA is closely related to the malignant phenotype of various tumors, including lung cancer, breast cancer, and kidney cancer.6-8 In recent years, many renal cancer-associated lncRNAs have been found to be up-regulated or down-regulated in renal cancer cells and have carcinogenic or tumor suppressor effects. LncRNA H19 was the earliest discovered lncRNA type, which was overexpressed in renal cancer cells and reduced the H19 expression in a tumor suppressive role.9 The loss of expression of lncRNA CCAT1 was observed in many malignant tumors and inhibited cell apoptosis in RCC.1,10 The expression level of lncRNA HOTAIR was significantly increased in renal cancer cells, and the knockout of HOTAIR by siRNA arrested the cell cycle in the G0/G1 phase and inhibited the malignant ability.11,12 Therefore, the key regulatory lncRNA has important application value as a diagnostic marker and a therapeutic target of RCC.
LncRNA LOC648987 is encoded from chr5 and is a newly discovered lncRNA type. Previous studies have shown that a variety of lncRNAs, including LOC648987, can be used as diagnostic biomarkers for lung adenocarcinoma and be regulated by methylation.13 However, the role of LOC648987 in other tumor types, such as RCC, has hardly been studied. Our study aimed to identify LOC648987 as a predictive tool for the diagnosis and the metastasis of human RCC and to reveal its role in the invasion and metastasis of RCC cells.
Materials and Methods
RCC Samples
A total of 50 primary renal cell carcinoma samples and corresponding adjacent tissues were surgically removed from the patients who had undergone radical cholecystectomy, without any prior radiotherapy or chemotherapy in Ningbo Beilun District People’s Hospital. All RCC and adjacent tissue samples were immediately frozen in liquid nitrogen and used for RNA extraction. Diagnoses of RCC, adjacent tissues, and lymph node metastasis were confirmed by histopathological examination, all clinical sample experiments were approved by the Ethics Committee of Ningbo Beilun District People’s Hospital.
Cell Lines and Materials
Renal cell carcinoma cell lines A704, ACHN, 786-O, 769-P, A498 and human accompaniment cell line 293 were all from the American Type Culture Collection. The number of cell passages is about 15 times. The cell lines were cultured in DMEM or RPMI 1640 supplemented with 10% fetal bovine serum and grown at 37° C, 5% CO2. Antibodies used in the study include: cyclin A (Cell Signaling Technology, #4656), CDK2 (Proteintech, 10122-1-AP), cyclin D1 (Proteintech, 26939-1-AP), E-cadherin (Abeam, ab1416), Vimentin (Abeam, ab8978) and MMP-9 (Proteintech, 10375-2-AP).
RT-PCR and shRNA
We isolated total RNA from frozen renal cell carcinoma tissue samples and cultured RCC cells using TRIzol reagent. RT-PCR of RNA reverse transcribed cDNA was performed using SYBR Premix Ex Taq (Takara). The primers presented below:
LOC648987: 5′-CTGGAATAAGGACCGTGAAT-3′ (forward), and 5′-TCGACAACCGCATCTCTTCG-3′ (reverse);
GAPDH: 5′-GTGGACATCCGCAAAGAC-3′ (forward), and 5′-AAAGGGTGTAACGCAACTA-3′ (reverse).
Specific shRNA against LOC648987 (shRNA#1
5′-GCCTGGTAAGGAAATCCATTTTGGCCTTGTAATTCTCACCCTCTCCCAACCCTTTTT-3′shRNA#2:
5′-CGTTCAGGGAAAGTTTGCGGCGCCGTGACGGATCGACTGGCACTGGGTGCCCCTTTTT-3′) was designed and synthesized by Invitrogen (Shanghai, China). A negative control shRNA was synchronously synthesized, then the fragment was subcloned into pFH1UGW vector (Addgene). LOC648987-shRNA and control shRNA were packaged separately for lentivirus, then infected with ACHN and 786-O cells, and stable knockdown cell lines were obtained by puromycin screening.
CCK-8 Analysis
ACHN and 786-O cells expressing the control group and shRNA knockdown LOC648987 were seeded into 96-well plates at a density of 104 cells per well and cultured for 7 days. Cell proliferation and viability were measured every other day. Specifically, 10 µL of CCK-8 solution (Dojindo Laboratories) was added to each well, and then cell viability was measured by using a 450 nm absorbance microplate reader.
Colony Formation Analysis
Eight hundred shNC and LOC648987-shRNA infected ACHN or 786-0 cells were seeded in 6-well plates and cultured in complete medium for 2 weeks. The medium was then discarded and the cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Finally, the stained cells were photographed and the number of colonies in each well was calculated.
Cell Cycle Analysis
ACHN and 786-O cells stably expressing shNC and LOC648987-shRNA were fixed with absolute ethanol for 24 h. The cells were washed twice with PBS and stained with PI / RNase staining buffer (BD Bioscience Pharmingen) for 15 min. The DNA content in the cell population was analyzed by FACS flow cytometry. Cell cycle data was analyzed by Flowjo V10 software (Tree Star, Ashland, OR).
Apoptosis Detection
ACHN and 786-O cells stably expressing shNC and LOC648987-shRNA were washed twice with PBS, and then incubated with FITC-Annexin V and PI mixture (BD Bioscience Pharmingen) for 15 min. Apoptosis analysis was performed using a FACS flow cytometer. Apoptosis data was analyzed by Flowjo V10 software (Tree Star, Ashland, OR).
Migration Detection
105 shNC and LOC648987-shRNA infected ACHN and 786-0 cells were added to 600 ul of serum-free medium and cultured in the upper chamber of transwell. 600 ul of complete medium was added to the lower chamber of the transwell. After the transwell was incubated at 37° C for 24 hours, the non-invasive cells in the upper chamber were gently removed with a cotton swab. Transwell-invaded cells stained with 0.1% crystal violet. The invading cells were photographed under a microscope.
Xenograft Tumor Experiment
5 × 106 ACHN cells stably expressing shNC and LOC648987-shRNA were subcutaneously injected into BALB/c nude mice. Each set of experiments consisted of 10 replicated nude mice. The volume of the tumor was measured by caliper and recorded every week. Four weeks later, nude mice were sacrificed, nude mice were photographed and tumor weight was counted. This study was approved by the Medical Ethical Committee of Ningbo Beilun District People’s Hospital.
Statistical Analysis
Comparison between two means was done by 2-tailed t-test or non-parametric 2-tailed Mann-Whitney t-test. Comparisons between 3 or more means were done by one-way ANOVA with Tukey’s multiple comparisons-test. Graphs were generated and statistical analysis were performed with Prism 7 (GraphPad) and P value <0.05 was considered to be statistically significant.
Results
High expression of lncRNA LOC648987 in Human RCC
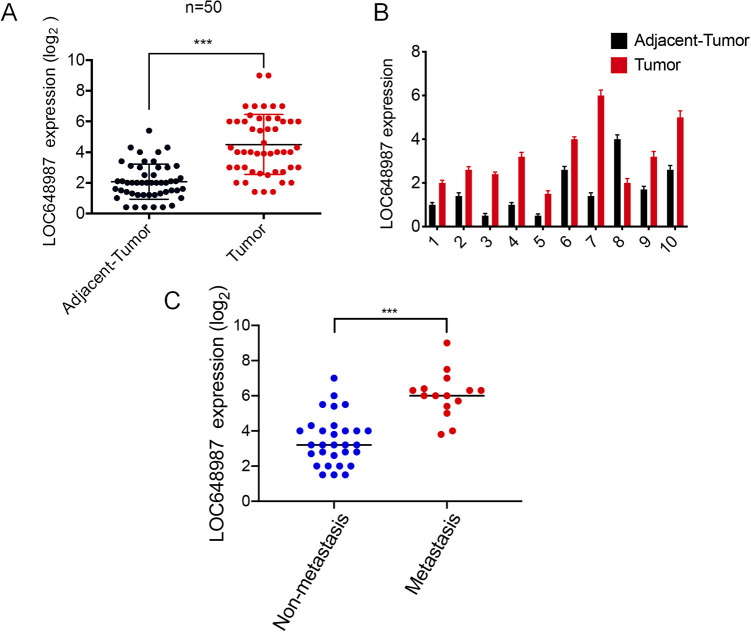
We first studied the clinically obtained human RCC samples (Table 1). We collected 50 RCCs and their corresponding adjacent tissues for lncRNA detection. The RT-PCR results showed that the lncRNA LOC648987 levels were highly expressed in RCC as compared to in the adjacent tissues (Figure 1A). From among these samples, we took 10 matched RCC tissues and their corresponding adjacent tissues, and the results showed that the expression level of LOC648987 in RCC was higher than in the paired paracancerous group (Figure 1B). At the same time, we divided the collected RCC tissues into the metastasis group and the non-metastasis group according to the patient’s tumor metastasis. The RT-PCR results showed that LOC648987 was highly expressed in metastatic RCC tissues (Figure 1C). Our results indicated that lncRNA LOC648987 was highly expressed in human RCC tissues and was associated with more severe tumor metastasis.
Table 1.
Clinical and Pathological Information of 50 Human RCC.
| Characteristics | N = 50 |
|---|---|
| Gender | |
| Male | 35 |
| Female | 15 |
| Age | |
| < 65 y | 26 |
| > 65 y | 24 |
| Tumor size | |
| < 4 cm | 25 |
| > 4 cm | 25 |
| Histological grade | |
| I-II | 17 |
| III-IV | 33 |
| T stage | |
| T 1-2 | 27 |
| T 3-4 | 23 |
| Metastasis* | (N = 45) |
| Absence | 30 |
| Presence | 15 |
* 5 patients with missing information on tumor metastasis.
Figure 1.
LncRNA LOC648987 is highly expressed in human renal cell carcinoma. A, Total RNA was extracted from 50 RCC tissues and adjacent tissues, and RNA levels of LOC648987 were detected by RT-PCR. B, Detection of RNA levels of LOC648987 in 10 paired RCC tissues and adjacent tissues randomly selected from 50 RCC patients by RT-PCR. C, 50 RCC tissues were divided into metastasis group and non-metastasis group, and the RNA level of LOC648987 was detected by RT-PCR. ***P < 0.001.
LncRNA LOC648987 Increases Proliferation of Human RCC Cell Lines
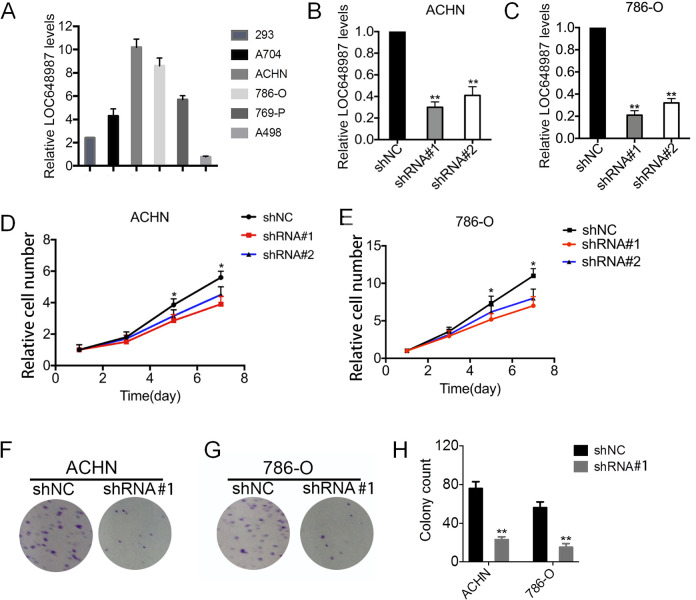
The effect of LOC648987 on the proliferation of RCC cell lines was investigated. We collected several human RCC cell lines, including A704, ACHN, 786-O, 769-P, and A498 cell lines, and human embryonic kidney cell line 293 cells, and examined the expression of LOC648987 in these cells by using RT-PCR. The results showed that LOC648987 was the most highly expressed in the ACHN and 786-O cell lines (Figure 2A). Therefore, we selected RCC cell lines ACHN and 786-O as the research models to knock down LOC648987 in these 2 cell lines. The significant knockdown of LOC648987 in ACHN (Figure 2B) and 786-O cells (Figure 2C) at the RNA level was verified. In the ACHN cells, the knockdown of LOC648987 using shRNA significantly slowed the proliferation of ACHN cells (Figure 2D). Similarly, the shRNA knockdown of LOC648987 inhibited the proliferation of the 786-O cells (Figure 2E). Colony formation assays showed that the number of clones in the ACHN (Figure 2F) and 786-O (Figure 2G) cell knockdown of LOC648987 by shRNA was reduced. The number of clones in the LOC648987-shRNA group was counted to show significant statistical differences from the shNC group (Figure 2H). Our results confirmed that lncRNA LOC648987 promotes the proliferation of RCC cells.
Figure 2.
LncRNA LOC648987 increases proliferation of RCC cell lines. A, RNA levels of LOC648987 in 293, A704, ACHN, 786-O, 769-P and A498 cells were detected by Western blotting. B and C, LOC648987-shRNA and negative control-shRNA lentivirus were infected into ACHN and 786-O cells. The knockdown efficiency of LOC648987 was detected by RT-PCR. D and E, ACHN and 786-O cells expressing LOC648987-shRNA cells and control shRNA were cultured in 96-well plates, and cell viability was measured using CCK-8 after 1, 3, 5 and 7 days of culture. F and G, ACHN and 786-O cells expressing LOC648987-shRNA cells and control shRNA were cultured in 6-well plates and subjected to colony formation assays. H, Count the number of cell clones in the LOC648987-shRNA groups and the shNC groups. **P < 0.01.
IncRNA LOC648987 Accelerates Cell Cycle Progression in RCC Cells
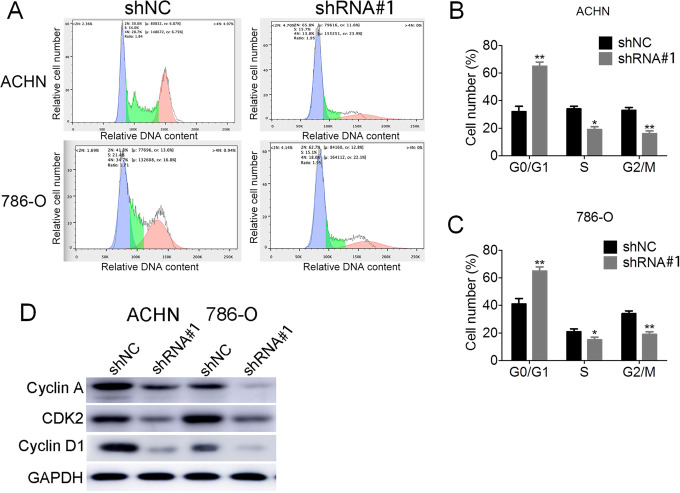
The acceleration of cell proliferation is closely related to cell cycle progression. We examined the cell cycle regulation in ACHN and 786-O cells expressing shNC and LOC648987-shRNA by flow cytometry (Figure 3A). Compared with the shNC control group, LOC648987-shRNA knocked down the ACHN (Figure 3B) and 786-O (Figure 3C) cells with an increase in G0/G1 and a decrease in the ratio of the S phase to the G2/M phase. This indicated that the knockdown of LOC648987 inhibited cell cycle progression. We then examined the expression of cell cycle-associated proteins before and after the knockdown of LOC648987. After the knockdown of LOC648987 by shRNA in the ACHN and 786-O cells, the expressions of cyclin A, CDK2, and cyclin D1 were significantly reduced (Figure 3D). Therefore, LOC648987 could accelerate the progression of the cell cycle of renal cancer cells.
Figure 3.
LncRNA LOC648987 accelerates the cell cycle progression of RCC cells. A, Cell cycle assays were performed by flow cytometry on ACHN and 786-O cells expressing LOC648987-shRNA and shNC. B and C, The results were analyzed using Flowjo software to calculate the ratio of G0/G1, S and G2/M phases in ACHN and 786-O cells. D, Cyclin A, CDK2 and cyclin D1 protein levels in ACHN and 786-O cells expressing LOC648987-shRNA and shNC were detected by Western blotting. *P < 0.05, **P < 0.01.
LncRNA LOC648987 Inhibits Apoptosis of RCC Cells
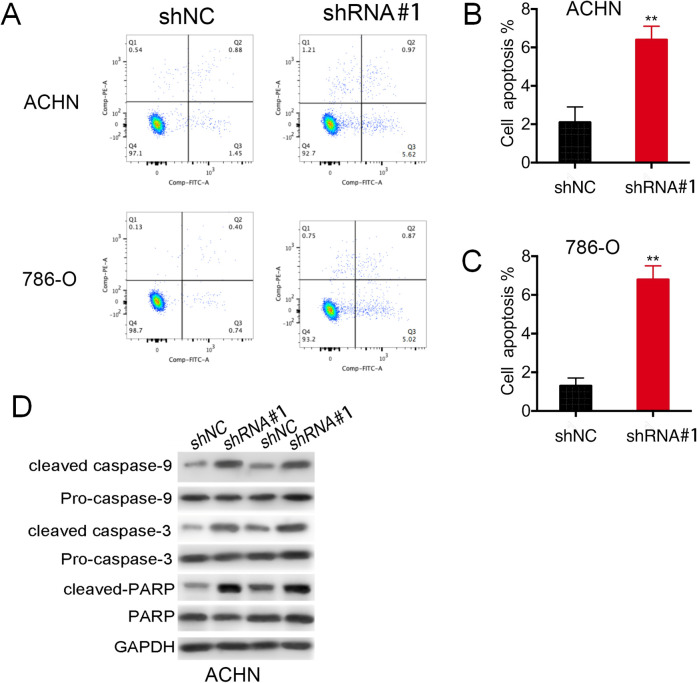
The cell proliferation inhibition by RCC cells expressed by LOC648987-shRNA was accompanied by apoptosis. We detected the apoptosis of the ACHN and 786-O cells expressing shNC and LOC648987-shRNA by using flow cytometry (Figure 4A). The proportion of apoptotic cells in the RCC lines ACHN (Figure 4B) and 786-O (Figure 4C) of the LOC648987 knockdown was significantly increased as compared to that in the control group. The apoptosis of tumor cells is a programmed cell death state in which caspase-3, caspase-9, and PARP are cleaved as the expression forms of this apoptosis. In the ACHN and 786-O cells, the expressions of caspase-3, cleaved caspase-9, and cleaved PARP were significantly increased in cells expressing LOC648987-shRNA (Figure 4D), as compared to those in the shNC control group. These data indicated that lncRNA LOC648987 inhibited the apoptosis in RCC cells.
Figure 4.
LncRNA LOC648987 inhibits apoptosis in RCC cells. A, Apoptosis detection by flow cytometry on ACHN and 786-O cells expressing LOC648987-shRNA and shNC. B and C, The results were analyzed by Flowjo software to calculate the proportion of apoptotic cells in the Q1, Q2 and Q3 quadrants of ACHN and 786-O cells. D, Protein levels of cleaved caspase-3, cleaved caspase-9 and cleaved PARP were detected by Western blotting in ACHN and 786-O cells expressing LOC648987-shRNA and shNC. **P < 0.01.
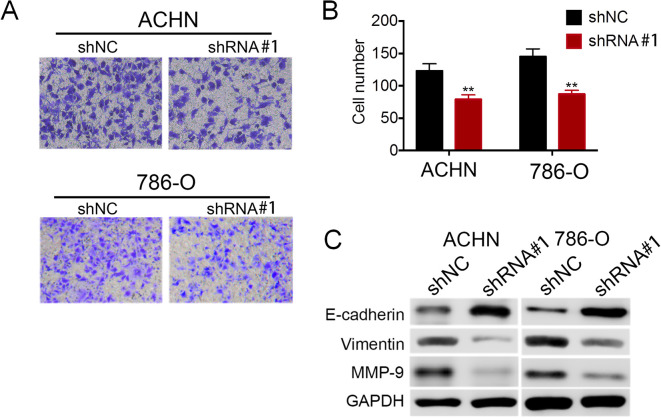
LncRNA LOC648987 Promotes Invasion of Human RCC Cells
LOC648987 has a regulatory effect on the migration and invasion of RCC cells. In the transwell cell invasion and metastasis assay, the invasive ability of the ACHN and 786-O cells expressing LOC648987-shRNA was significantly reduced as compared to that in the shNC control cells (Figure 5A). At the same time, the number of cells penetrating the transwell was counted, and the results showed that the invasive RCC cells were significantly reduced after the shRNA knockdown of LOC648987 (Figure 5B). As the invasion and metastasis of tumor cells is closely related to the epithelial–mesenchymal transition (EMT), we next examined the expression of EMT-related biomarkers in RCC cells. Western blotting showed that after the shRNA knockdown of LOC648987, the expression of the epithelial marker E-cadherin increased in the ACHN and 786-O cells, while the expressions of invasion markers vimentin and MMP-9 decreased (Figure 5C). These results implied that LOC648987 significantly promoted the invasion and metastasis of RCC cells, consistent with the results of the LOC648987 expression analysis (Figure 1C) of the clinical data of metastasized RCC patients.
Figure 5.
LncRNA LOC648987 promotes migration of RCC cells. A, ACHN and 786-O cells expressing LOC648987-shRNA and shNC were seeded into transwell, and invasive cells were photographed after 24 hours of culture. B, Count and statistics on invasive ACHN and 786-O cells. C, Protein levels of E-cadherin, Vimentin and MMP-9 in ACHN and 786-O cells expressing LOC648987-shRNA and shNC were detected by Western blotting. **P < 0.01.
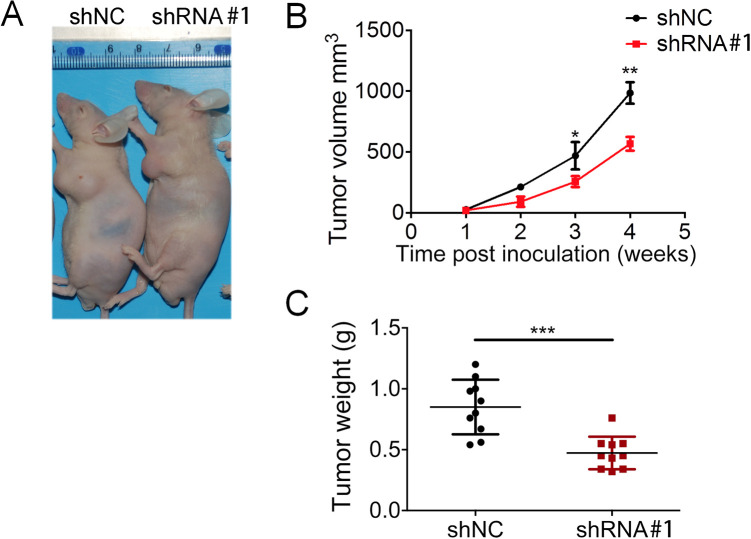
Promotion of lncRNA LOC648987 in the Tumorigenesis of RCC in Vivo
To investigate the role of LOC648987 in the development of RCC in vivo, we used a xenograft tumor model to perform subcutaneous tumorigenesis in nude mice. In vitro cultured stable LOC648987 knockdown (shRNA) ACHN cells and control ACHN cells (shNC) were injected subcutaneously into nude mice (10 mice per group). The results showed that the LOC648987-shRNA group showed a significant reduction in subcutaneous tumors at week 4 as compared to the control shNC group (Figure 6A). The growth volume of the tumors of nude mice bearing LOC648987-shRNA cells was significantly smaller than that of the control group (Figure 6B). After the mice were sacrificed, the tumors were removed and weighed, and the tumor weight expressing LOC648987-shRNA was significantly reduced as compared to that in the shNC group (Figure 6C). Therefore, we confirmed that LOC648987 played a role in promoting the tumorigenesis of renal cancer in vivo.
Figure 6.
LncRNA LOC648987 promotes tumor formation in nude mice model. A, ACHN cells expressing LOC648987-shRNA and shNC were subcutaneously implanted into nude mice. Tumor-bearing mice were photographed at 4-week. B, Tumor volumes of the LOC648987-shRNA group and the shNC group were recorded every week during tumor growth. C, Mice were sacrificed 4 weeks later, and the tumor weight of each group was measured after the tumor was taken out. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
RCC metastasis is one of the leading causes of the failure of RCC treatment. However, traditional surgical treatment has limited efficacy for metastatic RCC and progressive RCC and is not sensitive to radiation and chemotherapy.14 Although the clinical application of targeted drugs such as sorafenib and sunitinib improves the survival of patients with advanced RCC, there are still defects such as drug resistance.15 Therefore, the new RCC therapeutic target is a research finding. Many potential RCC molecular targets have also been discovered in recent years, including mRNA (such as HIF1-α and VEGF), miRNA (such as miR-21 and miR-155), and lncRNA.16-19 LncRNA has important clinical application value in the diagnosis, treatment, and prognosis of tumors, and has become the research focus of urinary system tumors.
LncRNAs are a class of RNA molecules that are greater than 200 nucleotides in length and do not encode a protein. LncRNA is highly heterogeneous and participates in the biological processes of a number of important diseases, particularly tumors.20 LncRNA acts as a proto-oncogene and a tumor suppressor gene in the biological regulation of tumors, such as p53 and NF-κB pathways.21 Although some lncRNAs such as MALAT1, NEAT1, and ATB have been studied in RCC, many lncRNAs have not been discovered, and there is currently no lncRNA with RCC specificity.22-24 Our study showed that lncRNA LOC648987 was a newly discovered RCC-associated lnRNA. LOC648987 was highly expressed in RCC tissues and promoted the proliferation of RCC cell lines in vitro. Therefore, we recommend lncRNA LOC648987 as a new biomarker for RCC diagnosis and treatment.
Our results indicated that lncRNA LOC648987 achieved RCC tumor metastasis by regulating EMT. The EMT process dedifferentiates epithelial cells and makes tumor cells more aggressive across the extracellular matrix and stromal cell layers into the vasculature. The activation of the EMT process enhanced tumor metastatic invasiveness, such as the upregulation of lncRNA SPRY4-IT1 expression in melanoma to promote tumor metastasis by inducing EMT processes.25 A variety of lncRNA types have been found to be involved in the regulation of tumor metastasis. In the hepatocellular carcinoma (HCC) cells, lncRNA CCAT2 increased tumor migration, whereas in highly metastatic HCC, the knockdown of CCAT2 reduced invasion.26 LncRNA HOTAIR regulated tumor metastasis by regulating the p53/Akt signaling pathway to regulate breast cancer cells.27 In addition, HOTAIR was directly related to metastasis such as glioma and was a negative prognostic factor for glioma.28 Our study revealed that the regulatory factor LOC648987 for RCC tumor metastasis upregulated the expression of vimentin and MMP-9 in RCC cells to promote EMT. Although our clinical samples are small and only come from the Chinese population, the RT-PCR results showed that the lncRNA LOC648987 levels were highly expressed in RCC as compared to in the adjacent tissues. At the same time, LOC648987 was highly expressed in metastatic RCC tissues. Therefore, LOC648987 might serve as a predictor of RCC progression and tumor metastasis.
Nevertheless, the specific regulatory mechanisms of LOC648987 in the development of RCC are not yet fully understood. As the current research on lncRNA is still in its infancy, although some lncRNAs have been discovered, there are still a large number of lncRNA functions and regulatory mechanisms to be studied. A small amount of lncRNA associated with tumor metastasis has been discovered, but it remains to be explored in clinical applications such as tumor diagnosis and treatment. LncRNA, including LOC648987, has good diagnostic and therapeutic prospects and is expected to bring a new era to the development of new RCC treatment strategies.
Acknowledgments
This project was supported by Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2019319797).
Authors’ Note: This study was approved by the Ethical Committee of Ningbo Beilun District People’s Hospital (approval no. 20180401). All patients provided written informed consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Received financial support from Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2019319797).
ORCID iD: Yaowu Su, MM  https://orcid.org/0000-0002-7366-812X
https://orcid.org/0000-0002-7366-812X
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clinc. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Bhatt JR, Finelli A. Landmarks in the diagnosis and treatment of renal cell carcinoma. Nat Rev Urol. 2014;11(9):517–525. [DOI] [PubMed] [Google Scholar]
- 3. Hsieh JJ, Cheng EH. The panoramic view of clear cell renal cell carcinoma metabolism: values of integrated global cancer metabolomics. Transl Androl Urol. 2016;5(6):984–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takezawa Y, Izumi K, Shimura Y. et al. Treatment outcome of low-dose interleukin-2 therapy in patients with metastatic renal cell carcinoma. Anticancer Res. 2016;36(9):4961–4964. [DOI] [PubMed] [Google Scholar]
- 5. Chen S, Liang H, Yang H. et al. Long non-coding RNAs: the novel diagnostic biomarkers for leukemia. Environ Toxicol Pharmacol. 2017;55:81–86. [DOI] [PubMed] [Google Scholar]
- 6. Ding X, Yuhan Z, Hongjian Y. et al. Long non-coding RNAs may serve as biomarkers in breast cancer combined with primary lung cancer. Oncotarget, 2017;8(35):58210–58221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qian H, Li C, Jiangping H. et al. The lncRNA MIR4435-2HG promotes lung cancer progression by activating β-catenin signalling. J Mol Med. 2018;96(8):1–12. [DOI] [PubMed] [Google Scholar]
- 8. Wang G, Zhang ZJ, Jian WG. et al. Novel long noncoding RNA OTUD6B-AS1 indicates poor prognosis and inhibits clear cell renal cell carcinoma proliferation via the Wnt/β-catenin signaling pathway. Mol Cancer. 2019;18(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L, Cai Y, Zhao X. et al. Down-regulated long non-coding RNA H19 inhibits carcinogenesis of renal cell carcinoma. Neoplasma. 2015;62(3):412–418. [DOI] [PubMed] [Google Scholar]
- 10. Chen S, Ma P, Li B. et al. LncRNA CCAT1 inhibits cell apoptosis of renal cell carcinoma through up-regulation of Livin protein. Mol Cell Biochem. 2017;434(1-2):1–8. [DOI] [PubMed] [Google Scholar]
- 11. Wu Y, Liu J, Zheng Y. et al. Suppressed expression of long non-coding RNA HOTAIR inhibits proliferation and tumourigenicity of renal carcinoma cells. Tumor Biol. 2014;35(12):11887–11894. [DOI] [PubMed] [Google Scholar]
- 12. Hong Q, Li O, Zheng W. et al. LncRNA HOTAIR regulates HIF-1α/AXL signaling through inhibition of miR-217 in renal cell carcinoma. Cell Death Dis. 2017;8(5):e2772. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Daugaard I, Dominguez D, Kjeldsen TE. et al. Identification and validation of candidate epigenetic biomarkers in lung adenocarcinoma. Sci Rep. 2016;6:35807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Emmanuel M, David P, Guillemette B. et al. Stereotactic radiation therapy in the strategy of treatment of metastatic renal cell carcinoma: a study of the Getug group. Eur J Cancer. 2018;98:38–47. [DOI] [PubMed] [Google Scholar]
- 15. Buchler T, Klapka R, Melichar B. et al. Sunitinib followed by sorafenib or vice versa for metastatic renal cell carcinoma—data from the Czech registry. Ann Oncol. 2012;23(2):395–401. [DOI] [PubMed] [Google Scholar]
- 16. Okabe T, Kumagai M, Ikeda M. et al. The impact of HIF1A/ARNT on the PER2 transcriptional activity in renal cancer cells. J Urology. 2013;189(4):e122. [Google Scholar]
- 17. Powles T, Staehler M, Ljungberg B. et al. Updated EAU guidelines for clear cell renal cancer patients who fail VEGF targeted therapy. Eur Urol. 2016;69(1):4–6. [DOI] [PubMed] [Google Scholar]
- 18. Wang G, Kwan CH, Lai MM. et al. Elevated levels of miR-146a and miR-155 in kidney biopsy and urine from patients with IgA nephropathy. Dis Markers. 2011;30(4):171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen J, Gu Y, Shen W. MicroRNA-21 functions as an oncogene and promotes cell proliferation and invasion via TIMP3 in renal cancer. Eur Rev Med Pharmacol Sci. 2017;21(20):4566–4576. [PubMed] [Google Scholar]
- 20. Silva A, Bullock M, Calin G. The clinical relevance of long non-coding RNAs in cancer. Cancers. 2015;7(4):2169–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eswar S, Ailin Z, Daniel F, Gupta S. Betulinic acid-mediated apoptosis in human prostate cancer cells involves p53 and nuclear factor-kappa B (NF-κB) Pathways. Molecules. 2017;22(2):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiao H, Tang K, Liu P. et al. LncRNA MALAT1 functions as a competing endogenous RNA to regulate ZEB2 expression by sponging miR-200 s in clear cell kidney carcinoma. Oncotarget. 2015;6(35):38005–38015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qi JJ, Liu YX, Lin L. High expression of long non-coding RNA ATB is associated with poor prognosis in patients with renal cell carcinoma. Eur Rev Med Pharmacol Sci. 2017;21(12):2835–2839. [PubMed] [Google Scholar]
- 24. Ning L, Li Z, Wei D. et al. LncRNA, NEAT1 is a prognosis biomarker and regulates cancer progression via epithelial-mesenchymal transition in clear cell renal cell carcinoma. Cancer Biomark. 2017;19(1):75–83. [DOI] [PubMed] [Google Scholar]
- 25. Zhang C-Y, Li R-K, Qi Y. et al. Upregulation of long noncoding RNA SPRY4-IT1 promotes metastasis of esophageal squamous cell carcinoma via induction of epithelial–mesenchymal transition. Cell Biol Toxicol. 2016;32(5):391–401. [DOI] [PubMed] [Google Scholar]
- 26. Zhou N, Si Z, Li T. et al. Long non-coding RNA CCAT2 functions as an oncogene in hepatocellular carcinoma, regulating cellular proliferation, migration and apoptosis. Oncology Lett. 2016;12(1):132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu Y, Lv F, Liang D. et al. HOTAIR may regulate proliferation, apoptosis, migration and invasion of MCF-7 cells through regulating the P53/Akt/JNK signaling pathway. Biomed Pharmacot. 2017;90:555–561. [DOI] [PubMed] [Google Scholar]
- 28. Zhou X, Ren Y, Zhang J. et al. HOTAIR is a therapeutic target in glioblastoma. Oncotarget. 2015;6(10):8353–8365. [DOI] [PMC free article] [PubMed] [Google Scholar]