Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) that is characterised pathologically by demyelination, gliosis, neuro-axonal damage and inflammation. Despite intense research, the underlying pathomechanisms driving inflammatory demyelination in MS still remain incompletely understood. It is thought to be caused by an autoimmune response towards CNS self-antigens in genetically susceptible individuals, assuming autoreactive T cells as disease-initiating immune cells. Yet, B cells were recognized as crucial immune cells in disease pathology, including antibody-dependent and independent effects. Moreover, myeloid cells are important contributors to MS pathology, and it is becoming increasingly evident that different cell types act in concert during MS immunopathology. This is supported by the finding that the beneficial effects of actual existing disease-modifying therapies cannot be attributed to one single immune cell-type, but rather involve immunological cooperation. The current strategy of MS therapies thus aims to shift the immune cell repertoire from a pro-inflammatory towards an anti-inflammatory phenotype, involving regulatory T and B cells and anti-inflammatory macrophages. Although no existing therapy actually exists that directly induces an enhanced regulatory immune cell pool, numerous studies identified potential net effects on these cell types. This review gives a conceptual overview on T cells, B cells and myeloid cells in the immunopathology of relapsing-remitting MS and discusses potential contributions of actual disease-modifying therapies on these immune cell phenotypes.
Keywords: B cells, immune network, immune regulation, inflammation, myeloid cells, relapsing-remitting multiple sclerosis, T cells
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) mainly affecting young adults. Despite intense research, the pathology of MS still remains incompletely understood. Traditionally, MS is considered as autoimmune disorder characterised by the infiltration of peripheral autoreactive immune cells into the CNS accompanied by the activation of innate immune mechanisms.1 The most widely accepted working model on the pathogenesis of MS starts with the escape of autoreactive T cells from clonal deletion in the thymus and dysfunctional regulatory mechanism in the periphery, before (re-)activation of these cells in lymphoid tissues by as yet unknown triggers. Finally, these autoreactive cells cross the blood–brain–barrier into the CNS, facilitating the damage of myelin and oligodendrocytes, ultimately resulting in gliosis, neuro-axonal damage and inflammation.2 The later stages of the disease are accompanied by compartmentalised inflammation, contributing to continuous inflammatory and degenerative changes in the CNS, hence driving disease progression.3,4 The autoimmune character of MS may be disputed by the Koch’s postulate that the diagnosis of an autoimmune disease requires the definitive identification of the autoantigen. Yet, the specific targets of autoreactive immune cells during MS are still lacking, and some studies only indicate myelin antigens as prominent candidates.5 Moreover, based on the observation that newly forming MS lesions spare inflammatory immune cells proposed an alternative idea of disease pathology, challenging the traditional concept of MS being an autoimmune disorder.6,7 The so-called ‘inside-out hypothesis’ claims that the initial loss of oligodendrocytes and myelin in the absence of peripheral inflammation leads to the release of CNS antigens. This triggers the development of autoimmune reactions against myelin components, ultimately resulting in neuroinflammation.8 Yet, the etiology of MS is unknown, and factors that either induce a primary inflammatory disease onset or a primary oligodendroglial pathology followed by inflammation are still not identified. MS etiology is multifactorial and seems to involve complex interactions of genetic and environmental factors. Particularly the prevalence of the MS-risk allele HLA-DR15 and many single nucleotide polymorphisms of genes that are important for the differentiation or effector function of pathogenic T cells strengthens the concept of an immune-mediated disease pathology.9,10 Moreover, extensive studies provide compelling evidence for a role of environmental factors in MS. The most consistent risk factors are childhood obesity, cigarette smoking and the infection with Epstein-Barr virus (EBV),11–13 whereas increased vitamin D levels and sunlight exposure are considered as beneficial factors in MS.14 Interestingly, the beneficial effects of vitamin D and its active metabolite are attributed to their immunomodulatory capacities affecting innate and adaptive immune cells.15,16 Moreover, EBV is suggested to cause MS in genetically susceptible individuals by infecting autoreactive B cells,17 linking environmental and genetic factors on the one hand, and highlighting the importance of the immune system in MS pathology on the other. Adding to this, there are various disease-modifying therapies available that significantly reduce relapse rates and the development of new brain lesions in relapsing-remitting MS (RRMS) patients, mainly by modulating peripheral immune cell activation or CNS infiltration. This immunomodulatory capacity of available MS drugs strengthens the concept that MS is an autoimmune disease where the initial event takes place outside the CNS, especially in the relapsing-remitting disease course. The most important immune cells targeted by disease-modifying therapies are T cells, B cells and, as a side-effect, also myeloid cells. This review thus focusses on the role of T cells, B cells and myeloid cells in the immunopathology of RRMS.
T cells in the immunopathology of MS
Considerable evidence from studies of multiple MS patients and the most commonly used animal model, experimental autoimmune encephalomyelitis (EAE),1 has contributed to the common view that MS is a T cell-mediated disease. This is in part due to the association of MS risk with variants in genes that are important for either the differentiation of pathogenic T cell subsets or the modulation of their effector function. Amongst the identified genes are, for instance, the interleukin (IL-) 2 and IL-7 receptor subunits IL-2RA and IL-7RA.9 In addition, variations in MHCII alleles provide a strong susceptibility to MS, possibly reflecting the presentation of specific CNS autoantigens to autoreactive, MHCII restricted CD4+ T cells.10 Myelin protein-derived antigens, such as myelin basic protein (MBP), proteolipid protein (PLP) and myelin oligodendrocyte glycoprotein (MOG), have been hypothesized to be the main autoreactive targets. Yet, these antigens were shown to be recognized by circulating CD4+ T cells in MS patients but also in healthy individuals, and there is conflicting evidence regarding potential differences in the frequency and avidity of these cells between the two groups.18,19 It has been shown that healthy individuals are likely to maintain regulatory mechanisms that keep these autoreactive T cells under control, a function that seems to be impaired in MS patients.20
The invasion of autoreactive CD4+ T cells into the CNS is considered to be the initial step of MS pathology, initiating inflammatory reactions and consequently neurodegenerative processes. Indeed, CD4+ T cells are found within CNS lesions and in the cerebrospinal fluid (CSF) of patients with MS.21 Classically, MS was thought to be a T helper (Th) 1-mediated autoimmune disease, while IL-4 producing Th2 cells were considered to exert a modulatory function with a protective role. This observation was supported by the finding of increased numbers of Th1 cells and elevated concentrations of the signature cytokine interferon gamma (IFN-γ) in CNS lesions of MS patients.21,22 Numerous studies in EAE,23–26 together with the finding that the administration of IFN-γ to MS patients exacerbated the disease,27 supported an important role of IFN-γ and Th1 cells in both EAE and MS pathogenesis. Moreover, Th1 cells express high levels of the α4β1 integrin VLA-4 that enables their infiltration into the CNS via VCAM-1 interaction.28 Blocking VLA-4 with the anti-α4 antibody natalizumab is a highly effective therapy in early MS, indicative of a pivotal role for Th1 cells in RRMS.29 However, further observations in mice revealed contradictory data, weakening the paradigm of Th1 cells in MS.30–33 Moreover, with the identification of IL-23 in EAE, IL-17-producing Th17 cells were also added to the list of factors potentially involved in disease pathogenesis.34,35 During past years, several studies in MS patients have provided evidence for a pivotal role of Th17 cells in disease pathogenesis. RRMS patients and patients with active disease show higher frequencies of IL-17-producing Th17 cells in the blood and active MS lesions,36–38 potentially correlating with disease progression.39 Furthermore, Th17 cells from MS patients show a highly pathogenic phenotype, with higher expression of costimulatory molecules and higher resistance to suppression.40 Th17 cells may also gain a Th1-type phenotype, co-expressing IFN-γ and IL-17.41 These Th1-like Th17 cells were found in CNS tissue from MS patients as well as peripheral blood and CSF of RRMS patients during relapse.42,43 Yet, the exact pathological role of these cells still needs to be identified. Numerous research studies have addressed the actual cause of increased autoreactive Th1 and Th17 cells in patients with MS. It was first suggested that MS pathology involves an abnormal balance between CNS-reactive effector T cells and regulatory T cells (Treg). The critical involvement of these Treg cells in MS was initially indicated by studies in EAE, showing that the adoptive transfer of Treg cells was sufficient to ameliorate the disease.44 In contrast, the depletion of Treg cells worsened EAE symptoms. However, MOG-specific Treg cells isolated from mice at different time points during EAE did not suppress MOG-specific effector T cells, either in vivo or in vitro, indicating an impaired suppressive capacity.45 Interestingly, this has also been demonstrated for MS patients, showing that Treg frequencies do not differ compared with healthy controls, whereas their suppressive capacity was shown to be impaired.20,46 The suppression of an augmented differentiation of pathogenic cells by Treg cells can either be induced via cell–cell contact mechanisms, modulation of antigen-presenting cells or via the secretion of anti-inflammatory cytokines, including IL-10.47 Yet, Treg cells from MS patients were shown to secrete less IL-10 but higher amounts of IFN-γ.48 This conversion to IFN-γ-producing Th1-like Treg cells might be one possible mechanism for the functional failure of Treg cells in MS patients. This change in phenotype and function can be induced by the pro-inflammatory cytokine IL-12, which is up-regulated in MS.49 IFN-γ-producing Treg cells are increased in the blood of MS patients compared with healthy controls.48 Moreover, in vitro data displayed a decline in their suppressive activity, as blocking IFN-γ in co-culture with Treg cells derived from RRMS patients restored their suppressive capability.48 Restoration of Treg function and a decrease of pathogenic T cells thus represents interesting targets in the therapy of MS. Indeed, some of the approved disease-modifying therapies target T cells. Yet, no drug is approved, either one that directly acts via Treg cell modulation or via specific Th1/Th17 depletion. In contrast, dietary factors have been shown to directly modulate this T cell balance during EAE and MS.50,51 While limiting the induction of Th17 cells during EAE, the short-chain fatty acid propionic acid (PA) increases the number of functionally active Treg cells, thereby ameliorating the disease.50 Of potential interest, supplementation of PA to therapy-naive MS patients and as an add-on to MS immunotherapy increased functionally competent Treg cells significantly. In line with this observation, MS patients receiving PA showed a reduced annual relapse rate together with reduced brain atrophy and a stabilisation of disability.51 These data support the relevance of Treg suppressive capacity during MS pathology and reveal short-chain fatty acids as interesting targets for the treatment of MS and potentially other autoimmune diseases. Moreover, various approved disease-modifying therapies also act via modulation of Treg cells, although most likely indirectly. For instance, glatiramer acetate, a first-line therapeutic for RRMS, was shown to increase Treg frequencies, correlating with an increased regulatory potential.52 Moreover, memory T cell numbers were shown to be reduced by dimethyl fumarate (DMF) treatment, whereas an increase of Treg cells was observed in the peripheral blood of RRMS patients treated with DMF.53 In addition, IFN-β therapy may shift the balance from an inflammatory Th1 phenotype to a more anti-inflammatory phenotype, characterized by an increase of Treg cells.54,55 Yet, the induction of Treg cells seems to be mediated via dendritic cells rather than via directly acting on CD4+ T cells, indicating the important relevance of other immune cells during the pathogenesis of MS. This is further supported by the notion that daclizumab, a monoclonal antibody targeting CD25 on activated T cells and Treg cells, is associated with an increased risk of secondary immune reactions. Although inhibiting the proliferation of activated T cells, daclizumab did not affect cells that express the low-affinity IL-2 receptor, such as natural killer cells,56 a fact that is now considered to contribute to severe immune reactions.
While providing a basic understanding of autoimmune pathomechanisms, a CD4+ T cell-centred model might be insufficient to describe the pathogenesis of MS. CD8+ T cells make up the majority of T cells in CNS infiltrates and at the edge of CNS lesions.57 CD8+ T cells can secrete IL-17, forming so-called Tc17 cells, which were shown to be increased in active lesions of MS patients. Interestingly, DMF treatment decreases the frequency of Tc17 cells instead of Th17 cells,58 indicating an important role for these cells in MS pathology. Yet, studies in EAE and MS patients indicate that Tc17 cells act via supporting Th17 cell pathogenicity,59 further strengthening the important role of Th17 cells in MS pathology. In summary, the actual concept of MS immunopathology suggests an imbalance of pro-inflammatory Th1, Th17 and Tc17 cells and a defective regulatory T cell pool in the periphery. This imbalance involves direct (cell–cell contact) or indirect (enhanced pro-inflammatory cytokine secretion) interaction with antigen-presenting cells, including macrophages, dendritic cells and B cells, strengthening the concept that different cell types act in concert during MS immunopathology.
B cells in the immunopathology of MS
It was long believed that MS is primarily a T-cell-mediated disease with an imbalance of pro- and anti-inflammatory cells driving CNS inflammation. Yet, recent findings also indicate an important role of B cells in disease pathology, including antibody-dependent and -independent effects. B cells were originally thought to contribute to the disease by differentiation towards antibody producing plasma cells after cell–cell contact and the resulting B cell activation. This autoantibody producing role of B cells was supported by the identification of oligoclonal bands (OCB) in the CSF of MS patients. OCB result from elevated immunoglobulin (Ig) G and IgM production by B cells differentiated towards plasma cells and represent a diagnostic hallmark in MS.60–62 More than 90% of MS patients show IgG OCB, whereas IgM OCB are only found in 30–40% of patients, potentially correlating with disease activity and therapy response.63–65 Within the CNS, antibody accumulation is associated with complement activation and demyelination, indicating that antibodies are directed against components of the CNS. Indeed, several studies identified numerous antibodies binding CNS structures, including MOG, MBP, neurons (neurofilament), astrocytes (KIR4.1), heat shock proteins and others.5,66–69 However, some of these autoantibodies can also be detected in healthy individuals, some could not be reproduced and the exact target antigens for antibodies in MS still remain unknown.70–72 Moreover, recent work identified antibodies that recognize intracellular self-proteins of cell debris, indicating that OCB may result from dead immune cells rather than representing a primary injury.73 Although the exact pathogenic role of B-cell-associated structures in the CNS remain controversial, increased numbers of B cells forming so-called ectopic lymphoid follicle-like aggregates within the meninges were described as associated with more aggressive forms of MS.74,75 These B-cell-rich lesions can also contain T cells and follicular dendritic cells that together contribute to increased microglial activation, and neuronal as well as oligodendroglial death in the cortex.76 The resulting cortical demyelination is now considered as an important contributor to the pathology of progressive MS.77,78 Interestingly, oligodendroglial and neuronal death was linked to soluble products of B cells.79,80 Supernatants of in vitro stimulated B cells isolated from RRMS patients but not controls induced cell death in rat oligodendrocytes and neurons.79,80 This effect was even present after the removal of immunoglobulins, suggesting antibody-independent effects of B cells, such as cytokine production and antigen-presentation, in MS pathology.
Normally, the development of autoreactive B cells is controlled by central and peripheral tolerance mechanisms during early B cell development, including suppression via Treg cells.81 Studies in MS patients demonstrated a defective peripheral tolerance in autoreactive B cell control, involving an impaired suppressive capacity of Treg cells in MS patients.82 This observed interaction of B cells and T cells coincides with the more recent finding that B cells affect MS disease via antibody-independent effects. Memory B cells can internalize, process and present different antigens via MHC class II molecules to antigen-specific CD4+ T cells.83,84 T cell activation further requires the interaction with co-stimulatory molecules expressed on B cells, including CD40, CD80 and CD86. Strong interaction of these molecules with their corresponding ligands induces a highly active state in T cells.85 Interestingly, researchers identified a higher expression of co-stimulatory molecules on B cells of MS patients compared with healthy controls,86 suggesting an enhanced antigen-presenting capacity in MS. Moreover, memory B cells mediate the proliferation of autoreactive T cells in a HLA-DR dependent manner, further supporting the pathogenic B cell–T cell interaction in MS.87 Studies in the EAE model additionally indicate the importance of co-inhibitory molecules expressed on B cells. These co-inhibitory molecules can downregulate T cell responses or induce Treg cell differentiation during EAE, thereby improving the disease.88,89 The importance of co-inhibitory molecules expressed on B cells, however, needs to be proven in humans. Recent work also identified a subclass of plasma cells with a potential regulatory function during MS independent from T cell interaction. Gut microbiota-specific IgA+ B cells were found to be enriched in the CSF and inflamed tissue of MS patients with active disease, suggesting their migration from the gut to the CNS during relapse.90 There is some evidence that these cells may exert regulatory functions via local IL-10 production, as observed in an EAE model.91
The most evident implication of antibody-independent contributions of B cells during MS pathogenesis result from the effectiveness of anti-CD20 therapies in patients with RRMS.92–95 The first B-cell-depleting clinical study testing rituximab – an anti-CD20 chimeric monoclonal antibody – decreased CNS inflammation and limited MS relapses.93 Since plasma cells or plasmablasts express no or little CD20, this success has been linked to autoantibody independent effects. The efficacy of anti-CD20 therapies seems to be mediated rather by reduced antigen-presentation and cytokine regulation, thereby limiting the stimulation of pathogenic T cells or myeloid cells. CD20 is expressed on a broad range of B cells, including immature, transitional, naïve and memory B cells. These B cells secrete pro-inflammatory cytokines, such as IL-6, IFN-γ, tumour necrosis factor alpha (TNFα) and granulocyte-macrophage colony-stimulating factor (GM-CSF), but also the anti-inflammatory cytokines IL-10, IL-35 and transforming growth factor beta (TGF-β). Interestingly, stimulated B cells isolated from untreated MS patients secrete less IL-10 and higher amounts of the pro-inflammatory cytokines IL-6 and GM-CSF,96,97 all cytokines that can induce Th1 or Th17 cell differentiation and inhibit Treg cell induction.98 In EAE, B cell-derived IL-6 increases disease pathogenesis by promoting the activation of Th1 cells and Th17 cells, which can be inhibited by treatment with CD20-depleting therapies.99 Recent studies identified GM-CSF-producing B cells in humans that are increased in MS patients compared with healthy controls and decreased after anti-CD20 therapy.100 This GM-CSF production might further enhance the pro-inflammatory response of myeloid cells during MS, highlighting that B cell depleting therapies might act via modulations of the B cell cytokine profile and their interaction with other immune cells. Besides reduced pro-inflammatory cytokine secretion, reconstituting B cells of patients treated with anti-CD20 therapy also produce higher levels of IL-10.96,101 In parallel, pro-inflammatory T cells and myeloid cells are decreased during the reconstitution phase, indicating a regulatory function of IL-10 secreting B cells. This property has already been demonstrated in EAE102,103 and studies in MS patients also indicate a regulatory function of IL10-secreting B cells in humans.104,105 Numerous studies identified an increase of these cells after treatment with disease-modifying therapies, including IFNβ, glatiramer acetate, fingolimod, rituximab and alemtuzumab.96,101,106–109 Whether the beneficial effects of these therapies can be (in part) directly linked to IL10-producing B cells still needs to be proven. Yet, a recent study demonstrated that reappearing B cells after cessation of rituximab treatment show an immature phenotype with high expression of CD25, co-stimulatory molecules and increased pro-inflammatory cytokine secretion, indicating a highly active phenotype.110 These data suggest that B cell reconstitution is an active process rather than a physiological regrowth of depleted B cells with a similar phenotype, demonstrating the importance of carefully monitoring anti-CD20-treated MS patients. Moreover, this study revealed a long-lasting effect of B cell depletion on T cells, indicating the importance of B cell–T cell interaction while confirming other studies showing a direct effect of rituximab and ocrelizumab on CD20-expressing CD4+ and CD8+ T cells.111–114 CD20+ T cells represent a highly active cell population characterized by enhanced production of pro-inflammatory cytokines (TNFα, IL1β and IL-17) that were found to be higher in RRMS and primary progressive MS compared with healthy controls.115 A recent study also identified an increased number of myelin-specific memory CD8+CD20+ T cells in MS patients that are significantly reduced following anti-CD20 treatment.114 These data indicate that the efficacy of CD20-depleting therapies are not related solely to antibody-independent functions of B cells but may also involve CD20+ T cell reduction, thus further strengthening the importance of T cells in the pathophysiology of MS. This concept is further supported by the finding that anti-CD20 therapies result in a significant reduction of CD4+ and CD8+ T cells, with a more pronounced effect in ocrelizumab-treated patients compared with rituximab treatment.116 In summary, B cells affect MS pathology by antibody-dependent and, more importantly, antibody-independent effects. These antibody-independent pathomechanisms include antigen-presentation and cytokine secretion, affecting mainly T cell phenotypes. Moreover, the defective suppression of Treg cells enhances autoreactive B cells during MS and, vice versa, defective Breg cells enhance the pro-inflammatory T cell pool, strengthening the concept of B cell–T cell interaction as important immunopathological factor. Yet, B cells are not considered the sole antigen-presenting cell type relevant in MS pathology and many data additionally point towards the involvement of myeloid cells in MS.
Myeloid cells in the immunopathology of MS
In addition to the established focus on autoreactive T cells and B lymphocytes, substantial evidence additionally points towards the involvement of myeloid cells in MS pathogenesis, including monocytes, macrophages and microglia. This finding is supported by the fact that macrophages are found in high numbers in MS lesions in RRMS, while microglia are present predominantly in progressive phases of the disease.117,118 Moreover, a recent study identified a lower magnetization transfer ratio (MTR) in white matter lesions associated with a lower density of macrophages, indicating a potential direct contribution of macrophages to tissue damage.119 In addition, studies investigating the occurrence of monocytes secreting IL-6, IL-12, TNF-α and IL-10 revealed that pro-inflammatory monocytes secreting IL-6 and IL-12 are higher in untreated MS patients compared with healthy controls.120 The higher percentage of IL-12 secreting monocytes was shown to correlate with disease activity and progression as measured by gadolinium-enhancing MRI and EDSS.121 These data suggest an important contribution of pro-inflammatory monocytes to MS pathology. On the other hand, studies also identified monocyte-derived macrophages with an anti-inflammatory phenotype in MS brain, potentially suppressing neuroinflammatory processes.122,123 These data demonstrate the heterogeneity of monocytes/myeloid cells, and add to the importance to determine the phenotype-associated functionality during MS pathogenesis rather than solely considering cell counts.
Circulating monocytes represent a heterogeneous cell population, which are divided into two main groups depending on the expression of the LPS receptor CD14 and the low-affinity FcγRIII CD16.124 Classical monocytes are defined as CD14++ CD16− cells, whereas non-classical monocytes are defined as CD14++ CD16+ cells.125 In addition, cells showing lower expression of CD14 but high expression of CD16 (CD14+ CD16++) are referred to as intermediate monocytes that, together with non-classical monocytes, make up around 10% of total monocytes in peripheral blood.125 It was shown in EAE that each monocyte population has distinct functionalities in the peripheral immune system and CNS pathology during neuroinflammation. Mouse Ly6Chi monocytes represent the equivalents of human CD16− classical monocytes, which are considered key players in monocyte subpopulations in MS.125,126 Probably due to the expression of the chemokine receptor CCR2, these cells emigrate from the bone marrow towards sites of inflammation,127 where they can differentiate towards pro-inflammatory macrophages or dendritic cells.128 Mice lacking CCR2 are resistant to EAE induction, which was linked to missing monocyte infiltration in the CNS and reduced antigen-induced T cell activation.129,130 In contrast, CCR2 negative but CX3CR1hi Ly6Clow monocytes, the counterparts of human CD16+ monocytes, may harbour a patrolling function in the peripheral immune compartment.131,132 Ly6Clow cells adhere mainly to endothelial surfaces, scanning for damage or the presence of pathogens and coordinating inflammatory processes (reviewed in Guilliams et al.133). This patrolling function has also been suggested to occur at the brain microvascular endothelial interface, indicating a potential importance at the blood–brain barrier and hence during MS pathology.134 In an in vitro transmigration assay, CD16+ monocytes were found to be enriched in the fraction adhering to the brain microvascular endothelium. Moreover, the CD16+ monocyte subset promoted CD4+ T cell trafficking via the endothelial barrier, suggesting that CD16+ monocytes contribute to the breakdown of the blood–brain barrier by promoting T cell entry into the CNS.134
These data confirm former studies demonstrating that monocyte frequencies are reduced in the CSF of RRMS patients but are increased at the meninges and the inflamed parenchyma.135,136 The importance of CD16+ monocytes during MS was further shown by the identification of increased expression of co-stimulatory markers such as CD40, CD86 and HLA-DR on CD16+ monocytes, and in vitro stimulation with LPS induced higher secretion of IL-6 and IL-12.137 Moreover, CD16+ cell numbers are increased in the peripheral blood of MS patients compared with healthy controls,138 although CD16+ monocyte frequencies seem to be influenced by disease-modifying therapies or disease duration.134,139 In contrast to treated MS patients, treatment-naïve patients had reduced frequencies of CD16+ monocytes in the peripheral blood,134 with therapy-naïve patients showing an early active MS phenotype compared with treated MS patients with a long disease duration. These data further add to the above-mentioned importance of determining monocyte functionality or phenotype rather than solely analysing cell counts. Moreover, circulating monocytes can differentiate towards macrophages upon entry into different tissues. Brain infiltrating monocytes/macrophages contribute to MS pathology via different mechanisms, which, together with the CNS resident monocytes, named microglia, may adopt a neuroinflammatory or neuroprotective role. Microglia can be found early in MS brains, forming so-called pre-active lesions that lack infiltrating leukocytes and demyelination.140 However, these microglial clusters were also found in healthy controls, albeit in lower numbers, and later studies identified that the lack of a homeostatic microglia population coincides with lesion and disease activity.141
Another study showed that, in active demyelinating MS lesions, although macrophages and activated microglia displayed predominantly pro-inflammatory characteristics, the majority of these cells co-expressed the markers of pro-inflammatory and anti-inflammatory macrophages, suggesting an intermediate activation status.142 These data indicate that the phenotype or activation state of microglia/macrophages is very diverse and contribute differentially to MS pathology. During early neuroinflammation, microglia/macrophages are suggested to conduct a beneficial function that later turns into a deleterious role with neurodegenerative contribution. This pro-inflammatory role has been linked to the antigen-presenting capacity of microglia/macrophages, which may re-activate CNS-infiltrating T cells after ingesting myelin and axonal components. This uptake promotes the expression of MHCII and co-stimulatory molecules,143 which, together with the secretion of pro-inflammatory cytokines and neurotoxic molecules, results in neuroinflammation and demyelination. In addition, pro-inflammatory macrophages can also suppress the expansion of Treg cells, thus inhibiting anti-inflammatory or regulatory processes during MS pathology and indicating the importance of monocyte/T cell interaction.144 However, phagocytosis of myelin debris is also essential to facilitate CNS repair and anti-inflammatory macrophages are necessary for efficient remyelination.145–147 In addition, the secretion of anti-inflammatory cytokines and neurotrophic factors by macrophages/microglia suppresses the disease-promoting activity of astrocytes and autoreactive T cells, thereby promoting remyelination processes and tissue repair.148–150 It is thus of high interest to shift the macrophage/monocyte pool from a pro-inflammatory towards and anti-inflammatory phenotype, suppressing neuroinflammation and promoting CNS repair.
Although no actually existing therapy directly addresses the monocyte/macrophage pool or phenotype, numerous data have revealed beneficial effects on these cell populations and further support their contribution to MS pathology. For instance, monocytes isolated from MS patients treated with glatiramer acetate, fingolimod, IFN-β or DMF show a less pro-inflammatory phenotype but enhanced anti-inflammatory characteristics.151–157 Glatiramer acetate was shown to induce increased IL-10 and TGF-β secretion in MS patient monocytes, but a decreased production of TNFα, IL-12 and IL-1β.151–153 Moreover, monocytes isolated from fingolimod-treated patients secreted lesser amounts of pro-inflammatory cytokines such as TNFα, IL-1β or IL-6,154,155 and IFN-β-treated monocytes produce less IL-1β in response to LPS stimulation.156 The first in vitro data with DMF demonstrated suppressed TNFα, IL-6 and IL-10 responses of human monocyte-derived macrophages and microglia to a pro-inflammatory stimulus.157 This less pro-inflammatory phenotype upon DMF treatment could also be observed in vivo, since monocytes from DMF-treated MS patients express reduced levels of mir-155,157 a micro-RNA that is known for its pro-inflammatory function. In addition to phenotypic changes, disease-modifying therapies might also affect monocyte/macrophage functionality. For instance, glatiramer acetate was shown to increase phagocytosis in both rat microglia and MS patient monocytes,153,158 with debris clearance necessary for remyelination.145 Additional studies suggest that the antigen-presenting capacities of monocytes are affected by disease-modifying therapies. Monomethyl fumarate, the active metabolite of DMF, was shown to inhibit the maturation of myeloid cells in vitro, characterized by reduced expression of MHCII and co-stimulatory molecules and a concomitant reduction in their capacity to activate T cells.159 These data add to the importance of the monocyte/T-cell interaction during MS pathology, and further data will be necessary to discriminate whether the beneficial effects of disease-modifying therapies can be solely linked to direct effects on B and T cells or are rather related indirectly to their side-effects on antigen-presenting cells such as monocytes/macrophages.
Summary
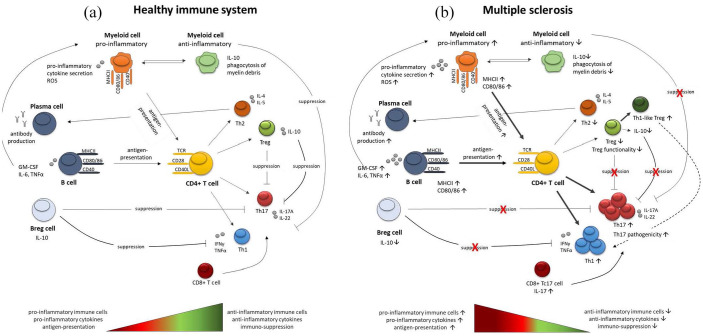
Inflammation in MS is characterised by pathogenic immune responses comprising T cells, B cells and myeloid cells. Depending on distinct activation states and the micromilieu, these different cell types act in concert to amplify or dampen pathogenic immune responses (Figure 1). The actual concept of MS immunopathology suggests an imbalance of pro-inflammatory immune cells and a defective regulatory immune cell pool in the periphery. This phenomenon is linked to the capacity of immune cells to perform a phenotype-switch, resulting in a defective suppressor-function of regulatory cells, and hence an increased infiltration of autoreactive adaptive immune cells into the CNS. Disease-modifying therapies approved for RRMS target autoreactive immune cells, thereby reducing relapses in early MS. However, if they do not substantially halt the disease, this process may result in a secondary progressive disease course. Such a progressive disease form is linked mainly to neurodegenerative processes. In addition, chronic inflammation by ongoing immune cell infiltration and re-activation of already resident cells within the CNS may enhance this process. Hence, compartmentalisation of inflammation also needs to be considered in progressive forms of MS. A goal for the future treatment of MS may thus be the simultaneous, early targeting of peripheral immune cell function and of CNS-intrinsic inflammation, along with combination therapy with neuroprotective or neuroregenerative compounds. Moreover, first clinical data indicate a potential benefit of dietary supplements as add-on therapies. Besides short-chain fatty acids,51 anti-oxidative compounds (reviewed in Plemel et al.) or coenzyme Q10 may represent potential supplements beneficially affecting MS disease.160,161 Yet, further clinical studies are needed to prove a relevant effect in clinical practice.
Figure 1.
Simplified overview of the immune network during health and disease. (a) Simplified overview of the interaction between T cells, B cells and myeloid cells in a healthy immune system. Upon stimulation, CD4+ T cells can differentiate towards anti-inflammatory Th2 and Treg cells or towards pro-inflammatory Th1 and Th17 cells, depending on the surrounding micro milieu. T cell stimulation can be induced by the interaction with B cells or myeloid cells. Besides their antigen-presenting capacity, B cells also differentiate towards plasma cells, affecting immune responses via antibody secretion. A newly identified Breg subset can suppress enhanced pro-inflammatory Th1 and Th17 differentiation via IL-10 secretion. Moreover, B cell cytokines can directly affect the myeloid cell phenotype, inducing pro-inflammatory or anti-inflammatory myeloid cells. In a healthy immune system, autoreactive immune responses are suppressed via different mechanisms, including IL-10 secretion from Treg cells, Breg cells and anti-inflammatory myeloid cells, maintaining a balance between pro- and anti-inflammatory immune cells. (b) Simplified overview of the interaction between T cells, B cells and myeloid cells in MS. Pro-inflammatory Th1 and Th17 cell responses are increased in MS patients, showing higher secretion of pro-inflammatory cytokines. Moreover, the activation state of pro-inflammatory myeloid cells, secreting high amounts of ROS, as well as autoantibodies produced by plasma cells, and activated B cells are increased in MS patients. This shift towards a pro-inflammatory immune cell pool is induced by disturbed regulatory mechanisms, including defective Treg responses, decreased Breg cells and less anti-inflammatory myeloid cells.
Breg, regulatory B cells; IL, interleukin; MS, multiple sclerosis; ROS, reactive oxygen species; Th, T helper; Treg, regulatory T cells.
Footnotes
Author contributions: Both authors made a substantial contribution to the data collection and the drafting of the manuscript and reviewed and accepted the contents of the manuscript prior to its submission.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ralf A. Linker  https://orcid.org/0000-0002-8740-3106
https://orcid.org/0000-0002-8740-3106
Contributor Information
Stefanie Haase, Neuroimmunologie, Klinik und Poliklinik für Neurologie, Universitätsklinik Regensburg, Franz-Josef-Strauss Allee, Regensburg, 93053, Germany.
Ralf A. Linker, Department of Neurology, University Hospital Regensburg, Regensburg, Germany
References
- 1. Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 2006; 129: 1953–1971. [DOI] [PubMed] [Google Scholar]
- 2. Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 3. Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol 2018; 9: 3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Monaco S, Nicholas R, Reynolds R, et al. Intrathecal inflammation in progressive multiple sclerosis. Int J Mol Sci. Epub ahead of print 3 November 2020. DOI: 10.3390/ijms21218217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Genain CP, Cannella B, Hauser SL, et al. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med 1999; 5: 170–175. [DOI] [PubMed] [Google Scholar]
- 6. Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 2004; 55: 458–468. [DOI] [PubMed] [Google Scholar]
- 7. Henderson APD, Barnett MH, Parratt JDE, et al. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol 2009; 66: 739–753. [DOI] [PubMed] [Google Scholar]
- 8. Stys PK, Zamponi GW, van Minnen J, et al. Will the real multiple sclerosis please stand up? Nat Rev Neurosci 2012; 13: 507–514. [DOI] [PubMed] [Google Scholar]
- 9. International Multiple Sclerosis Genetics Consortium, Hafler DA, Compston A, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 2007; 357: 851–862. [DOI] [PubMed] [Google Scholar]
- 10. Sawcer S, Hellenthal G, Pirinen M, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011; 476: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munger KL, Bentzen J, Laursen B, et al. Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult Scler 2013; 19: 1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann Neurol 2007; 61: 504–513. [DOI] [PubMed] [Google Scholar]
- 13. Ascherio A, Munger KL. Epstein–Barr virus infection and multiple sclerosis: a review. J Neuroimmune Pharmacol 2010; 5: 271–277. [DOI] [PubMed] [Google Scholar]
- 14. Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol 2010; 9: 599–612. [DOI] [PubMed] [Google Scholar]
- 15. Colotta F, Jansson B, Bonelli F. Modulation of inflammatory and immune responses by vitamin D. J Autoimmun 2017; 85: 78–97. [DOI] [PubMed] [Google Scholar]
- 16. Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. Epub ahead of print 15 July 2020. DOI: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pender MP. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol 2003; 24: 584–588. [DOI] [PubMed] [Google Scholar]
- 18. Hellings N, Barée M, Verhoeven C, et al. T-cell reactivity to multiple myelin antigens in multiple sclerosis patients and healthy controls. J Neurosci Res 2001; 63: 290–302. [DOI] [PubMed] [Google Scholar]
- 19. Bielekova B, Sung M-H, Kadom N, et al. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J Immunol 2004; 172: 3893–3904. [DOI] [PubMed] [Google Scholar]
- 20. Viglietta V, Baecher-Allan C, Weiner HL, et al. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med 2004; 199: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Traugott U, Reinherz EL, Raine CS. Multiple sclerosis: distribution of T cells, T cell subsets and Ia-positive macrophages in lesions of different ages. J Neuroimmunol 1983; 4: 201–221. [DOI] [PubMed] [Google Scholar]
- 22. Lock C, Hermans G, Pedotti R, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 2002; 8: 500–508. [DOI] [PubMed] [Google Scholar]
- 23. Ando DG, Clayton J, Kono D, et al. Encephalitogenic T cells in the B10.PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol 1989; 124: 132–143. [DOI] [PubMed] [Google Scholar]
- 24. Waldburger KE, Hastings RC, Schaub RG, et al. Adoptive transfer of experimental allergic encephalomyelitis after in vitro treatment with recombinant murine interleukin-12. Preferential expansion of interferon-gamma-producing cells and increased expression of macrophage-associated inducible nitric oxide synthase as immunomodulatory mechanisms. Am J Pathol 1996; 148: 375–382. [PMC free article] [PubMed] [Google Scholar]
- 25. Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002; 415: 536–541. [DOI] [PubMed] [Google Scholar]
- 26. Bettelli E, Sullivan B, Szabo SJ, et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med 2004; 200: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Panitch HS, Hirsch RL, Schindler J, et al. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology 1987; 37: 1097–1102. [DOI] [PubMed] [Google Scholar]
- 28. Baron JL, Madri JA, Ruddle NH, et al. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med 1993; 177: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hutchinson M. Natalizumab: a new treatment for relapsing remitting multiple sclerosis. Ther Clin Risk Manag 2007; 3: 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gran B, Chu N, Zhang G-X, et al. Early administration of IL-12 suppresses EAE through induction of interferon-gamma. J Neuroimmunol 2004; 156: 123–131. [DOI] [PubMed] [Google Scholar]
- 31. Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest 2002; 110: 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang G-X, Gran B, Yu S, et al. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol 2003; 170: 2153–2160. [DOI] [PubMed] [Google Scholar]
- 33. Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol 1996; 156: 5–7. [PubMed] [Google Scholar]
- 34. Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005; 6: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matusevicius D, Kivisäkk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 1999; 5: 101–104. [DOI] [PubMed] [Google Scholar]
- 37. Durelli L, Conti L, Clerico M, et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann Neurol 2009; 65: 499–509. [DOI] [PubMed] [Google Scholar]
- 38. Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 2008; 172: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arellano G, Acuña E, Reyes LI, et al. Th1 and Th17 cells and associated cytokines discriminate among clinically isolated syndrome and multiple sclerosis phenotypes. Front Immunol 2017; 8: 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brucklacher-Waldert V, Stuerner K, Kolster M, et al. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 2009; 132: 3329–3341. [DOI] [PubMed] [Google Scholar]
- 41. Sie C, Korn T, Mitsdoerffer M. Th17 cells in central nervous system autoimmunity. Exp Neurol 2014; 262: 18–27. [DOI] [PubMed] [Google Scholar]
- 42. Kebir H, Ifergan I, Alvarez JI, et al. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann Neurol 2009; 66: 390–402. [DOI] [PubMed] [Google Scholar]
- 43. Cao Y, Goods BA, Raddassi K, et al. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci Transl Med 2015; 7: 287ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kohm AP, Carpentier PA, Anger HA, et al. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol 2002; 169: 4712–4716. [DOI] [PubMed] [Google Scholar]
- 45. Korn T, Reddy J, Gao W, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med 2007; 13: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haas J, Hug A, Viehöver A, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol 2005; 35: 3343–3352. [DOI] [PubMed] [Google Scholar]
- 47. Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev 2014; 259: 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1–like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med 2011; 17: 673–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nicoletti F, Patti F, Cocuzza C, et al. Elevated serum levels of interleukin-12 in chronic progressive multiple sclerosis. J Neuroimmunol 1996; 70: 87–90. [DOI] [PubMed] [Google Scholar]
- 50. Haghikia A, Jörg S, Duscha A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 2015; 43: 817–829. [DOI] [PubMed] [Google Scholar]
- 51. Duscha A, Gisevius B, Hirschberg S, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. Epub ahead of print 5 March 2020. DOI: 10.1016/j.cell.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 52. Hong J, Li N, Zhang X, et al. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci U S A 2005; 102: 6449–6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gross CC, Schulte-Mecklenbeck A, Klinsing S, et al. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. Epub ahead of print 10 December 2015. DOI: 10.1212/NXI.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mirandola SR, Hallal DEM, Farias AS, et al. Interferon-beta modifies the peripheral blood cell cytokine secretion in patients with multiple sclerosis. Int Immunopharmacol 2009; 9: 824–830. [DOI] [PubMed] [Google Scholar]
- 55. Chen M, Chen G, Deng S, et al. IFN-β induces the proliferation of CD4+CD25+Foxp3+ regulatory T cells through upregulation of GITRL on dendritic cells in the treatment of multiple sclerosis. J Neuroimmunol 2012; 242: 39–46. [DOI] [PubMed] [Google Scholar]
- 56. Wiendl H, Gross CC. Modulation of IL-2Rα with daclizumab for treatment of multiple sclerosis. Nat Rev Neurol 2013; 9: 394–404. [DOI] [PubMed] [Google Scholar]
- 57. Hauser SL, Bhan AK, Gilles F, et al. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol 1986; 19: 578–587. [DOI] [PubMed] [Google Scholar]
- 58. Lückel C, Picard F, Raifer H, et al. IL-17 + CD8 + T cell suppression by dimethyl fumarate associates with clinical response in multiple sclerosis. Nat Commun 2019; 10: 5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huber M, Heink S, Pagenstecher A, et al. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest. Epub ahead of print 10 December 2012. DOI: 10.1172/JCI63681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kabat EA, Freedman DA. A study of the crystalline albumin, gamma globulin and total protein in the cerebrospinal fluid of 100 cases of multiple sclerosis and in other diseases. Am J Med Sci 1950; 219: 55–64. [DOI] [PubMed] [Google Scholar]
- 61. Siritho S, Freedman MS. The prognostic significance of cerebrospinal fluid in multiple sclerosis. J Neurol Sci 2009; 279: 21–25. [DOI] [PubMed] [Google Scholar]
- 62. Kabat EA, Glusman M, Knaub V. Quantitative estimation of the albumin and gamma globulin in normal and pathologic cerebrospinal fluid by immunochemical methods. Am J Med 1948; 4: 653–662. [DOI] [PubMed] [Google Scholar]
- 63. Villar LM, Sádaba MC, Roldán E, et al. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J Clin Invest 2005; 115: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sola P, Mandrioli J, Simone AM, et al. Primary progressive versus relapsing-onset multiple sclerosis: presence and prognostic value of cerebrospinal fluid oligoclonal IgM. Mult Scler 2011; 17: 303–311. [DOI] [PubMed] [Google Scholar]
- 65. Villar LM, Casanova B, Ouamara N, et al. Immunoglobulin M oligoclonal bands: biomarker of targetable inflammation in primary progressive multiple sclerosis. Ann Neurol 2014; 76: 231–240. [DOI] [PubMed] [Google Scholar]
- 66. Xiao BG, Linington C, Link H. Antibodies to myelin-oligodendrocyte glycoprotein in cerebrospinal fluid from patients with multiple sclerosis and controls. J Neuroimmunol 1991; 31: 91–96. [DOI] [PubMed] [Google Scholar]
- 67. Wajgt A, Górny M. CSF antibodies to myelin basic protein and to myelin-associated glycoprotein in multiple sclerosis. Evidence of the intrathecal production of antibodies. Acta Neurol Scand 1983; 68: 337–343. [DOI] [PubMed] [Google Scholar]
- 68. Srivastava R, Aslam M, Kalluri SR, et al. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med 2012; 367: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Brennan KM, Galban-Horcajo F, Rinaldi S, et al. Lipid arrays identify myelin-derived lipids and lipid complexes as prominent targets for oligoclonal band antibodies in multiple sclerosis. J Neuroimmunol 2011; 238: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wardemann H, Yurasov S, Schaefer A, et al. Predominant autoantibody production by early human B cell precursors. Science 2003; 301: 1374–1377. [DOI] [PubMed] [Google Scholar]
- 71. Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity 2008; 28: 18–28. [DOI] [PubMed] [Google Scholar]
- 72. Navas-Madroñal M, Valero-Mut A, Martínez-Zapata MJ, et al. Absence of antibodies against KIR4.1 in multiple sclerosis: a three-technique approach and systematic review. PLoS One 2017; 12: e0175538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brändle SM, Obermeier B, Senel M, et al. Distinct oligoclonal band antibodies in multiple sclerosis recognize ubiquitous self-proteins. Proc Natl Acad Sci U S A 2016; 113: 7864–7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pitzalis C, Jones GW, Bombardieri M, et al. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol 2014; 14: 447–462. [DOI] [PubMed] [Google Scholar]
- 75. Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007; 130: 1089–1104. [DOI] [PubMed] [Google Scholar]
- 76. Magliozzi R, Howell OW, Reeves C, et al. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 2010; 68: 477–493. [DOI] [PubMed] [Google Scholar]
- 77. Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 2011; 134: 2755–2771. [DOI] [PubMed] [Google Scholar]
- 78. Serafini B, Rosicarelli B, Magliozzi R, et al. Detection of ectopic B-cell follicles with Germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathology 2004; 14: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lisak RP, Benjamins JA, Nedelkoska L, et al. Secretory products of multiple sclerosis B cells are cytotoxic to oligodendroglia in vitro. J Neuroimmunol 2012; 246: 85–95. [DOI] [PubMed] [Google Scholar]
- 80. Lisak RP, Nedelkoska L, Benjamins JA, et al. B cells from patients with multiple sclerosis induce cell death via apoptosis in neurons in vitro. J Neuroimmunol 2017; 309: 88–99. [DOI] [PubMed] [Google Scholar]
- 81. Meffre E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann N Y Acad Sci 2011; 1246: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kinnunen T, Chamberlain N, Morbach H, et al. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J Clin Invest 2013; 123: 2737–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Walters SN, Webster KE, Daley S, et al. A role for intrathymic B cells in the generation of natural regulatory T cells. J Immunol 2014; 193: 170–176. [DOI] [PubMed] [Google Scholar]
- 84. Barnett LG, Simkins HMA, Barnett BE, et al. B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation. J Immunol 2014; 192: 3607–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev 1996; 153: 85–106. [DOI] [PubMed] [Google Scholar]
- 86. Genç K, Dona DL, Reder AT. Increased CD80+ B cells in active multiple sclerosis and reversal by interferon beta-1b therapy. J Clin Invest 1997; 99: 2664–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jelcic I, Al Nimer F, Wang J, et al. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell 2018; 175: 85–100.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bodhankar S, Galipeau D, Vandenbark AA, et al. PD-1 interaction with PD-L1 but not PD-L2 on B-cells mediates protective effects of estrogen against EAE. J Clin Cell Immunol 2013; 4: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ray A, Basu S, Williams CB, et al. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol 2012; 188: 3188–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pröbstel A-K, Zhou X, Baumann R, et al. Gut microbiota–specific IgA+ B cells traffic to the CNS in active multiple sclerosis. Sci Immunol. Epub ahead of print 20 November 2020. DOI: 10.1126/sciimmunol.abc7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rojas OL, Pröbstel A-K, Porfilio EA, et al. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell 2019; 176: 610–624.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008; 358: 676–688. [DOI] [PubMed] [Google Scholar]
- 93. Bar-Or A, Calabresi PAJ, Arnold D, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 2008; 63: 395–400. [DOI] [PubMed] [Google Scholar]
- 94. Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011; 378: 1779–1787. [DOI] [PubMed] [Google Scholar]
- 95. Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376: 221–234. [DOI] [PubMed] [Google Scholar]
- 96. Duddy M, Niino M, Adatia F, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 2007; 178: 6092–6099. [DOI] [PubMed] [Google Scholar]
- 97. Bar-Or A, Fawaz L, Fan B, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol 2010; 67: 452–461. [DOI] [PubMed] [Google Scholar]
- 98. Li R, Rezk A, Li H, et al. Antibody-independent function of human B cells contributes to antifungal T cell responses. J Immunol 2017; 198: 3245–3254. [DOI] [PubMed] [Google Scholar]
- 99. Barr TA, Shen P, Brown S, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6–producing B cells. J Exp Med 2012; 209: 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li R, Rezk A, Miyazaki Y, et al. Proinflammatory GM-CSF–producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med 2015; 7: 310ra166–310ra166. [DOI] [PubMed] [Google Scholar]
- 101. Kim Y, Kim G, Shin H-J, et al. Restoration of regulatory B cell deficiency following alemtuzumab therapy in patients with relapsing multiple sclerosis. J Neuroinflammation 2018; 15: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fillatreau S, Sweenie CH, McGeachy MJ, et al. B cells regulate autoimmunity by provision of IL-10. Nat Immunol 2002; 3: 944–950. [DOI] [PubMed] [Google Scholar]
- 103. Yoshizaki A, Miyagaki T, DiLillo DJ, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 2012; 491: 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol 2008; 64: 187–199. [DOI] [PubMed] [Google Scholar]
- 105. Okada Y, Ochi H, Fujii C, et al. Signaling via toll-like receptor 4 and CD40 in B cells plays a regulatory role in the pathogenesis of multiple sclerosis through interleukin-10 production. J Autoimmun 2018; 88: 103–113. [DOI] [PubMed] [Google Scholar]
- 106. Schubert RD, Hu Y, Kumar G, et al. IFN-β treatment requires B cells for efficacy in neuroautoimmunity. J Immunol 2015; 194: 2110–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ireland SJ, Guzman AA, O’Brien DE, et al. The effect of glatiramer acetate therapy on functional properties of B cells from patients with relapsing-remitting multiple sclerosis. JAMA Neurol 2014; 71: 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Miyazaki Y, Niino M, Fukazawa T, et al. Suppressed pro-inflammatory properties of circulating B cells in patients with multiple sclerosis treated with fingolimod, based on altered proportions of B-cell subpopulations. Clin Immunol 2014; 151: 127–135. [DOI] [PubMed] [Google Scholar]
- 109. Blumenfeld S, Staun-Ram E, Miller A. Fingolimod therapy modulates circulating B cell composition, increases B regulatory subsets and production of IL-10 and TGFβ in patients with multiple sclerosis. J Autoimmun 2016; 70: 40–51. [DOI] [PubMed] [Google Scholar]
- 110. Nissimov N, Hajiyeva Z, Torke S, et al. B cells reappear less mature and more activated after their anti-CD20–mediated depletion in multiple sclerosis. Proc Natl Acad Sci U S A 2020; 117: 25690–25699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wilk E, Witte T, Marquardt N, et al. Depletion of functionally active CD20+ T cells by rituximab treatment. Arthritis Rheum 2009; 60: 3563–3571. [DOI] [PubMed] [Google Scholar]
- 112. Palanichamy A, Jahn S, Nickles D, et al. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J Immunol 2014; 193: 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gingele S, Jacobus TL, Konen FF, et al. Ocrelizumab depletes CD20+ T cells in multiple sclerosis patients. Cells. Epub ahead of print 28 December 2018. DOI: 10.3390/cells8010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sabatino JJ, Wilson MR, Calabresi PA, et al. Anti-CD20 therapy depletes activated myelin-specific CD8+ T cells in multiple sclerosis. Proc Natl Acad Sci U S A 2019; 116: 25800–25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. von Essen MR, Ammitzbøll C, Hansen RH, et al. Proinflammatory CD20+ T cells in the pathogenesis of multiple sclerosis. Brain 2019; 142: 120–132. [DOI] [PubMed] [Google Scholar]
- 116. Capasso N, Nozzolillo A, Scalia G, et al. Ocrelizumab depletes T-lymphocytes more than rituximab in multiple sclerosis. Mult Scler Relat Disord 2021; 49: 102802. [DOI] [PubMed] [Google Scholar]
- 117. Kornek B, Lassmann H. Neuropathology of multiple sclerosis-new concepts. Brain Res Bull 2003; 61: 321–326. [DOI] [PubMed] [Google Scholar]
- 118. Lucchinetti C, Brück W, Parisi J, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 2000; 47: 707–717. [DOI] [PubMed] [Google Scholar]
- 119. Moccia M, van de Pavert S, Eshaghi A, et al. Pathologic correlates of the magnetization transfer ratio in multiple sclerosis. Neurology 2020; 95: e2965–e2976. [DOI] [PubMed] [Google Scholar]
- 120. Kouwenhoven M, Teleshova N, Özenci V, et al. Monocytes in multiple sclerosis: phenotype and cytokine profile. J Neuroimmunol 2001; 112: 197–205. [DOI] [PubMed] [Google Scholar]
- 121. Makhlouf K, Weiner HL, Khoury SJ. Increased percentage of IL-12+ monocytes in the blood correlates with the presence of active MRI lesions in MS. J Neuroimmunol 2001; 119: 145–149. [DOI] [PubMed] [Google Scholar]
- 122. Boven LA, Van Meurs M, Van Zwam M, et al. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 2006; 129: 517–526. [DOI] [PubMed] [Google Scholar]
- 123. Zhang Z, Zhang Z-Y, Schittenhelm J, et al. Parenchymal accumulation of CD163+ macrophages/microglia in multiple sclerosis brains. J Neuroimmunol 2011; 237: 73–79. [DOI] [PubMed] [Google Scholar]
- 124. Wong KL, Yeap WH, Tai JJY, et al. The three human monocyte subsets: implications for health and disease. Immunol Res 2012; 53: 41–57. [DOI] [PubMed] [Google Scholar]
- 125. Ziegler-Heitbrock L, Hofer TPJ. Toward a refined definition of monocyte subsets. Front Immunol. Epub ahead of print 4 February 2013. DOI: 10.3389/fimmu.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ingersoll MA, Spanbroek R, Lottaz C, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 2010; 115: e10–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 2006; 7: 311–317. [DOI] [PubMed] [Google Scholar]
- 128. Delneste Y, Charbonnier P, Herbault N, et al. Interferon-gamma switches monocyte differentiation from dendritic cells to macrophages. Blood 2003; 101: 143–150. [DOI] [PubMed] [Google Scholar]
- 129. Izikson L, Klein RS, Charo IF, et al. Resistance to experimental autoimmune encephalomyelitis in mice lacking the Cc chemokine receptor (Ccr2). J Exp Med 2000; 192: 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Fife BT, Huffnagle GB, Kuziel WA, et al. Cc chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med 2000; 192: 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Carlin LM, Stamatiades EG, Auffray C, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 2013; 153: 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007; 317: 666–670. [DOI] [PubMed] [Google Scholar]
- 133. Guilliams M, Mildner A, Yona S. Developmental and functional heterogeneity of monocytes. Immunity 2018; 49: 595–613. [DOI] [PubMed] [Google Scholar]
- 134. Waschbisch A, Schröder S, Schraudner D, et al. Pivotal role for CD16 + monocytes in immune surveillance of the central nervous system. J Immunol 2016; 196: 1558–1567. [DOI] [PubMed] [Google Scholar]
- 135. Kowarik MC, Grummel V, Wemlinger S, et al. Immune cell subtyping in the cerebrospinal fluid of patients with neurological diseases. J Neurol 2014; 261: 130–143. [DOI] [PubMed] [Google Scholar]
- 136. Han S, Lin YC, Wu T, et al. Comprehensive immunophenotyping of cerebrospinal fluid cells in patients with neuroimmunological diseases. J Immunol 2014; 192: 2551–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chuluundorj D, Harding SA, Abernethy D, et al. Expansion and preferential activation of the CD14+CD16+ monocyte subset during multiple sclerosis. Immunol Cell Biol 2014; 92: 509–517. [DOI] [PubMed] [Google Scholar]
- 138. Gjelstrup MC, Stilund M, Petersen T, et al. Subsets of activated monocytes and markers of inflammation in incipient and progressed multiple sclerosis. Immunol Cell Biol 2018; 96: 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Haschka D, Tymoszuk P, Bsteh G, et al. Expansion of neutrophils and classical and nonclassical monocytes as a hallmark in relapsing-remitting multiple sclerosis. Front Immunol. Epub ahead of print 29 April 2020. DOI: 10.3389/fimmu.2020.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Singh S, Metz I, Amor S, et al. Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol 2013; 125: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zrzavy T, Hametner S, Wimmer I, et al. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 2017; 140: 1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Vogel DY, Vereyken EJ, Glim JE, et al. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation 2013; 10: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol 1998; 90: 113–121. [DOI] [PubMed] [Google Scholar]
- 144. Wu C, Rauch U, Korpos E, et al. Sialoadhesin-positive macrophages bind regulatory T cells, negatively controlling their expansion and autoimmune disease progression. J Immunol 2009; 182: 6508–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Robinson S, Miller RH. Contact with central nervous system myelin inhibits oligodendrocyte progenitor maturation. Dev Biol 1999; 216: 359–368. [DOI] [PubMed] [Google Scholar]
- 146. Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 2018; 359: 684–688. [DOI] [PubMed] [Google Scholar]
- 147. Miron VE, Boyd A, Zhao J-W, et al. M2 microglia/macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 2013; 16: 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hikawa N, Takenaka T. Myelin-stimulated macrophages release neurotrophic factors for adult dorsal root ganglion neurons in culture. Cell Mol Neurobiol 1996; 16: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Rothhammer V, Borucki DM, Tjon EC, et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018; 557: 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Du L, Zhang Y, Chen Y, et al. Role of microglia in neurological disorders and their potentials as a therapeutic target. Mol Neurobiol 2017; 54: 7567–7584. [DOI] [PubMed] [Google Scholar]
- 151. Kim HJ, Ifergan I, Antel JP, et al. Type 2 monocyte and microglia differentiation mediated by glatiramer acetate therapy in patients with multiple sclerosis. J Immunol 2004; 172: 7144–7153. [DOI] [PubMed] [Google Scholar]
- 152. Burger D, Molnarfi N, Weber MS, et al. Glatiramer acetate increases IL-1 receptor antagonist but decreases T cell-induced IL-1beta in human monocytes and multiple sclerosis. Proc Natl Acad Sci U S A 2009; 106: 4355–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Pul R, Moharregh-Khiabani D, Škuljec J, et al. Glatiramer acetate modulates TNF-α and IL-10 secretion in microglia and promotes their phagocytic activity. J Neuroimmune Pharmacol 2011; 6: 381–388. [DOI] [PubMed] [Google Scholar]
- 154. Luessi F, Kraus S, Trinschek B, et al. FTY720 (fingolimod) treatment tips the balance towards less immunogenic antigen-presenting cells in patients with multiple sclerosis. Mult Scler 2015; 21: 1811–1822. [DOI] [PubMed] [Google Scholar]
- 155. Di Dario M, Colombo E, Govi C, et al. Myeloid cells as target of fingolimod action in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2015; 2: e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Guarda G, Braun M, Staehli F, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 2011; 34: 213–223. [DOI] [PubMed] [Google Scholar]
- 157. Michell-Robinson MA, Moore CS, Healy LM, et al. Effects of fumarates on circulating and CNS myeloid cells in multiple sclerosis. Ann Clin Transl Neurol 2016; 3: 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Pul R, Morbiducci F, Škuljec J, et al. Glatiramer acetate increases phagocytic activity of human monocytes in vitro and in multiple sclerosis patients. PLoS One 2012; 7: e51867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mazzola MA, Raheja R, Regev K, et al. Monomethyl fumarate treatment impairs maturation of human myeloid dendritic cells and their ability to activate T cells. Mult Scler 2019; 25: 63–71. [DOI] [PubMed] [Google Scholar]
- 160. Plemel JR, Juzwik CA, Benson CA, et al. Over-the-counter anti-oxidant therapies for use in multiple sclerosis: a systematic review. Mult Scler 2015; 21: 1485–1495. [DOI] [PubMed] [Google Scholar]
- 161. Moccia M, Capacchione A, Lanzillo R, et al. Coenzyme Q10 supplementation reduces peripheral oxidative stress and inflammation in interferon-β1a-treated multiple sclerosis. Ther Adv Neurol Disord 2019; 12: 1756286418819074. [DOI] [PMC free article] [PubMed] [Google Scholar]