Abstract
Exhaled nitric oxide fraction (FeNO) is an indicator of allergic airway inflammation. However, it is unknown how asthma, allergic rhinitis (AR) and allergic sensitisation relate to FeNO, particularly among adolescents and in overlapping conditions. We sought to determine the associations between asthma, AR, and aeroallergen immunoglobulin (Ig)E and FeNO in adolescents.
We measured FeNO among 929 adolescents (aged 11–16 years) in Project Viva, an unselected prebirth cohort in Massachusetts, USA. We defined asthma as ever asthma physician diagnosis plus wheezing in the past year or taking asthma medications in the past month, AR as a physician diagnosis of hay fever or AR, and aeroallergen IgE as any IgE >0.35 IU·mL−1 among 592 participants who provided blood samples. We examined associations of asthma, AR and IgE with percent difference in FeNO in linear regression models adjusted for sex, race/ethnicity, age and height, maternal education and smoking during pregnancy, and household/neighbourhood demographics.
Asthma (14%) was associated with 97% higher FeNO (95% CI 70–128%), AR (21%) with 45% higher FeNO (95% CI 28–65%), and aeroallergen IgE (58%) with 102% higher FeNO (95% CI 80–126%) compared to those without each condition, respectively. In the absence of asthma or AR, aeroallergen IgE was associated with 75% higher FeNO (95% CI 52–101), while asthma and AR were not associated with FeNO in the absence of IgE.
The link between asthma and AR with FeNO is limited to those with IgE-mediated phenotypes. FeNO may be elevated in those with allergic sensitisation alone, even in the absence of asthma or AR.
Short abstract
While asthma, allergic rhinitis (AR) and allergic sensitisation are associated with higher FENO, asthma and AR in the absence of aeroallergen IgE are not associated with FENO. When elevated in asthma or AR, FENO suggests allergic sensitisation. https://bit.ly/3bGgr0r
Introduction
Exhaled nitric oxide fraction (FeNO) is an indicator of allergic airway inflammation and can aid in the diagnosis of allergic asthma [1]. The influence of concurrent allergic rhinitis (AR) and allergic sensitisation to environmental allergens on FeNO measurements is not well-known in adolescents. How each of these (asthma, AR and aeroallergen immunoglobulin (Ig)E) relate to FeNO is important not only for interpretation of FeNO in clinical use but also for a greater understanding of the factors that lead to elevated FeNO. We sought to understand the associations of asthma, AR, and aeroallergen IgE with FeNO in an adolescent population.
Asthma and AR are common and contribute to significant morbidity, with poorer quality of life, missed days of school, healthcare costs and emergency room visits for asthma exacerbations, resulting from difficulty sleeping, fatigue, and changes in mood and cognition [2]. AR and asthma share epidemiological overlap. AR occurs in >75% of patients with asthma, and asthma is seen in up to 40% of those with AR [3]. In those with both AR and asthma, AR may present first and there is a risk factor for subsequent development of asthma [4–7].
Asthma and AR are each known to be associated with elevated FeNO in both adults and children. Nitric oxide (NO) is an intercellular messenger known to mediate a variety of processes, including immune function and inflammation [8]. In the lung, NO acts as a signalling molecule in bronchial and vascular dilatation, ciliary kinesis, and neurotransmission in the non-adrenergic and non-cholinergic systems [9]. In allergic inflammation, T-helper-2 cells, type 2 innate lymphoid cells, mast cells and eosinophils produce cytokines, like interleukin (IL)-4 and IL-13, with downstream effects including the activation of inducible NO synthase (iNOS) and thus elevated NO levels. Therefore, FeNO is considered a marker of type 2 airway inflammation, which occurs with AR in the upper airway or allergic asthma in the lower airway [9–13]. Elevation of FeNO may indicate not only allergic asthma or AR but also elevations in circulating IgE against environmental allergens. In previous work in this same Project Viva cohort, an unselected pre-birth cohort in Massachusetts, USA, we have found differences in the nasal epigenome in association with asthma, AR, aeroallergen IgE and FeNO that are annotated with genes implicated in type 2 inflammatory responses [14]. Currently, the American Thoracic Society recommends FeNO in the diagnosis of eosinophilic airway inflammation, and suggests that it may be used to support the diagnosis of asthma where the diagnosis is less clear [1]. FeNO, however, may also be elevated in AR or allergic sensitisation to environmental allergens [15, 16]. Therefore, we sought to elucidate the contributors to FeNO and supplement our understanding of its diagnostic utility. In this study, we examined the relationships of asthma, AR, and aeroallergen IgE with FeNO in an adolescent population in Project Viva, a cohort not selected on the basis of asthma or allergy.
Materials and methods
Study design and participants
Between 1999 and 2002 we recruited women in early pregnancy into Project Viva from eight obstetric offices of Atrius Harvard Vanguard Medical Associates, a multi-specialty group practice in eastern Massachusetts. Exclusion criteria included multiple gestation, inability to answer questions in English, and gestational age ≥22 weeks at recruitment. Details of recruitment and retention are available elsewhere [17]. Of the 2128 infants, we included in this analysis those participants with FeNO measurements at an in-person visit in early adolescence, which totalled 929. Compared with the 929 included participants, the 1199 excluded participants were less likely to have college-educated mothers (59% versus 71%) and more likely to have mothers who smoked during pregnancy (15% versus 9%). However, maternal age at enrolment, gestational age at delivery, and sex and race/ethnicity were similar. Those 1199 were excluded due to either missing FeNO or no early adolescent visit. The median adolescent age was 12.9 years with a range of 11.9–16.6 years; there were no participants older than this due to cohort inception dates.
Asthma and AR
We defined current asthma as a maternal report of ever asthma diagnosis plus wheeze symptoms in the past 12 months or use of asthma medications in the past month, reported on an early teen questionnaire in keeping with the Study of Asthma and Allergies in Childhood questionnaire [18]. We used as a comparison group with no asthma diagnosis, no wheezing and no use of asthma medications or wheezing in the past 12 months. Participants were defined as having AR if they reported ever receiving a physician diagnosis of hay fever or AR. Both current asthma and AR were reported by parental questionnaire at the early teen follow-up visit.
Aeroallergen IgE
Trained research phlebotomists collected blood from participants at the early teen visit, which we centrifuged and stored at −80C. We measured plasma IgE against Dermatophagoides farina (dust mite), cat or dog dander, Aspergillus fumigatus (mould), Alternaria alternata (plant fungi), common ragweed, oak, ryegrass, or silver birch. Allergen extract-specific IgE antibodies were measured by ImmunoCap (Thermo Fisher Scientific/Phadia, Kalamazoo, Michigan); a widely used in vitro sandwich immunoassay, as described previously [19, 20]. We defined aeroallergen IgE as having IgE against any of these outdoor or indoor allergens >0.35 IU·mL−1. We defined “perennial IgE” as having IgE >0.35 IU·mL−1 against any of the following: D. farina (dust mite), cat or dog dander, A. fumigatus (mould) or A. alternata (plant fungi). We defined “seasonal IgE” as having any IgE >0.35 IU·mL−1 against any of the following: common ragweed, oak, ryegrass or silver birch.
Measurement of FeNO
Exhaled NO levels were measured twice for each participant with a portable electrochemical device (NIOX MINO; Aerocrine AB); this has been validated by chemiluminescence technology, with an accuracy of ±5 ppb [21]. Prior to each measurement, participants breathed in through an NO scrubbing filter and exhaled into room air twice. This was done in keeping with prior studies using FeNO; the ambient air NO was not measured [22–24]. On the third breath, participants inhaled through the filter and exhaled into the FeNO analyser. The last 3 s of the exhalation were utilised for FeNO measurement; this ensures lower rather than upper airway measurement. Nose clips were not used.
Statistical analysis
Potential confounders were selected a priori, based on known or suspected associations with asthma or allergy. Model 1 adjusted for sex and age and height at the early teen visit. Model 2 additionally adjusted for race/ethnicity, maternal education and smoking during pregnancy, median value of owner-occupied housing and education (% with a bachelor degree) based on census tract of home residence at time of mid-childhood visit from 2000, and household income and any smokers at home at the early teen visit. We additionally adjusted for body mass index in model 3.
We averaged the two FeNO measurements and included the log-transformed value as a continuous outcome in linear regression models. We present effect estimates as % change (95% CI) in FeNO, calculated as (exponentiated (β)–1)×100. In secondary analyses, we examined FeNO in categories (<20, 20–≤35 and >35 ppb) based on American Thoracic Society guidelines [1] using multinomial logistic regression models. We examined the associations of asthma, AR, and aeroallergen IgE with FeNO using separate linear and logistic regression models. We repeated linear regression models for the associations of seasonal and perennial IgE with FeNO.
To examine associations of overlapping conditions of asthma, AR and aeroallergen IgE, we derived an eight-category exposure based on the combination of these three exposures. We ran linear regression models with this eight-category exposure and used no aeroallergen IgE, no AR, no asthma as the reference category. We performed all analyses using SAS 9.4 (SAS Institute).
Results
Study population
The characteristics of the 929 study participants included in any analysis and their mothers are shown in table 1. Reported smoking in the home was rare (12%) and mean reported annual household income was $109 000. Children were predominantly white (64%) and their average age was 13 years. Mean±sdFeNO was 26±27 ppb and 19% of study participants had exhaled NO levels above the upper limit of normal for this age group (35 ppb) [1].
TABLE 1.
Participant characteristics (N=929)
| Subjects n | 929 |
| Child | |
| Sex | |
| Male | 466 (50) |
| Female | 463 (50) |
| Race/ethnicity | |
| Black | 154 (17) |
| Hispanic | 40 (4) |
| Asian | 28 (3) |
| White | 593 (64) |
| Other or >1 race/ethnicity | 113 (12) |
| Early teen visit | |
| Age years | 13±1 |
| Age at visit years | |
| 11.9 to <13 | 505 (54.4) |
| 13.0 to <15.0 | 374 (40.3) |
| 15.0 to 16.6 | 50 (5.4) |
| Height cm | 160±9 |
| BMI percentile category % | |
| <5th | 30 (3) |
| 5−<85th | 634 (68) |
| >85th | 262 (28) |
| Exhaled nitric oxide ppb | 26±27 |
| Exhaled nitric oxide | |
| <20 ppb | 553 (60) |
| 20−≤35 ppb | 196 (21) |
| >35 ppb | 180 (19) |
| Current asthma | |
| No | 682 (86) |
| Yes | 115 (14) |
| Ever allergic rhinitis | |
| No | 690 (79) |
| Yes | 179 (21) |
| Any aeroallergen IgE >0.35 kU·L−1 | |
| No | 247 (42) |
| Yes | 345 (58) |
| Annual household income at early teen visit $1000 | 109±44 |
| Any smokers at home at early teen visit | 114 (12) |
| Mother/family | |
| Pregnancy smoking status | |
| Never | 653 (71) |
| Former | 188 (20) |
| During pregnancy | 85 (9) |
| College graduate | |
| No | 264 (29) |
| Yes | 662 (71) |
| Median value owner-occupied housing $1000# | 263±149 |
| Percent with education higher than a bachelor's degree# | 42±20 |
Data are presented as n (%) or mean±sd, unless otherwise stated. BMI: body mass index; Ig: immunoglobulin. #: based on census tract, mid-childhood.
Overall, 115 (14%) out of 797 participants reported asthma while 179 (21%) out of 869 reported AR. Aeroallergen IgE >0.35 IU·mL−1 was detected in 345 (58%) out of 592 of those providing blood samples with IgE results. Mean±sd FeNO in those with asthma was 48.4±43.0 ppb compared to 21.8±20.4 ppb in those without asthma. Mean±sd FeNO in those with AR was 35.7±32.8 ppb compared to 23.5±24.9 ppb without AR. Participants with aeroallergen IgE had mean±sd FeNO 36.2±34.2 ppb compared to 14.8±9.5 ppb in those without IgE.
Associations of each condition with FeNO are shown in table 2, where model 1 is parsimoniously adjusted and model 2 is fully adjusted, with similar results. Asthma was common in those with FeNO >35 ppb; 35% of teens with FeNO >35 ppb had asthma versus 7% among participants with FeNO <20 ppb and 18% among participants with FeNO 20–≤35 ppb. Of the three conditions, the presence of aeroallergen IgE and asthma diagnosis each had a similar association with FeNO, while the magnitude of the association was half as great for AR. Specifically, those with asthma had a 97% higher FeNO (95% CI 70–128%) compared to those without asthma. Those with AR had a 45% higher FeNO (95% CI 28–65%) compared to those without AR, and those with aeroallergen IgE had 102% higher FeNO (95% CI 80–126%) compared to those without aeroallergen IgE. Perennial IgE was associated with 119% higher FeNO (95% CI 97–144) while seasonal IgE was associated with 71% higher FeNO (95% CI 52–93). Similar results were seen in logistic regression analyses for the clinical thresholds of FeNO. Asthma was associated with eight times higher odds (95% CI 5–14) of FeNO >35 ppb, AR was associated with three times higher odds (95% CI 2–5) of FeNO >35 ppb and aeroallergen IgE was associated with 28 times higher odds (95% CI 12–63) of FeNO >35 ppb compared to those without each condition (table 2).
TABLE 2.
Associations of asthma, allergic rhinitis (AR) and aeroallergen immunoglobulin (Ig)E with exhaled nitric oxide fraction (FeNO) using linear and multinomial logistic regression models
| Model 1# | Model 2¶ | |||||
|
Linear regression % difference in FeNO (95% CI) |
Logistic regression+ OR (95% CI) |
Linear regression % difference in FeNO (95% CI) |
Logistic regression+ OR (95% CI) |
|||
| 20–≤35 ppb | >35 ppb | 20–≤35 ppb | >35 ppb | |||
| Asthma versus no asthma | 97 (73–124) | 3 (2–5) | 8 (5–13) | 97 (70–128) | 3 (1–5) | 8 (5–14) |
| AR versus no AR | 48 (32–66) | 2 (1–3) | 3 (2–5) | 45 (28–65) | 2 (1–3) | 3 (2–5) |
| Aeroallergen IgE versus no aeroallergen IgE | 99 (79–121) | 4 (3–7) | 26 (12–59) | 102 (80–126) | 4 (3–7) | 28 (12–63) |
Current asthma: ever having an asthma diagnosis plus wheezing in the past year or taking asthma medications in the past month; no asthma: no asthma: no wheezing and no use of asthma medications or wheezing in the past 12 months; AR: ever having a diagnosis of hay fever or AR; no AR: never having a diagnosis of hay fever or AR; aeroallergen IgE; having any aeroallergen IgE >0.35 IU·mL−1; no aeroallergen IgE: IgE≤0.35 IU·mL−1. #: adjusted for sex and current age and height; ¶: model 1 additionally adjusted for race/ethnicity, maternal education and smoking during pregnancy, median value owner-occupied housing and percent≥bachelor's degree (census tract, mid-childhood), and household income and any smokers at home at early teen visit; +: reference group is <20 ppb.
We found similar associations when stratified by sex (table S2). Since steroids can affect FeNO, we ran a sensitivity analysis, excluding participants who used oral or inhaled corticosteroids within 72 h of FeNO testing (n=22) and found similar associations between asthma, AR and aeroallergen IgE with higher FeNO. Finally, we adjusted for body mass index and found similar associations with asthma, AR and aeroallergen IgE with FeNO in both linear and logistic regression models (table S3).
Overlapping associations of allergy, asthma and aeroallergen IgE with FeNO
To examine associations of overlapping conditions of asthma, AR and aeroallergen IgE, we derived an eight-category exposure based on the combination of these three exposures among 476 participants with non-missing values for the three exposures. The reference category was no asthma, no AR and no aeroallergen IgE (figure 1, table S1). We found that, in the absence of asthma or AR, aeroallergen IgE alone (n=173, 36%) was associated with 75% higher FeNO (95% CI 52–101%). Asthma in the absence of AR and aeroallergen IgE (n=10, 2%), AR in the absence of asthma and aeroallergen IgE (n=14, 3%), and asthma and AR in the absence of aeroallergen IgE (n=6, 1%) were uncommon and not associated with FeNO. The presence of all three exposures (asthma, AR and aeroallergen IgE (n=32, 7%)) was associated with 272% higher FeNO (95% CI 189–379%) compared to having none of these (figure 1).
FIGURE 1.
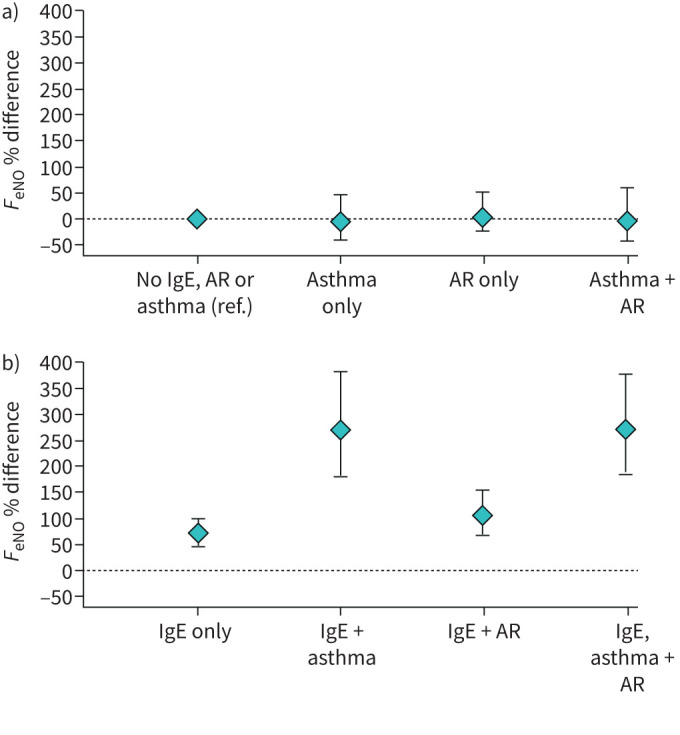
Percent difference in exhaled nitric oxide fraction (FeNO) relative to reference group a) without detectable aeroallergen immunoglobulin (Ig)E and b) with aeroallergen IgE. AR: allergic rhinitis.
Discussion
In this study of adolescents, we found, as expected, that asthma and allergy were each associated with higher FeNO. However, this association was absent among those without detectable aeroallergen IgE. Additionally, aeroallergen IgE was associated with elevated FeNO even in the absence of asthma or AR.
The association between asthma and elevated FeNO among adolescents in this study is consistent with the well-established connection between allergic asthma and airway inflammation as measured by FeNO. Exhaled NO >35 ppb in children strongly supports the diagnosis of asthma and can be used as a diagnostic tool [1]. FeNO is a marker of an allergic asthma phenotype, characterised by elevations in type 2 cytokines IL-4, IL-5 and IL-13 and eosinophils in sputum, serum, and bronchial biopsy [25–27].
However, we found that this association between asthma and increased FeNO was seen only when aeroallergen IgE was detected. In the absence of aeroallergen IgE, the association between asthma and FeNO was lost. Our results suggest that asthma is only associated with FeNO in the presence of allergic sensitisation, and that FeNO is more likely to indicate the presence of aeroallergen IgE. We found that FeNO >35 ppb had a positive predictive value of only 35% for the diagnosis of asthma, compared to a positive predictive value of 94% for the presence of aeroallergen IgE. Our findings contrast with a study of 1156 children in France, which found that non-atopic asthmatics had higher FeNO than non-atopic children without asthma: average FeNO 13.4 ppb in non-atopic children with asthma versus 10.6 ppb in non-atopic children without asthma [28]. However, our findings are consistent with other birth cohorts, including one from the Netherlands, which observed higher FeNO only among young adults with atopic asthma, defined as asthma plus allergic sensitisation with positive serum IgE against 10 common aeroallergens, but not non-atopic asthma [29]. The Isle of Wight birth cohort in England, UK, similarly found that atopy (measured by skin-prick testing) was associated with higher FeNO, while the level of FeNO did not differ between non-atopic teens with and without asthma [30]. The authors concluded, as we do, that FeNO is a biomarker for atopy rather than asthma.
Our study also confirmed the association between AR and higher FeNO among adolescents, which has been well established among adults and children [31–35]. Similar to our findings in asthma, we found that this association between AR and FeNO was observed only in the presence of aeroallergen IgE. Other studies have also found that those with atopy and rhinitis have higher FeNO than those with rhinitis but no atopy [28, 36, 37]. This same large cohort of 1156 children in France found that non-asthmatic atopic participants (as determined by skin-prick test to common allergens) with rhinitis had significantly higher FeNO than non-atopic participants with rhinitis (20.7±13 versus 12.5±6.4 ppb) [28]. Likewise, in an adult population examining atopic participants identified by skin-prick testing, Gratziou et al. [37] found that FeNO was significantly higher in atopic rhinitis than in those with non-atopic rhinitis (13.3±1.3 versus 5.8±1.2 ppb).
One explanation of our findings is that asthma in the setting of low aeroallergen IgE is mediated by a non-allergic inflammatory pathway. This distinct non-allergic asthma phenotype represents a subset of asthmatics that may have more severe and more difficult to control asthma [38, 39]. Rather than allergic, type 2-mediated pathways, these non-atopic asthmatics may have neutrophilic airway inflammation [40]. This may be determined, in part, by genetic factors, and those with allergic and non-allergic asthma have distinct HLA haplotypes [41]. Environmental exposures such as particulate air pollution or childhood viral infections may also contribute to non-allergic asthma, in a process mediated through neutrophilic inflammation [42]. A similar neutrophilic process may be occurring in our participants with asthma or AR and no significant serum IgE.
We found that aeroallergen IgE is associated with elevated FeNO even in the absence of asthma or AR. A few studies have similarly found that atopy is associated with FeNO regardless of symptoms. For example, both Choi et al. [36] and Franklin et al. [43] examined children with asthma and atopy and found that atopy increases FeNO regardless of asthma diagnosis. This has been similarly observed among Pacific Islander adults, where positive skin-prick testing to house dust mite was associated with higher exhaled and nasal NO even in the absence of asthma symptoms [44]. In a study comparing asthmatic and healthy children, Barreto et al. [45] found that even in those without respiratory symptoms, atopy (defined by skin-prick testing) and peripheral eosinophil count were associated with significantly higher FeNO compared to those without atopy or eosinophilia. In a population of school children, van Amsterdam et al. [46] similarly found that allergic sensitisation was associated with higher FeNO even in those without wheeze, although the association was significantly augmented in those with wheeze.
Our findings suggest that IgE is an important factor in the elevated FeNO observed in asthma and AR in adolescents. While the exact mechanism by which IgE may influence FeNO is not entirely clear, the pathophysiology by which each are elevated in asthma and AR has been previously explored. IgE may increase FeNO along with inflammatory cytokines inducing iNOS. In immediate hypersensitivity reactions, IgE cross-links to the FcεR1 receptor on mast cells, resulting in mast cell degranulation, inflammatory cytokine release, and activation of inflammatory cells at sites sensitive to the inciting allergy. Local IgE-mediated mechanisms may explain the localised reactions in skin, nasal turbinates, airways, and gut in eczema, rhinitis, asthma, and food allergy, respectively. In allergic asthma and AR specifically, the inflammatory cytokines that occur as a result of IgE-FcεR1 cross-linking may induce iNOS, resulting in the increased FeNO observed in AR and asthma [47, 48]. The upregulated eosinophils in type 2 inflammation of asthma and AR may also exert direct oxidative damage and promote the continued release of inflammatory cytokines that activate iNOS, increasing NO in exhaled air [28, 45, 49].
Our study is one of the largest studies conducted in a well-characterised adolescent age group that examines the associations of asthma, allergy and IgE with airway inflammation as measured by FeNO. However, we acknowledge limitations to this study. Specifically, this is a cross sectional study evaluating FeNO, asthma and AR at a single time-point in adolescence. Asthma and AR vary widely in symptoms, severity and control, and so associations may change depending on different time-points in the disease trajectory. Additionally, steroid use may reduce FeNO although we had similar findings when excluding participants with any steroid use within 72 h of testing. In addition, there were very few participants who had asthma or AR but no aeroallergen IgE. This may represent the fact that the majority of those with asthma or AR in this study had an allergic asthma phenotype. There were, however, a large portion of our participants (36%) who did not have asthma or AR but who did have aeroallergen IgE. Finally, we did not include those with previous or non-active asthma for those without wheeze in the last 12 months. Similarly, we did not include IgE positivity to non-tested allergens such as food-specific IgE. Future work may include these other allergens. We would also explore the comparison of FeNO with blood eosinophils as a more widely available biomarker than FeNO.
Our study confirms the expected pattern of FeNO elevation among adolescents with asthma and AR and implicates IgE-mediated pathways in the association between asthma, AR and FeNO. Our findings suggest that the presence of aeroallergen IgE was critical to the FeNO elevations observed in those with asthma or AR. Without aeroallergen IgE, that relationship was lost. Aeroallergen IgE can be considered a marker for elevated FeNO, since FeNO was detected whenever aeroallergen IgE was present, regardless of the presence of clinical disease. Furthermore, even if FeNO is elevated, it does not necessarily indicate asthma or AR, and patients with asthma and no aeroallergen IgE may not have an elevated FeNO. Therefore, this study places FeNO into perspective as a marker predominantly for sensitisation rather than for clinical disease, and further raises the need to identify alternative markers for airway inflammation in those children and adolescents with non-IgE-mediated asthma or AR.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
TABLE S1 Associations of overlapping aeroallergen IgE, allergic rhinitis, and asthma with FENO 00945-2020.tableS1 (57.7KB, pdf)
TABLE S2 Associations of asthma, allergic rhinitis, and aeroallergen IgE with FENO using linear regression models, stratified by sex 00945-2020.tableS2 (67.2KB, pdf)
TABLE S3 Associations of asthma, allergic rhinitis, and aeroallergen IgE with FENO using linear and multinomial logistic regression models 00945-2020.tableS3 (68.9KB, pdf)
Footnotes
Author contributions: B.M. Flashner drafted the manuscript. M.B. Rice contributed to the drafting and revision of the manuscript. S.L. Rifas-Shiman conducted the analyses. E. Oken, C.A. Camargo Jr, T.A.E. Platts-Mills, L. Workman, A.A. Litonjua and D.R. Gold contributed to the revision of the manuscript.
Support statement: This work was supported by the US National Institutes of Health (K23ES026204, R01 HD034568, R01AI102960, UH3OD023286), the American Thoracic Society Foundation and the American Lung Association. Funding information for this article has been deposited with the Crossref Funder Registry.
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: B.M. Flashner has nothing to disclose.
Conflict of interest: S.L. Rifas-Shiman reports grants from the US National Institutes of Health during the conduct of the study.
Conflict of interest: E. Oken reports grants from the US National Institutes of Health during the conduct of the study.
Conflict of interest: C.A. Camargo Jr has nothing to disclose.
Conflict of interest: T.A.E. Platts-Mills has nothing to disclose.
Conflict of interest: L. Workman has nothing to disclose.
Conflict of interest: A.A. Litonjua has nothing to disclose.
Conflict of interest: D.R. Gold reports grants from the US National Institutes of Health during the conduct of the study.
Conflict of interest: M.B. Rice reports grants from the US National Institutes of Health during the conduct of the study.
References
- 1.Dweik RA, Boggs PB, Erzurum SC, et al. . An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011; 184: 602–615. doi: 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leynaert B, Neukirch C, Liard R, et al. . Quality of life in allergic rhinitis and asthma: a population-based study of young adults. Am J Respir Crit Care Med 2000; 162: 1391–1396. doi: 10.1164/ajrccm.162.4.9912033 [DOI] [PubMed] [Google Scholar]
- 3.Guerra S, Sherrill DL, Martinez FD, et al. . Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol 2002; 109: 419–425. doi: 10.1067/mai.2002.121701 [DOI] [PubMed] [Google Scholar]
- 4.Settipane RJ, Hagy GW, Settipane GA. Long-term risk factors for developing asthma and allergic rhinitis: a 23- year follow-up study of college students. Allergy Proc 1994; 15: 21–25. doi: 10.2500/108854194778816634 [DOI] [PubMed] [Google Scholar]
- 5.Leynaert B, Bousquet J, Neukirch C, et al. . Perennial rhinitis: an independent risk factor for asthma in nonatopic subjects: results from the European Community Respiratory Health Survey. J Allergy Clin Immunol 1999; 104: 301–304. doi: 10.1016/S0091-6749(99)70370-2 [DOI] [PubMed] [Google Scholar]
- 6.Linneberg A, Henrik Nielsen N, Frølund L, et al. . The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy Study. Allergy 2002; 57: 1048–1052. doi: 10.1034/j.1398-9995.2002.23664.x [DOI] [PubMed] [Google Scholar]
- 7.Huovinen E, Kaprio J, Laitinen LA, et al. . Incidence and prevalence of asthma among adult Finnish men and women of the Finnish Twin Cohort from 1975 to 1990, and their relation to hay fever and chronic bronchitis. Chest 1999; 115: 928–936. doi: 10.1378/chest.115.4.928 [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ, Liew FY. Nitric oxide and asthmatic inflammation. Immunol Today 1995; 16: 128–130. doi: 10.1016/0167-5699(95)80128-6 [DOI] [PubMed] [Google Scholar]
- 9.Duong-Quy S. Clinical utility of the exhaled nitric oxide (NO) measurement with portable devices in the management of allergic airway inflammation and asthma. J Asthma Allergy 2019; 12: 331–341. doi: 10.2147/JAA.S190489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silvestri M, Spallarossa D, Frangova Yourukova V, et al. . Orally exhaled nitric oxide levels are related to the degree of blood eosinophilia in atopic children with mild-intermittent asthma. Eur Respir J 1999; 13: 321–326. doi: 10.1034/j.1399-3003.1999.13b17.x [DOI] [PubMed] [Google Scholar]
- 11.Gandhi NA, Bennett BL, Graham NMH, et al. . Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov 2016; 15: 35–50. doi: 10.1038/nrd4624 [DOI] [PubMed] [Google Scholar]
- 12.Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol 2003; 111: 1171–1183. doi: 10.1067/mai.2003.1592 [DOI] [PubMed] [Google Scholar]
- 13.Feng CH, Miller MD, Simon RA. The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol Allergy 2012; 26: 187–190. doi: 10.2500/ajra.2012.26.3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardenas A, Sordillo JE, Rifas-Shiman SL, et al. . The nasal methylome as a biomarker of asthma and airway inflammation in children. Nat Commun 2019; 10: 3095. doi: 10.1038/s41467-019-11058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HB, Eckel SP, Kim JH, et al. . Exhaled NO: determinants and clinical application in children with allergic airway disease. Allergy Asthma Immunol Res 2016; 8: 12–21. doi: 10.4168/aair.2016.8.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olin AC, Alving K, Torén K. Exhaled nitric oxide: relation to sensitisation and respiratory symptoms. Clin Exp Allergy 2004; 34: 221–226. doi: 10.1111/j.1365-2222.2004.01888.x [DOI] [PubMed] [Google Scholar]
- 17.Oken E, Baccarelli AA, Gold DR, et al. . Cohort profile: project viva. Int J Epidemiol 2015; 44: 37–48. doi: 10.1093/ije/dyu008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asher MI, Keil U, Anderson HR, et al. . International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995; 8: 483–491. doi: 10.1183/09031936.95.08030483 [DOI] [PubMed] [Google Scholar]
- 19.Wilson JM, Workman L, Schuyler AJ, et al. . Allergen sensitisation in a birth cohort at midchildhood: Focus on food component IgE and IgG4 responses. J Allergy Clin Immunol 2018; 141: 419–423.e5. doi: 10.1016/j.jaci.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng C, Cardenas A, Rifas-Shiman SL, et al. . Epigenome-wide association study of total serum immunoglobulin E in children: A life course approach. Clin Epigenetics 2018; 10: 55. doi: 10.1186/s13148-018-0488-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalili B, Boggs PB, Bahna SL. Reliability of a new hand-held device for the measurement of exhaled nitric oxide. Allergy 2007; 62: 1171–1174. doi: 10.1111/j.1398-9995.2007.01475.x [DOI] [PubMed] [Google Scholar]
- 22.Menou A, Babeanu D, Paruit HN, et al. . Normal values of offline exhaled and nasal nitric oxide in healthy children and teens using chemiluminescence. J Breath Res 2017; 11: 36008. doi: 10.1088/1752-7163/aa76ef [DOI] [PubMed] [Google Scholar]
- 23.Alving K, Janson C, Nordvall L. Performance of a new hand-held device for exhaled nitric oxide measurement in adults and children. Respir Res 2006; 7: 67. doi: 10.1186/1465-9921-7-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sordillo JE, Webb T, Kwan D, et al. . Allergen exposure modifies the relation of sensitisation to fraction of exhaled nitric oxide levels in children at risk for allergy and asthma. J Allergy Clin Immunol 2011; 127: 1165–1172.e5. doi: 10.1016/j.jaci.2011.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strunk RC, Szefler SJ, Phillips BR, et al. . Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol 2003; 112: 883–892. doi: 10.1016/j.jaci.2003.08.014 [DOI] [PubMed] [Google Scholar]
- 26.Bettiol J, Bartsch P, Louis R, et al. . Cytokine production from peripheral whole blood in atopic and nonatopic asthmatics: relationship with blood and sputum eosinophilia and serum IgE levels. Allergy 2000; 55: 1134–1141. doi: 10.1034/j.1398-9995.2000.00711.x [DOI] [PubMed] [Google Scholar]
- 27.Lemière C, Ernst P, Olivenstein R, et al. . Airway inflammation assessed by invasive and noninvasive means in severe asthma: Eosinophilic and noneosinophilic phenotypes. J Allergy Clin Immunol 2006; 118: 1033–1039. doi: 10.1016/j.jaci.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 28.Jouaville LF, Annesi-Maesano I, Nguyen LT, et al. . Interrelationships among asthma, atopy, rhinitis and exhaled nitric oxide in a population-based sample of children. Clin Exp Allergy 2003; 33: 1506–1511. doi: 10.1046/j.1365-2222.2003.01800.x [DOI] [PubMed] [Google Scholar]
- 29.van Asch CJJ, Balemans WAF, Rovers MM, et al. . Atopic disease and exhaled nitric oxide in an unselected population of young adults. Ann Allergy, Asthma Immunol 2008; 100: 59–65. doi: 10.1016/S1081-1206(10)60406-1 [DOI] [PubMed] [Google Scholar]
- 30.Scott M, Raza A, Karmaus W, et al. . Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax 2010; 65: 258–262. doi: 10.1136/thx.2009.125443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kharitonov SA, Rajakulasingam K, O'Connor B, et al. . Nasal nitric oxide is increased in patients with asthma and allergic rhinitis and may be modulated by nasal glucocorticoids. J Allergy Clin Immunol 1997; 99: 58–64. [DOI] [PubMed] [Google Scholar]
- 32.Arnal JF, Didier A, Rami J, et al. . Nasal nitric oxide is increased in allergic rhinitis. Clin Exp Allergy 1997; 27: 358–362. doi: 10.1111/j.1365-2222.1997.tb00719.x [DOI] [PubMed] [Google Scholar]
- 33.Martin U, Bryden K, Devoy M, et al. . Increased levels of exhaled nitric oxide during nasal and oral breathing in subjects with seasonal rhinitis. J Allergy Clin Immunol 1996; 97: 768–772. doi: 10.1016/S0091-6749(96)80154-0 [DOI] [PubMed] [Google Scholar]
- 34.Henriksen AH, Sue-Chu M, Holmen TL, et al. . Exhaled and nasal NO levels in allergic rhinitis: relation to sensitisation, pollen season and bronchial hyperresponsiveness. Eur Respir J 1999; 13: 301–306. doi: 10.1034/j.1399-3003.1999.13b14.x [DOI] [PubMed] [Google Scholar]
- 35.Cardinale F, de Benedictis FM, Muggeo V, et al. . Exhaled nitric oxide, total serum IgE and allergic sensitisation in childhood asthma and allergic rhinitis. Pediatr Allergy Immunol 2005; 16: 236–242. doi: 10.1111/j.1399-3038.2005.00265.x [DOI] [PubMed] [Google Scholar]
- 36.Choi BS, Kim KW, Lee YJ, et al. . Exhaled nitric oxide is associated with allergic inflammation in children. J Korean Med Sci 2011; 26: 1265–1269. doi: 10.3346/jkms.2011.26.10.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gratziou C, Lignos M, Dassiou M, et al. . Influence of atopy on exhaled nitric oxide in patients with stable asthma and rhinitis. Eur Respir J 1999; 14: 897–901. doi: 10.1034/j.1399-3003.1999.14d28.x [DOI] [PubMed] [Google Scholar]
- 38.Peters SP. Asthma phenotypes: nonallergic (Intrinsic) asthma. J Allergy Clin Immunol Pract 2014; 2: 650–652. doi: 10.1016/j.jaip.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 39.Hekking PPW, Bel EH. Developing and emerging clinical asthma phenotypes. J Allergy Clin Immunol Pract 2014; 2: 671–680. doi: 10.1016/j.jaip.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 40.Drews AC, Pizzichini MMM, Pizzichini E, et al. . Neutrophilic airway inflammation is a main feature of induced sputum in nonatopic asthmatic children. Allergy 2009; 64: 1597–1601. doi: 10.1111/j.1398-9995.2009.02057.x [DOI] [PubMed] [Google Scholar]
- 41.Takejima P, Agondi RC, Rodrigues H, et al. . Allergic and nonallergic asthma have distinct phenotypic and genotypic features. Int Arch Allergy Immunol 2017; 172: 150–160. doi: 10.1159/000458151 [DOI] [PubMed] [Google Scholar]
- 42.Douwes J, Gibson P, Pekkanen J, et al. . Non-eosinophilic asthma: importance and possible mechanisms. Thorax 2002; 57: 643–648. doi: 10.1136/thorax.57.7.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franklin PJ, Turner SW, Le Souëf PN, et al. . Exhaled nitric oxide and asthma: complex interactions between atopy, airway responsiveness, and symptoms in a community population of children. Thorax 2003; 58: 1048–1052. doi: 10.1136/thorax.58.12.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moody A, Fergusson W, Wells A, et al. . Increased nitric oxide production in the respiratory tract in asymptomatic Pacific Islanders: an association with skin prick reactivity to house dust mite. J Allergy Clin Immunol 2000; 105: 895–899. doi: 10.1067/mai.2000.105318 [DOI] [PubMed] [Google Scholar]
- 45.Barreto M, Villa MP, Monti F, et al. . Additive effect of eosinophilia and atopy on exhaled nitric oxide levels in children with or without a history of respiratory symptoms. Pediatr Allergy Immunol 2005; 16: 52–58. doi: 10.1111/j.1399-3038.2005.00220.x [DOI] [PubMed] [Google Scholar]
- 46.van Amsterdam JGC, Janssen NAH, de Meer G, et al. . The relationship between exhaled nitric oxide and allergic sensitisation in a random sample of school children. Clin Exp Allergy 2003; 33: 187–191. doi: 10.1046/j.1365-2222.2003.01597.x [DOI] [PubMed] [Google Scholar]
- 47.Gould HJ, Sutton BJ, Beavil AJ, et al. . The biology of IgE and the basis of allergic disease. Annu Rev Immunol 2003; 21: 579–628. doi: 10.1146/annurev.immunol.21.120601.141103 [DOI] [PubMed] [Google Scholar]
- 48.Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol 2007; 7: 365–378. doi: 10.1038/nri2072 [DOI] [PubMed] [Google Scholar]
- 49.Thomassen MJ, Raychaudhuri B, Dweik RA, et al. . Nitric oxide regulation of asthmatic airway inflammation with segmental allergen challenge. J Allergy Clin Immunol 1999; 104: 1174–1182. doi: 10.1016/S0091-6749(99)70010-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
TABLE S1 Associations of overlapping aeroallergen IgE, allergic rhinitis, and asthma with FENO 00945-2020.tableS1 (57.7KB, pdf)
TABLE S2 Associations of asthma, allergic rhinitis, and aeroallergen IgE with FENO using linear regression models, stratified by sex 00945-2020.tableS2 (67.2KB, pdf)
TABLE S3 Associations of asthma, allergic rhinitis, and aeroallergen IgE with FENO using linear and multinomial logistic regression models 00945-2020.tableS3 (68.9KB, pdf)


