Abstract
Purpose
The purpose of this study was to determine the epidemiologic characteristics and survival of patients with primary brain and other central nervous system (CNS) tumors in Korea and to compare our findings with those from the United States.
Materials and Methods
We collected data on primary brain and CNS tumors diagnosed between 2007 and 2016 from the Korea Central Cancer Registry. The age-standardized incidence rates (ASRs) and 5-year relative survival rates (RSRs) were evaluated. We applied the classification and definitions of the Central Brain Tumor Registry of the United States to our analysis for direct comparison with United States data.
Results
A total of 115,050 primary brain and CNS tumors were identified, and the ASR of all tumors was 22.01 per 100,000 individuals, which was lower than the 23.41 in the United States. However, the ASR of malignant tumors was significantly lower herein (4.27) than in the United States (7.08). Meningeal tumors were the most common histologic group among all tumors (ASR, 8.32). The 5-year RSR of all primary brain and other CNS tumors was 86.4%, and that of all malignant tumors was 44.1%, which was higher than the 35.8% observed in the United States. Among malignant tumors, glioblastomas had the lowest 5-year RSR (12.1%).
Conclusion
In Korea, malignant brain and other CNS tumors have a lower incidence and better survival outcome.
Key words: Epidemiology, Brain neoplasms, Incidence, Survival rate
Introduction
Primary central nervous system tumors (PCNSTs) are rare, but are associated with high morbidity and mortality. In addition, PCNSTs are a heterogeneous group of benign and malignant tumors in terms of histomorphology, genetics, and behavior. According to the International Classification of Diseases for Oncology, third edition (ICD-O-3), PCNSTs involve more than 100 subtypes of tumors, including not only those located in the brain and spine, but also those found in the meninges, pituitary gland, pineal gland, and nerves [1]. The prognosis of malignant PCNSTs is poor because of their invasive histologic characteristics; however, that of some benign PCNSTs is also poor owing to their inoperable location.
Only a few population-based registries have collected and disseminated the epidemiologic data of PCNSTs worldwide, most of which are regional registries and do not collect non-malignant PNCSTs data. The Central Brain Tumor Registry of the United States (CBTRUS) and Surveillance, Epidemiology, and End Results (SEER) program in the United States are the largest registries and have reported their data regularly since 2010. The reports from the CBTRUS include incidence and survival data of non-malignant as well as malignant PCNSTs [2,3].
The Korea Central Cancer Registry (KCCR) was established in 1980 as a nationwide and hospital-based cancer registry by the Korean Ministry of Health and Welfare. It was expanded into a nationwide and population-based cancer registry in 1999. To date, the KCCR is the largest national population-based cancer registry in Asia. Since the first epidemiologic report of PCNSTs from the KCCR was published in 2010, which described PCNSTs diagnosed in 2005, three articles have been published, each with data for a single year [4–6].
In this study, we aimed to report the nationwide incidence and survival of PCNSTs diagnosed from 2007 to 2016 in Korea and compare the results with those in the United States. It is the first nationwide, population-based, multi-year epidemiologic study covering both malignant and non-malignant PCNSTs in Asia.
Materials and Methods
1. Data sources
We used national cancer registry data from the KCCR. The KCCR was established in 1980 by the Korean Ministry of Health and Welfare and has become a population-based national cancer registry that includes information about over 98% of patients newly diagnosed with cancer in Korea [7]. The KCCR has collected data not only on malignant PCNSTs, but also on non-malignant tumors since 2005.
This study included cases of malignant and non-malignant PCNSTs diagnosed from 2007 to 2016 in Korea. Cases within the first 2 years after the KCCR started registering non-malignant tumors were excluded owing to reporting bias because relatively small numbers of non-malignant tumors were collected between 2005 and 2006. In a period of 10 years, a total of 115,050 patients were diagnosed with PCNSTs according to the ICD-O-3: brain (ICD-O-3: C71.0–C71.9); meninges (C70.0–C70.9); spinal cord, cranial nerves, and other parts of the CNS (C72.0–C72.9); pituitary gland and pineal gland (C75.1–C75.3); and olfactory region of the nasal cavity (C30.0 [9522–9523]), along with the World Health Organization (WHO) classification for brain tumors. Histologic grouping was performed using the classification of the CBTRUS [2].
We utilized the data from patients with PCNSTs diagnosed from 2007 to 2016 for survival analysis. We excluded cases that could not be followed up owing to mismatched personal identification numbers, cases that were not first primary sites, and patients identified by death certificate only. As a result, 112,133 cases of PCNSTs diagnosed between 2007 and 2016 were included in the survival analysis. Passive follow-up was performed using the mortality database from Statistics Korea until December 31, 2017 [8].
2. Statistical analysis
Age-specific incidence rates, age-standardized incidence rates (ASRs), and male-to-female sex incidence rate ratios (M:F rate ratios) were calculated with their corresponding 95% confidence intervals (CIs). The age-specific incidence rates were calculated according to age group (children [0–14 years], adolescents and young adults [15–39 years], adults [40–64 years], seniors [≥ 65 years], and adults and seniors [≥ 40 years]) by dividing the total number of cases in a given period by the entire population and were expressed per 100,000 population. The ASRs were age-adjusted to the United States standard population in 2000 to directly compare with those in the United States from the most recent CBTRUS report, i.e., 2012–2016 [2].
The annual percent change (APC) for the incidence rates was calculated using a linear model according to the following formula: (exp(b)−1)×100, where b is the slope of the regression of the natural logarithm of the ASR in a calendar year [3]. The M:F rate ratio was calculated by dividing the age-adjusted incidence rates of the male patients by those of the female patients. All analyses were conducted by location, histologic diagnoses, and age groups.
Relative survival rates (RSRs) were calculated as the ratios of the observed survival of the patients with cancer to the expected survival of the general population, which was calculated using the standard life table provided by Statistics Korea [8]. The RSRs were estimated using the Ederer II method.
All statistical analyses were conducted using SAS ver. 9.4 (SAS Institute Inc., Cary, NC).
Results
1. Overall incidence
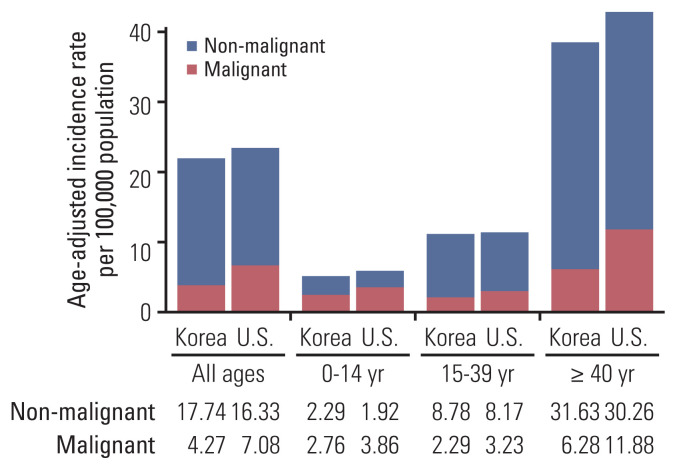
Between 2007 and 2016, 115,050 PCNSTs were diagnosed in a population of 50.2 million in 2012 [8]. Among them, 21,411 cases were malignant (18.6%). The median patient age at diagnosis was 55.0 years. The overall ASR was 22.01 per 100,000 individuals (95% CI, 21.88 to 22.14). The overall M:F rate ratio was 0.66:1 (1.27:1 for malignant tumors and 0.56:1 for non-malignant tumors). The ASR, median patient age, and M:F rate ratio by histology are summarized in Table 1. Approximately 49.2% of the PCNSTs were diagnosed by histologic confirmation (72.8% of malignant tumors and 43.8% of non-malignant tumors). A comparison of the overall ASRs between the two countries is shown in Fig. 1. The overall ASR in Korea was similar to that in the United States (ASR, 23.41 [95% CI, 23.34 to 23.49]); however, the ASR of all malignant tumors in Korea was lower than that in the United States (4.27% vs. 7.08%). Conversely, the histologic confirmation rate for malignant tumors in Korea was lower than that in the United States (72.8% vs. 84.4%); however, that for non-malignant tumors in Korea was similar to that in the United States (43.8% vs. 44.1%).
Table 1.
Epidemiologic data of primary CNS tumors
| Histological group | 10-Year total (%) | Median age (yr) | ASR (95% CI)a) | Sex ratio: male/female |
|---|---|---|---|---|
| Tumors of neuroepithelial tissue | 16,012 (13.9) | 49.0 | 3.17 (3.12–3.22) | 1.23* |
| Pilocytic astrocytoma | 702 (0.6) | 16.0 | 0.16 (0.15–0.17) | 0.90 |
| Diffuse astrocytoma | 1,193 (1.0) | 44.0 | 0.23 (0.22–0.24) | 1.35* |
| Anaplastic astrocytoma | 979 (0.9) | 50.0 | 0.19 (0.18–0.20) | 1.50* |
| Unique astrocytoma variants | 390 (0.3) | 43.5 | 0.08 (0.07–0.09) | 1.23* |
| Glioblastoma | 5,796 (5.0) | 60.0 | 1.11 (1.08–1.14) | 1.36* |
| Oligodendroglioma | 756 (0.7) | 45.0 | 0.14 (0.13–0.15) | 1.07 |
| Anaplastic oligodendroglioma | 580 (0.5) | 50.0 | 0.11 (0.10–0.11) | 1.21* |
| Oligoastrocytic tumors | 349 (0.3) | 43.0 | 0.06 (0.06–0.07) | 1.08 |
| Ependymal tumors | 1,259 (1.1) | 43.0 | 0.24 (0.23–0.26) | 1.07 |
| Glioma malignant, NOS | 1,599 (1.4) | 47.0 | 0.33 (0.31–0.34) | 1.15* |
| Choroid plexus tumors | 237 (0.2) | 34.0 | 0.05 (0.04–0.06) | 1.01 |
| Other neuroepithelial tumors | 33 (0.0) | 46.0 | 0.01 (0.00–0.01) | 0.75 |
| Neuronal and mixed neuronal-glial tumors | 1,147 (1.0) | 31.0 | 0.23 (0.22–0.25) | 1.11 |
| Tumors of the pineal region | 211 (0.2) | 42.0 | 0.04 (0.04–0.05) | 1.00 |
| Embryonal tumors | 781 (0.7) | 9.0 | 0.19 (0.18–0.21) | 1.24* |
| Tumors of cranial and spinal nerves | 13,109 (11.4) | 53.0 | 2.43 (2.39–2.47) | 0.89* |
| Nerve sheath tumors | 13,101 (11.4) | 53.0 | 2.43 (2.39–2.47) | 0.89* |
| Other tumors of cranial and spinal nerves | 8 (0.0) | 60.5 | 0.00 (0.00–0.00) | 1.11 |
| Tumors of meninges | 43,572 (37.9) | 60.0 | 8.32 (8.24–8.40) | 0.36* |
| Meningioma | 41,438 (36.0) | 61.0 | 7.92 (7.84–8.00) | 0.34* |
| Mesenchymal tumors | 725 (0.6) | 46.0 | 0.14 (0.13–0.15) | 0.97 |
| Primary melanocytic lesions | 58 (0.1) | 52.0 | 0.01 (0.01–0.01) | 1.41 |
| Other neoplasms related to the meninges | 1,351 (1.2) | 48.0 | 0.25 (0.24–0.26) | 1.27* |
| Lymphomas and hematopoietic neoplasms | 2,033 (1.8) | 62.0 | 0.40 (0.38–0.41) | 1.41* |
| Lymphoma | 1,942 (1.7) | 63.0 | 0.38 (0.36–0.39) | 1.40* |
| Other hematopoietic neoplasms | 91 (0.1) | 17.0 | 0.02 (0.02–0.03) | 1.54* |
| Germ cell tumors and cysts | 1,083 (0.9) | 17.0 | 0.24 (0.22–0.25) | 2.52* |
| Germ cell tumors, cysts, and heterotopias | 1,083 (0.9) | 17.0 | 0.24 (0.22–0.25) | 2.52* |
| Tumors of sellar region | 24,250 (21.1) | 48.0 | 4.53 (4.47–4.59) | 0.71* |
| Tumors of the pituitary | 22,751 (19.8) | 48.0 | 4.24 (4.18–4.29) | 0.70* |
| Craniopharyngioma | 1,499 (1.3) | 44.0 | 0.29 (0.28–0.31) | 0.95 |
| Unclassified tumors | 14,991 (13.0) | 55.0 | 2.93 (2.88–2.98) | 0.86* |
| Hemangioma | 3,418 (3.0) | 47.0 | 0.64 (0.62–0.66) | 0.94 |
| Neoplasm, unspecified | 11,458 (10.0) | 57.0 | 2.26 (2.22–2.30) | 0.83* |
| All other | 115 (0.1) | 66.0 | 0.02 (0.02–0.03) | 1.13 |
| Total | 115,050 (100) | 55.0 | 22.01 (21.88–22.14) | 0.66* |
ASR, age-standardized incidence rate; CI, confidence interval; CNS, central nervous system; NOS, not otherwise specified.
A statistically significant difference between sexes.
ASRs were age-adjusted to the United States standard population in 2000.
Fig. 1.
Comparison of incidence of primary brain and central nervous system tumors by age groups between Korea and the United States. Our data was from the KCCR, 2007–2016; U.S. data was from CBTRUS, 2012–2016. We standardized to U.S. 2000 population to directly compare our incidences to those of the United States. CBTRUS, Central Brain Tumor Registry of the United States; KCCR, Korean Central Cancer Registry.
2. Distribution of tumors by histology
Among all PCNSTs (n=115,050; ASR, 22.01), meningeal tumors were the most prevalent histologic group (37.9% of all PCNSTs; ASR, 8.32), followed by sellar region tumors (21.1%; ASR, 4.53) and neuroepithelial tumors (13.9%; ASR, 3.17). Meningiomas comprised the most frequently reported histology (36.0%; ASR, 7.92), followed by pituitary tumors (19.8%; ASR, 4.24), nerve sheath tumors (11.4%; ASR, 2.43), and glioblastomas (5.0%; ASR, 1.11). Among malignant tumors (n=21,411; ASR, 4.27), neuroepithelial tumors were the most common histologic group (66.6% of all malignant PCNSTs; ASR, 2.82), followed by lymphomas and hematopoietic neoplasms (9.5%; ASR, 0.40) and germ cell tumors and cysts (3.9%; ASR, 0.19). Because only 2% of meningeal tumors and 0.2% of sellar region tumors were malignant, they accounted for a small proportion of malignant PCNSTs, although they occupied nearly half of all PCNSTs. In the CBTRUS, the most prevalent histologic group was meningeal tumors (38.8% of all PCNSTs; ASR, 8.83), similar to ours, but followed by neuroepithelial tumors (27.7%; ASR, 6.56) and sellar region tumors (17.5%; ASR, 4.27). Unlike in Korea, glioblastomas were the third most common PCNST histology in the United States (14.6% of all PCNSTs; ASR, 3.22), following meningiomas (37.6%; ASR, 8.56) and pituitary tumors (16.8%; ASR, 4.08).
Ninety-two percent of the neuroepithelial tumors were gliomas. Among gliomas, glioblastomas were the most pre-valent tumors (ASR, 1.11), accounting for 39% of gliomas, followed by unspecified malignant gliomas (ASR, 0.33), ependymal tumors (ASR, 0.24), diffuse astrocytoma (ASR, 0.23), and anaplastic astrocytoma (ASR, 0.19). Ninety-one percent of the gliomas were malignant. The incidence rate of neuroepithelial tumors was two times higher in the United States than in Korea (ASR, 6.56 vs. 3.17). In particular, the incidence rate of glioblastomas was three times higher in the United States than in Korea (ASR, 3.22 vs. 1.11).
We directly compared the four clinically important non-malignant histologies, i.e., vestibular schwannoma, pituitary adenoma, and WHO grade I and II meningiomas. A comparison is shown in S1 Table. The median patient age was the lowest with pituitary adenoma (49.0 years) and the highest with WHO grade I meningioma (61.0 years) histologies. WHO grade I meningioma most predominantly occurred in the female patients (M:F rate ratio, < 0.5). In addition, the age-specific incidence rates increased with age for all four histologies; especially, those of WHO grade I meningioma increased most significantly with age. The increasing pattern of the incidence rates with age and the incidence rates of selected non-malignant tumors were similar between Korea and the United States.
3. Distribution of tumors by location
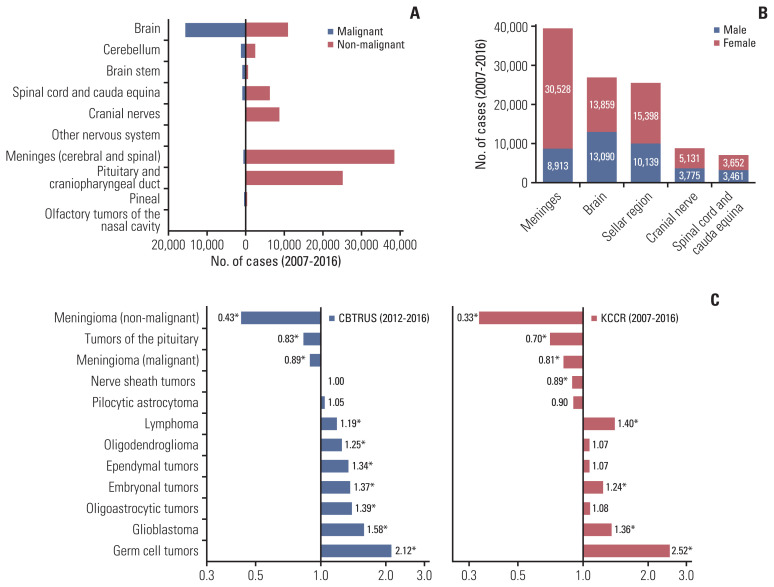
The meninges (34.3%) were the most common location of PCNSTs, followed by the cerebrum (including the frontal, temporal, parietal, and occipital lobes; ventricle; and other parts of the brain; 23.4%), sellar region (22.2%), cranial nerves (7.7%), and spinal cord and cauda equina (6.2%). Meningeal tumors and sellar region tumors were more likely to be non-malignant (malignant: non-malignant ratio, < 0.02:1), whereas cerebral, brain stem, and pineal gland tumors were more likely to be malignant (malignant: non-malignant ratio, > 1:1) (Fig. 2A). Meningeal tumors (M:F rate ratio, 0.36:1) and sellar region tumors (M:F rate ratio, 0.71:1) developed more frequently in the female patients (Fig. 2B). Similarly, in the CBTRUS, the meninges were the most common location of PCNSTs (37.7%), followed by the cerebrum (29.2%) and sellar region (18%).
Fig. 2.
Distribution of primary brain and other CNS tumors by location, sex and malignancy. (A) The distribution of all types of primary brain and other CNS tumors by location and behavior. (B) The distribution of all types of primary brain and other CNS tumors by location and sex. (C) Comparison of M:F rate ratio by histology between the United States and Korea. CBTRUS, Central Brain Tumor Registry of the United States; CNS, central nervous system; KCCR, Korean Central Cancer Registry; M:F rate ratio, male-to-female incidence rate ratio. *A statistically significant difference between sexes.
4. Distribution and incidence of tumors according to age and sex
The age-specific incidence rates according to age and histology are shown in S2 Table, and the comparison of the age-specific incidence rates between Korea and the United States is presented in Fig. 1. The incidence rate of PCNSTs increased with age and was the highest among the seniors aged ≥ 65 years (ASR, 59.91). Tumors in the children aged 0–14 years were most likely to be malignant (malignant:non-malignant ratio, > 1:1). Among the children and adolescents and young adults, non-malignant PCNSTs were slightly more prevalent in Korea than in the United States. In contrast, malignant tumors were slightly more common in the United States than in Korea. Conversely, the incidence rate of non-malignant PCNSTs among the adults and seniors aged ≥ 40 years was similar between the two countries; however, the incidence rate of malignant tumors in the United States was nearly twice as high as that in Korea (11.88 vs. 6.28).
The five most prevalent histologies for each age group of the two countries are compared in Table 2. Among the children aged 0–14 years, the first (pilocytic astrocytoma; ASR, 1.02) and second most frequent tumors (malignant glioma, not otherwise specified; ASR, 0.80) were gliomas in the United States and embryonal tumors (ASR, 0.69) and germ cell tumors (ASR, 0.50) were the most common, followed by gliomas, in Korea. Notably, the incidence rate of germ cell tumors among the children aged 0–14 years was significantly higher in Korea than in the United States (ASR, 0.50 [95% CI, 0.45 to 0.55] vs. 0.22 [95% CI, 0.20 to 0.24]). Among the adolescents and young adults aged 15–39 years, pituitary tumors were the most common, followed by meningiomas and nerve sheath tumors, in both countries. Among the adults and seniors aged ≥ 40 years, the incidence rate of glioblastoma was remarkably lower in Korea than in the United States (ASR, 2.12 [95% CI, 2.06 to 2.18] vs. 6.95 [95% CI, 6.89 to 7.01]).
Table 2.
The most common primary CNS tumor histologies by age group (Korea vs. United States)a)
| Rank | Children (0–14 yr) | Adolescents and young adults (15–39 yr) | Adults (≥ 40 yr) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Histology | Rate (95% CI)b) | Histology | Rate (95% CI) | Histology | Rate (95% CI) | |
| Korea, KCCR 2007–2016 | ||||||
|
| ||||||
| 1 | Embryonal tumors | 0.69 (0.63–0.75) | Tumors of the pituitary | 3.87 (3.78–3.96) | Meningioma | 16.26 (16.10–16.42) |
|
| ||||||
| 2 | Germ cell tumors, cysts and heterotopias | 0.50 (0.45–0.55) | Meningioma | 1.33 (1.28–1.38) | Tumors of the pituitary | 6.43 (6.33–6.53) |
|
| ||||||
| 3 | Pilocytic astrocytoma | 0.40 (0.35–0.44) | Nerve sheath tumors | 1.31 (1.25–1.36) | Nerve sheath tumors | 4.39 (4.31–4.47) |
|
| ||||||
| 4 | Glioma malignant, NOS | 0.32 (0.28–0.36) | Hemangioma | 0.52 (0.49–0.55) | Glioblastoma | 2.12 (2.06–2.18) |
|
| ||||||
| 5 | Tumors of the pituitary | 0.31 (0.27–0.35) | Glioblastoma | 0.34 (0.32–0.37) | Hemangioma | 0.94 (0.90–0.98) |
|
| ||||||
| U.S., CBTRUS 2012–2016 | ||||||
|
| ||||||
| 1 | Pilocytic astrocytoma | 1.02 (0.98–1.06) | Tumors of the pituitary | 3.82 (3.76–3.87) | Meningioma | 18.30 (18.21–18.40) |
|
| ||||||
| 2 | Glioma malignant, NOS | 0.80 (0.77–0.84) | Meningioma | 1.86 (1.82–1.90) | Glioblastoma | 6.95 (6.89–7.01) |
|
| ||||||
| 3 | Embryonal tumors | 0.75 (0.72–0.78) | Nerve sheath tumors | 1.03 (1.00–1.06) | Tumors of the pituitary | 6.18 (6.13–6.24) |
|
| ||||||
| 4 | Neuronal and mixed neuronal-glial tumors | 0.42 (0.40–0.44) | Glioblastoma | 0.53 (0.51–0.55) | Nerve sheath tumors | 3.68 (3.63–3.72) |
|
| ||||||
| 5 | Tumors of the pituitary | 0.32 (0.30–0.34) | Pilocytic astrocytoma | 0.44 (0.42–0.45) | Lymphoma | 0.91 (0.89–0.93) |
CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; CNS, central nervous system; KCCR, Korean Central Cancer Registry; NOS, not otherwise specified.
Histologic group of unspecified neoplasm was excluded from this table. The incidence rate of unspecified neoplasm was 0.74, 1.03, and 3.76 in the 0–14, 15–39, and ≥ 40 years old groups, respectively,
Rate indicated the age-specific incidence rate.
The M:F rate ratio was the highest for malignant hemangioma in Korea (M:F rate ratio, 3.38); however, this finding was not significant. Among the tumors that showed a significantly noticeable M:F rate ratio, malignant germ cell tumors occurred most predominantly in the male patients (M:F rate ratio, 3.02), whereas non-malignant meningioma occurred most predominantly in the female patients (M:F rate ratio, 0.33). A comparison of the M:F rate ratio for selected histologies and histologic groups between both countries is shown in Fig. 2C. The pattern of the selected histologic groups was quite similar between the two countries; however, we found minimal variations.
5. Time trends of tumors
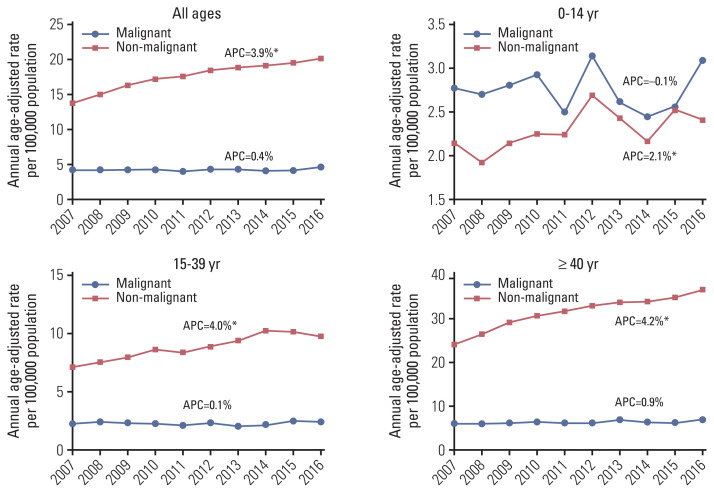
The time trends of the incidence rate of PCNSTs are shown in Fig. 3. For malignant tumors, there was no significant change in the age-specific incidence rates in each age group. However, there was a significant increasing trend in the overall incidence rate of non-malignant PCNSTs (APC, 3.9%). The 10-year increasing trend for non-malignant tumors in Korea was similar to that in the United States between 2000 and 2016 (APC, 2.2%). Conversely, the incidence rate of malignant tumors in the United States between 2000 and 2016 (APC, −0.4%) significantly decreased, which differed to that in Korea.
Fig. 3.
Incidence trend of primary brain and other central nervous system tumors by behavior and age group. *A statistically significant annual percent changes (APC).
6. Survival outcomes by histology
For the survival analysis, 112,133 of the 115,050 PCNSTs (97%) were included. Table 3 lists the survival of selected malignant PCNSTs, and S3 Table shows the survival outcomes of all tumors. The 5-year RSR of all PCNSTs was 86.4%, whereas that of all malignant tumors was 44.1%. Overall, female patients showed the more favorable survival outcome than male patients for malignant PCNSTs (5-year RSR, 45.8 vs. 42.6). The 5-year RSRs ranged from 12.1% to 99.3% according to histology, and glioblastomas had the lowest RSR (12.1%), followed by malignant primary melanocytic lesions (23.4%), anaplastic astrocytoma (26.2%), and lymphoma (34.5%). The histologies with the most unfavorable outcomes in Korea were similar to those in the United States: glioblastoma (5-year RSR, 6.8%), malignant primary melanocytic lesions (5-year RSR, 28.9%), anaplastic astrocytoma (5-year RSR, 30.2%), and lymphoma (5-year RSR, 35.3%). The 5-year overall RSR of malignant PCNSTs was higher in Korea than in the United States (44.1% vs. 35.8%).
Table 3.
One-, two-, five-, and ten-year relative survival rates for selected malignant primary CNS tumors by histologya)
| Histologic group | Sex | No. of cases | Relative survival rate | |||
|---|---|---|---|---|---|---|
| 1-Year rate (95% CI) | 2-Year rate (95% CI) | 5-Year rate (95% CI) | 10-Year rate (95% CI) | |||
| Germ cell tumors, cysts and heterotopias (malignant) | Total | 737 | 95.4 (93.7–96.7) | 92.8 (90.6–94.5) | 89.4 (86.8–91.5) | 86.8 (83.6–89.5) |
| Male | 573 | 95.7 (93.7–97.1) | 92.4 (89.9–94.4) | 89.2 (86.2–91.7) | 87.4 (83.7–90.3) | |
| Female | 164 | 94.6 (89.8–97.2) | 94.0 (89.0–96.7) | 89.8 (83.8–93.7) | 85.1 (77.1–90.5) | |
| Ependymal tumors (malignant) | Total | 884 | 94.9 (93.1–96.2) | 91.4 (89.2–93.2) | 83.3 (80.3–85.9) | 81.2 (77.3–84.6) |
| Male | 457 | 94.6 (92.0–96.5) | 90.5 (87.2–93.0) | 79.4 (74.7–83.5) | 77.3 (71.6–82.2) | |
| Female | 427 | 95.1 (92.5–96.8) | 92.4 (89.3–94.6) | 87.3 (83.3–90.5) | 85.3 (79.9–89.6) | |
| Nerve sheath tumors (malignant) | Total | 89 | 91.6 (83.4–96.1) | 88.7 (79.7–94.2) | 81.4 (70.1–89.3) | 82.1 (69.9–90.7) |
| Male | 45 | 93.9 (81.2–98.4) | 87.6 (73.3–94.9) | 78.0 (60.6–89.1) | 77.8 (58.8–90.6) | |
| Female | 44 | 89.3 (75.4–95.9) | 90.1 (76.0–96.6) | 85.2 (68.5–94.4) | 87.1 (70.0–96.4) | |
| Oligodendroglioma | Total | 749 | 94.0 (92.0–95.5) | 88.2 (85.6–90.4) | 78.6 (75.0–81.8) | 67.7 (62.3–72.6) |
| Male | 388 | 95.3 (92.5–97.1) | 88.5 (84.7–91.5) | 78.5 (73.3–82.9) | 70.7 (63.6–76.8) | |
| Female | 361 | 92.6 (89.3–95.0) | 87.9 (83.9–91.0) | 78.7 (73.4–83.1) | 64.5 (56.0–72.0) | |
| Neuronal and mixed neoronal-glial tumors (malignant) | Total | 203 | 86.8 (81.2–90.9) | 78.6 (72.0–83.8) | 70.5 (62.7–77.1) | 56.7 (44.7–67.4) |
| Male | 115 | 88.6 (81.0–93.4) | 79.7 (70.7–86.4) | 75.1 (64.7–83.1) | 59.4 (43.1–73.2) | |
| Female | 88 | 84.4 (74.9–90.6) | 77.1 (66.5–84.8) | 64.5 (52.0–74.7) | 53.2 (35.4–68.6) | |
| Meningioma (malignant) | Total | 461 | 89.2 (85.8–92.0) | 79.4 (75.1–83.2) | 68.9 (63.4–73.9) | 52.7 (43.8–61.2) |
| Male | 190 | 88.8 (83.0–93.0) | 77.4 (70.1–83.4) | 64.4 (55.3–72.6) | 43.5 (28.9–58.2) | |
| Female | 271 | 89.5 (85.0–92.9) | 80.8 (75.1–85.4) | 71.7 (64.8–77.9) | 59.4 (49.5–68.5) | |
| Oligoastrocytic tumors | Total | 345 | 93.4 (90.1–95.6) | 82.8 (78.3–86.5) | 66.3 (60.4–71.5) | 45.2 (35.1–54.9) |
| Male | 180 | 93.3 (88.4–96.3) | 82.4 (75.8–87.4) | 66.2 (58.0–73.3) | 46.5 (33.0–59.1) | |
| Female | 165 | 93.5 (88.4–96.4) | 83.2 (76.4–88.1) | 66.3 (57.7–73.7) | 44.0 (29.4–57.8) | |
| Embryonal tumors (malignant) | Total | 720 | 80.4 (77.3–83.1) | 68.1 (64.5–71.4) | 56.4 (52.5–60.1) | 50.3 (46.1–54.5) |
| Male | 415 | 81.3 (77.2–84.8) | 67.3 (62.5–71.7) | 56.9 (51.7–61.7) | 50.9 (45.4–56.2) | |
| Female | 305 | 79.1 (74.1–83.3) | 69.1 (63.5–74.0) | 55.7 (49.6–61.5) | 49.5 (42.6–56.1) | |
| Diffuse astrocytoma | Total | 1,174 | 84.8 (82.6–86.8) | 72.2 (69.5–74.7) | 55.7 (52.5–58.8) | 42.3 (38.1–46.3) |
| Male | 668 | 85.6 (82.6–88.1) | 73.3 (69.7–76.6) | 57.1 (52.8–61.2) | 38.9 (32.9–44.9) | |
| Female | 506 | 83.8 (80.3–86.8) | 70.7 (66.4–74.6) | 53.8 (48.8–58.5) | 46.0 (40.5–51.3) | |
| Anaplastic oligodendroglioma | Total | 577 | 87.0 (83.9–89.6) | 71.5 (67.5–75.2) | 52.0 (47.3–56.5) | 35.1 (28.4–41.8) |
| Male | 309 | 85.5 (81.0–89.1) | 68.3 (62.5–73.4) | 46.0 (39.5–52.3) | 31.1 (23.0–39.6) | |
| Female | 268 | 88.7 (84.2–92.0) | 75.2 (69.4–80.1) | 58.6 (51.8–64.9) | 39.4 (28.8–49.9) | |
| Glioma malignant, NOS | Total | 1,568 | 71.3 (68.9–73.5) | 59.8 (57.2–62.3) | 51.0 (48.2–53.7) | 46.2 (42.7–49.5) |
| Male | 820 | 70.0 (66.6–73.0) | 58.9 (55.3–62.3) | 50.4 (46.6–54.2) | 43.5 (38.6–48.4) | |
| Female | 748 | 72.7 (69.3–75.8) | 60.8 (57.1–64.3) | 51.6 (47.6–55.5) | 49.1 (44.4–53.6) | |
| Lymphoma | Total | 1,895 | 65.5 (63.3–67.6) | 51.6 (49.2–53.9) | 34.5 (32.0–37.0) | 22.6 (19.3–26.1) |
| Male | 1,035 | 64.7 (61.6–67.6) | 51.9 (48.6–55.0) | 34.0 (30.6–37.3) | 25.4 (21.5–29.5) | |
| Female | 860 | 66.5 (63.2–69.6) | 51.2 (47.7–54.6) | 35.1 (31.5–38.9) | 19.6 (14.6–25.1) | |
| Anaplastic astrocytoma | Total | 964 | 71.4 (68.4–74.2) | 46.8 (43.5–50.0) | 26.2 (23.1–29.4) | 17.5 (14.2–21.1) |
| Male | 567 | 70.0 (65.9–73.6) | 46.2 (41.9–50.4) | 26.4 (22.3–30.6) | 17.5 (13.0–22.7) | |
| Female | 397 | 73.4 (68.7–77.5) | 47.6 (42.5–52.6) | 26.0 (21.3–30.9) | 17.5 (12.8–22.9) | |
| Primary melanocytic lesions (malignant) | Total | 37 | 60.0 (42.4–73.8) | 37.4 (21.9–52.9) | 23.4 (10.2–39.9) | 24.6 (10.7–42.0) |
| Male | 21 | 67.1 (42.8–83.1) | 47.7 (25.3–67.3) | 22.4 (6.2–45.3) | 24.1 (6.7–48.6) | |
| Female | 16 | 50.5 (24.8–71.8) | 23.6 (6.7–46.5) | 24.0 (6.8–47.2) | 24.3 (6.9–47.7) | |
| Glioblastoma | Total | 5,754 | 59.3 (58.0–60.6) | 30.4 (29.2–31.7) | 12.1 (11.1–13.1) | 6.6 (5.6–7.7) |
| Male | 3,148 | 57.7 (55.9–59.4) | 27.6 (26.0–29.3) | 10.3 (9.1–11.6) | 5.0 (3.7–6.5) | |
| Female | 2,606 | 61.2 (59.3–63.1) | 33.8 (32.0–35.7) | 14.2 (12.7–15.8) | 8.4 (6.8–10.2) | |
| Total, malignant tumorsb) | Total | 20,427 | 72.3 (71.7–72.9) | 56.9 (56.2–57.6) | 44.1 (43.3–44.8) | 37.1 (36.2–38.1) |
| Male | 11,041 | 71.4 (70.6–72.3) | 55.4 (54.4–56.3) | 42.6 (41.5–43.6) | 35.7 (34.5–37.0) | |
| Female | 9,386 | 73.3 (72.4–74.2) | 58.8 (57.8–59.8) | 45.8 (44.7–46.9) | 38.8 (37.4–40.2) | |
CI, confidence interval; CNS, central nervous system; NOS, not otherwise specified.
Sorted in descending order of 5-year relative survival rate,
Relative survival rate of overall malignant tumors, including histologies even not listed in this table.
Discussion
To our knowledge, the KCCR is the largest PCNST registry in Asia, and our study is the first nationwide, population-based, multi-annual epidemiologic study of PCNSTs in Asia, including both malignant and non-malignant tumors. Our analyses showed that there were some differences in the incidence and survival outcomes of PCNSTs between Korea and the United States. In particular, the incidence rate of malignant tumors was lower in Korea than in the United States, whereas that of non-malignant tumors was slightly higher in Korea for each age group. The ASR of glioblastoma was three times lower in Korea than in the United States. However, the incidence rate of germ cell tumors among children in Korea was more than twice as high as that in the United States. The patterns of the RSRs for malignant tumors of the two countries were quite similar; however, the overall RSRs for malignant PCNSTs in Korea were slightly higher than those in the United States.
The overall ASRs of PCNSTs in Korea and the United States were similar (ASR, 22.01 vs. 23.41); however, large geographical variations in the ASRs of PCNSTs have been reported, i.e., from 5.9 to 23.4 [9–19]. In a comprehensive report of cancer registries from 185 countries, mostly subnational registries, the incidences of malignant PCNSTs were 3.9 and 3.1 in men and women, respectively [20]. Despite the heterogeneity in the registration quality among the registries and differences in the reported period, we found that the incidence rates of malignant tumors in European countries and the United States were higher than those in Asian countries (ASR: the United States, 7.1; Austria, 8.8; Korea, 4.3, Taiwan, 3.82; Iran, 2.7) [12,14,21].According to Sant et al. [11], the ASRs of malignant tumors in 72 European registries ranged from 5.7 to 11.0 and averaged 7.9; these were age-adjusted to the European standard population. The relatively lower incidence rate of malignant PCNSTs was observed not only in east Asian countries, but also in Iran and western Asian countries. Although the apparent cause is unclear, several reports suggest that disparities in ethnicity, lifestyle, environment, hereditary factors, and population composition may be responsible for this finding. In this study, the ASR of malignant tumors among Koreans (ASR, 4.27) was close to that among Asian/Pacific Islanders (ASR, 4.25) and Blacks (ASR, 4.48) from the CBTRUS, but was different from that of Whites (ASR, 7.58) and Hispanics (ASR, 5.66). Ward et al. [22] showed that in terms of access to healthcare in the United States, Asian/Pacific Islanders were statistically closer to Whites than Blacks or Hispanics. Although Whites had similar socioeconomic states to Asian/Pacific Islanders, Whites showed higher incidence and mortality rates for most cancers, except for stomach and liver cancers, than did Asian/Pacific Islanders. This may be attributed to the relatively high smoking and obesity rates in Whites; however, the proportion of individuals who had these lifestyle risk factors was high in Blacks and Hispanics. Therefore, something that goes beyond socioeconomic abilities or lifestyle could be the cause of the lower incidence of malignant PCNSTs in Korea than in the United States, particularly among Whites; the difference in ethnicity-based genetic vulnerability is a potential explanation.
The proportion of histologically confirmed cases among all tumors was not followed up annually in this study. However, by analyzing previous reports from the KCCR, we revealed that the histologic confirmation rate of all PCNSTs decreased from 2005 to 2013 (2005, 58%; 2010, 52%; 2013, 48%) [4–6]. The histologic confirmation rate of glioma slightly decreased (2005, 86%; 2010, 84%; 2013, 82%), whereas that of meningioma significantly decreased (2005, 59%; 2010, 46%; 2013, 39%). Thus, considering that 81% of all PCNSTs were non-malignant, it is assumed that the decrease in the histologic confirmation rate of all tumors mainly involved non-malignant tumors. The indication of surgical resection for non-malignant tumors has changed over time. With the development of imaging techniques and radiosurgery as a treatment modality for non-malignant brain tumors, many small and stable tumors that do not cause neurological symptoms have been treated without surgical procedures. In Korea, patients with brain tumors have a relatively good access to radiosurgery because the national health insurance covers them. According to Korean statistics between 2007 and 2016, the number of Gamma Knife radiosurgery performed nationwide increased gradually from 3012 to 4996 [8]. As of June 2020, 39 radiosurgery-dedicated devices (Gamma Knife, n=21; Cyberknife, n=9; and Novalis Tx, n=9) are in operation in Korea. In addition, considering radiosurgery-compatible LINACs, radiosurgery is available in most cancer treatment hospitals. Therefore, the rapid spread of radiosurgery-dedicated or -compatible devices and good accessibility to the same are presumed to be important factors for the declining histologic confirmation rate of non-malignant PCNSTs in Korea.
The increasing incidence rate of PCNSTs from 2007 to 2016, mainly involving non-malignant tumors, may be explained by the development of imaging techniques and the increase in the number of screening tests performed. The trend is similar to that in most developed countries, such as France and the United States [2,18].
Survival outcomes vary depending on the histologic type, treatment modality, and diagnosis and treatment accessibility. We found that the 5-year RSR for malignant PCNSTs in Korea was 44.1%, which was higher than the 35.8% revealed when analyzing the US SEER 2001–2015 data. Sant et al. [11] analyzed 72 European registries from 24 countries and reported that the 5-year RSR for malignant tumors was 19.9%. By region, the United Kingdom and Ireland had the lowest at 15.6%, and Northern Europe the highest at 25.1% [11]. In Asia, Chien et al. [14] reported that the 5-year RSR for 14 selected malignant PCNSTs was 34.3% and that for glioblastoma was 9.8% from the Taiwan Cancer Registry. Even a few countries that do not report survival rates for overall malignant PCNSTs have reported survival rates for glioblastoma. In Europe, for glioblastoma diagnosed since 2005 when the Stupp regimen was introduced, Austria had the 4-year RSR of 1.0% and Sweden with the 5-year RSR of 5.4% [23,24]. The reason why Korea has a relatively better survival rate for malignant PCNSTs than the United States may be explained by the good accessibility to diagnostics and treatment because of higher medical insurance coverage in Korea, i.e., 99% (the United States, 84%) [25]. In addition, Korean National Health Insurance has a unique additional coverage program only for severe or rare diseases; most PCNSTs are indicated. Korean patients with PCNSTs pay only 5% of the cost of diagnostics and treatment supported by the program. Another explanation is that glioblastoma accounted for a relatively low proportion of malignant PCNSTs in Korea. As glioblastoma shows the most unfavorable survival outcome, the higher the proportion of glioblastoma among malignant PCNSTs, the lower the 5-year RSR for all malignant tumors. The proportion of glioblastoma among all malignant tumors in the United States was nearly twice that in Korea (48.3% [59,164 of 122,569 for 5 years] vs. 27.1% [5,796 of 21,411 for 10 years]). In addition, the 5-year RSR for glioblastomas in Korea was higher than that in the United States (12.1% vs. 6.8%). It may be intricately caused by factors, such as intrinsic genetic factors, tumor genetics, and accessibility to treatment. The CBTRUS had not reported the RSRs according to ethnicity; conversely, Thakkar et al. [26] reported that Asian/Pacific Islanders showed more favorable outcomes for glioblastomas than did Whites or Blacks. This suggests that genetic factors have a significant influence on the survival rate of glioblastoma. However, for most of the other malignant PCNSTs, unlike glioblastoma, the 5-year RSRs in the United States were higher than those in Korea: malignant primary melanocytic lesions (28.9% vs. 23.4%), anaplastic astrocytoma (30.2% vs. 26.2%), lymphoma (35.3% vs. 34.5%), and unspecified malignant glioma (54.1% vs. 51.0%). Although many malignant PCNSTs other than glioblastomas showed more favorable outcomes in the United States, a low proportion of glioblastomas among malignant PCNSTs in Korea may be the reason why the survival outcome of all malignant tumors was better in Korea.
The relatively high proportion of unspecified neoplasms is a limitation of this study (10.0%, 11,458 of 115,050). In the United States, unspecified neoplasms accounted for only 3.6% of all PCNSTs. The relatively lower histologic confirmation rate in Korea may be an explanation. Some physicians registered cases on the KCCR as unspecified neoplasms rather than specific histologies suspected in imaging findings when there was no histologic confirmation. However, the histologic confirmation rate for all tumors was not much different between Korea and the United States (49.2% vs. 56.3%). This suggests that a nearly three times higher proportion of unspecified neoplasms in Korea cannot be explained by a relatively lower histologic confirmation rate. The distribution of histology may be another possible explanation. The proportion of non-malignant tumors among all PCNSTs was higher in Korea than in the United States (81% vs. 70%). Another limitation of the study is that risk factors affecting brain tumorigenesis were not collected and analyzed. This should be investigated in further studies.
In conclusion, we analyzed the nationwide statistics for PCNSTs to determine their epidemiologic characteristics and survival outcomes in Korea based on our nationwide registry, which is the largest in Asia, with high-quality data. We also directly compared the KCCR data with the CBTRUS data. We found that low incidence of malignant tumors, increasing trend of non-malignant tumors, and better survival outcomes of malignant tumors were the characteristic features of PCNSTs in Korea.
Acknowledgments
This work was supported by research grants (1910131) from the National Cancer Center, Korea. The funding source played no role in the study’s design, data collection and analysis, writing of the report, or decision to submit the report for publication. The authors wish to express our sincere thanks to all neurosurgeons, neuropathologists, neuroradiologists, neuro-oncologists, and health information managers (tumor registrars) who contributed to registering all cases of PCNSTs.
Footnotes
Ethical Statement
This study was conducted in accordance with the ethical standards set forth in the 1964 World Medical Association Declaration of Helsinki. To maintain confidentiality of personal information, we anonymously processed all data collected from the individuals.
Author Contributions
Conceived and designed the analysis: Kang H, Park CK, Yoo H, Jung KW.
Collected the data: Ha J, Won YJ.
Contributed data or analysis tools: Ha J, Won YJ, Jung KW.
Performed the analysis: Kang H, Song SW, Ha J, Won YJ, Jung KW.
Wrote the paper: Kang H, Song SW.
Review the manuscript: Park CK, Yoo H, Jung KW.
Conflicts of Interest
Conflicts of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
References
- 1.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International Classification of Diseases for Oncology (ICD-O) 3rd ed. Geneva: World Health Organization; 2000. [Google Scholar]
- 2.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21:v1–100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER cancer statistics review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 4.Lee CH, Jung KW, Yoo H, Park S, Lee SH. Epidemiology of primary brain and central nervous system tumors in Korea. J Korean Neurosurg Soc. 2010;48:145–52. doi: 10.3340/jkns.2010.48.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung KW, Ha J, Lee SH, Won YJ, Yoo H. An updated nationwide epidemiology of primary brain tumors in republic of Korea. Brain Tumor Res Treat. 2013;1:16–23. doi: 10.14791/btrt.2013.1.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dho YS, Jung KW, Ha J, Seo Y, Park CK, Won YJ, et al. An updated nationwide epidemiology of primary brain tumors in Republic of Korea, 2013. Brain Tumor Res Treat. 2017;5:16–23. doi: 10.14791/btrt.2017.5.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019;51:417–30. doi: 10.4143/crt.2019.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korean Statistical Information Service [Internet] Daejeon: Korean Statistics; 2019. [cited 2020 Jan 10]. Available from: http://kosis.kr. [Google Scholar]
- 9.Kaneko S, Nomura K, Yoshimura T, Yamaguchi N. Trend of brain tumor incidence by histological subtypes in Japan: estimation from the Brain Tumor Registry of Japan, 1973–1993. J Neurooncol. 2002;60:61–9. doi: 10.1023/a:1020239720852. [DOI] [PubMed] [Google Scholar]
- 10.Wohrer A, Waldhor T, Heinzl H, Hackl M, Feichtinger J, Gruber-Mosenbacher U, et al. The Austrian Brain Tumour Registry: a cooperative way to establish a population-based brain tumour registry. J Neurooncol. 2009;95:401–11. doi: 10.1007/s11060-009-9938-9. [DOI] [PubMed] [Google Scholar]
- 11.Sant M, Minicozzi P, Lagorio S, Borge Johannesen T, Marcos-Gragera R, Francisci S, et al. Survival of European patients with central nervous system tumors. Int J Cancer. 2012;131:173–85. doi: 10.1002/ijc.26335. [DOI] [PubMed] [Google Scholar]
- 12.Jazayeri SB, Rahimi-Movaghar V, Shokraneh F, Saadat S, Ramezani R. Epidemiology of primary CNS tumors in Iran: a systematic review. Asian Pac J Cancer Prev. 2013;14:3979–85. doi: 10.7314/apjcp.2013.14.6.3979. [DOI] [PubMed] [Google Scholar]
- 13.de Robles P, Fiest KM, Frolkis AD, Pringsheim T, Atta C, St Germaine-Smith C, et al. The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro Oncol. 2015;17:776–83. doi: 10.1093/neuonc/nou283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chien LN, Gittleman H, Ostrom QT, Hung KS, Sloan AE, Hsieh YC, et al. Comparative brain and central nervous system tumor incidence and survival between the United States and Taiwan based on population-based registry. Front Public Health. 2016;4:151. doi: 10.3389/fpubh.2016.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miranda-Filho A, Pineros M, Soerjomataram I, Deltour I, Bray F. Cancers of the brain and CNS: global patterns and trends in incidence. Neuro Oncol. 2017;19:270–80. doi: 10.1093/neuonc/now166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darlix A, Zouaoui S, Rigau V, Bessaoud F, Figarella-Branger D, Mathieu-Daude H, et al. Epidemiology for primary brain tumors: a nationwide population-based study. J Neurooncol. 2017;131:525–46. doi: 10.1007/s11060-016-2318-3. [DOI] [PubMed] [Google Scholar]
- 17.Lin YJ, Chiu HY, Chiou MJ, Huang YC, Wei KC, Kuo CF, et al. Trends in the incidence of primary malignant brain tumors in Taiwan and correlation with comorbidities: a population-based study. Clin Neurol Neurosurg. 2017;159:72–82. doi: 10.1016/j.clineuro.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Pouchieu C, Gruber A, Berteaud E, Menegon P, Monteil P, Huchet A, et al. Increasing incidence of central nervous system (CNS) tumors (2000–2012): findings from a population based registry in Gironde (France) BMC Cancer. 2018;18:653. doi: 10.1186/s12885-018-4545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura H, Makino K, Yano S, Kuratsu JI Kumamoto Brain Tumor Research Group. Epidemiological study of primary intracranial tumors: a regional survey in Kumamoto prefecture in southern Japan--20-year study. Int J Clin Oncol. 2011;16:314–21. doi: 10.1007/s10147-010-0178-y. [DOI] [PubMed] [Google Scholar]
- 20.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 21.Woehrer A, Hackl M, Waldhor T, Weis S, Pichler J, Olschowski A, et al. Relative survival of patients with non-malignant central nervous system tumours: a descriptive study by the Austrian Brain Tumour Registry. Br J Cancer. 2014;110:286–96. doi: 10.1038/bjc.2013.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 23.Woehrer A. Brain tumour epidemiology in Austria and the Austrian brain tumour registry. Clin Neuropathol. 2013;32:269–85. doi: 10.5414/NP300600. [DOI] [PubMed] [Google Scholar]
- 24.Bruhn H, Strandeus M, Milos P, Hallbeck M, Vrethem M, Lind J. Improved survival of Swedish glioblastoma patients treated according to Stupp. Acta Neurol Scand. 2018;138:332–7. doi: 10.1111/ane.12966. [DOI] [PubMed] [Google Scholar]
- 25.Glied S, Jackson A. The future of the affordable care act and insurance coverage. Am J Public Health. 2017;107:538–40. doi: 10.2105/AJPH.2017.303665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23:1985–96. doi: 10.1158/1055-9965.EPI-14-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.