Significance
Mosquitoes are major disease vectors that require a gut microbiota for development into adults under some conditions but not others. To determine the underlying mechanisms for this variation, we determined that dietary riboflavin rapidly decays under most environmental conditions that mosquitoes experience, which makes larvae reliant on their gut microbiota for this essential micronutrient. Our findings illustrate the value of being able to both control and manipulate dietary nutrients, microbiota composition, and environmental conditions in studying the role of gut microbes in animal biology. Our results also suggest environmental conditions are an understudied variable in why the gut microbiota is essential for provisioning certain essential vitamins in aquatic organisms like mosquito larvae.
Keywords: insect, microbiota, respiration, growth, molting
Abstract
We previously determined that several diets used to rear Aedes aegypti and other mosquito species support the development of larvae with a gut microbiota but do not support the development of axenic larvae. In contrast, axenic larvae have been shown to develop when fed other diets. To understand the mechanisms underlying this dichotomy, we developed a defined diet that could be manipulated in concert with microbiota composition and environmental conditions. Initial studies showed that axenic larvae could not grow under standard rearing conditions (27 °C, 16-h light: 8-h dark photoperiod) when fed a defined diet but could develop when maintained in darkness. Downstream assays identified riboflavin decay to lumichrome as the key factor that prevented axenic larvae from growing under standard conditions, while gut community members like Escherichia coli rescued development by being able to synthesize riboflavin. Earlier results showed that conventional and gnotobiotic but not axenic larvae exhibit midgut hypoxia under standard rearing conditions, which correlated with activation of several pathways with essential growth functions. In this study, axenic larvae in darkness also exhibited midgut hypoxia and activation of growth signaling but rapidly shifted to midgut normoxia and arrested growth in light, which indicated that gut hypoxia was not due to aerobic respiration by the gut microbiota but did depend on riboflavin that only resident microbes could provide under standard conditions. Overall, our results identify riboflavin provisioning as an essential function for the gut microbiota under most conditions A. aegypti larvae experience in the laboratory and field.
Diet crucially affects the health of all animals (1). Most animals have a gut microbiota that can also affect host health both positively and negatively (2–6). However, understanding of the mechanisms underlying the effects of the gut microbiota remains a major challenge. This is because animals often consume complex or variable diets, and harbor large, multimember microbial communities that can result in many interactions that hinder identification of the factors responsible for particular host responses (2, 6–11). Metaanalyses and multiomic approaches can provide inferential insights on how diet–microbe or microbe–microbe interactions affect hosts (11–18), but functional support can be difficult to generate if proposed mechanisms cannot be studied experimentally (2, 14). Thus, study systems where hosts can be reared on defined diets with or without a microbiota of known composition can significantly advance mechanistic insights by providing the means to control and manipulate dietary, microbial, and environmental variables that potentially affect a given host response (19–21).
Mosquitoes are best known as insects that blood feed on humans and other vertebrates. Only adult-stage female mosquitoes blood feed, which is required for egg formation by most species (22). Blood feeding has also led to several mosquitoes evolving into vectors that can transmit disease-causing microbes between hosts (22). In contrast, the juvenile stages of all mosquitoes are aquatic, with most species feeding on detritivorous diets (22–24). Larvae hatch from eggs with no gut microbiota but quickly acquire relatively low-diversity communities from the environment by feeding (25). Most gut community members are aerobic or facultatively anaerobic bacteria in four phyla (Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria), although other microbes, such as fungi and apicomplexans, have also been identified (25–39). Gut community composition also commonly varies within and between species as a function of where larvae develop, diet, and other variables (28–30, 32, 34, 40–42).
Aedes aegypti has a worldwide distribution in tropical and subtropical regions, and is the primary vector of the agents that cause yellow fever, dengue fever, and lymphatic filariasis in humans (43). Preferentially living in urban habitats, females lay eggs in water-holding containers with microbial communities, and larvae molt through four instars before pupating and emerging as adults (30, 35, 41, 43). Conventionally reared cultures with a gut microbiota are usually maintained in the laboratory under conditions that mimic natural habitats with rearing temperatures of 25 to 28 °C and a 12- to 16-h light: 8- to 12-h dark photoperiod (44–46). Most insects that require microbial partners for survival live on nutrient-poor diets where microbes provision nutrients that cannot be synthesized or produced in sufficient abundance by the host (3). Mosquito larvae can experience resource limitations in the field (23–25), but in the laboratory are reared on undefined, nutrient-rich diets, such as rodent chow, fish food flakes, or mixtures of materials like liver powder, fish meal, and yeast extract (44–46). Nonetheless, our previous studies indicated that axenic A. aegypti as well as other species consume but fail to grow beyond the first instar when fed several diets that support the development of nonsterile, conventionally reared larvae (30, 47–49). Escherichia coli and several other bacteria identified as gut community members could colonize the gut (producing monoxenic, gnotobiotic larvae) and rescue development, but feeding axenic larvae dead bacteria could not (30, 35, 47). The presence of a gut microbiota in conventional and gnotobiotic but not axenic larvae was also associated with midgut hypoxia and activation of several signaling pathways with growth functions (50, 51). Finally, our own previous results using a strain of E. coli susceptible to ampicillin (50), and more recently a method for clearing an auxotrophic strain of E. coli from gnotobiotic larvae (52), both showed that the proportion of individuals that develop into adults correlates with the duration that larvae have living bacteria in their gut.
Altogether, the preceding results suggested that A. aegypti and several other mosquitoes require a gut microbiota for development. In contrast, another recent study showed that axenic A. aegypti larvae develop into adults, albeit more slowly than larvae with a gut microbiota, when fed diets comprised of autoclaved bovine liver powder (LP) and brewer’s yeast (Saccharomyces cerevisiae) extract (YE) or autoclaved LP, YE, and E. coli (EC) embedded in agar (53). This latter finding suggests the undefined dietary components used provide factors larvae require for development into adults, whereas a gut microbiota was also required to provide these factors under the conditions in which our own previous studies were conducted. The goal of this study was to identify what these factors are. Toward this end, we first assessed the growth of axenic A. aegypti when fed diets containing autoclaved LP, YE, and EC under different conditions. We then used this information to develop a defined diet that allowed us to systematically manipulate nutrient, microbial, and environmental variables. We report that the instability of riboflavin is a key factor underlying why A. aegypti larvae require a gut microbiota under most conditions experienced in the laboratory and field.
Results
Axenic Larvae Develop when Fed Autoclaved LP, YE, and EC but Only in Darkness.
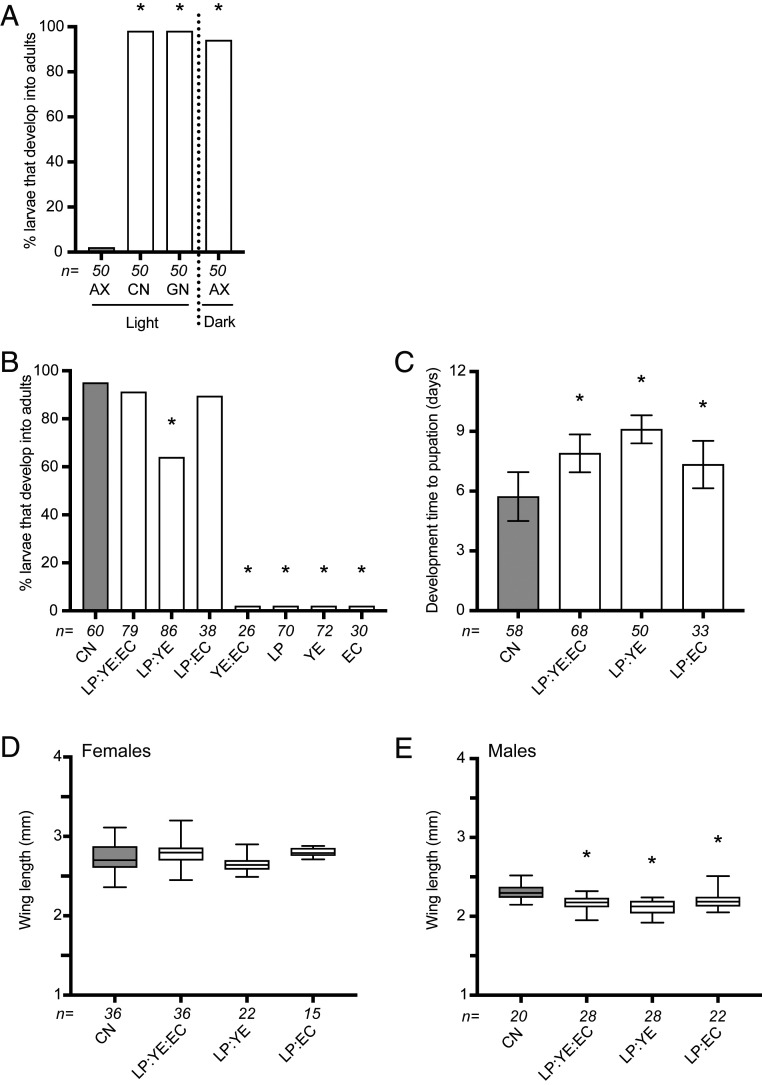
All of our own previous studies in which axenic larvae failed to grow beyond the first instar (30, 47–49) were conducted under previously stated environmental conditions that are usually used to rear A. aegypti in the laboratory (44–46). We thus prepared an LP:YE:EC agar diet as described previously (53) using commercially purchased LP and YE plus E. coli K12 MG1655 (SI Appendix, Table S1), and then tested whether axenic A. aegypti developed under what we henceforth refer to as “standard rearing conditions” (27 °C, 16-h light: 8-h dark photoperiod) by placing five newly hatched first instars per culture well containing 5 mL of water and 1.2 g of diet in an environmental chamber. Results showed that no axenic larvae grew beyond the first instar, although most conventional larvae with a laboratory gut microbiota and gnotobiotic larvae with only living E. coli K12 MG1655 developed into adults (Fig. 1A). Axenic larvae also failed to grow beyond the first instar when fed LP:YE:EC agar and maintained in our insectary or on a laboratory bench. These outcomes were thus the same as we had previously observed when larvae were fed other diets (30, 48) but differed from results showing that axenic A. aegypti do develop when fed LP:YE:EC agar (53). However, environmental conditions in which axenic larvae developed into adults when fed LP:YE:EC agar were also unstated (53). We thus examined whether this was an important variable by feeding axenic larvae LP:YE:EC agar in a cell-culture incubator at 27 °C that maintained larvae in darkness. This alteration resulted in more than 90% of axenic larvae that were fed 1.2 g of LP:YE:EC agar in 5 mL of water developing into adults (Fig. 1A). A similar proportion of conventional larvae fed rat chow diet that was primarily used in earlier studies (30, 47) also developed into adults when maintained in darkness but development times to pupation were shorter and adult male sizes were slightly larger (Fig. 1 A–D). In contrast, no axenic larvae maintained in a cell-culture incubator that were fed rat chow diet or fish food that had also been used in previous studies (48) grew beyond the first instar, although both readily supported the development of conventional larvae (SI Appendix, Fig. S1A).
Fig. 1.
Development of axenic A. aegypti larvae fed different combinations of autoclaved LP, YE, and EC in agar. (A) Percentage of axenic (AX) larvae that develop into adults when fed LP:YE:EC agar in an environmental chamber under standard rearing conditions (light) versus a cell-culture incubator that maintained larvae in darkness. The percentage of conventional (CN) and gnotobiotic (GN) larvae inoculated with living E. coli that developed into adults under standard rearing conditions is also shown. The number below each bar indicates the total number of larvae assayed per treatment group. Asterisks (*) indicate treatment groups that significantly differed from the control (AX light) as determined by pairwise Fisher’s exact tests (P < 0.01). (B) Percentage of larvae that develop into adults when fed LP, YE, or EC together (LP:YE:EC), as paired combinations (LP:YE, LP:EC, YE:EC) or alone (LP, YE, EC). The total number of larvae assayed for each treatment below each bar and asterisks (*) are as defined in A, with the CN treatment serving as the control. (C) Mean development time ± SD (days) to pupation. Numbers below each bar indicates the total number of larvae assayed while an asterisk (*) indicates treatment groups that significantly differed from the positive control (CN) as determined by ANOVA and a post hoc Dunnett’s means comparison test (P < 0.01). (D) Box and whiskers plots showing mean wing length of adult females that emerged when fed different diets. (E) Box and whiskers plots showing mean wing lengths of adult males. Samples sizes and asterisks are as defined in C with the CN treatment serving as the control.
Focusing on LP:YE:EC agar, the proportion of larvae that developed into adults was unchanged when diet amount was increased but decreased when diet amount was reduced (SI Appendix, Fig. S1B). We therefore used 1.2-g agar plugs to assess whether subcomponents of LP:YE:EC agar also supported development in darkness. Larvae fed LP:EC agar exhibited similar survival, development times, and adult sizes as larvae fed LP:YE:EC agar (Fig. 1 B–E). Survival rates were lower and development times were longer for larvae fed LP:YE agar, while no larvae developed beyond the third instar when fed YE:EC agar or agar containing LP, YE, or EC alone (Fig. 1 B–E). Taken together, these results suggested the combination of LP:EC or LP:YE:EC agar provided factors in darkness that resulted in most axenic larvae developing into adults but each component alone did not. We further concluded that: 1) factors in LP:EC or LP:YE:EC agar that larvae require were adversely affected by standard rearing conditions; 2) factors in rat chow diet and fish food diet that larvae require were already inadequate since axenic larvae were unable to develop in either light or dark conditions; and 3) both living E. coli K12 MG1655 in gnotobiotic larvae and community members present in conventional larvae can provide these factors.
Development of Axenic Larvae Is Also Supported by a Defined Diet.
Unambiguously identifying the factors that enabled axenic larvae to develop in darkness when fed autoclaved LP:EC or LP:YE:EC agar could be very difficult given the complex makeup of these components and inability to manipulate them. These constraints could also hinder understanding of how even a well-characterized bacterium like E. coli K12 MG1655 rescues development under standard conditions. We thus assessed whether a defined diet could be produced that also supported the development of axenic larvae. Like many animals, most insects are unable to de novo-synthesize essential amino acids and B vitamins, which must be acquired from consumed food or microbial partners (54). Unlike vertebrates, insects also lack the ability to de novo-synthesize sterols (54). We thus developed a liquid holidic (H) medium that contained defined components, including cholesterol, essential amino acids, and the B vitamins insects are thought to require (SI Appendix, Table S2), but lacked other components, such as vitamins A, C, and D that vertebrates require but not insects (54).
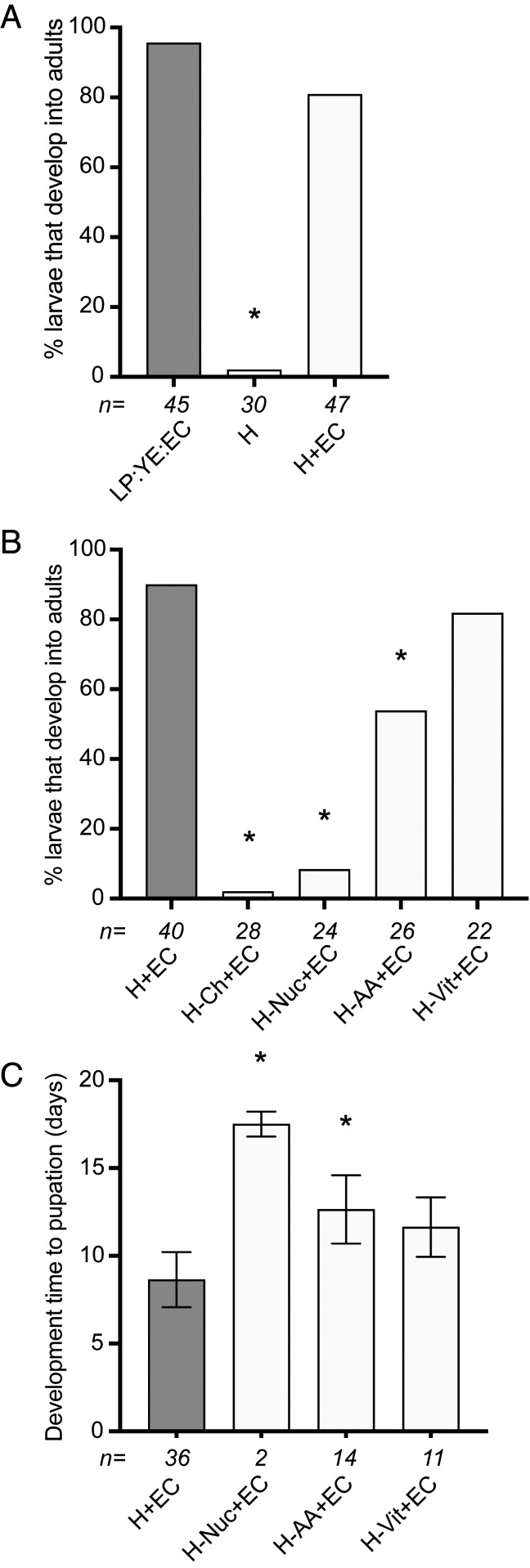
No axenic larvae grew beyond the second instar when fed H medium alone in a cell-culture incubator (Fig. 2A). However, a similar proportion of larvae developed into adults when fed either 1 mL of H medium plus 1.2 g of EC agar in 4 mL of water or 1.2 g of LP:YE:EC agar in 5 mL of water (Fig. 2A). This outcome strongly suggested that H medium provided the factors present in LP or LP:YE that enabled axenic larvae to develop when combined with autoclaved EC. To assess what these factors were, we repeated the previous assay but used H medium in which major categories of nutrients were omitted. No larvae developed beyond the third instar when larvae were fed H medium without cholesterol plus 1.2 g of EC agar (Fig. 2B). This finding was consistent with both LP and YE containing sterols because each derives from eukaryotic sources, while most bacteria including E. coli K12 MG1655 lack sterols due to their inability to synthesize or uptake them (55–57). Omitting the nucleosides (inosine and uridine), the carboxylic sugar myo-inositol, and choline chloride in H medium resulted in a smaller proportion of larvae becoming adults that also exhibited longer development times to pupation (Fig. 2 B and C). Omitting all of the amino acids in H medium also reduced survivorship to adulthood and increased development times (Fig. 2 B and C), whereas omitting all of the B vitamins did not significantly alter either parameter when compared with larvae fed complete H medium plus EC agar (Fig. 2 B and C).
Fig. 2.
Development of axenic A. aegypti larvae fed H medium with or without EC agar. (A) Percentage of larvae that develop into adults when fed H medium alone (H) versus H medium plus EC (H+EC). Larvae fed LP:YE:EC agar served as a positive control. (B) Percentage of larvae that developed into adults when fed H medium without different classes of nutrients (no cholesterol [−Ch], nucleosides and metabolites [−Nuc], amino acids [−AA], or vitamins [−Vit]) plus EC agar. Larvae fed complete H medium plus EC agar (H+EC) served as the positive control. (C) Mean development time ± SD (days) to pupation of larvae fed H medium without different nutrients plus EC or complete H+EC (control). Numbers below bars and asterisks (*) in each graph are defined as in Fig. 1 (P < 0.01).
With the exception of cholesterol, genome and pathway analysis through EcoCyc (58) indicated that E. coli K12 MG1655 can synthesize all of the organic components in H medium including the B vitamins (SI Appendix, Fig. S2). We thus concluded that axenic larvae developed when all of the vitamins in H medium were omitted because sufficient amounts of each remained present in 1.2 g of EC agar despite autoclaving. In contrast, reductions in the proportion of larvae that developed into adults when free amino acids and nucleosides were omitted likely reflected insufficient abundance in autoclaved EC relative to requirements for the number of larvae placed per culture well. We further hypothesized that H medium alone failed to support development because amino acid amounts were insufficient to produce the large amounts of protein required for development into adults. However, increasing free amino acid concentrations in H medium up to solubility limits in water (SI Appendix, Table S3) resulted in all larvae rapidly dying due to high osmotic pressure that mosquito larvae cannot tolerate (54).
We therefore examined whether a purified protein could replace EC since E. coli biomass is also predominantly protein (21). Commercially available bovine lactalbumin (LA) is high in essential amino acids but has low water solubility (59), which was conducive to producing LA agar with a similar colloidal consistency as EC agar. Axenic larvae failed to grow beyond the first instar when fed 1.2 g of LA agar in 5 mL of water, but similar proportions of axenic larvae developed into adults when fed LA or EC agar plus 1 mL of H medium (Fig. 3A). Development time to pupation, adult size, and number of eggs females laid after consuming a blood meal also did not differ between larvae fed LA or EC agar plus H medium (Fig. 3 B–E). However, females fed these diets as larvae laid smaller numbers of eggs than conventionally reared females fed rat chow diet or axenic females that were fed LP:YE:EC agar as larvae (SI Appendix, Fig. S3A). All larvae fed LA agar plus H medium without cholesterol developed into adults, whereas no larvae developed beyond the first instar when fed LA agar plus H medium without vitamins (Fig. 3F). These outcomes were opposite what occurred when larvae were fed H medium plus EC agar (Fig. 2B), but were consistent with LA being known to bind cholesterol (60) but not any of the B vitamins in H medium. We confirmed that the LA used in this study contained 1.25 mg of cholesterol per gram of protein. LA also binds fatty acids (61) but no differences were detected in the proportion of larvae that developed into adults when fed H medium plus LA or H medium plus delipidated LA (SI Appendix, Fig. S3B).
Fig. 3.
Development of axenic A. aegypti larvae fed 1.2 g of LA agar with or without 1 mL of H medium. (A) Percentage of larvae that develop into adults when fed LA agar, H medium plus LA agar (H+LA), or H+EC agar (control). (B) Mean development time ± SD (days) to pupation of larvae fed H+LA or H+EC (control). (C) Box and whiskers plots showing mean wing length of adult females. (D) Box and whiskers plots showing mean wing length of adult females. (E) Number of eggs laid by adult females after consuming a blood meal ± 95% confidence interval. (F) Percentage of larvae that developed into adults when fed H medium without cholesterol (−Ch), all nucleosides and metabolites (−Nuc), amino acids (−AA), or vitamins (−Vit) plus LA agar. Larvae fed complete H+LA served as the control. Numbers below each treatment bar or mean and asterisks are defined as in Fig. 1 (P < 0.01).
Riboflavin Instability Is a Key Factor Underlying the Requirement for a Gut Microbiota Under Most Conditions Larvae Experience in the Laboratory and Field.
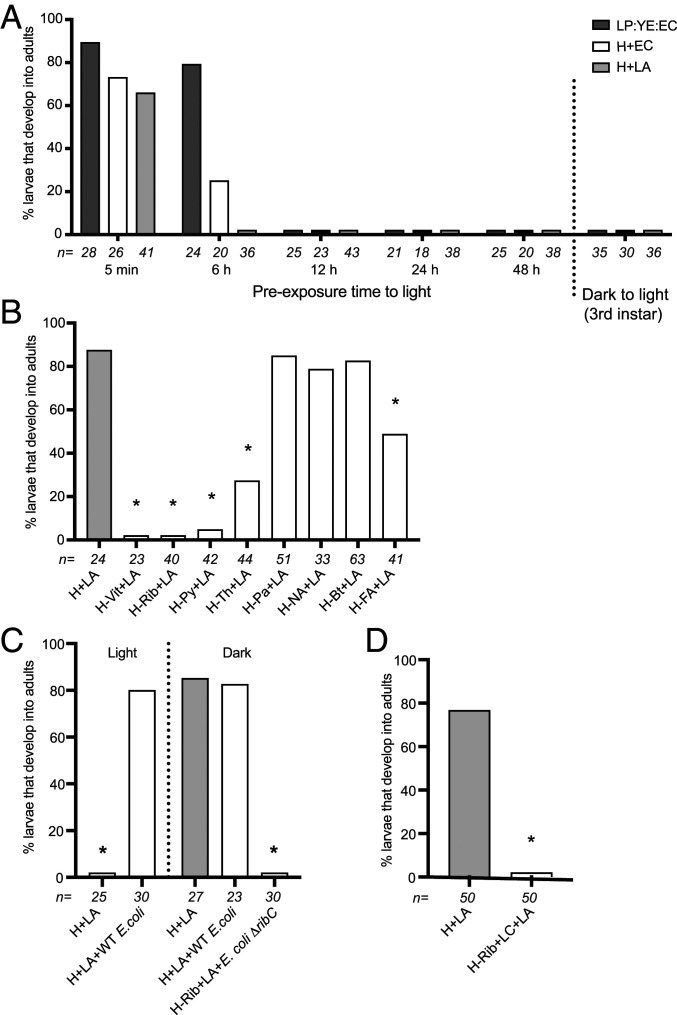
As found for LP:YE:EC agar, no axenic larvae fed H medium plus EC or LA agar grew beyond the first instar when maintained in an environmental chamber or our insectary under standard conditions. We thus directly tested whether axenic larvae fail to grow under standard rearing conditions due to light exposure adversely affecting diet, axenic larvae, or both. No larvae developed beyond the first instar if culture wells containing LP:YE:EC agar, H medium plus EC agar, or H medium plus LA agar were preincubated in an environmental chamber for more than 6 h before adding axenic larvae and transfer to a cell-culture incubator (Fig. 4A). Akin to earlier results showing that clearing gnotobiotic larvae of their microbiota arrests development (50, 52), feeding axenic larvae each diet in a cell-culture incubator until they molted to the third instar before transferring to an environmental chamber also resulted in larvae arresting (Fig. 4A). In contrast, preincubating newly hatched axenic larvae for 24 h before transfer to culture plates and feeding in a cell-culture incubator resulted in most individuals developing into adults (SI Appendix, Fig. S4A). Thus, preexposure of diet but not axenic first instars to standard rearing conditions resulted in larvae not growing beyond the first instar.
Fig. 4.
Development of axenic larvae when fed diets under standard rearing conditions. (A) Percentage of larvae that develop into adults when fed LP:YE:EC agar, H+EC agar, or H+LA agar that was preincubated in an environmental chamber for 5 min to 48 h before transfer to a cell-culture incubator. On the right side of the graph is also shown the percentage of larvae that develop into adults if maintained in a cell-culture incubator until the third instar and then transferred to an environmental chamber. (B) Percentage of larvae that develop into adults when fed H+LA agar that was preincubated in an environmental chamber for 24 h followed by addition of an equivalent amount of all but one of the vitamins in complete H medium before transfer to a cell-culture incubator (all vitamins but: riboflavin [H-Rib+LA], pyroxidine [H-Py+LA], thiamine [H-Th+LA], calcium pantothenate [H-Pa+LA], biotin [H-Bt+LA], or folic acid [H-FA+LA]). Larvae fed H+LA agar that was not preexposed to light served as the positive control while larvae fed H+LA agar that was preincubated in an environmental chamber for 24 h with no replacement vitamins added (H-Vit+LA) served as the negative control. (C) Percentage of gnotobiotic larvae that develop into adults when maintained in an environmental chamber (light) or cell-culture incubator (dark), fed H+LA agar and inoculated with E. coli K12 MG1655 (wild-type E. coli). Axenic larvae fed H+LA agar under light or dark conditions served as controls. The percentage of gnotobiotic larvae that develop into adults when maintained in a cell-culture incubator (dark), fed H+LA in which riboflavin is omitted (H-Rib+LA), and inoculated with E. coli K12 BSV13 (E. coli ΔribC) is also shown. (D) Percentage of axenic larvae that develop into adults when fed H+LA agar (control) versus H+LA in which riboflavin was replaced with lumichrome (H-Rib+LC+LA). Numbers below bars and asterisks (*) in each graph are as defined in Fig. 1 (P < 0.01).
Average illuminance in the environmental chamber was 4,650 lx when lights were on while illuminance in our insectary was similar (4,500 lx). Since some vitamins in H medium are photosensitive (62), we preincubated culture plates containing 1 mL of H medium plus LA agar and 4 mL of water in the environmental chamber for 24 h. We then added the equivalent amount of each vitamin present in 1 mL of H medium plus axenic larvae and transferred the culture plate to a cell-culture incubator. Most larvae developed into adults when all of the vitamins in light preexposed culture wells were replaced, whereas no larvae developed beyond the first instar when no replacement vitamins were added (Fig. 4B). Adding replacement amounts of the other major components in H medium before transfer to darkness also resulted in no larvae molting beyond the first instar (SI Appendix, Fig. S4B), which overall supported that developmental arrest was due to loss of one or more vitamins. We thus repeated the previous experiment but for each treatment replaced all but one of the vitamins in H medium. All axenic larvae remained first instars when no replacement riboflavin was added. Most larvae molted to third or fourth instars but did not pupate when no replacement thiamine, pyridoxine or folic acid was added, while most larvae developed into adults when no calcium pantothenate, nicotinic acid, or biotin was added (Fig. 4B).
We also assessed whether vitamin deficiency was responsible for axenic larvae not growing beyond the first instar, even in darkness when fed rat chow diet or fish food by either adding riboflavin alone or all of the B vitamins in H medium to these undefined diets. Adding no vitamins resulted in larvae remaining first instars when fed either diet, while adding only riboflavin also resulted in no larvae developing into adults but most larvae molted to the third instar (SI Appendix, Fig. S4 C and D). In contrast, adding all of the vitamins in H medium resulted in most larvae developing into adults, which indicated that rat chow diet and fish food had other B vitamin deficiencies in addition to riboflavin that prevented axenic larvae from being able to develop into adults.
However, the preceding results overall indicated that only when riboflavin was not replaced did larvae remain first instars when fed LP:YE:EC agar or H medium plus EC or LA agar under standard conditions, or rat chow diet or fish food as used in previous studies under either standard conditions or darkness. We thus concluded that riboflavin was the key factor that prevented larvae from growing beyond the first instar in all previous studies we had conducted with A. aegypti (30, 47, 48). It had already been determined that wild-type E. coli K12 MG1655 synthesizes riboflavin continuously, which is both retained in cells and secreted (63). We thus added living E. coli K12 MG1655 to culture wells containing H plus LA agar in 4 mL of water, which grew to an average density of 1 × 108 colony forming units per milliliter (SI Appendix, Fig. S4E) while sampling confirmed that all larvae contained living E. coli in their midgut as determined in previous studies (30, 47). Inoculation of axenic larvae with E. coli K12 MG1655 produced monoxenic, gnotobiotic larvae that near fully developed into adults when fed H medium plus LA agar and maintained in either an environmental (light) or cell-culture (dark) incubator (Fig. 4C). In contrast, E. coli K12 strain BSV13, which is a riboflavin auxotroph due to deletion of the ribC gene (64) (SI Appendix, Fig. S2), could not rescue development when larvae were fed H medium without riboflavin plus LA agar in darkness (Fig. 4C).
Riboflavin can degrade into several products, including lumichrome, depending on light, pH, and other factors (65). Adding 1 mL of H medium plus 1.2 g of LA agar to 4 mL of water resulted in a pH of 6.5 while water alone had a pH of 6.8 due to low-level absorption of atmospheric carbon dioxide (66). Under these conditions, HPLC analysis showed that riboflavin remained unchanged after 24 h in a cell-culture incubator but decayed within 9 h in the environmental chamber, with the only major degradation peak having a retention time that matched a lumichrome standard (SI Appendix, Fig. S5). HPLC/MS analysis confirmed this degradation product had an m/z of 243 (M+H)+, which corresponded to the molecular cation of lumichrome (SI Appendix, Fig. S6). Feeding axenic larvae in darkness LA agar plus H medium in which riboflavin was replaced with lumichrome also resulted in no larvae growing beyond the first instar (Fig. 4D). Thus, photodegradation of dietary riboflavin to primarily lumichrome resulted in rapid developmental arrest of axenic larvae that living but not dead E. coli could rescue under standard conditions.
Midgut Hypoxia and Associated Activation of Signaling Pathways Required for Growth Are Riboflavin Dependent.
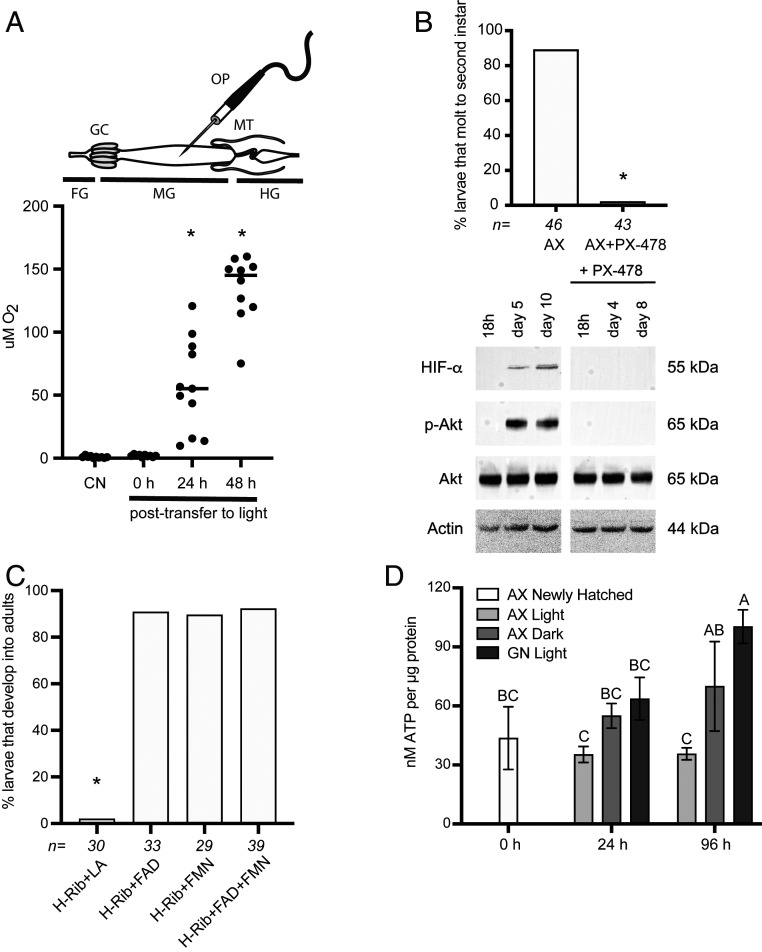
A fluorescent reporter assay previously indicated the midgut lumen was hypoxic in conventional and gnotobiotic larvae inoculated with only E. coli K12 MG1655 but was normoxic in axenic larvae that do not grow under standard conditions (50). Many cellular responses to hypoxia involve conserved hypoxia-inducible transcription factors (HIFs), which are α/β heterodimers that activate HIF-responsive genes (67, 68). HIFs are also often constitutively expressed at the mRNA level, whereas at the protein level HIF-α is targeted for degradation under normoxia by a prolyl hydroxylase but remains stabilized under hypoxia (67). HIFs were similarly behaved in A. aegypti larvae, which resulted in degradation of HIF-α in normoxic axenic larvae but stabilization in hypoxic conventional and gnotobiotic larvae, which as previously noted was associated with activation of insulin–insulin growth factor signaling (IIS) and other pathways with growth functions (51, 69). A specific oral inhibitor (FG4592) of the prolyl hydroxylase that is required for degradation of HIF-α under normoxia further stimulated axenic larvae to molt, whereas a specific oral inhibitor of HIF-α (PX-478) resulted in conventional and gnotobiotic larvae developmentally arresting as occurred in axenic larvae (50, 51).
These findings originally suggested to us that aerobic respiration by the microbiota reduced midgut oxygen levels, which in turn functioned as a signal that stabilized HIFs and activated pathways with growth functions in conventional and gnotobiotic larvae that develop, whereas these events do not occur in axenic larvae that remained first instars (50, 51). However, this interpretation cannot be fully correct, given evidence that axenic larvae can grow provided riboflavin does not degrade. We therefore reassessed the assumption that midgut hypoxia was due to microbial respiration by using a microsensor to quantitatively measure luminal oxygen levels in conventional third instars with a gut microbiota, axenic third instars fed H medium plus LA that were maintained in darkness, and axenic third instars fed H medium plus LA that were moved from darkness to light that, as previously determined, developmentally arrested (Fig. 4A). Strikingly, oxygen levels were near-identically hypoxic in conventional and axenic larvae in darkness, but rapidly shifted to normoxia when axenic larvae were moved to standard conditions (Fig. 5A). HIF-α was also stabilized and the IIS pathway activated in axenic larvae in darkness, while PX-478 inhibited the growth of axenic larvae in darkness, which was associated with destabilization of HIF-α and inhibition of the IIS pathway (Fig. 5B), as previously determined to occur in conventional and gnotobiotic larvae under standard conditions (51). Thus, luminal hypoxia was clearly not due to microbial respiration given luminal oxygen levels were the same in axenic larvae in darkness and conventional larvae. However, these results strongly suggested gut hypoxia was riboflavin-dependent, which under standard conditions could only be provided by resident microbes like wild-type E. coli. That developing axenic larvae in darkness exhibited HIF stabilization and IIS activation but were developmentally arrested by PX-478 further suggested signaling events downstream of riboflavin acquisition were required for growth by both axenic larvae in darkness and larvae with a gut microbiota under standard rearing conditions.
Fig. 5.
Riboflavin, riboflavin metabolites, and pharmacological agents affect midgut hypoxia, HIF-α stabilization, activation of the IIS pathway, and growth. (A) Oxygen concentration in the central midgut lumen of conventional third instars (CN) (control) or axenic (AX) third instars fed complete H+LA in darkness (0 h), or axenic third instars fed H+LA 24 or 48 h after transfer from a cell-culture incubator to an environmental chamber. Above the graph is shown a schematic of the digestive tract with the borders of the fore- (FG), mid- (MG), and hindgut (HG) indicated by the gastric caecae (GC) and Malpighian tubules (MT). Measures were taken by inserting the oxygen probe (OP) into the central lumen of the midgut midway between the GC and MT. Data points for each treatment indicate measures from individual larvae. Asterisks (*) indicate treatments that significantly differed from the CN control as determined by ANOVA and a post hoc Dunnett’s test (P < 0.01). (B) The upper graph shows the proportion of axenic larvae in darkness that molted to the second instar when fed and maintained in the absence (AX) or presence of 50 μM PX-478 (AX+PX-478). Asterisk (*) indicates larvae treated with PX-478 differed from the control (Fisher’s exact test). The lower immunoblots show larval extracts from axenic larvae that were maintained in the absence or presence of 50 µM PX-478 that were probed with antibodies to HIF-α, the phosphorylated form of Akt (p-Akt), the nonphosphorylated form of Akt (Akt), or actin (loading control). Note that the IIS pathway is activated in the absence of PX-478 as evidenced by detection of p-Akt, whereas in the presence of PX-478 the IIS pathway remains nonactivated, as evidenced by detection of only nonphosphorylated Akt. Molecular masses of each target indicated to the right. (C) Percentage of axenic larvae that develop into adults when fed H+LA in which riboflavin was replaced with FAD (H-Rib+FAD+LA), FMN (H-Rib+FMN+LA), or FAD and FMN (H-Rib+FAD & FMN+LA). H medium without riboflavin plus LA (H-Rib+LA) served as the negative control. Numbers above bars and asterisks (*) are as defined in Fig. 1 (P < 0.01). (D) Mean ATP levels (±SD) per microgram of protein in axenic (AX) or gnotobiotic (GN) larvae. Newly hatched axenic larvae served as the 0 h point. ATP levels were first measured in newly hatched axenic larvae.(AX Newly Hatched). ATP levels were then measured at 24 and 96 h after placing axenic larvae in culture wells containing complete H+LA that that were maintained in an environmental chamber (AX Light) or cell-culture incubator (AX Dark), or culture wells containing H+LA and E. coli K12 MG1655 to produce gnotobiotic larvae that were maintained in an environmental chamber (GN Light). Three independent replicates comprised of 20 larvae were processed per treatment. Different letters above a bar indicate samples differed after ANOVA and a Tukey–Kramer honest significant difference test (P < 0.01).
Animals and bacteria like E. coli K12 MG1655 convert riboflavin to flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN), which are cofactors for many flavoproteins, including several enzymes with essential functions in respiratory metabolism (70). Unlike lumichrome, axenic larvae in darkness developed into adults when riboflavin in H medium was replaced with FAD and/or FMN, which supported that FAD and FMN deficiency likely underlies developmental arrest in the absence of riboflavin (Fig. 5C). Since FAD- and FMN-associated enzymes are especially important for energy production, we measured ATP levels, which increased in axenic larvae maintained in darkness and gnotobiotic larvae inoculated with E. coli K12 MG1655, but not in axenic larvae that were maintained under standard rearing conditions where dietary riboflavin rapidly decayed and larvae developmentally arrested as first instars (Fig. 5D).
Discussion
The goal of this study was to disentangle the factors underlying why larval stage A. aegypti and other species of mosquitoes required a gut microbiota for growth in several previous studies (30, 47–52), but not others where larvae were fed undefined but nutrient-rich diets containing LP, YE, and EC (53). We show that similar proportions of axenic A. aegypti from our own culture develop into adults when fed LP:EC or LP:YE:EC agar in darkness, which indicated that the growth-supporting activities of these diets are generalizable and not restricted to a particular population or genotype of A. aegypti. That larvae develop when fed a defined diet like H plus LA agar also indicated that A. aegypti does not require a factor uniquely present in LP:EC or LP:YE:EC agar. Systematic manipulation of H medium identified several nutrients that enable LP or LP:YE to complement EC in supporting the growth of axenic larvae. However, our results indicate the rapid decay of riboflavin is the primary factor underlying why newly hatched axenic larvae remain first instars or older larvae developmentally arrest under standard rearing conditions. Four lines of evidence support this conclusion. First, each diet we studied only supported the growth of axenic larvae in darkness. Second, riboflavin rapidly degrades to primarily lumichrome under standard conditions, which results in newly hatched axenic larvae as well as later instars rapidly arresting. Third, axenic larvae also developmentally arrest when riboflavin is omitted or the primary product of riboflavin decay (lumichrome) is added to H medium plus LA in darkness, but are rescued by FAD or FMN. Fourth, wild-type E. coli K12 MG1655 that synthesizes riboflavin continuously (63) rescues development under standard rearing conditions, whereas an E. coli auxotroph defective for riboflavin synthesis does not even in darkness. Conservation of the riboflavin biosynthetic pathway in many bacteria (71) is further consistent with conventional larvae and several individual gut community members in addition to E. coli being able to rescue the development of axenic larvae under standard conditions (30, 47, 48).
Studies dating to the 1930s describe efforts to produce defined diets for rearing mosquito larvae, although in most cases growth and adult emergence were poor (72–74). Insights into how diet composition or microbes affected outcomes were also unclear in these early studies because no culture-dependent or -independent methods were reported for assessing sterility. In addition to monitoring for sterility and microbiota composition in gnotobiotic larvae in every assay, our approach to diet development differed from past efforts in the sense that our first goal was to determine whether an H medium could provide insights into: 1) what LP or LP:YE likely provide that enable larvae in darkness to grow when combined with autoclaved EC, and 2) whether autoclaved EC could be replaced by a protein source when larvae were also fed H medium. Our second goal was then to determine how specific nutrients and resident microbes interact under different environmental conditions to affect host development. In formulating H medium, we initially used nutrient amounts that were similar to previously reported diets for the mosquito Culex pipiens and Drosophila melanogaster (19, 75). Initial efforts resulted in very poor survival but improved by modifying salt, trace metal, amino acid, and nucleoside composition. In the case of amino acids, for example, we included the nine essential amino acids plus alanine but as found in studies with other insects (54), further assays suggested the addition of select nonessential amino acids improved performance. These modifications also enabled replacement of autoclaved EC with autoclaved LA, which supported our hypothesis that free amino acid levels in H medium promote development when fed to axenic larvae with EC agar but alone were insufficient for growth into adults. However, we reemphasize that the primary purpose in developing a defined diet for this study was to facilitate identification of the factors underlying why larvae required a gut microbiota for development under standard rearing conditions. Thus, additional refinements could potentially further improve the defined diet we developed in terms of, for example, adult performance, where egg production was lower for axenic versus conventional females.
It has long been hypothesized that gut microbes are sources of B vitamins in different animals, including insects (17, 76–80, 81, 82). In contrast, whether diet or the gut microbiota is the primary source of these vitamins is unclear in most species. Our results indicate several B vitamins A. aegypti requires for development decay in LP:YE:EC agar, H medium plus EC agar, or H medium plus LA agar under standard rearing conditions. Our results also indicate that rodent chow-based diets and fish food that are commonly used to rear mosquito larvae (44–46) are also vitamin-deficient due to storage or other factors. As previously stated, we focused this study on riboflavin because only omission of this micronutrient from H medium resulted in newly hatched axenic larvae not being able to grow beyond the first instar, which was characteristic of axenic larvae in all previous studies we had conducted where larvae were maintained under standard conditions (30, 47–51). Studies of human and domestic animal nutrition generally conclude that adequate amounts of dietary riboflavin from plants or other sources remain available after cooking because riboflavin is relatively stable to heat at neutral to acidic conditions (70, 83). This would also appear to be the case for LP:YE:EC agar if used in darkness. In contrast, our results show that riboflavin near fully degrades to lumichrome within 9 h under standard rearing conditions for most mosquito larvae, which results in axenic first instars never molting and later instars rapidly arresting in the absence of resident microbes. Illuminance in our insectary averages 4,500 lx, but outdoor illuminance typically cycles from 0 to 100,000 lx (84), which suggests decay of dietary riboflavin may be more rapid in some field habitats but could also be slower in others, such as tree holes or cisterns. Vertebrates lack the ability to store appreciable amounts of riboflavin (85), which our results suggest is also the case for A. aegypti given how rapidly axenic larvae developmentally arrest in the absence of riboflavin. Altogether, our results suggest the environmental instability of several B vitamins may be an underrecognized variable in vitamin provisioning by gut microbes generally and to aquatic organisms, like mosquito larvae, in particular. While bacteria like E. coli can secrete riboflavin via flavin transporters (63, 86), digestion of ingested microbes by host animals may also be a means of acquisition.
Like other animals, insects including A. aegypti encode FAD synthase and riboflavin kinase, which convert riboflavin to FAD and FMN that are cofactors for hundreds of flavoproteins (70). Known functions for these flavoproteins further range from energy metabolism to conversion of other B vitamins into cofactors (70, 71). The physiological effects of riboflavin deficiency have been much less studied in insects than mammals but are likely wide-ranging. Given how quickly riboflavin loss causes axenic A. aegypti to developmentally arrest suggests the effects of riboflavin deficiency are also likely severe in other mosquito species. That riboflavin deficiency prevents ATP levels from rising is also consistent with the conserved, essential roles of FAD and FMN in energy production, which could play a central role in disabling metabolism, downstream signaling through HIFs, and activation of key pathways that regulate the growth of larvae into adults.
Luminal hypoxia in the midgut, hindgut, or both is known to occur in other insects besides mosquitoes (87, 88). Parallel studies in mammals also document the transformation of the gut in early life from normoxia to hypoxia or anoxia, which mirrors shifts in the gut microbiota from a predominance of aerobic and facultatively anaerobic bacteria to an abundance of anaerobes (89). It has been assumed in both insects and mammals that respiration by the microbiota is the primary driver of intestinal hypoxia (87–89). We had assumed this as well in our own previous studies with A. aegypti (50), but results reported here indicate this is incorrect. Recent studies also show that axenic and conventionally reared mice exhibit near identical low oxygen levels in the distal intestine (89). In contrast, the mechanisms responsible for maintaining luminal hypoxia in the absence of a microbiota remain unclear. Studies conducted in a closed vessel to simulate the mouse gut during digestion showed that chemical oxidation of dietary proteins or lipids reduced oxygen levels, but reaction rates were too slow to fully account for the low oxygen levels maintained in the lumen (90). In contrast, other studies in vertebrates show that oxygen consumption by the colon during digestion is larger than the amount of oxygen blood flow provides, which suggests metabolism by the gut itself may result in consumption of luminal oxygen (89). Our results in A. aegypti also align with respiratory metabolism being an important determinant, since luminal hypoxia was maintained when riboflavin was available but normoxia rapidly ensued upon riboflavin depletion. That PX-478 destabilizes HIF-α and IIS activation and arrests the growth of both axenic larvae in darkness and conventional/gnotobiotic larvae in light (51) further supports that gut hypoxia and activation of growth-associated signaling pathways are linked downstream to riboflavin availability.
In conclusion, we show in this study that resident microbes are an essential source of riboflavin and other B vitamins under most conditions A. aegypti larvae experience in the laboratory and field. Our results further indicate that midgut hypoxia is not due to oxygen consumption by the microbiota but does depend on riboflavin, which could only be provided by E. coli or other gut community members under standard rearing conditions. The effects of PX-478 treatment further suggests riboflavin-dependent stabilization of HIF-α and activation of the IIS pathway are also required for growth of both axenic larvae in darkness or larvae with a gut microbiota under standard rearing conditions. Our results generally highlight the value of being able to concurrently control and manipulate diet, microbiota, and environmental conditions when trying to understand the mechanisms underlying gut microbiota functions. That well-characterized bacteria like E. coli K12 MG1655 can colonize and rescue development of A. aegypti is another potential tool in characterizing other functions of the gut microbiota in mosquito biology.
Materials and Methods
Mosquitoes.
The University of Georgia strain (UGAL) of A. aegypti (51), which was maintained in an insectary at 27 °C with 16-h light: 8-h dark photoperiod, was used in the study. Conventional larvae were hatched from unsterilized eggs and reared in 1-L pans by feeding them either rat chow diet which consisted of equal parts powdered rat chow (LabDiet), lactalbumin (MP), and dead torula yeast (Frontier Scientific Services) (1:1:1 [wt/wt]) or fish food (TetraColor Tropical Granules, Tetra). Both diets were stored at −20 °C after preparation or purchase. Adults were maintained in large population cages where they were provided water and a 10% sucrose solution in water ad libitum. Adult females were blood-fed using membrane feeders and commercially purchased sterile, defibrinated rabbit blood (Hemostat Laboratories).
Other Media.
LP, YE, and EC agar diets were prepared similarly to previous descriptions (53) (SI Appendix, Table S1). Each diet was stored at 4 °C in darkness before use. Preparation of H medium and LA agar are summarized in SI Appendix, Table S2. In brief, H medium stock solutions were prepared in milliQ water, except cholesterol, which was prepared in absolute ethanol. Stock solutions were filter-sterilized using 0.22-μm filters, and stored at 4 °C in darkness in disposable plasticware. Stock solutions were then used to prepare 100-mL aliquots of H medium that were also stored at 4 °C in darkness. H medium components were omitted by replacing particular stock solutions with water, preparing stock solutions in which particular components were omitted, or preparing stock solutions in which riboflavin was replaced with lumichrome, FAD, FMN, or FAD and FMN (Cayman).
Rearing Assays.
Axenic A. aegypti larvae were obtained by hatching surface-sterilized eggs as previously described (30). All rearing assays were conducted in an environmental chamber (Percival) or insectary (16-h light: 8-h dark photoperiod), or a cell-culture incubator (no photoperiod) at 27 °C using six-well sterile culture plates (Corning). Five milliliters of sterile water, 4 mL of sterile water plus 1 mL of H medium or 0.3 to 1.8 g of agar-embedded diet components were added per well followed by five axenic larvae per well, with all assays independently replicated a minimum of five times. For gnotobiotic treatments, wild-type E. coli K12 MG1655 and BSV13 (64) obtained from the Yale University E. coli Genetic Stock Center were grown in Luria Broth (LB) to an optical density of 1.0 at 600 nm, which equals 1 × 106 cells per microliter (30). E. coli K12 strain BSV13 was also Sanger sequenced across the ribC locus, which confirmed inactivation of this gene. All bacteria were pelleted by centrifugation, resuspended in sterile water, and added to six-well plates at a density of ∼1 × 105 cells per culture well containing a given diet and axenic larvae to produce monoxenic, gnotobiotic larvae (30, 47). A dilution series was performed to measure colony forming units per milliliter in cultures containing gnotobiotic larvae. For conventional treatments, a 50-µL drop of water from rearing pans of our general culture was added per well containing 5 mL of water and axenic larvae followed by feeding rat chow diet or fish food, as also used in the general culture. Depending on treatment, the total number of larvae that developed into adults, development time to pupation, adult size as estimated by wing length, and number of eggs laid by individual females after consuming a blood meal from a membrane feeder were determined using previously established methods (47). Water from culture wells, homogenized larvae, or homogenized adults were assessed for contamination or successful inoculation by culturing on LB or YPD (RPI) agar and PCR after DNA extraction using taxon-specific or 16S rRNA gene primers, as previously described (30, 47, 48). Illuminance in environmental chambers and the insectary was measured using an iPhone (Apple) and Lux Light Meter Pro software.
Delipidation of LA and Cholesterol Determination.
Lipids including cholesterol were extracted using 20 mL of 2:1 chloroform-methanol per gram of LA (91). After solvent removal by filtration, the LA was rinsed with a second volume of solvent and after drying was used to make LA agar, as described above. The amount of cholesterol present in the solvent fraction was then determined using the Amplex red assay kit (ThermoFisher).
Riboflavin Stability.
Five milliliters of milliQ water containing riboflavin (100 μg/mL) was added to wells of six-well culture plates that were placed in either the environmental chamber or cell-culture incubator used in bioassays. A Beckman 126/166 HPLC unit equipped with a diode array detector and a Phenomenex Luna C18 (2) column (100 A; 250 × 4.6 mm) was used to monitor riboflavin and its degradation products by collecting samples from culture plates at different time points and injecting them (100 μL) onto the column. Samples were then isocratically eluted with 50% methanol-50% water (HPLC grade, Fisher) at 1 mL/min as monitored at 224 nm (92). Riboflavin and the primary degradation product, lumichrome, were identified by comparison with standards followed by fraction collection and introduction into a Bruker Esquire 3000 Plus ion trap using alternating positive and negative mode via loop injections with methanol as the carrier solvent at a flow of 5.4 μL/mL.
Midgut Luminal Oxygen Levels.
Oxygen levels in the midgut lumen of third instars were measured using Clark-type oxygen microelectrodes at room temperature (OX-10; Unisense). Electrodes were polarized and calibrated using CAL 300 chambers (Unisense) containing water saturated with air or an anoxic solution of 0.1 M sodium hydroxide and 0.1 M sodium ascorbate. Calibration was carried out for more than 2 h before data collection. For each larva, the digestive tract was explanted by dissection in water followed immediately by positioning the microelectrode lengthwise to midpoint of the midgut and insertion into the central lumen using a manual micromanipulator and Leica stereomicroscope to monitor tip position. Oxygen levels were recorded at 1-s intervals using UniAmp and SensorTrace Rate software (Unisense). Each sample could be measured within 30 s, which was followed by water replacement after each dissection. A minimum of four third instars were measured per treatment.
HIF-α and Phosphorylated Akt.
A 10-mM stock of PX-478 (Selleckchem) was prepared in water followed by use at a working concentration (50 μM) that had previously been determined to inhibit the growth of conventional and gnotobiotic larvae (51). Axenic larvae fed in darkness with or without 50 μM PX-478 were collected in Proprep protein extraction buffer (Intron Biotechnology) containing 10× protease/phosphatase inhibitor mixture (Halt; ThermoFisher) with protein concentrations determined using Coomassie Plus Protein Reagent (ThermoFisher). Samples were resuspended in Laemmli buffer with mercaptoethanol (10 µM), electrophoresed (100 µg per lane) on 4 to 20% Tris⋅HCl gels (Bio-Rad), followed by transfer to polyvinyl difluoride (PVDF; ThermoFisher). After blocking in 5% nonfat dry milk in PBS+0.1%Tween20 for 1 h, blots were probed with rabbit anti-HIF-α (1:5,000) (51), phospho-Drosophila Akt (Ser505) (1:1,000; 4054 Cell Signaling Technology), Akt (1:1,000; 9272 Cell Signaling Technology), or anti-Actin (1:1,000; A2103 Sigma Aldrich), which was used as a loading control. Samples were then washed and probed with a peroxidase-conjugated goat anti-rabbit secondary antibody (Jackson; 1:5,000) followed by visualization using a chemiluminescent substrate (Clarity Western ECL Substrate, Bio-Rad) and Syngene imaging system.
ATP Measurement.
Newly hatched axenic larvae were added to culture wells containing H medium plus LA followed by placement in an environmental chamber or cell-culture incubator. Twenty whole larvae were then collected at specific times, gently blotted to remove residual water, and weighed followed by addition of 40 μL of a chaotropic buffer (6 M guanidine HCl, 100 mM Tris [pH 7.8], 4 mM EDTA) per milligram of tissue that stabilizes adenylates (93). After addition of beads, samples were homogenized using a FastPrep-24 homogenizer (MP Bio) at 4.0 m/s for 30 s. Protein concentration of supernatants were determined using a commercially available kit (Bio-Rad) before storing samples at −80 °C. ATP concentrations were then determined using a luminescent ATP detection kit (Abcam) following the manufacturer’s instructions. Each treatment was replicated in triplicate followed by determination of average ATP concentration.
Genome and Data Analysis.
For the E. coli K12 MG1655 genome, gene prediction and annotation were performed using EcoCyc (58). Statistical analyses were performed using JMP 11 (SAS). The proportion of larvae in different treatments that developed into adults were pooled across replicates and analyzed by pairwise Fisher’s exact tests that compared treatments with a positive control. Development time to pupation, adult sizes, number of eggs laid, luminal oxygen levels, and ATP concentrations were analyzed by one-way ANOVA followed by post hoc Tukey-Kramer honest significant difference or Dunn’s Test that compared treatment with the positive control.
Supplementary Material
Acknowledgments
We thank Ms. L. South for assistance with rearing of mosquitoes; Dr. D. Phillips in the Georgia mass spectrometry core facility for running samples; Dr. V. G. Martinson for comments on the manuscript; and Dr. B. Steven for sharing that axenic larvae, which developed into adults in earlier studies, were maintained in darkness. This work was supported by NIH Grant R01AI106892, United States Department of Agriculture National Institute of Food and Agriculture Hatch Project GEO00772, and the Pulliam endowment (to M.R.S.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2101080118/-/DCSupplemental.
Data Availability
All study data are included in the article and SI Appendix.
References
- 1.Simpson S. J., Raubenheimer D., The Nature of Nutrition (Princeton University Press, Princeton, 2012). [Google Scholar]
- 2.Sommer F., Bäckhed F., The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 11, 227–238 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Engel P., Moran N. A., The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 37, 699–735 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Hooper L. V., Littman D. R., Macpherson A. J., Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libertucci J., Young V. B., The role of the microbiota in infectious diseases. Nat. Microbiol. 4, 35–45 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Carding S., Verbeke K., Vipond D. T., Corfe B. M., Owen L. J., Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 26, 26191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rakoff-Nahoum S., Foster K. R., Comstock L. E., The evolution of cooperation within the gut microbiota. Nature 533, 255–259 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnúsdóttir S., Thiele I., Modeling metabolism of the human gut microbiome. Curr. Opin. Biotechnol. 51, 90–96 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Zelezniak A., et al., Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proc. Natl. Acad. Sci. U.S.A. 112, 6449–6454 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David L. A., et al., Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J., et al., Dietary history contributes to enterotype-like clustering and functional metagenomic content in the intestinal microbiome of wild mice. Proc. Natl. Acad. Sci. U.S.A. 111, E2703–E2710 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heintz-Buschart A., et al., Integrated multi-omics of the human gut microbiome in a case study of familial type 1 diabetes. Nat. Microbiol. 2, 16180 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Heintz-Buschart A., Wilmes P., Human gut microbiome: Function matters. Trends Microbiol. 26, 563–574 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Brüssow H., Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 13, 423–434 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng H., et al., Division of labor in honey bee gut microbiota for plant polysaccharide digestion. Proc. Natl. Acad. Sci. U.S.A. 116, 25909–25916 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jing T.-Z., Qi F.-H., Wang Z.-Y., Most dominant roles of insect gut bacteria: Digestion, detoxification, or essential nutrient provision? Microbiome 8, 38 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong A. C.-N., Dobson A. J., Douglas A. E., Gut microbiota dictates the metabolic response of Drosophila to diet. J. Exp. Biol. 217, 1894–1901 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould A. L., et al., Microbiome interactions shape host fitness. Proc. Natl. Acad. Sci. U.S.A. 115, E11951–E11960 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piper M. D., et al., A holidic medium for Drosophila melanogaster. Nat. Methods 11, 100–105 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sannino D. R., Dobson A. J., Edwards K., Angert E. R., Buchon N., The Drosophila melanogaster microbiota provisions thiamine to its host. MBio 9, e00155-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henriques S. F., et al., Metabolic cross-feeding in imbalanced diets allows gut microbes to improve reproduction and alter host behaviour. Nat. Commun. 11, 4236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clements A. N., The Biology of Mosquitoes, Vol. 1. Development, Nutrition, and Reproduction (Chapman & Hall, New York, 1992). [Google Scholar]
- 23.Briegel H., Physiological bases of mosquito ecology. J. Vector Ecol. 28, 1–11 (2003). [PubMed] [Google Scholar]
- 24.Merritt R. W., Dadd R. H., Walker E. D., Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu. Rev. Entomol. 37, 349–376 (1992). [DOI] [PubMed] [Google Scholar]
- 25.Strand M. R., Composition and functional roles of the gut microbiota in mosquitoes. Curr. Opin. Insect Sci. 28, 59–65 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favia G., et al., Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc. Natl. Acad. Sci. U.S.A. 104, 9047–9051 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Gilbreath T. M. 3rd, Kukutla P., Yan G., Xu J., Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS One 6, e24767 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boissière A., et al., Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 8, e1002742 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gimonneau G., et al., Composition of Anopheles coluzzii and Anopheles gambiae microbiota from larval to adult stages. Infect. Genet. Evol. 28, 715–724 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Coon K. L., Vogel K. J., Brown M. R., Strand M. R., Mosquitoes rely on their gut microbiota for development. Mol. Ecol. 23, 2727–2739 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muturi E. J., Kim C. H., Bara J., Bach E. M., Siddappaji M. H., Culex pipiens and Culex restuans mosquitoes harbor distinct microbiota dominated by few bacterial taxa. Parasit. Vectors 9, 18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duguma D., et al., Developmental succession of the microbiome of Culex mosquitoes. BMC Microbiol. 15, 140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buck M., et al., Bacterial associations reveal spatial population dynamics in Anopheles gambiae mosquitoes. Sci. Rep. 6, 22806 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muturi E. J., Bara J. J., Rooney A. P., Hansen A. K., Midgut fungal and bacterial microbiota of Aedes triseriatus and Aedes japonicus shift in response to La Crosse virus infection. Mol. Ecol. 25, 4075–4090 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Thongsripong P., et al., Mosquito vector-associated microbiota: Metabarcoding bacteria and eukaryotic symbionts across habitat types in Thailand endemic for dengue and other arthropod-borne diseases. Ecol. Evol. 8, 1352–1368 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chandler J. A., Liu R. M., Bennett S. N., RNA shotgun metagenomic sequencing of northern California (USA) mosquitoes uncovers viruses, bacteria, and fungi. Front. Microbiol. 6, 185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belda E., et al., Preferential suppression of Anopheles gambiae host sequences allows detection of the mosquito eukaryotic microbiome. Sci. Rep. 7, 3241 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steyn A., Roets F., Botha A., Yeasts associated with Culex pipiens and Culex theileri mosquito larvae and the effect of selected yeast strains on the ontogeny of Culex pipiens. Microb. Ecol. 71, 747–760 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Bozic J., et al., Mosquitoes can harbour yeasts of clinical significance and contribute to their environmental dissemination. Environ. Microbiol. Rep. 9, 642–648 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Osei-Poku J., Mbogo C. M., Palmer W. J., Jiggins F. M., Deep sequencing reveals extensive variation in the gut microbiota of wild mosquitoes from Kenya. Mol. Ecol. 21, 5138–5150 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Coon K. L., Brown M. R., Strand M. R., Mosquitoes host communities of bacteria that are essential for development but vary greatly between local habitats. Mol. Ecol. 25, 5806–5826 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dada N., et al., Comparative assessment of the bacterial communities associated with Aedes aegypti larvae and water from domestic water storage containers. Parasit. Vectors 7, 391 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powell J. R., Tabachnick W. J., History of domestication and spread of Aedes aegypti—A review. Mem. Inst. Oswaldo Cruz 108 (suppl. 1), 11–17 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bond J. G., et al., Efficiency of two larval diets for mass-rearing of the mosquito Aedes aegypti. PLoS One 12, e0187420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puggioli A., et al., Efficiency of three diets for larval development in mass rearing Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 50, 819–825 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Kauffman E., et al., Rearing of Culex spp. and Aedes spp. mosquitoes. Bio Protoc. 7, 2542 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coon K. L., Brown M. R., Strand M. R., Gut bacteria differentially affect egg production in the anautogenous mosquito Aedes aegypti and facultatively autogenous mosquito Aedes atropalpus (Diptera: Culicidae). Parasit. Vectors 9, 375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valzania L., et al., Both living bacteria and eukaryotes in the mosquito gut promote growth of larvae. PLoS Negl. Trop. Dis. 12, e0006638 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coon K. L., Valzania L., Brown M. R., Strand M. R., Predaceous Toxorhynchites mosquitoes require a living gut microbiota to develop. Proc. Biol. Sci. 287, 20192705 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coon K. L., et al., Bacteria-mediated hypoxia functions as a signal for mosquito development. Proc. Natl. Acad. Sci. U.S.A. 114, E5362–E5369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valzania L., Coon K. L., Vogel K. J., Brown M. R., Strand M. R., Hypoxia-induced transcription factor signaling is essential for larval growth of the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 115, 457–465 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romoli O., Schönbeck J. C., Hapfelmeier S., Gendrin M., Production of germ-free mosquitoes via transient colonisation allows stage-specific investigation of host-microbiota interactions. Nat. Commun. 12, 942 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Correa M. A., Matusovsky B., Brackney D. E., Steven B., Generation of axenic Aedes aegypti demonstrate live bacteria are not required for mosquito development. Nat. Commun. 9, 4464 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dadd R. H., Insect nutrition: Current developments and metabolic implications. Annu. Rev. Entomol. 18, 381–420 (1973). [DOI] [PubMed] [Google Scholar]
- 55.Huang Z., London E., Cholesterol lipids and cholesterol-containing lipid rafts in bacteria. Chem. Phys. Lipids 199, 11–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei J. H., Yin X., Welander P. V., Sterol synthesis in diverse bacteria. Front. Microbiol. 7, 990 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nes W. D., Biosynthesis of cholesterol and other sterols. Chem. Rev. 111, 6423–6451 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keseler I. M., et al., The EcoCyc database: Reflecting new knowledge about Escherichia coli K-12. Nucleic Acids Res. 45, D543–D550 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ebner K., Brodbeck U., Biological role of alpha-lactalbumin: A review. J. Dairy Sci. 51, 317–322 (1968). [DOI] [PubMed] [Google Scholar]
- 60.Ansbacher S., Supplee G. C., The cholesterol content and the antirachitic activation of milk constituents. J. Biol. Chem. 105, 391–404 (1934). [Google Scholar]
- 61.Cawthern K. M., Narayan M., Chaudhuri D., Permyakov E. A., Berliner L. J., Interactions of alpha-lactalbumin with fatty acids and spin label analogs. J. Biol. Chem. 272, 30812–30816 (1997). [DOI] [PubMed] [Google Scholar]
- 62.Schnellbaecher A., Binder D., Bellmaine S., Zimmer A., Vitamins in cell culture media: Stability and stabilization strategies. Biotechnol. Bioeng. 116, 1537–1555 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X., Wang Q., Qi Q., Identification of riboflavin: Revealing different metabolic characteristics between Escherichia coli BL21(DE3) and MG1655. FEMS Microbiol. Lett. 362, fnv071 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Bandrin S. V., Rabinovich P. M., Stepanov A. I., [3 linkage groups of the genes of riboflavin biosynthesis in Escherichia coli]. [in Russian] Genetika 19, 1419–1425 (1983). [PubMed] [Google Scholar]
- 65.Sheraz M. A., Kazi S. H., Ahmed S., Anwar Z., Ahmad I., Photo, thermal and chemical degradation of riboflavin. Beilstein J. Org. Chem. 10, 1999–2012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vazquez G., Antorrena G., Navaza J. M., Santos V., Absorption of CO2 by water and surfactant solutions in the presence of induced Marangoni effect. Chem. Eng. Sci. 51, 3317–3324 (1996). [Google Scholar]
- 67.Semenza G. L., Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE 2007, cm8 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Ward J. P. T., Oxygen sensors in context. Biochim. Biophys. Acta 1777, 1–14 (2008). [DOI] [PubMed] [Google Scholar]
- 69.Vogel K. J., Valzania L., Coon K. L., Brown M. R., Strand M. R., Transcriptome sequencing reveals large-scale changes in axenic Aedes aegypti larvae. PLoS Negl. Trop. Dis. 11, e0005273 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCormick D. B., “Riboflavin” in Present Knowledge in Nutrition, Erdman J. W., Macdonald I. A., Zeisel S. H., Eds (Wiley-Blackwell, Oxford, ed. 10, 2012), pp. 280–292. [Google Scholar]
- 71.García-Angulo V. A., Overlapping riboflavin supply pathways in bacteria. Crit. Rev. Microbiol. 43, 196–209 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Trager W., On the nutritional requirements of mosquito larvae (Aedes aegypti). Am. J. Hyg. 22, 475–493 (1935). [Google Scholar]
- 73.Lea A. O., Dimond J. B., DeLong D. M., A chemically defined medium for rearing Aedes aegypti larvae. J Econ Ent 49, 313–315 (1956). [Google Scholar]
- 74.Nayar J., A method of rearing salt-marsh mosquito larvae in a defined sterile medium. Ann. Entomol. Soc. Am. 59, 1283–1285 (1966). [Google Scholar]
- 75.Dadd R. H., Kleinjan J. E., Chemically defined dietary media for larvae of the mosquito Culex pipiens (Diptera: Culicidae): Effects of colloid texturizers. J. Med. Entomol. 13, 285–291 (1976). [DOI] [PubMed] [Google Scholar]
- 76.LeBlanc J. G., et al., Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 24, 160–168 (2013). [DOI] [PubMed] [Google Scholar]
- 77.Qi B., Kniazeva M., Han M., A vitamin-B2-sensing mechanism that regulates gut protease activity to impact animal’s food behavior and growth. eLife 6, e26243 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salem H., et al., Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proc. Biol. Sci. 281, 20141838 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Snyder A. K., Rio R. V., “Wigglesworthia morsitans” folate (vitamin B9) biosynthesis contributes to tsetse host fitness. Appl. Environ. Microbiol. 81, 5375–5386 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blatch S. A., Meyer K. W., Harrison J. F., Effects of dietary folic acid level and symbiotic folate production on fitness and development in the fruit fly Drosophila melanogaster. Fly (Austin) 4, 312–319 (2010). [DOI] [PubMed] [Google Scholar]
- 81.Douglas A. E., The B vitamin nutrition of insects: The contributions of diet, microbiome and horizontally acquired genes. Curr. Opin. Insect Sci. 23, 65–69 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Sannino D. R., Dobson A. J., Edwards K., Angert E. R., Buchon N., The Drosophila melanogaster gut microbiota provisions thiamine to its host. MBio 9, e00155–e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miquel Becker E., Christensen J., Frederiksen C. S., Haugaard V. K., Front-face fluorescence spectroscopy and chemometrics in analysis of yogurt: Rapid analysis of riboflavin. J. Dairy Sci. 86, 2508–2515 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Knoop M., et al., Daylight: What makes the difference. Light. Res. Technol. 52, 423–442 (2020). [Google Scholar]
- 85.Suwannasom N., Kao I., Pruß A., Georgieva R., Bäumler H., Riboflavin: The health benefits of a forgotten natural vitamin. Int. J. Mol. Sci. 21, 950 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McAnulty M. J., Wood T. K., YeeO from Escherichia coli exports flavins. Bioengineered 5, 386–392 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brune A., Emerson D., Breznak J. A., The termite gut microflora as an oxygen sink: Microelectrode determination of oxygen and pH gradients in gut of lower and higher termites. Appl. Environ. Microbiol. 61, 2681–2687 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ceja-Navarro J. A., et al., Compartmentalized microbial composition, oxygen gradients and nitrogen fixation in the gut of Odontotaenius disjunctus. ISME J. 8, 6–18 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng L., Kelly C. J., Colgan S. P., Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: Cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 309, C350–C360 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Friedman E. S., et al., Microbes vs. chemistry in the origin of the anaerobic gut lumen. Proc. Natl. Acad. Sci. U.S.A. 115, 4170–4175 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Folch J., Lees M., Sloane Stanley G. H., A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509 (1957). [PubMed] [Google Scholar]
- 92.Guo J., Lu Y., Dong H., HPLC-MS analysis of the riboflavin crude product of semisynthesis. J. Chromatogr. Sci. 44, 552–556 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Ravn M. V., Campbell J. B., Gerber L., Harrison J. F., Overgaard J., Effects of anoxia on ATP, water, ion and pH balance in an insect (Locusta migratoria). J. Exp. Biol. 222, jeb190850 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.