Abstract
The potential of endoscopic evaluation in the management of inflammatory bowel diseases (IBD) has undoubtedly grown over the last few years. When dealing with IBD patients, histological remission (HR) is now considered a desirable target along with symptomatic and endoscopic remission, due to its association with better long-term outcomes. Consequently, the ability of endoscopic techniques to reflect microscopic findings in vivo without having to collect biopsies has become of upmost importance. In this context, a more accurate evaluation of inflammatory disease activity and the detection of dysplasia represent two mainstay targets for IBD endoscopists. New diagnostic technologies have been developed, such as dye-less chromoendoscopy, endomicroscopy, and molecular imaging, but their real incorporation in daily practice is not yet well defined. Although dye-chromoendoscopy is still recommended as the gold standard approach in dysplasia surveillance, recent research questioned the superiority of this technique over new advanced dye-less modalities [narrow band imaging (NBI), Fuji intelligent color enhancement (FICE), i-scan, blue light imaging (BLI) and linked color imaging (LCI)]. The endoscopic armamentarium might also be enriched by new video capsule endoscopy for monitoring disease activity, and high expectations are placed on the application of artificial intelligence (AI) systems to reduce operator-subjectivity and inter-observer variability. The goal of this review is to provide an updated insight on contemporary knowledge regarding new endoscopic techniques and devices, with special focus on their role in the assessment of disease activity and colorectal cancer surveillance.
Keywords: artificial intelligence, capsule enteroscopy, confocal laser endomicroscopy, dye-chromoendoscopy, endocytoscopy, inflammatory bowel diseases, molecular imaging, virtual chromoendoscopy
Introduction
Endoscopy plays a crucial role in the management of patients with inflammatory bowel diseases (IBD), as it represents the mainstay of both initial diagnostic assessment and evaluation of disease activity.1,2 To date, endoscopic remission is the main treatment target as mucosal healing (MH) is associated with better long-term efficacy outcomes.3–5 However, there is growing evidence that, in addition to MH, the objective of attaining histological remission (HR) might result in further improved outcomes.6,7 In fact, histological disease activity can be detected in up to 40% of patients with endoscopic remission and is associated with a greater risk of clinical relapse.8
It is currently recommended that endoscopic procedures should be performed with high definition white-light endoscopy (HD-WLE) in order to ensure optimal evaluation of the mucosa.2 Importantly, residual inflammatory activity persists even if HD-WLE identifies a normal-appearing mucosa.9 Besides the certain discordance between HD-WLE and histopathology, there is a multitude of histological scoring systems, which makes disease activity estimation more complex.10 Of note, in recent years, several methods have been introduced to ameliorate the evaluation of the mucosa by endoscopists. New diagnostic devices, such as confocal laser endomicroscopy (CLE), have been developed to better predict histopathology, but their real incorporation in daily practice is not yet well defined.11 The use of artificial intelligence (AI) systems to standardize operator-subjectivity in IBD and molecular imaging have also come to light as captivating areas of reasearch.12 In addition, endoscopic surveillance is fundamental in the prevention of colorectal cancer in IBD and the use of modalities such as chromoendoscopy, which involves the spraying of topical dyes to enhance visualization of the mucosa has become the gold standard for dysplasia detection.13 The Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients (SCENIC) recommendations highlighted the superiority of HD-WLE over standard definition (SD)-WLE and, most importantly, put emphasis on the preferential use of dye-chromoendoscopy (DCE) over WLE, in guiding targeted biopsies.14 Despite the authoritative position of the SCENIC consensus, debate is still ongoing, as further research has produced contradictory results, with a recent meta-analysis failing to show a significant superiority of DCE over HD-WLE in dysplasia identification [relative risk (RR) = 1.36; 95% confidence interval (CI): 0.84–2.18].15 Moreover, long examination time, need for adequate training, and additional costs for dye spraying might limit the wider application of this technique.16,17 In this context, dye-less chromoendoscopy or virtual chromoendoscopy (VCE) is emerging for its advantageous role in IBD surveillance.18 In this review, we aim to summarize the current evidence on the most recent endoscopic techniques and devices in two cornerstones of IBD management: evaluation of disease activity and colorectal cancer surveillance. The review also focused on the strengths and limitations of the new advances to provide physicians with an updated insight on what is feasible and effective in a real-life setting.
Methods
We searched the PubMed, Ovid MEDLINE and EMBASE databases using the following keywords ‘endoscopy’, ‘dye-chromoendoscopy’, ‘virtual chromoendoscopy’, ‘endocytoscopy’, ‘confocal laser endomicroscopy’, ‘capsule enteroscopy’, ‘artificial intelligence’, ‘molecular imaging’ individually or in combination with ‘IBD’, ‘Inflammatory Bowel Disease(s)’, ‘Crohn’s disease’, ‘CD’, ‘Ulcerative Colitis’, or ‘UC’. The search focused on full text papers published in English up to December 2020. Abstracts were selected when relevant. No publication date restrictions were imposed. Finally, articles were included in this review if they were relevant, while additional publications were identified through their reference lists. Ethics approval and informed consent were not required for this review.
Endoscopic technologies for the assessment of mucosal inflammation
Endoscopy plays a key role in both Crohn’s disease (CD) and ulcerative colitis (UC). However, in patients with CD inflammation is extended to deeper layers and additional cross-sectional imaging techniques are required to assess transmural healing.19 Rapid advancements in endoscopic technologies including DCE, VCE, endocytoscopy (EC), and CLE have enabled a more precise assessment of mucosal inflammation (Figure 1).18
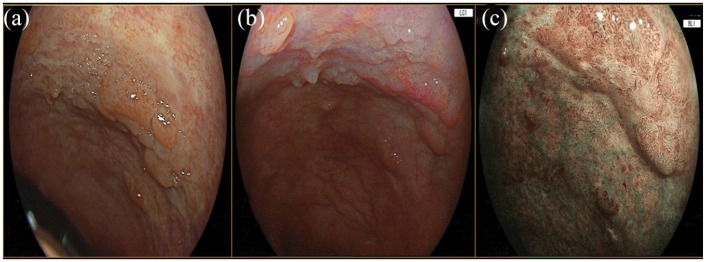
Figure 1.
Rectum flat elevated lesion (IIa + IIb according to the Paris classification), pit Patter IIIL–IV according to the Kudo classification. (a) WLE. (b) LCI. (c) BLI. Histologic examination: high grade dysplasia.
Blue light imaging; LCE, linked color imaging; WLE, white-light endoscopy.
Dye-chromoendoscopy
DCE was introduced more than a decade ago. In this procedure, dye agents (e.g., methylene blue and indigo carmine) are applied to improve the detection of the mucosal surface and any potential abnormalities.20 The interest in DCE derives from the clear benefit in the adenoma detection rate (ADR) in the context of IBD surveillance (Figure 2).21 Several studies have demonstrated the role of this technology in accurately determining mucosal inflammation.22–24 A paradigmatic trial randomized 263 clinically inactive, UC patients at a 1:1 ratio to undergo conventional colonoscopy or colonoscopy with DCE.24 A significantly improved correlation between endoscopic assessment of disease extent and histologic data was observed in the group of patients undergoing DCE (89% versus 52%, p < 0.0001). This technique was also able to better predict the degree of inflammatory activity, as a higher percentage of agreement between endoscopic and histopathologic findings was described in the DCE arm (87% versus 54%, p < 0.0002).24
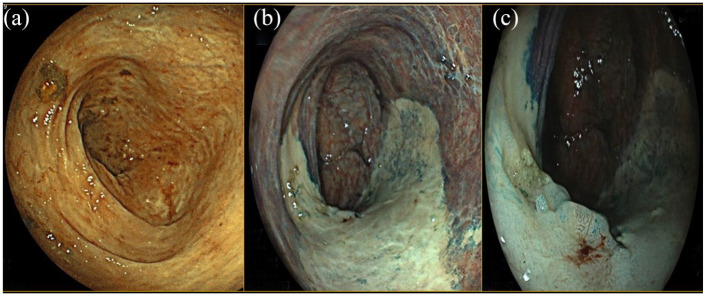
Figure 2.
Descending colon flat lesion (IIb according to the Paris classification), pit Patter IIIL according to the Kudo classification at the same specific points. (a) WLE. (b) DCE. (c) Detail with DCE. Histologic examination: biopsies on the white area compatible with chronic inflammation; biopsies on the lesion compatible with low grade dysplasia.
DEC, dye-chromoendoscopy; WLE, white-light endoscopy.
Dye-less chromoendoscopy
Unlike DCE, which utilizes staining agents and has a long procedure time, dye-less chromoendoscopy or VCE [narrow band imaging (NBI), Fuji intelligent color enhancement (FICE), i-scan, blue light imaging (BLI), and linked color imaging (LCI)] can be performed easily without complex equipment (Table 1).25
Table 1.
Dye-less chromoendoscopy techniques.
| Modality | Company | Description | Digital pre-image processing | Digital post-image processing |
|---|---|---|---|---|
| NBI | Olympus, Japan | It uses an optic filter that reduces the light spectrum emitted from the endoscope, thus being absorbed by hemoglobin and providing an enhanced image of mucosal vascularity | No | No |
| FICE | Fujinon, Japan | Starting from white-light endoscopic images from the video processor, FICE mathematically processes the image by emphasizing certain ranges of wavelengths (post-image processing) | No | Yes |
| i-SCAN | Pentax, Japan | It uses digital post-processing to enhance real-time vascular and surface images | No | Yes |
| i-SCAN OE | Pentax, Tokyo, Japan | It incorporates a pre-processor OE to gain better visualization of mucosal vascular pattern morphology | Yes | Yes |
| BLI | Fujifilm, Japan | It enhances superficial vascularity through the not-filtered emission of a short wavelength blue light that is selectively absorbed by hemoglobin | No | Yes |
| LCI | Fujifilm, Japan | By increasing shades of reddish and whitish tones through the BLI light, LCI uses both pre-processing and post-processing technology to increase slight differences in the red region of the mucosa that are related to inflammation and neoplasia. | Yes | Yes |
BLI, blue light imaging; FICE, flexible imaging color enhancement; LCI, linked color imaging; NBI, narrow band imaging; OE, optical enhancement; VCE, virtual chromoendoscopy.
NBI uses an optic filter that reduces the light spectrum emitted from the endoscope, thus being absorbed by hemoglobin and providing an enhanced image of the mucosal vascularity.26 FICE and i-scan belong to DCE. A digital post-processing system is used to enhance the real-time vascular and surface images.25 Among most advanced developments in light emitting technologies, BLI is based on the not-filtered emission of short wavelength blue light, which is selectively absorbed by hemoglobin.27 By increasing shades of reddish and whitish tones through the BLI light, an image-enhanced endoscope called LCI, increases slight differences in the red region of the mucosa that are related to inflammation and neoplasia (Figure 3).28 A prospective study compared structural and vascular alterations of Peyer’s paths (PPs) between 60 IBD patients and 23 healthy controls (HC) through the use of NBI.29 IBD patients had significant abnormalities in the vessels of PPs (e.g., branch-like structures) compared with HC (p < 0.001).29 When compared with conventional HD-WLE, NBI was shown to be more accurate in assessing the mucosal vascular pattern (p = 0.0001), which was correlated with the inflammatory histological features in patients with quiescent UC.30 Danese et al.31 suggested that NBI colonoscopy might be a useful tool for the in vivo detection of angiogenesis in IBD.23 In fact, a significant increase in vessel density in inflamed areas was identified in endoscopically normal NBI-positive areas compared with NBI-negative areas (12 ± 1 vessels/field versus 18 ± 2 vessels/field, respectively; p < 0.05).31 A more recent study reinforced the previous findings on vascular patterns, by showing a correlation between magnified NBI and histological disease activity and prognosis.32 In particular, Sasanuma et al.32 focused on the shapes of capillary vessels with magnifying NBI and reported that patients with vessels shaped like bare branches had a greater risk of recurrence compared with those showing a honeycomb-like appearance [odds ratio (OR) = 14.2, 95% CI: 3.3–60.9]. A randomized study comparing i-scan and HD-WLE in 78 IBD patients reported that i-scan correlated better with histologic findings in terms of extent (92.3% versus 48.7%, p = 0.0009) and degree of disease activity (89.7% versus 53.9%, p = 0.066).33 Accordingly, Iacucci et al. developed and validated a new endoscopic score, the Paddington International Virtual Chromoendoscopy Score (PICaSSO),34 for assessing the severity of mucosal inflammation in UC by using the i-scan. PICaSSO assesses mucosal (e.g., crypts, erosions, ulcerations) and vascular (e.g., vessels appearance, bleeding) findings and is associated with the HR.34 Here a particularly positive interobserver agreement was found among experts (kappa = 0.92 and 0.89 in the pre and post evaluations, respectively). Furthermore, the correlation between i-scan optical enhancement (OE) magnification and two histological scores has been evaluated recently.35 The overall i-scan OE score correlated moderately with two histologic scores used in UC: Robarts Histopathology Index (RHI) (r = 0.61, p < 0.01) and ECAP (Extent, Chronicity, Activity, Plus additional findings) (r = 0.70, p < 0.001).35 Notably, a strong correlation between magnification endoscopy with i-scan OE and histological inflammation was established also in CD patients.36 In this population, i-scan OE plus magnification endoscopy demonstrated to precisely identify histological inflammation, defined by the modified Riley score (r = 0.74, 95% CI: 0.58–0.85; p < 0.05).36 The first study using LCI in UC patients showed that endoscopic assessment with LCI strongly correlated with Matts histopathological grade (p = 0.001).37 The interobserver agreement for LCI was excellent (kappa > 0.8) between experts and non-experts.37 Furthermore, LCI is a promising tool for the prediction of clinical relapse in UC, as the average of the LCI-index (based on the redness of the lesion in the region of interest) in relapsing patients was statistically higher than that in non-relapsing (47.23 versus 31.91, p = 0.0003).37
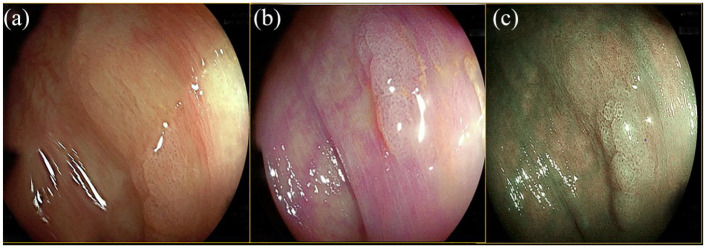
Figure 3.
Flat elevated lesion in the ascending colon (IIa according to the Paris classification), pit pattern serrated (IIO according to the Kudo classification). (a) WLE. (b) LCI. (c) BLI. Histologic examination: serrated lesion without dysplasia.
Blue light imaging; LCE, linked color imaging; WLE, white-light endoscopy.
Endocytoscopy
Endocytoscopy (EC) is an endoscopic imaging technique that allows histological analysis of in vivo optical biopsies during the endoscopic procedure itself, in order to evaluate inflammatory disease activity.18 In EC, the microscopic visualization of the superficial mucosal layer can extend up to 50 μm in depth. In a pilot study involving 40 IBD (UC = 21, CD = 19) subjects, a concordance of 100% between EC and histopathology assessment (Riley score) of bowel disease activity was found and the intraobserver agreement between two investigators was almost perfect (kappa = 0.76–0.88).38 Nakazato et al. hypothesized that EC was able to differentiate among patients in endoscopic remission, those in HR (defined as Geboes score ⩽ 2) from patients with histologically active disease.39 The agreement between EC remission and HR was substantial in patients with a Mayo endoscopic score of 0 (kappa = 0.72). The authors therefore suggested considering the possibility of evaluating histological activity through EC without collecting biopsy specimens.39 Ueda et al. recently stratified EC appearance into four groups (A, B, C, and D), according to crypt alterations in UC patients.40 They found significant differences in terms of relapse among the different groups, since patients with disruptive or hidden pits (group D) had higher recurrence rates compared with individuals without crypt abnormalities or with milder EC appearance (75% versus 0, 50% and 40% in group A, B, and C respectively; p < 0.005).40 Interestingly, a pilot study combined the EC system with the dye-less chromoendoscopy NBI (EC-NBI).41 A strong correlation between EC-NBI capillary appearance and histological activity according to Geboes index was found (r = 0.871, p < 0.01).41
Confocal laser endomicroscopy
Confocal laser endomicroscopy (CLE) is an additional endomicroscopic technique that allows for tissue analysis with a maximum depth of imaging of 250 μm.42 In UC patients in remission, CLE revealed a significant rate of mucosal pathologic abnormalities such as impaired and distorted crypt regeneration, sustained inflammation, and abnormal vascular patterns (all p < 0.001).43 Interestingly, the Erlangen group investigated the ability of CLE to evaluate and define MH during biological therapy with anti-TNF, aiming at validating the first CLE MH score for UC.44 The endomicroscopic MH score (eMHs), which includes number of crypts, lumen deformity and leakage, and vascular endothelial permeability, showed high sensitivity, specificity, and accuracy values (100%, 93.75%, and 94.44%, respectively) and a good correlation with the histological Gupta score (r = 0.82, p < 0.0001) and the endoscopic Mayo subscore (r = 0.81%, p < 0.0001).44 All patients with an eMHs score < 1 were in remission (Partial Mayo score 0) after 3 years, suggesting that CLE and eMHs were valuable tools for prediction of a deep sustained remission.44 The above-mentioned PICaSSO score and a new probe-based CLE (pCLE) grading system have been tested recently for their ability to approximate histopathologic results and therefore predict HR (defined as RHI ⩽ 6).45 Preliminary results found that a PICaSSO of ⩽4 and a pCLE of ⩽10 predicted HR with accuracy of 92.7% (95% CI: 84.8–97.3) and 95.1% (95% CI: 88.0–98.7), respectively.45 A recent prospective observational study focusing on CD patients evaluated the role of CLE in predicting clinical outcomes.46 The presence of both discontinuous crypt architecture and focal cryptitis was described in 63.3% of patients (n = 31, CLE+ group).46 After 12 months of follow up, the CLE+ group showed an increased incidence of medical treatment escalation (RR = 3.27, p < 0.001) and transmural lesions (RR = 1.70, p = 0.025) compared with CLI–, suggesting that CLE might represent an attractive prognostic tool.46 The most relevant studies on available techniques for the evaluation of IBD activity are summarized in Table 2.
Table 2.
Most relevant studies on available techniques for the assessment of mucosal inflammation.
| Author | Study design | Population | Endoscopic technique | Outcome | Results | Authors’ conclusions |
|---|---|---|---|---|---|---|
| Matsumoto et al.22 | Prospective cohort study | 41 UC | DCE with indigo carmine | Determination of UC severity | Patients with visible NWP and CO had lower grade of histologic inflammation than those in whom both findings could not be visualized (grade ⩽2 versus ⩾3, p = 0.017) | Good estimation of disease activity with DCE |
| Ibarra-Palomino et al.23 | Prospective cohort study | 25 UC | DCE with indigo carmine or methylene blue | Determination of UC extension and severity, as well as inter-observer variability | Fair agreement among endoscopic and pathologic diagnoses of each observer | DCE increases endoscopic-pathologic agreement for assessment of severity |
| Kiesslich et al.24 | RCT | 263 UC Randomized 1:1 to conventional colonoscopy or DCE |
Blue-aided DCE | Prediction of inflammation extent and severity between the two groups | DCE group showed better correlation between the endoscopic assessment of degree (p < 0.001) and extent (89% versus 52%; p < 0.001) of colonic inflammation and the histopathologic findings | DCE permits more accurate diagnosis of the extent and severity of the inflammatory activity compared with conventional colonoscopy |
| Hiyama et al.29 | Prospective cohort study | 17 CD, 43 UC, 23 HC | NBI-ME | Evaluation of endoscopic changes of PPs | IBD patients had a high prevalence of having branch-like structures (p < 0.001) and significantly higher vascularity lesions (p < 0.05) than HC | NBI-ME demonstrates microstructure and microvascular alterations of PPs |
| Kudo et al.30 | Prospective cohort study | 30 inactive or mildly active UC | NBI | Significance of MVP classification and possible correlation between MVP and the histologic grade of inflammation | MVP determination was significantly different between conventional colonoscopy and NBI colonoscopy (p = 0.001). Histologic inflammation was more frequently observed in segments with an obscure MVP than in those with a clear MVP |
NBI valuable for determining inflammation grade |
| Danese et al.31 | Prospective cohort study | 8 UC, 6 CD | NBI | Investigation whether NBI colonoscopy could detect in vivo angiogenesis in IBD patients with colonic inflammation | Endoscopically normal areas NBI+ showed a significant increase in angiogenesis (12 ± 1 vessels/field versus 18 ± 2 vessels/field, p < 0.05) compared with NBI- | NBI may allow in vivo imaging of intestinal angiogenesis |
| Sasanuma et al.32 | Prospective cohort study | 52 UC | NBI | Evaluation of the efficiency of magnified NBI findings of MH and their relationship with histological activity and prognosis | BV-BB had a higher risk of recurrence than those showing BV-H (OR = 14.2 (95% CI: 3.3–60.9) | Good relationship between NBI and histological activity. Magnifying NBI observation is also effective for UC follow-up |
| Neumann et al.33 | RCT | 39 UC 39 CD Randomized 1:1 to HD-WLE (group A) or VCE (group B) |
VCE with i-scan | Comparison between VCE and HD-WLE in the assessment of disease severity and extent | VCE showed better agreement than WLE with the histologic findings in extent (92.3% versus 48.7%, p = 0.0009) and degree (89.7% versus 53.9%, p = 0.066) of inflammatory activity | VCE with i-scan significantly improves the diagnosis of severity and extent of mucosal inflammation |
| Iacucci et al.34 | Prospective cohort study | 41 UC | VCE with i-scan OE | Investigation of the use of i-scan OE in the assessment of inflammatory changes | The overall i-scan OE score correlated with both RHI (r = 0.61, p < 0.01) and ECAP (r = 0.70, p < 0.001) | i-scan OE accurately identifies mucosal inflammation, and correlates well with histological scores |
| Klenske et al.36 | Prospective cohort study | 30 UC, 52 CD | VCE with i-scan OE plus ME | Evaluation of i-scan OE plus ME in the assessment of histologic inflammation | i-scan OE plus ME showed strong correlation with histopathologic scoring in both UC (RHI: r = 0.83, NHI: r = 0.78, p < 0.05) and CD (mRI: r = 0.74, p < 0.05) with high accuracy, sensitivity, and specificity | i-scan OE plus ME shows strong correlation with histologic inflammation |
| Uchiyama et al.37 | Prospective cohort study | 52 UC | LCI | Investigation of the efficacy of LCI for diagnosing mucosal inflammation in UC | The LCI index strongly correlated with the histopathological Matts score. Non-relapse rates significantly correlated with LCI classification (p = 0.0055) | LCI may be a novel approach for the evaluation of colonic mucosa and predictive of clinical outcome |
| Neumann et al.38 | Prospective cohort study | 21 UC, 19 CD | EC | Determination the reliability of EC for the discrimination of mucosal inflammatory cells and disease activity | Interobserver agreement was substantial (κ = 0.61–0.78), intraobserver agreement was substantial to almost perfect (κ = 0.76–0.88). Concordance between EC and histopathology for grading intestinal disease activity was 100% | EC has the potential to improve both in vivo diagnosis and clinical management |
| Nakazato et al.39 | Prospective cohort study | 64 UC | EC | EC ability to assess HR (Geboes score ⩽ 2) | Agreement between EC remission and HR remission was substantial in patients with a Mayo endoscopic score of 0 (κ = 0.72) | EC can be used to assess HR |
| Ueda et al.40 | Prospective cohort study | 32 UC | EC | Evaluation of the association of an EC classification with microscopic features and disease relapse | Patients with disruptive or disappeared pits (group D) had higher recurrence rates (75% versus 0, 50% and 40% in group A, B and C respectively, p < 0.005) during the follow-up period | EC is reliable for the assessment of microscopic inflammatory features. An EC stratification can predict the disease relapse |
| Maeda et al.47 | Retrospective cohort study | 52 UC | EC-NBI | Assessment of the efficacy of EC-NBI for evaluating the severity of inflammation | Strong correlation between the EC-NBI findings and the histological assessment (r = 0.871, p < 0.01) | EC-NBI findings can be effective in the on-site evaluation of inflammatory activity |
| Macé et al.43 | Prospective cohort study | 6 UC-IR, 6 HC | CLE | Feasibility of measurements of mucosal microvascular permeability using CLE in patients with UC-IR | CLE detected mucosal pathologic abnormalities such as impaired and distorted crypt regeneration, persistent inflammation, and abnormal vascular patterns when compared with controls (all p < 0.001 versus normal mucosa) | CLE may serve as a new gold standard for the assessment of MH in UC |
| Hundorfean et al.44 | Prospective cohort study | 23 UC | CLE | Establishment and validation of an eMHs based on CLE | The eMHs showed high sensitivity, specificity, and accuracy values (100%; 93.75% and 94.44%, respectively) and good correlation with the histological Gupta score (rs = 0.82, p < 0.001) | CLE can assess MH accurately based on the newly developed and validated eMHs |
| Iacucci et al.48 | Prospective cohort study | 82 UC | VCE and CLE | Evaluation of the ability of PICaSSO and a pCLE grading system to predict HR (RHI ⩽ 6) | A PICaSSO of ⩽4 and pCLE of ⩽10 predicted HR with accuracy of 92.7% (95% CI: 84.8–97.3) and 95.1% (95% CI: 88.0–98.7), respectively | The new VCE PICaSSO score and pCLE score can predict HR accurately |
| Tontini et al.46 | Prospective cohort study | 49 CD | CLE | Evaluation of the value of CLE for prediction of clinical outcomes | CLE+ group showed an increased incidence of medical treatment escalation (RR = 3.27, p < 0.001) and transmural lesions (RR = 1.70, p = 0.025), | CLE reveals CD-related features of mucosal inflammation and allows for early prediction of relevant clinical outcomes |
BV-BB, blood vessels shaped like bare branches; BV-H, honeycomb-like blood vessels; CD, Crohn’s disease; CI, confidence interval; CLE, confocal laser endomicroscopy; CO, cryptal opening; DCE, dye-chromoendoscopy; EC, endocytoscopy; ECAP, Extent; Chronicity; Activity; Plus additional findings; eMHs, endomicroscopic MH score; HC, healthy controls; HR, histological remission; IBD, inflammatory bowel diseases; IR, in remission; κ, kappa; LCI, linked color imaging; ME, magnifying endoscopy; MH, mucosal healing; MPV, mucosal vascular pattern; mRI, modified Riley index; NHI, Nancy histology index; NWP, network pattern; OE, optical enhancement; pCLE, probe-based CLE; PICaSSO, Paddington International Virtual Chromoendoscopy Score; PP, Peyer paths; RHI, Robarts histopathology index; RCT, randomized controlled trial; RR, relative risk; UC, ulcerative colitis; WLE, white light endoscopy.
Endoscopic technologies for the detection of dysplasia
Patients with longstanding colonic IBD have an increased risk of developing colorectal cancer (estimated prevalence is approximately 3.5% in both longstanding UC and CD).49,50 Subsequently, 8–10 years after disease onset, IBD patients are enrolled in surveillance colonoscopy programmes.2
At the moment DCE is recognized as the gold standard method for dysplasia surveillance in IBD, as it was demonstrated to be superior to WLE for dysplasia detection.14
Dye-chromoendoscopy
A prospective study by Picco et al. reported that indigo carmine CE for UC surveillance resulted in higher rates of dysplasia detection as compared with WLE (21.3% versus 9.3%, p = 0.007).51 The superiority of DCE over HD-WLE for identifying dysplasia was confirmed by a recent trial that assigned IBD patients randomly to HD-DCE with indigo carmine (n = 152) or HD-WLE (n = 153).52 Dysplastic lesions were identified in a more significant number of patients with HD-DCE (17 versus 7, respectively; p = 0.032).52 However, there is contradictory evidence concerning superiority of DCE over HD-WLE (RR = 1.36; 95% CI, 0.84–2.18), and additional studies are needed to support these data.15
Dye-less chromoendoscopy
The first results from studies evaluating the effectiveness of VCE in revealing dysplastic lesions in IBD are now becoming available.15 To compare the performance of DCE with NBI for the detection of neoplastic lesions in patients with long-standing UC, a multicenter prospective study randomized 1:1 131 patients to DCE (n = 66) or NBI (n = 65).53 After targeting suspicious lesions and surrounding mucosa, no significant difference was found in neoplasia detection between NBI and DCE with a mean number of neoplastic lesions per colonoscopy of 0.47 and 0.32, respectively.53 In addition, per lesion analysis revealed a neoplasia detection of 17% and 16% for NBI, respectively.53 The authors concluded that, given the longer withdrawal time for DCE, the gold standard might be replaced by NBI.53 A meta-analysis included 10 randomized controlled trials with the aim of comparing DCE with other endoscopic techniques (SD-WLE, HD-WLE, NBI).54 DCE was able to identify more dysplastic lesions compared with SD-WLE (RR = 2.12; 95% CI: 1.15–3.91).54 No significant difference was found between DCE and HD-WLE (RR = 1.42; 95% CI: 0.80–2.52) or NBI (RR = 1.05; 95% CI: 0.64–1.71).54 The advantages of new VCE techniques are sustained by yet another meta-analysis that confirmed no significant differences among DCE, HD-WLE, NBI, and FICE in detecting neoplasia during IBD surveillance, and also supported the use of Full spectrum HD-WLE.55 Full-spectrum endoscopy is characterized by supplementary lateral camera lenses in addition to the standard forward-viewing camera. According to Iannone et al.,55 this new colonoscope showed higher odds of detecting neoplastic (OR = 3.22, 95% CI: 0.55–18.98) and non-polypoid lesions (OR = 18.04, 95% CI: 0.49–668.36) in IBD patients.
Two studies by Hoffman et al.56,57 demonstrated that i-scan imaging was superior to standard colonoscopy (neoplasia detection rate: 38 % versus 13 %, p < 0.0001) and equal to DCE in identifying neoplastic lesions (number of neoplastic lesions: 11 versus 11). More recently, a comparative study by Iacucci et al.48 proved that i-scan and HD-WLE were not inferior to DCE in detecting neoplastic lesions. Adenoma, dysplasia (polypoid and non-polypoid), and adenocarcinoma detection rates were similar in the three-arms study (number of total lesions: 42 versus 27 versus 23 with HD, DCE, and VCE, respectively; p = 0.84).48 Along these lines, the VIRTUOSO trial randomized 188 longstanding IBD patients (>8 years after disease onset) to VCE with i-scan OE or HD-WLE during colorectal cancer surveillance.58 The neoplasia detection rate was not statistically different between the two groups (14.9% versus 23.4% with i-scan OE and HD-WLE, respectively, p = 0.14).58
In addition, an updated meta-analysis conducted by El-Dallal et al. strengthened the concept that dye-less chromoendoscopy, including both NBI and i-scan, was not statistically different in colorectal cancer screening compared with DCE (RR = 0.77; 95% CI: 0.55–1.08) or HD-WLE (RR 0.72; 95% CI: 0.45–1.15).59
A new classification of lesions discovered during IBD surveillance, FACILE, (Frankfurt Advanced Chromoendoscopic IBD LEsions classification) was recently proposed.60 It integrates four endoscopic findings (e.g., morphology, surface and vessel pattern, and inflammation), focusing on predictors of dysplasia (e.g., flat shape, irregular vessel and surface pattern, and signs of inflammation).60 Finally, data about the use of either BLI or LCI to identify neoplastic lesions in patients with IBD are not available yet. However, these newer-generation endoscopic devices proved to be promising in gastrointestinal tumor surveillance in the general population.61–63 A prospective randomized study including 245 patients proved that BLI was more accurate in predicting the histopathology of colon polyps when compared with HD-WLE (92% versus 84%, p = 0.011).61
Endocytoscopy and CLE
At the moment, consistent evidence from using EC and CLE for dysplasia detection is lacking, probably due to the intrinsic difficulties of obtaining clear images during breathing and cardiac motion.64 A prospective pilot study in 2011 aimed to evaluate feasibility and diagnostic accuracy of pCLE in UC surveillance.65 Even though accuracy, specificity, and sensitivity of pCLE were 81%, 82%, and 65%, respectively, these performance measures were inferior to those provided by NBI/HD-WLE in real time (92%, 89% and 100%, respectively).65 In line with these findings, a study assessing the diagnostic value of CE combined with integrated CLE (iCLE) reported a relatively good accuracy (86.7%, 95% CI: 78.1–95.3 versus 80.3%, 95% CI: 70.7–89.9), but a very poor sensitivity (42.9%, 95% CI: 11.8–79.8 versus 28.6%, 95% CI: 5.1–69.7).11 The most relevant studies on available technologies for cancer surveillance in IBD are summarized in Table 3.
Table 3.
Most relevant studies on available techniques for dysplasia detection in IBD.
| Author | Study design | Population | Endoscopic technique | Outcome | Results | Authors’ Conclusions |
|---|---|---|---|---|---|---|
| Picco et al.51 | Prospective cohort study | 586 images (266 WLE and 320 DCE) from 75 UC | WLE and DCE | Interobserver variability in the detection of dysplastic lesions, dysplasia detection rates | Interobserver agreement for lesions was high (κ = 0.91 and 0.86 for WLE and CE, respectively). Dysplasia was found in 9.3% with WLE compared with 21.3% with WLE and DCE (p = 0.007) |
Adoption of DCE into general endoscopic practice should be encouraged |
| Alexandersson et al.52 | RCT | 186 UC, 116 CD, 3 indetermined colitis randomized 1:1 to HD-DCE group or HD-WLE group | HD-DCE vs. HD-WLE | To compare the detection rates of dysplasia using HD-DCE plus random biopsies vs. HD-WLE plus random biopsies | Dysplastic lesions were detected in 17 patients with HD-DCE vs. 7 patients with HD-WLE (p < 0.032) | HD-DCE with random biopsies is superior to HD-WLE with random biopsies |
| Feuerstein et al.15 | Meta-analysis of 10 studies (6 RCTs) | 494 IBD | DCE compared with SD and HD-WLE | To compare the number of patients in whom dysplasia was identified using a per patient analysis in RCTs and analyzed separately for non-RCTs. | DCE was more effective at identifying dysplasia than SD-WLE (RR, 2.12; 95% CI:1.15–3.91), but it was not more effective compared with HD-WLE (RR, 1.36; 95% CI:0.84–2.18) | DCE appears superior to non-chromoendoscopy only when compared with SD-WLE and in non-RCT studies |
| Bisschops et al.53 | RCT | 131 UC randomized to DCE or VCE with NBI | DCE vs. NBI | To compare the performance of DCE to VCE for the detection of neoplastic lesions | Mean number of neoplastic lesions per colonoscopy (0.47 versus 0.32 for DCE and NBI, respectively; p = 0.992). Neoplasia detection rate was not different between DCE (21.2%) and NBI (21.5%) (OR = 1.02, p = 0.964). |
NBI may replace DCE for surveillance |
| Iannone et al.54 | Meta-analysis of 10 RCTs | 1500 IBD | DCE vs. SD-WLE/HD-WLE/NBI | To review current evidence on the comparative benefits and harms of DCE vs. all other endoscopic techniques in dysplasia surveillance | Likelihood of detecting patients with dysplasia with DCE was higher compared with other techniques (RR, 1.37; 95% CI: 1.04–1.79). Subgroup analyses confirmed this effect only when DCE was compared with SD-WLE (RR, 2.12; 95% CI:1.15–3.91). |
Data from existing RCTs show no difference between DCE and HD-WLE, NBI |
| Iacucci et al.50 | RCT | 129 UC, 136 CD, 5 indeterminate colitis | HD-WLE vs. DCE vs. and VCE (i-scan) | To compare HD-WLE, DCE and VCE (i-scan) for surveillance to detect colonic neoplastic lesions | Adenoma, dysplasia and adenocarcinoma detection rates were similar in the three-arms study (number of total lesions: 42 vs. 27 vs. 23 with HD, DCE and VCE, respectively, p = 0.84) | VCE or HD-WLE is not inferior to DCE for detection of colonic neoplastic lesions |
| Kandiah et al.58 | RCT | 188 IBD randomized to HD-WLE or VCE with i-scan OE | HD-WLE vs. VCE (i-scan) | To compare HD-WLE with i-scan OE for detection of neoplasia | NDR was not significantly different for HD-WLE (24.2%) and VCE with i-scan (14.9%) (p = 0.14) | VCE with i-scan and HD-WLE do not differ significantly in the detection of neoplasia |
| El-Dallal et al.59 | Meta-analysis of 11 RCTs | 1328 IBD | VCE vs. HD-WLE/DCE | To compare the efficacy of VCE vs. HD-WLE or DCE through a meta-analysis and rating the quality of evidence | VCE, including both NBI and i-scan, was not statistically different compared with DCE (RR = 0.77; 95% CI: 0.55–1.08) or HD-WLE (RR 0.72; 95% CI: 0.45–1.15) in detecting dysplasia | VCE or only HD-WLE might be considered for CRC screening |
| Iannone et al.55 | Systematic review with network meta-analysis of 18 RCTs | 2638 IBD | SD-WLE, HD-WLE, DCE, NBI, i-scan, autofluorescence, FICE and FUSE | To compare endoscopic techniques for dysplasia surveillance | Full spectrum HD-WLE showed higher odds of detecting patients with both neoplastic (OR = 3.22) and non-polypoid lesions (OR = 18.04) | DCE, HD-WLE, NBI, FICE and Full spectrum HD-WLE may be comparable for dysplasia surveillance. Full spectrum HD-WLE may represent the first-line approach. |
| Van Den Broek et al.65 | Prospective cohort study | 22 UC | pCLE | To evaluate feasibility and diagnostic accuracy of pCLE in UC surveillance | The sensitivity, specificity, and accuracy of blinded pCLE were 65%,82%, and 81%, respectively compared with 100%, 89%, and 92% for real-time endoscopic diagnosis with NBI and HD-WLE endoscopy | pCLE is feasible with reasonable diagnostic accuracy, but there is little possible gain as an add-on test alongside NBI/HD-WLE endoscopy |
| Wanders et al.11 | Prospective cohort study | 61 CD | DCE plus iCLE | To evaluate diagnostic accuracy of DCE plus iCLE for differentiating dysplastic vs. non-dysplastic lesions | DCE plus iCLE for differentiating lesions had good accuracy and specificity (86.7% and 92.4, respectively), but poor sensitivity (42.9%) | iCLE has limited applicability in daily practice as a surveillance strategy |
CD, Crohn’s disease; CLE, confocal laser endomicroscopy; CRC, colorectal cancer; DCE, dye-chromoendoscopy; FICE Fujinon intelligent color enhancement; FUSE, full-spectrum endoscopy; HD, high definition; IBD, inflammatory bowel diseases; iCLE, integrated CLE; ns, not significant; NDR, neoplasia detection rate; OE, optical enhancement; OR, odds ratio; pCLE, probe-based CLE; RCT, randomized controlled trial; RR, relative risk; SD, standard definition; UC, ulcerative colitis; VCE, virtual chromoendoscopy; WLE, white light endoscopy.
What is next: capsule endoscopy, artificial intelligence, and molecular imaging
Capsule endoscopy
Small bowel capsule endoscopy (SBCE) is a particularly sensitive instrument used to detect mucosal alterations in the small bowel.2 It should be considered for patients with clinical suspicion of CD who have already undergone an endoscopy, as stated in more recent guidelines.2 Since the introduction of the first wireless capsule endoscopy (CE) in the 2000s, several advances, such as improved resolution systems and new types of capsules, have been made to further increase diagnostic yield.66 Several clinical trials have investigated the potential role of SBCE in MH assessment.67,68 A prospective, multicenter, case-series included 40 patients who underwent SBCE before therapy initiation and after achievement of clinical response.67 The number of large ulcers detected through SBCE was significantly reduced after treatment (8.3 ± 1.4 versus 5 ± 0.8; respectively, p = 0.01), suggesting that SBCE was a valuable method not only for CD diagnosis, but also for disease activity assessment.67 Another study demonstrated the effectiveness of SBCE in assessing small bowel treatment response in CD patients with active disease. At week 52, 42% of subjects achieved complete mucosal healing (absence of ulcers) and deep remission (Harvey–Bradshaw Index ⩽ 5 + C-reactive protein ⩽5 mg/l or fecal calprotectin ⩽50 μg/g).68 More recently Ben-Horin et al. supported the use of CE for the monitoring of quiescent CD patients,69 as CE was able to predict disease flares within 6 months in a cohort of 61 patients [area under the curve (AUC) of 0.79, % 95 CI: 0.65–0.89; p = 0.011]. In this context, the pan-enteric video capsule system through Pillcam Crohn’s Capsule (Medtronic) constitutes a very promising innovation.70 In a pilot study including 41 patients with suspected or established CD, the Pillcam Crohn’s Capsule was performed successfully in all 41 videos, allowing for an overall evaluation of the whole intestine without risk of capsule retention.70 Moreover, thanks to a new integrated reading system, this video capsule permitted the recording and further comparison of patients’ images over time, thus monitoring disease activity and extent under treatment.71 It is particularly relevant in the pediatric population, where this type of “pan-endoscopy” was shown to effectively monitor mucosal inflammation and successfully guide a treat-to target strategy (twofold increase in the rate of MH and deep remission from baseline to week 54; p < 0.05).72
Artificial intelligence
The broad term AI generally refers to the ability of a computer to perform functions and reasoning that are typical of the human mind.73 AI tools are used in the field of endoscopy, and studies evaluating AI systems for endoscopic support have shown good results.74,75 This can be highly valued also in IBD, where the endoscopic assessment can be influenced by operator subjectivity.76 A computer-aided diagnosis (CAD) system in use with EC was developed to predict persistent histologic inflammation in UC patients.47 CAD provided good performance measures, showing sensitivity, specificity, and accuracy of 74% (95% CI: 65–81%), 97% (95% CI: 95–99%), and 91% (95% CI: 83–95%), respectively.47 A CAD system based on GoogLeNet architecture was applied to identify normal mucosa (Mayo endoscopic sub-score 0) and MH (Mayo endoscopic sub-score 0–1) in an independent test set of 3981 images from 114 UC patients.77 This new system performed well, with AUCs of 0.86 and 0.98 in the two groups, respectively.77 Moreover, a computer algorithm based on the integration of pixel color data from the redness map and vascular pattern recognition, called red density (RD), was tested in a validation cohort of 29 UC patients.78 In this latter study, Bossuyt et al. found a correlation between RD score and endoscopic (Mayo endoscopic subscore and UC Endoscopic Index of Severity scores with r = 0.76, p < 0.0001 and r = 0.74, p < 0.0001, respectively) and histological scoring systems (RHI, r = 0.74, p < 0.0001),78 suggesting that this tool might overcome interobserver variability and subjectivity for disease activity assessment. Deep learning convolutional neural networks (DCNN) belong to the AI field aiming to obtain automatically detailed imaging features, by using multiple network layers without human perceptual biases.79 Given input data, DCNN manage to autonomously classify images and find the most relevant and useful ones for solving problems.73 Su et al. conceived of an automatic quality control system (AQCS) based on DCNN and investigated whether it could improve the identification of abnormal lesions such as adenomas and polyps in a large cohort of 659 non-IBD patients.80 The ADR (0.289 versus 0.165, p < 0.001) and the mean number of adenomas (0.367 versus 0.178, p < 0.001) were significantly higher in the AQCS group when compared with the control.80
Taking advantage of convolutional neural networks, a fully automated video analysis system was developed recently, aimed at generating Mayo endoscopic scores automatically.81 The analysis of 264 videos from a multicenter clinical trial proved that this system was able to distinguish remission from endoscopic activity in more than 80% of videos reviewed, with a positive level of agreement with gastroenterologist scoring (kappa = 0.84).81
Molecular imaging
Molecular imaging is based on the topical or intravenous administration of specific and label structures and the subsequent visualization of targeted structures through simultaneous confocal imaging.82 Molecular imaging with fluorescent labeled antibody for membrane-bound TNF (mTNF) in conjunction with CLE can be used for in vivo assessment of mTNF-expressing cells in CD inflamed mucosa.83 In a single-arm clinical study including 25 CD patients, subjects with high numbers of mTNF(+) cells had significantly higher short-term response rates (92%) after 12 weeks of an anti-TNF therapy when compared with patients in the low-mTNF group (15%).83 Moreover, the clinical response in the high-mTNF group was sustained over a 1-year follow-up period, and was associated with MH at follow-up endoscopy.83 Similarly, the topical application of fluorescein-labeled antiadhesion (α4β7) molecule antibody was tested in five CD patients with active disease.84 α4β7+ cells were observed during ex vivo confocal microscopy in only two patients responding to vedolizumab (VDZ). On the contrary, no α4β7+ were detected in the three non-responders to VDZ.84
Multiphoton imaging is a molecular imaging technique that provides both high cellular resolution and great depth of penetration into the tissue without the need for labels.85 An endoscopic application of this system, named multiphoton endomicroscopy (MPEM), has recently been proven to yield label-free in vivo histopathologic results in experimental colitis models.86
Discussion
Progress in endoscopy has paved the way for a more accurate approximation of histopathologic results. Histological modifications, inflammation, and healing are well reflected by endoscopic images provided by cutting-edge techniques. Furthermore, with the introduction of new advanced endoscopic modalities, such as VCE, most dysplastic lesions are visible and a smaller number of biopsy specimens is required, making surveillance less cumbersome for both patients and clinicians.53,87 Among image enhancement techniques, the available evidence suggests that dye-less chromoendoscopy is not inferior to the gold standard DCE in terms of neoplastic lesions detection.48,53,54,59 Moreover, dye-less chromoendoscopy modalities offer the advantage of requiring less procedure time without additional spray-agent costs (median withdrawal time: 15 versus 20 min with VCE and DCE, respectively).53,88 To date, no comparative studies are available between the different VCE techniques and there is no standardized training to evaluate the expertise of operators. The use of one method rather than another is currently linked to cost and the preferences of individual centers. Head-to-head trials between the different VCE modalities are necessary to define whether one approach is in fact superior to the other. Future data well may result in this modern methodology becoming the routine approach for MH assessment and colorectal cancer screening in IBD.
EC and CLE are also available, and they provide the clear advantage of allowing in vivo estimation of inflammatory activity.38,89 These methods could make endoscopic examinations less invasive by reducing the risk of complications related to biopsies. However, they are not yet widely adopted in clinical practice due to the associated costs and the prolonged time required by the procedure (an average increase of approximately 30–40 min).65 Less expensive confocal probes and economic analyses are eagerly awaited to overcome this significant disadvantage. Moreover, there is no standardized training and the level of expertise among operators is not high, so further studies are needed to increase the use of these techniques in clinical practice.
In addition, SBCE might be seen not only as a diagnostic tool in patients with suspected small bowel CD and normal endoscopy and magnetic resonance enterography (MRE), but also as an accurate investigation method to evaluate MH in small-bowel CD patients. Capsule retention leading to an urgent endoscopic or surgical intervention remains one of the main concerns of this device; as a result, it is not considered in patients with known or suspected stenotic disease or with a history of bowel obstruction. Of note, the new Pillcam Crohn’s capsule with a pan-enteric video system is a promising and safe tool for the assessment of MH in selected populations such as pediatric patients in order to avoid endoscopy deep sedation and biopsy-related complications. The use of pan-enteric CE for activity monitoring should be encouraged in centers where video capsule is a routine examination, and the staff are adequately trained to read images. Due to its likely cost-effectiveness (mean total 20-year cost per patient: £38,043 versus £42,266 with pan-enteric CE and colonoscopy ± MRE, respectively) it may well represent a valid alternative to colonoscopy combined with MRE.90
AI represents a revolutionary technique in the endoscopy field, providing an objective and accurate evaluation of disease severity. AI offers enormous potential to improve the quality of endoscopic procedures and ultimately to reduce the number of biopsy specimens.91 The use of AI in endoscopy is also expected to increase the ADR and help non-expert endoscopists in the challenging differentiation between inflammatory and neoplastic lesions, although this will need to be confirmed in studies involving IBD patients. Finally, implementing molecular imaging in endoscopy could provide physicians with a real-time in vivo view of targeted biomarkers, but it is still a research field that requires further validation [ClinicalTrials.gov identifier: NCT02852850].
Conclusion
The goal of HR in IBD management has certainly influenced new advances in the endoscopic field. Endoscopy is now asked to accurately describe inflammatory disease activity and promptly identify suspected lesions during the ongoing procedure, thus getting as close as possible to microscopic findings. Dye-less chromoendoscopy has already demonstrated to be feasible and cost-effective in a real-life setting, whereas endomicroscopy systems still appear as complex and costly techniques. New pan-enteric video capsules might enrich the endoscopic armamentarium for monitoring disease in specific populations. Further clinical studies are expected to confirm the great expectations placed on AI tools and molecular imaging.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: V. Solitano, F. D’Amico, A. Zilli, L. Loy, D. Gilardi, S. Radice, and C. Correale declare no conflict of interest. M. Allocca received consulting fees from Nikkiso Europe and lecture fees from Janssen and Pfizer. G. Fiorino received consultancy fees from Ferring, MSD, AbbVie, Takeda, Janssen, Amgen, Sandoz, Samsung Bioepis, and Celltrion. S. Danese has served as a speaker, consultant, and advisory board member for Schering-Plough, AbbVie, Actelion, Alphawasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson and Johnson, Millenium Takeda, MSD, Nikkiso Europe GmbH, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, UCB Pharma, and Vifor. L. Peyrin-Biroulet has served as a speaker consultant and advisory board member for Merck, Abbvie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Hospira/Pfizer, Celltrion, Takeda, Biogaran, Boerhinger-Ingelheim, Lilly, HAC- Pharma, Index Pharmaceuticals, Amgen, Sandoz, Forward Pharma GmbH, Celgene, Biogen, Lycera, Samsung Bioepis, and Theravance. F. Furfaro received consulting fees form MSD and Abbvie and lecture fees from Janssen and Pfizer.
ORCID iD: Federica Furfaro  https://orcid.org/0000-0002-4308-7295
https://orcid.org/0000-0002-4308-7295
Contributor Information
Virginia Solitano, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy.
Ferdinando D’Amico, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; IBD Center, Humanitas Clinical and Research Center, IRCCS, Rozzano, Milan, Italy.
Mariangela Allocca, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; IBD Center, Humanitas Clinical and Research Center, IRCCS, Rozzano, Milan, Italy.
Gionata Fiorino, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; IBD Center, Humanitas Clinical and Research Center, IRCCS, Rozzano, Milan, Italy.
Alessandra Zilli, IBD Center, Humanitas Clinical and Research Center, IRCCS, Rozzano, Milan, Italy.
Laura Loy, IBD Center, Humanitas Clinical and Research Center, IRCCS, Rozzano, Milan, Italy.
Daniela Gilardi, IBD Center, Humanitas Clinical and Research Center, IRCCS, Rozzano, Milan, Italy.
Simona Radice, IBD Center, Humanitas Clinical and Research Center, IRCCS, Rozzano, Milan, Italy.
Carmen Correale, IBD Center, Humanitas Clinical and Research Center, IRCCS, Rozzano, Milan, Italy.
Silvio Danese, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; IBD Center, Humanitas Clinical and Research Center, IRCCS, Rozzano, Milan, Italy.
Laurent Peyrin-Biroulet, Department of Gastroenterology and Inserm NGERE U1256, University Hospital of Nancy, University of Lorraine, Vandoeuvre-lès-Nancy, France.
Federica Furfaro, IBD Center, Humanitas Clinical and Research Center, IRCCS, Via Manzoni 56, Rozzano, Milan, 20089, Italy.
References
- 1. Negreanu L, Voiosu T, State M, et al. Endoscopy in inflammatory bowel disease: from guidelines to real life. Therap Adv Gastroenterol 2019; 12: 1756284819865153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019; 13: 144–164. [DOI] [PubMed] [Google Scholar]
- 3. Frøslie KF, Jahnsen J, Moum BA, et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 2007; 133: 412–422. [DOI] [PubMed] [Google Scholar]
- 4. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015; 110: 1324–1338. [DOI] [PubMed] [Google Scholar]
- 5. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011; 141: 1194–1201. [DOI] [PubMed] [Google Scholar]
- 6. Gupta A, Yu A, Peyrin-Biroulet L, et al. Treat to target: the role of histologic healing in inflammatory bowel diseases, a systematic review and meta-analysis. Clin Gastroenterol Hepatol. Epub ahead of print 30 September 2020. DOI: 10.1016/j.cgh.2020.09.046. [DOI] [PubMed] [Google Scholar]
- 7. Yoon H, Jangi S, Dulai PS, et al. Incremental benefit of achieving endoscopic and histologic remission in patients with ulcerative colitis: a systematic review and meta-analysis. Gastroenterology 2020; 159: 1262–1275.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bessissow T, Lemmens B, Ferrante M, et al. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol 2012; 107: 1684–1692. [DOI] [PubMed] [Google Scholar]
- 9. Bryant RV, Winer S, Travis SP, et al. Systematic review: histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis 2014; 8: 1582–1597. [DOI] [PubMed] [Google Scholar]
- 10. Neri B, Mossa M, Scucchi L, et al. Review article: histological scores in inflammatory bowel disease. J Dig Dis 202; 22: 9–22. [DOI] [PubMed] [Google Scholar]
- 11. Wanders LK, Kuiper T, Kiesslich R, et al. Limited applicability of chromoendoscopy-guided confocal laser endomicroscopy as daily-practice surveillance strategy in Crohn’s disease. Gastrointest Endosc 2016; 83: 966–971. [DOI] [PubMed] [Google Scholar]
- 12. Bossuyt P, Vermeire S, Bisschops R. Scoring endoscopic disease activity in IBD: artificial intelligence sees more and better than we do. Gut 2020; 69: 788–789. [DOI] [PubMed] [Google Scholar]
- 13. Kiesslich R, Von Bergh M, Hahn M, et al. Chromoendoscopy with indigocarmine improves the detection of adenomatous and nonadenomatous lesions in the colon. Endoscopy 2001; 33: 1001–1006. [DOI] [PubMed] [Google Scholar]
- 14. Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015; 148: 639–651.e28. [DOI] [PubMed] [Google Scholar]
- 15. Feuerstein JD, Rakowsky S, Sattler L, et al. Meta-analysis of dye-based chromoendoscopy compared with standard- and high-definition white-light endoscopy in patients with inflammatory bowel disease at increased risk of colon cancer. Gastrointest Endosc 2019; 90: 186–195.e1. [DOI] [PubMed] [Google Scholar]
- 16. Resende RH, Ribeiro IB, de Moura DTH, et al. Surveillance in inflammatory bowel disease: is chromoendoscopy the only way to go? A systematic review and meta-analysis of randomized clinical trials. Endosc Int Open 2020; 8: E578–E590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaltenbach TR, Soetikno RM, DeVivo R, et al. Optimizing the quality of endoscopy in inflammatory bowel disease: focus on surveillance and management of colorectal dysplasia using interactive image- and video-based teaching. Gastrointest Endosc 2017; 86: 1107–1117.e1. [DOI] [PubMed] [Google Scholar]
- 18. Iacucci M, Furfaro F, Matsumoto T, et al. Advanced endoscopic techniques in the assessment of inflammatory bowel disease: new technology, new era. Gut 2019; 68: 562–572. [DOI] [PubMed] [Google Scholar]
- 19. Castiglione F, Imperatore N, Testa A, et al. One-year clinical outcomes with biologics in Crohn’s disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther 2019; 49: 1026–1039. [DOI] [PubMed] [Google Scholar]
- 20. Subramanian V, Mannath J, Ragunath K, et al. Meta-analysis: the diagnostic yield of chromoendoscopy for detecting dysplasia in patients with colonic inflammatory bowel disease. Aliment Pharmacol Ther 2011; 33: 304–312. [DOI] [PubMed] [Google Scholar]
- 21. Tontini GE, Vecchi M, Neurath MF, et al. Review article: newer optical and digital chromoendoscopy techniques vs. dye-based chromoendoscopy for diagnosis and surveillance in inflammatory bowel disease. Aliment Pharmacol Ther 2013; 38: 1198–1208. [DOI] [PubMed] [Google Scholar]
- 22. Matsumoto T, Kuroki F, Mizuno M, et al. Application of magnifying chromoscopy for the assessment of severity in patients with mild to moderate ulcerative colitis. Gastrointest Endosc 1997; 46: 400–405. [DOI] [PubMed] [Google Scholar]
- 23. Ibarra-Palomino J, Barreto-Zúñiga R, Elizondo-Rivera J, et al. Application of chromoendoscopy to evaluate the severity and interobserver variation in chronic non-specific ulcerative colitis. Rev Gastroenterol México 2002; 67: 236–240. [PubMed] [Google Scholar]
- 24. Kiesslich R, Fritsch J, Holtmann M, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003; 124: 880–888. [DOI] [PubMed] [Google Scholar]
- 25. Neumann H, Fujishiro M, Wilcox CM, et al. Present and future perspectives of virtual chromoendoscopy with i-Scan and optical enhancement technology. Dig Endosc 2014; 26(Suppl. 1): 43–51. [DOI] [PubMed] [Google Scholar]
- 26. Neumann H, Neurath MF, Mudter J. New endoscopic approaches in IBD. World J Gastroenterol 2011; 17: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshida N, Yagi N, Inada Y, et al. Ability of a novel blue laser imaging system for the diagnosis of colorectal polyps. Dig Endosc 2014; 26: 250–258. [DOI] [PubMed] [Google Scholar]
- 28. Shinozaki S, Osawa H, Hayashi Y, et al. Linked color imaging for the detection of early gastrointestinal neoplasms. Therap Adv Gastroenterol 2019; 12: 1756284819885246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hiyama S, Iijima H, Shinzaki S, et al. Narrow band imaging with magnifying endoscopy for Peyer’s patches in patients with inflammatory bowel disease. Digestion 2013; 87: 269–280. [DOI] [PubMed] [Google Scholar]
- 30. Kudo T, Matsumoto T, Esaki M, et al. Mucosal vascular pattern in ulcerative colitis: observations using narrow band imaging colonoscopy with special reference to histologic inflammation. Int J Colorectal Dis 2009; 24: 495–501. [DOI] [PubMed] [Google Scholar]
- 31. Danese S, Fiorino G, Angelucci E, et al. Narrow-band imaging endoscopy to assess mucosal angiogenesis in inflammatory bowel disease: a pilot study. World J Gastroenterol 2010; 16: 2396–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sasanuma S, Ohtsuka K, Kudo S, et al. Narrow band imaging efficiency in evaluation of mucosal healing/relapse of ulcerative colitis. Endosc Int Open 2018; 6: E518–E523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neumann H, Vieth M, Günther C, et al. Virtual chromoendoscopy for prediction of severity and disease extent in patients with inflammatory bowel disease: a randomized controlled study. Inflamm Bowel Dis 2013; 19: 1935–1942. [DOI] [PubMed] [Google Scholar]
- 34. Iacucci M, Daperno M, Lazarev M, et al. Development and reliability of the new endoscopic virtual chromoendoscopy score: the PICaSSO (Paddington International Virtual ChromoendoScopy ScOre) in ulcerative colitis. Gastrointest Endosc 2017; 86: 1118–1127.e5. [DOI] [PubMed] [Google Scholar]
- 35. Iacucci M, Kiesslich R, Gui X, et al. Beyond white light: optical enhancement in conjunction with magnification colonoscopy for the assessment of mucosal healing in ulcerative colitis. Endoscopy 2017; 49: 553–559. [DOI] [PubMed] [Google Scholar]
- 36. Klenske E, Atreya R, Hartmann A, et al. Magnification endoscopy with optical chromoendoscopy shows strong correlation with histologic inflammation in patients with inflammatory bowel disease. Endosc Int Open 2019; 7: E1018–E1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uchiyama K, Takagi T, Kashiwagi S, et al. Assessment of endoscopic mucosal healing of ulcerative colitis using linked colour imaging, a novel endoscopic enhancement system. J Crohns Colitis 2017; 11: 963–969. [DOI] [PubMed] [Google Scholar]
- 38. Neumann H, Vieth M, Neurath MF, et al. Endocytoscopy allows accurate in vivo differentiation of mucosal inflammatory cells in IBD: a pilot study. Inflamm Bowel Dis 2013; 19: 356–362. [DOI] [PubMed] [Google Scholar]
- 39. Nakazato Y, Naganuma M, Sugimoto S, et al. Endocytoscopy can be used to assess histological healing in ulcerative colitis. Endoscopy 2017; 49: 560–563. [DOI] [PubMed] [Google Scholar]
- 40. Ueda N, Isomoto H, Ikebuchi Y, et al. Endocytoscopic classification can be predictive for relapse in ulcerative colitis. Medicine (Baltimore) 2018; 97: e0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maeda Y, Ohtsuka K, Kudo SE, et al. Endocytoscopic narrow-band imaging efficiency for evaluation of inflammatory activity in ulcerative colitis. World J Gastroenterol 2015; 21: 2108–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neumann H, Fuchs FS, Vieth M, et al. Review article: in vivo imaging by endocytoscopy. Aliment Pharmacol Ther 2011; 33: 1183–1193. [DOI] [PubMed] [Google Scholar]
- 43. Macé V, Ahluwalia A, Coron E, et al. Confocal laser endomicroscopy: a new gold standard for the assessment of mucosal healing in ulcerative colitis. J Gastroenterol Hepatol 2015; 30: 85–92. [DOI] [PubMed] [Google Scholar]
- 44. Hundorfean G, Chiriac MT, Mihai S, et al. Development and validation of a confocal laser endomicroscopy-based score for in vivo assessment of mucosal healing in ulcerative colitis patients. Inflamm Bowel Dis 2018; 24: 35–44. [DOI] [PubMed] [Google Scholar]
- 45. Iacucci M, Cannatelli R, Gui SX, et al. P254 re-defining the concept of endoscopic and histological healing by using electronic virtual chromoendoscopy and probe confocal endomicroscopy in ulcerative colitis. J Crohns Colitis 2019; 13: S223–S224. [Google Scholar]
- 46. Tontini GE, Mudter J, Vieth M, et al. Prediction of clinical outcomes in Crohn’s disease by using confocal laser endomicroscopy: results from a prospective multicenter study. Gastrointest Endosc 2018; 87: 1505–1514.e3. [DOI] [PubMed] [Google Scholar]
- 47. Maeda Y, Kudo SE, Mori Y, et al. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest Endosc 2019; 89: 408–415. [DOI] [PubMed] [Google Scholar]
- 48. Iacucci M, Kaplan GG, Panaccione R, et al. A randomized trial comparing high definition colonoscopy alone with high definition dye spraying and electronic virtual chromoendoscopy for detection of colonic neoplastic lesions during IBD surveillance colonoscopy. Am J Gastroenterol 2018; 113: 225–234. [DOI] [PubMed] [Google Scholar]
- 49. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001; 48: 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gillen CD, Andrews HA, Prior P, et al. Crohn’s disease and colorectal cancer. Gut 1994; 35: 651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Picco MF, Pasha S, Leighton JA, et al. Procedure time and the determination of polypoid abnormalities with experience: implementation of a chromoendoscopy program for surveillance colonoscopy for ulcerative colitis. Inflamm Bowel Dis 2013; 19: 1913–1920. [DOI] [PubMed] [Google Scholar]
- 52. Alexandersson B, Hamad Y, Andreasson A, et al. High-definition chromoendoscopy superior to high-definition white-light endoscopy in surveillance of inflammatory bowel diseases in a randomized trial. Clin Gastroenterol Hepatol 2020; 18: 2101–2107. [DOI] [PubMed] [Google Scholar]
- 53. Bisschops R, Bessissow T, Joseph JA, et al. Chromoendoscopy versus narrow band imaging in UC: a prospective randomised controlled trial. Gut 2018; 67: 1087–1094. [DOI] [PubMed] [Google Scholar]
- 54. Iannone A, Ruospo M, Wong G, et al. Chromoendoscopy for surveillance in ulcerative colitis and Crohn’s disease: a systematic review of randomized trials. Clin Gastroenterol Hepatol 2017; 15: 1684–1697.e11. [DOI] [PubMed] [Google Scholar]
- 55. Iannone A, Ruospo M, Palmer SC, et al. Systematic review with network meta-analysis: endoscopic techniques for dysplasia surveillance in inflammatory bowel disease. Aliment Pharmacol Ther 2019; 50: 858–871. [DOI] [PubMed] [Google Scholar]
- 56. Hoffman A, Sar F, Goetz M, et al. High definition colonoscopy combined with i-Scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy 2010; 42: 827–833. [DOI] [PubMed] [Google Scholar]
- 57. Hoffman A, Kagel C, Goetz M, et al. Recognition and characterization of small colonic neoplasia with high-definition colonoscopy using i-Scan is as precise as chromoendoscopy. Dig Liver Dis 2010; 42: 45–50. [DOI] [PubMed] [Google Scholar]
- 58. Kandiah K, Subramaniam S, Thayalasekaran S, et al. Multicentre randomised controlled trial on virtual chromoendoscopy in the detection of neoplasia during colitis surveillance high-definition colonoscopy (the VIRTUOSO trial). Gut. Epub ahead of print 19 November 2020. DOI: 10.1136/gutjnl-2020-320980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. El-Dallal M, Chen Y, Lin Q, et al. Meta-analysis of virtual-based chromoendoscopy compared with dye-spraying chromoendoscopy standard and high-definition white light endoscopy in patients with inflammatory bowel disease at increased risk of colon cancer. Inflamm Bowel Dis 2020; 26: 1319–1329. [DOI] [PubMed] [Google Scholar]
- 60. Iacucci M, McQuaid K, Gui XS, et al. A multimodal (FACILE) classification for optical diagnosis of inflammatory bowel disease associated neoplasia. Endoscopy 2019; 51: 133–141. [DOI] [PubMed] [Google Scholar]
- 61. Rondonotti E, Paggi S, Amato A, et al. Blue-light imaging compared with high-definition white light for real-time histology prediction of colorectal polyps less than 1 centimeter: a prospective randomized study. Gastrointest Endosc 2019; 89: 554–564.e1. [DOI] [PubMed] [Google Scholar]
- 62. Diao W, Huang X, Shen L, et al. Diagnostic ability of blue laser imaging combined with magnifying endoscopy for early esophageal cancer. Dig Liver Dis 2018; 50: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 63. Sakamoto T, Inoki K, Takamaru H, et al. Efficacy of linked colour imaging in magnifying chromoendoscopy with crystal violet staining: a pilot study. Int J Colorectal Dis 2019; 34: 1341–1344. [DOI] [PubMed] [Google Scholar]
- 64. Sasajima K, Kudo SE, Inoue H, et al. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest Endosc 2006; 63: 1010–1017. [DOI] [PubMed] [Google Scholar]
- 65. Van Den Broek FJC, Van Es JA, Van Eeden S, et al. Pilot study of probe-based confocal laser endomicroscopy during colonoscopic surveillance of patients with longstanding ulcerative colitis. Endoscopy 2011; 43: 116–122. [DOI] [PubMed] [Google Scholar]
- 66. Hilmi I, Kobayashi T. Capsule endoscopy in inflammatory bowel disease: when and how. Intest Res 2020; 18: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Efthymiou A, Viazis N, Mantzaris G, et al. Does clinical response correlate with mucosal healing in patients with Crohn’s disease of the small bowel? A prospective, case-series study using wireless capsule endoscopy. Inflamm Bowel Dis 2008; 14: 1542–1547. [DOI] [PubMed] [Google Scholar]
- 68. Hall B, Holleran G, Chin JL, et al. A prospective 52week mucosal healing assessment of small bowel Crohn’s disease as detected by capsule endoscopy. J Crohns Colitis 2014; 8: 1601–1609. [DOI] [PubMed] [Google Scholar]
- 69. Ben-Horin S, Lahat A, Amitai MM, et al. Assessment of small bowel mucosal healing by video capsule endoscopy for the prediction of short-term and long-term risk of Crohn’s disease flare: a prospective cohort study. Lancet Gastroenterol Hepatol 2019; 4: 519–528. [DOI] [PubMed] [Google Scholar]
- 70. Eliakim R, Spada C, Lapidus A, et al. Evaluation of a new pan-enteric video capsule endoscopy system in patients with suspected or established inflammatory bowel disease – feasibility study. Endosc Int Open 2018; 6: E1235–E1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Eliakim R, Yablecovitch D, Lahat A, et al. A novel PillCam Crohn’s capsule score (Eliakim score) for quantification of mucosal inflammation in Crohn’s disease. United Eur Gastroenterol J 2020; 8: 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Oliva S, Aloi M, Viola F, et al. A treat to target strategy using panenteric capsule endoscopy in pediatric patients with Crohn’s disease. Clin Gastroenterol Hepatol 2019; 17: 2060–2067.e1. [DOI] [PubMed] [Google Scholar]
- 73. Le Berre C, Sandborn WJ, Aridhi S, et al. Application of artificial intelligence to Gastroenterology and Hepatology. Gastroenterology 2020; 158: 76–94.e2. [DOI] [PubMed] [Google Scholar]
- 74. Parasa S, Wallace M, Bagci U, et al. Proceedings from the first global artificial intelligence in Gastroenterology and Endoscopy Summit. Gastrointest Endosc 2020; 92: 938–945.e1. [DOI] [PubMed] [Google Scholar]
- 75. Luo H, Xu G, Li C, et al. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol 2019; 20: 1645–1654. [DOI] [PubMed] [Google Scholar]
- 76. de Groen PC. Using artificial intelligence to improve adequacy of inspection in gastrointestinal endoscopy. Tech Gastrointest Endosc 2019; 22: 71–79. [Google Scholar]
- 77. Ozawa T, Ishihara S, Fujishiro M, et al. Novel computer-assisted diagnosis system for endoscopic disease activity in patients with ulcerative colitis. Gastrointest Endosc 2019; 89: 416–421.e1. [DOI] [PubMed] [Google Scholar]
- 78. Bossuyt P, Nakase H, Vermeire S, et al. Automatic, computer-aided determination of endoscopic and histological inflammation in patients with mild to moderate ulcerative colitis based on red density. Gut 2020; 69: 1778–1786. [DOI] [PubMed] [Google Scholar]
- 79. Lecun Y, Bengio Y, Hinton G. Deep learning. Nature 2015; 521: 436–444. [DOI] [PubMed] [Google Scholar]
- 80. Su JR, Li Z, Shao XJ, et al. Impact of a real-time automatic quality control system on colorectal polyp and adenoma detection: a prospective randomized controlled study (with videos). Gastrointest Endosc 2020; 91: 415–424.e4. [DOI] [PubMed] [Google Scholar]
- 81. Yao H, Najarian K, Gryak J, et al. Fully automated endoscopic disease activity assessment in ulcerative colitis. Gastrointest Endosc. Epub ahead of print 15 August 2020. DOI: 10.1016/j.gie.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 82. Goetz M, Wang TD. Molecular imaging in gastrointestinal endoscopy. Gastroenterology 2010; 138: 828–833.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Atreya R, Neumann H, Neufert C, et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat Med 2014; 20: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rath T, Bojarski C, Neurath MF, et al. Molecular imaging of mucosal α4β7 integrin expression with the fluorescent anti-adhesion antibody vedolizumab in Crohn’s disease. Gastrointest Endosc 2017; 86: 406–408. [DOI] [PubMed] [Google Scholar]
- 85. Dilipkumar A, Al-Shemmary A, Kreiß L, et al. Label-free multiphoton endomicroscopy for minimally invasive in vivo imaging. Adv Sci 2019; 6: 1801735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kreiß L, Thoma OM, Dilipkumar A, et al. Label-free in vivo histopathology of experimental colitis via 3-channel multiphoton endomicroscopy. Gastroenterology 2020; 159: 832–834. [DOI] [PubMed] [Google Scholar]
- 87. Picot J, Rose M, Cooper K, et al. Virtual chromoendoscopy for the real-time assessment of colorectal polyps in vivo: a systematic review and economic evaluation. Health Technol Assess 2017; 21: 1–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. González-Bernardo O, Riestra S, Vivas S, et al. Chromoendoscopy with indigo carmine vs virtual chromoendoscopy (iSCAN 1) for neoplasia screening in patients with inflammatory bowel disease: a prospective randomized study. Inflamm Bowel Dis. Epub ahead of print 10 November 2020. DOI: 10.1093/ibd/izaa291. [DOI] [PubMed] [Google Scholar]
- 89. Neumann H, Vieth M, Atreya R, et al. Assessment of Crohn’s disease activity by confocal laser endomicroscopy. Inflamm Bowel Dis 2012; 18: 2261–2269. [DOI] [PubMed] [Google Scholar]
- 90. Lobo A, Torrejon Torres R, McAlindon M, et al. Economic analysis of the adoption of capsule endoscopy within the British NHS. Int J Qual Health Care 2020; 32: 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sinagra E, Rossi F, Conoscenti G, et al. Use of artificial intelligence in improving adenoma detection rate during colonoscopy: might both endoscopists and pathologists be further helped. World J Gastroenterol 2020; 26: 5911–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]