Abstract
Since the coronavirus disease 2019 (COVID-19) pandemic, Brazil has the third-highest number of confirmed cases and the second-highest number of recovered patients. SARS-CoV-2 detection by real-time RT-PCR is the gold standard but requires a certified laboratory infrastructure with high-cost equipment and trained personnel. However, for large-scale testing, diagnostics should be fast, cost-effective, widely available, and deployed for the community, such as serological tests based on lateral flow immunoassay (LFIA) for IgM/IgG detection. We evaluated three different commercial point-of-care (POC) LFIAs for anti-SARS-CoV-2 IgM and IgG detection in capillary whole blood of 100 healthcare workers (HCW) from São Paulo university hospital previously tested by RT-PCR: (1) COVID-19 IgG/IgM BIO (Bioclin, Brazil), (2) Diagnostic Kit for IgM/IgG Antibody to Coronavirus (SARS-CoV-2) (Livzon, China), and (3) SARS-CoV-2 Antibody Test (Wondfo, China). A total of 84 positives and 16 negatives HCW were tested. The data was also analyzed by the number of days post symptoms (DPS) in three groups: <30 (n=26), 30–59 (n=42), and >59 (n=16). The observed sensibility was 85.71%, 47.62%, and 44.05% for Bioclin, Wondfo, and Livzon, respectively, with a specificity of 100% for all LFIA. Bioclin was more sensitive (p<0.01), regardless of the DPS. Thus, the Bioclin may be used as a POC test to monitor SARS-CoV-2 seroconversion in HCW.
Keywords: SARS-CoV-2, COVID-19, Point-of-care, Lateral flow immunoassay, Healthcare workers
Introduction
After 10 months since the coronavirus disease 2019 (COVID-19) pandemic [1], caused by the severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2), Brazil reached, until late January 2021, the third place in the number of confirmed cases, accounting for more than 9.1 million cases and 222 thousand deaths [2]. However, it is the second country with the highest number of recovered patients (more than 8.4 million) [3].
The molecular detection of SARS-CoV-2 by real-time Reverse Transcription-Polymerase Chain Reaction (RT-PCR) is the gold standard test but requires a certified laboratory infrastructure with high-cost equipment and trained personnel. This structure is suitable, and of paramount importance, for the diagnostic of hospitalized patients, as well as healthcare workers (HCW). However, for large-scale testing, RT-PCR is not the best option. Therefore, COVID-19 diagnostic tests should be fast, cost-effective, widely available, and deployed for the community. In general, those requisites are achieved by serological tests based on lateral flow immunoassay (LFIA). Many LFIAs have been described for the detection of IgM and IgG immunoglobulins against SARS-CoV-2 or detection of SARS-CoV-2 viral proteins (antigen tests) [4]. Many of LFIAs for SARS-CoV-2 antibody detection are manufactured using the nucleocapsid (N) or spike (S) protein or N and S combined [5]. These tests should also be used to support RT-PCR results, especially since antibody responses can assist in the prognosis of patients [6]. Immunoglobulin response against viral infection begins with an early and transient IgM production, followed by a longer and lasting IgG response. In patients with COVID-19, the production of IgM and IgG could be simultaneous and detected after 2 days of symptoms onset and could reach in some patients a plateau level after 6 days [7, 8]. Particularly, the IgM response could last for more than 6 months [9]. Moreover, the immunoglobulin levels are in most cases correlated positively with the severity of COVID-19 although the antibody response could be delayed in critical patients compared to non-critical cases [10].
In the present study, we evaluated the sensitivity of three different commercial point-of-care (POC) LFIAs for anti-SARS-CoV-2 antibody detection in 100 HCW from São Paulo university hospital in Brazil, with confirmed tests (positive or negative) for COVID-19 by real-time RT-PCR assay.
Material and methods
Three commercial POC LFIAs for detection of anti-SARS-CoV-2 IgG and IgM were tested: (1) COVID-19 IgG/IgM BIO (Bioclin, Brazil), (2) Diagnostic Kit for IgM/IgG Antibody to Coronavirus (SARS-CoV-2) (Livzon, China), and (3) SARS-CoV-2 Antibody Test (Wondfo, China). Bioclin and Livzon LFIAs independently detect IgG and IgM, whereas Wondfo detects IgG and IgM combined.
In brief, these tests detect IgG and IgM immunoglobulins anti-SARS-CoV-2, in a lateral flow assay, that react with colloidal gold particles conjugated with SARS-CoV-2 antibodies, which in turn are captured by antibodies against human IgM and IgG present in the Test Region (T), resulting in a dark-colored test band (positive result). A Control Region (C), present before T region, indicates a valid test when a dark-colored band is also generated, due to the reaction between human immunoglobulins and human anti-immunoglobulin antibodies fixed in the C region or invalid otherwise. The declared combined sensitivities (IgG/IgM) of the manufacturer’s LFIAs are 96.3%, 90.6%, and 86.43% for Bioclin, Livzon, and Wondfo, respectively.
A total of 100 HCW from the São Paulo university hospital, previously tested for SARS-CoV-2 infection by real-time RT-PCR with the GeneFinder COVID-19 Plus RealAmp Kit (Osang Healthcare, Korea), in the period of March to June 2020, were enrolled in the study. From them, 84 were confirmed positive and 16 negative.
The indicated volume of finger-prick capillary whole blood for each test, collected preferably from the skin of annular fingertip with a lancing device, was pipetted immediately into the cassette sample wells, following the addition of sample diluent according to the manufacturer instructions. All LFIAs were tested simultaneously at the moment of blood draw of each investigated HCW. Results were read up to 15 min to confirm negative results. The LFIAs were performed from April to July 2020.
The sensitivity was calculated as the proportion of positive results of LFIAs in relation to the positive RT-PCR confirmed cases and specificity was calculated as the proportion of LFIAs negative results in relation to the negative RT-PCRs. The 95% confidence intervals (CI) of sensitivity and specificity proportions were calculated by the modified Wald method. The results were also analyzed according to the number of days post symptoms (DPS), distributed in three distinct groups: <30 (n=26), 30–59 (n=42), and >59 (n=16). The proportion of results accounted for IgM and IgG, alone or combined, regarding DPS, and the pairwise comparison within LIFAs was analyzed by Cochran’s Q and McNemar tests, for a p-value <0.05. The analysis was made using software R version 4.0.2 [11].
The study was approved by the São Paulo hospital Research Ethics Committee (CEP n. 34371020.5.0000.5505).
Results
The age of investigated HCWs varied from 20 to 67 years (mean = 37.45, median = 36). The overall sensitivity of IgM and IgG detection, individually or combined, are described in Table 1.
Table 1.
Sensitivity of LFIAs results from 84 positive RT-PCR HCW for SARS-CoV-2
| LFIA | Sensitivity in % (95% CI) | ||
|---|---|---|---|
| IgG/IgM | IgM | IgG | |
| Bioclin | 85.71 (76.52–91.79) | 54.76 (44.14–64.97) | 85.71 (76.52–91.79) |
| Livzon | 44.05 (33.92–54.70) | 29.76 (21.01–40.29) | 35.71 (26.28–46.40) |
| Wondfo | 47.62 (37.28–58.17) | N.A. | N.A. |
HCW healthcare workers, N.A. not available
Bioclin LFIA showed an overall sensitivity of 85.71% (72/84), followed by Wondfo with 47.62% (40/84), and Livzon with 44.05% (37/84). In comparison to the 16 negative RT-PCR individuals, the sensitivity of all LFIAs was 100% (77.31 to 100%, 95% CI).
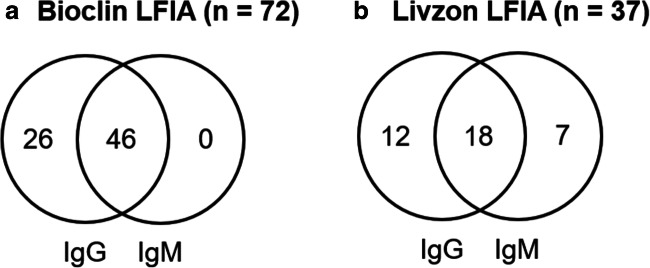
The results showing the overlap between individual IgG and IgM reactivity for Bioclin and Livzon are shown in Fig. 1.
Fig. 1.
Venn diagram showing the overlap between individual IgG and IgM reactivity for Bioclin and Livzon LFIAs. a, Bioclin. b, Livzon
The results, according to the groups of DPS (<30, 30–59, and >59), are depicted in Table 2.
Table 2.
Comparison of LFIAs results in time groups according to the days post symptoms (DPS)
| Antibody | DPS | HCW | Number (%) | p-value | ||
|---|---|---|---|---|---|---|
| Bioclin | Wondfo | Livzon | ||||
| IgM/IgG1 | <30 | 26 | 22 (84.62) | 14 (53.85) | 13 (50.00) | 0.0022* |
| 30–59 | 42 | 38 (90.48) | 21 (50.00) | 17 (40.48) | <0.001* | |
| >59 | 16 | 12 (75.00) | 5 (31.25) | 7 (43.75) | 0.0131* | |
| IgM2 | <30 | 26 | 19 (73.08) | NA | 11 (42.31) | 0.0047* |
| 30–59 | 42 | 21 (50.00) | NA | 11 (26.19) | 0.03892* | |
| >59 | 16 | 6 (37.50) | NA | 3 (18.75) | 0.0833 | |
| IgG2 | <30 | 26 | 22 (84.62) | NA | 10 (38.46) | 0.0005* |
| 30–59 | 42 | 38 (90.48) | NA | 15 (35.71) | <0.001* | |
| >59 | 16 | 12 (75.00) | NA | 5 (31.25) | 0.0081 | |
DPS days post symptoms, HCW healthcare workers
*Significant for p<0.05
1Cochran’s Q test, p<0.05
2McNemar test, p<0.05
The Bioclin LFIA was significantly more sensitive, in comparison to Livzon and Wondfo, regardless of the DPS or detection of IgM and IgG combined (Cochran’s Q test, p<0.05). The post hoc analysis of pairwise comparisons, with the McNemar test, also has shown that Bioclin was more sensitive than Livzon for IgM and IgG individually, and no differences were observed between Livzon and Wondfo regardless of the DPS and immunoglobulin class (Table 2).
The proportion of positive results for each LFIA test along the analyzed DPS have not shown any significant difference for the overall IgM/IgG detection (Bioclin, p=0.316; Livzon, p=0.744; Wondfo, p=0.33), although the sensibility of Wondfo LFIA dropped to 31.25% after 60 DPS. The same was observed for IgG (Bioclin, p=0.316; Livzon, p=0.894) and IgM (Bioclin, p=0.054; Livzon, p=0.208) alone, although Bioclin is likely to be more sensitive for IgM in the group of <30 (p=0.054).
We also observed in the Wondfo LFIA test a trace of red blood cells in all lateral flow test cassettes which made reading difficult in some positive results when a faint but visible T line was present.
Discussion
In the present study, we analyzed three different commercial LFIAs for the detection of anti-SARS-CoV-2 IgG and IgM in HCW. For the POC test format, capillary whole blood is more suitable than serum or plasma and does not require a laboratory infrastructure for venous blood draw and serum/plasma separation. In the three evaluated LFIAs, the recommended volume of capillary whole blood by the manufacturers is twice the volume of serum or plasma.
The use of POC-based tests for rapid antibody detection can be helpful in identifying patients at different stages of infection, due to the early production of IgM followed by IgG response, although, in patients with COVID-19, the response of IgM and IgG could be simultaneous [7, 8]. Our results demonstrated that overall sensitivity achieved by Bioclin LFIA (85.71%) with whole blood samples is compared to those obtained with serum or plasma for Wondfo (from 71.7 to 85.8%) [12–14] and Livzon (86.7%) [15], in contrast to Livzon and Wondfo LFIAs which showed sensitivities below 50%.
Similar to the results here described, Santos et al. [16] have shown, for capillary whole blood, a sensitivity of 55% for the Wondfo LFIA test in HCWs, while the sensitivity in serum samples was much higher (96%). A better sensitivity for capillary whole blood with Wondfo LFIA test was reported by Silveira et al. [13] at 77.1% in 83 volunteers with positive RT-PCR results at least 10 days before the LFIA test. In a larger study with hospitalized patients, Costa et al. [12] evaluated the Wondfo LFIA, in serum samples or plasma, and obtained a sensitivity of 85.8%. In another evaluation of the Wondfo LFIA, Wu et al. [17] have shown a sensibility of 75.8% in serum samples. In a Brazilian study accessing the performance of 12 serological tests for COVID-19 diagnosis, Cota et al. [14] described an overall sensitivity for Wondfo LFIA at 71.7% in serum from symptomatic patients with confirmed SARS-CoV-2 infection. In the same manner, the Livzon LFIA, when tested in serum samples of hospitalized patients, presented a sensibility of 80% for IgM and 86.7 for IgG, with a specificity of 95% and 100%, respectively.
In summary, for LFIA, antibody detection is more effective in plasma or serum samples than in whole blood, although, for POC format and large-scale testing, finger-prick capillary whole blood is more appropriate, and therefore, choosing a more sensitive test for this type of sample is of paramount importance. In this regard, Hallal et al. [18] extrapolated the sensitivity of Wondfo LFIA at 84.8%, based on pooled results of three validation studies using plasma or serum, with sensitivities varying from 81.5 to 100%, and one using whole blood (77.1%), in two nationwide surveys on the SARS-CoV-2 antibody prevalence in Brazil but using finger-prick whole blood. On that account, antibody prevalence could have been considerably underestimated.
The majority of LFIAs for SARS-CoV-2 antibody detection are manufactured using the N or S proteins or both combined. In terms of sensitivity, tests based on N or S antigen, in general, seem to be equivalent. However, the combined use of N and S antigens has presented a better sensitivity in comparison with N and S alone [5]. In this regard, it was not possible to analyze or infer any result concerning sensitivity differences between these antigens since the manufacturers of the analyzed LFIA do not provide this information.
An advantage of the present study is due to the fact that all LFIAs were carried out simultaneously at the time of blood draw of each HCW. On the other hand, a limitation of the study was the impossibility of follow-up on each HCW to observe possible variations in the detection of IgM and IgG over time or to expand the study to include hospitalized patients or low-income individuals from the general community.
Conclusion
Bioclin LFIA demonstrated high sensitivity and specificity for IgG detection (85.71%), and a reasonable detection of IgM (54.76%), with the use of capillary whole blood in HCW. On the other hand, Livzon and Wondfo LFIAs had an overall sensitivity below 53.85% considering all analyzed conditions (DPS and/or IgG/IgM). Thus, the Bioclin LFIA may be a suitable POC test to monitor SARS-CoV-2 seroconversion in HCW.
Acknowledgements
J.M.A.C. and L.K.S.L. are fellows of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil. D.D.C. is a fellow of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. We are grateful to Anderson Scorsato for the statistical support.
Author contribution
Danielle D. Conte: validation, investigation, data curation, and writing–original draft. Joseane M. A. Carvalho: investigation and data curation. Luciano K. de Souza Luna: validation, investigation, formal analysis, writing (review and editing), and visualization. Klinger S. Faíco-Filho: investigation. Ana H. S. Perosa: investigation. Nancy Bellei: conceptualization, methodology, resources, supervision, writing (review and editing), project administration, and funding acquisition.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Coronavirus disease 2019 (COVID-19), Situation Report –51 2020. Available from: https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/.
- 2.WHO. Weekly epidemiological update - 2 February 2021: World Health Organization; 2021 [cited 2021 February 09]. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update%2D%2D-2-february-2021.
- 3.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vashist SK (2020) In vitro diagnostic assays for COVID-19: recent advances and emerging trends. Diagnostics (Basel) 10(4). 10.3390/diagnostics10040202 [DOI] [PMC free article] [PubMed]
- 5.Wang H, Ai J, Loeffelholz MJ, Tang YW, Zhang W. Meta-analysis of diagnostic performance of serology tests for COVID-19: impact of assay design and post-symptom-onset intervals. Emerg Microbes Infect. 2020;9(1):2200–2211. doi: 10.1080/22221751.2020.1826362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen L, Wang C, Zhao J, Tang X, Shen Y, Lu M, Ding Z, Huang C, Zhang J, Li S, Lan J, Wong G, Zhu Y. Delayed specific IgM antibody responses observed among COVID-19 patients with severe progression. Emerg Microbes Infect. 2020;9(1):1096–1101. doi: 10.1080/22221751.2020.1766382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, Liao P, Qiu JF, Lin Y, Cai XF, Wang DQ, Hu Y, Ren JH, Tang N, Xu YY, Yu LH, Mo Z, Gong F, Zhang XL, Tian WG, Hu L, Zhang XX, Xiang JL, du HX, Liu HW, Lang CH, Luo XH, Wu SB, Cui XP, Zhou Z, Zhu MM, Wang J, Xue CJ, Li XF, Wang L, Li ZJ, Wang K, Niu CC, Yang QJ, Tang XJ, Zhang Y, Liu XM, Li JJ, Zhang DC, Zhang F, Liu P, Yuan J, Li Q, Hu JL, Chen J, Huang AL. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020;71:2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, Cipolla M, Viant C, Barnes CO, Bram Y, Breton G, Hägglöf T, Mendoza P, Hurley A, Turroja M, Gordon K, Millard KG, Ramos V, Schmidt F, Weisblum Y, Jha D, Tankelevich M, Martinez-Delgado G, Yee J, Patel R, Dizon J, Unson-O’Brien C, Shimeliovich I, Robbiani DF, Zhao Z, Gazumyan A, Schwartz RE, Hatziioannou T, Bjorkman PJ, Mehandru S, Bieniasz PD, Caskey M, Nussenzweig MC. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu J, Wu C, Li X, Zhang G, Jiang Z, Li X, Zhu Q, Liu L. Profile of IgG and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:2255–2258. doi: 10.1093/cid/ciaa489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Team RC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 12.Costa SF, Buss L, Espinoza EPS, Vieira JM, Jr, de Oliveira da Silva LC, de Souza RM, Neto LP, Porto APM, Lazari C, dos Santos VA, da Silva Duarte A, Nastri AC, da Costa Leite GF, Manuli E, de Oliveira MS, Zampelli DB, Pastore Junior L, Segurado AC, Levin AS, Sabino E. Performance of a qualitative rapid chromatographic immunoassay to diagnose COVID-19 in patients in a middle-income country. J Clin Virol. 2020;131:104592. doi: 10.1016/j.jcv.2020.104592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silveira MF, Barros AJD, Horta BL, Pellanda LC, Victora GD, Dellagostin OA, Struchiner CJ, Burattini MN, Valim ARM, Berlezi EM, Mesa JM, Ikeda MLR, Mesenburg MA, Mantesso M, Dall’Agnol MM, Bittencourt RA, Hartwig FP, Menezes AMB, Barros FC, Hallal PC, Victora CG. Population-based surveys of antibodies against SARS-CoV-2 in Southern Brazil. Nat Med. 2020;26(8):1196–1199. doi: 10.1038/s41591-020-0992-3. [DOI] [PubMed] [Google Scholar]
- 14.Cota G, Freire ML, de Souza CS, Pedras MJ, Saliba JW, Faria V, Alves LL, Rabello A, Avelar DM. Diagnostic performance of commercially available COVID-19 serology tests in Brazil. Int J Infect Dis. 2020;101:382–390. doi: 10.1016/j.ijid.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuaillon E, Bollore K, Pisoni A, Debiesse S, Renault C, Marie S, et al. Detection of SARS-CoV-2 antibodies using commercial assays and seroconversion patterns in hospitalized patients. J Infect. 2020;81(2):e39–e45. doi: 10.1016/j.jinf.2020.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos VAD, Rafael MM, Sabino EC, Duarte A. Sensitivity of the Wondfo One Step COVID-19 test using serum samples. Clinics (Sao Paulo) 2020;75:e2013. doi: 10.6061/clinics/2020/e2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu JL, Tseng WP, Lin CH, Lee TF, Chung MY, Huang CH, Chen SY, Hsueh PR, Chen SC. Four point-of-care lateral flow immunoassays for diagnosis of COVID-19 and for assessing dynamics of antibody responses to SARS-CoV-2. J Infect. 2020;81:435–442. doi: 10.1016/j.jinf.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallal PC, Hartwig FP, Horta BL, Silveira MF, Struchiner CJ, Vidaletti LP, Neumann NA, Pellanda LC, Dellagostin OA, Burattini MN, Victora GD, Menezes AMB, Barros FC, Barros AJD, Victora CG. SARS-CoV-2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys. Lancet Glob Health. 2020;8(11):e1390–e13e8. doi: 10.1016/S2214-109X(20)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]