Significance
“Dead clade walking” refers to fossil groups that suffer major drops in their biodiversity at a mass extinction but do not completely disappear from the fossil record. Why these groups were able to survive but not rediversify remains a relative mystery. Controls on the timing of their eventual extinction are additionally unclear. By gauging the frequency and cause of dead clades walking, we may be able to better understand how mass extinction events have shaped the evolution of animal lineages over Earth history.
Keywords: mass extinction, recovery, biodiversity, macroevolution
Abstract
D. Jablonski [Proc. Natl. Acad. Sci. U.S.A. 99, 8139–8144 (2002)] coined the term “dead clades walking” (DCWs) to describe marine fossil orders that experience significant drops in genus richness during mass extinction events and never rediversify to previous levels. This phenomenon is generally interpreted as further evidence that the macroevolutionary consequences of mass extinctions can continue well past the formal boundary. It is unclear, however, exactly how long DCWs are expected to persist after extinction events and to what degree they impact broader trends in Phanerozoic biodiversity. Here we analyze the fossil occurrences of 134 skeletonized marine invertebrate orders in the Paleobiology Database (paleobiodb.org) using a Bayesian method to identify significant change points in genus richness. Our analysis identifies 70 orders that experience major diversity losses without recovery. Most of these taxa, however, do not fit the popular conception of DCWs as clades that narrowly survive a mass extinction event and linger for only a few stages before succumbing to extinction. The median postdrop duration of these DCW orders is long (>30 Myr), suggesting that previous studies may have underestimated the long-term taxonomic impact of mass extinction events. More importantly, many drops in diversity without recovery are not associated with mass extinction events and occur during background extinction stages. The prevalence of DCW orders throughout both mass and background extinction intervals and across phyla (>50% of all marine invertebrate orders) suggests that the DCW pattern is a major component of macroevolutionary turnover.
The evolutionary history of life preserved in the fossil record reflects a continuous interplay between origination and extinction, punctuated by occasional intervals of intense global environmental and biological upheaval such as the so-called “Big Five” mass extinction (ME) events (1–3). These events not only drive sudden spikes in extinction relative to the origination rate but also influence the taxonomic composition of the postextinction world by vacating niches for colonization by new clades (4). Despite the disproportionate concentration of major macroevolutionary transitions at the Big Five ME events, many questions remain about which biotic and environmental factors impact selectivity and the timing of survivors’ recovery (4–6).
Here we examine a special pattern of selectivity and recovery known as “dead clades walking” (DCWs) (7, 8) wherein certain clades survive through a ME event but never rediversify and maintain lowered levels of taxonomic richness until finally becoming extinct. The driver of DCWs remains uncertain, although numerous mechanisms have been hypothesized (8): 1) biological or environmental bottlenecks that limit genetic or ecological variety and/or geographic range which stunts recovery (9–11), 2) continued environmental stressors related to the ME trigger (12–15), and 3) outcompetition by other clades during the recovery interval (16–19). Alternatively, apparent DCW patterns have been proposed to represent an artifact left by stratigraphic reworking, inconsistent preservation potential, or taxonomic errors (20–23). Regardless of cause, the existence of DCWs suggests that the macroevolutionary consequences of MEs may lag beyond the event by millions of years.
The DCW pattern was first quantified by contrasting the survivorship of clades in the stages immediately preceding and following the Big Five MEs (8). This analysis demonstrated that many boundary-crossing clades continued to exhibit elevated taxonomic attrition during the postextinction stages. Although the initial analysis did not preclude the possibility that some clades would “persist at low diversity for protracted periods without succumbing to final extinction” (8), DCWs have since evolved in the popular consciousness to imply a short-lived postextinction phenomenon (12, 13, 24). Nevertheless, some others have compared the DCW pattern to instances of taxa which persevere at low diversity for tens to hundreds of millions of years [e.g., Devonian eurypterids (11) and strophomenids (25), and Triassic parareptiles (19)]. Determining the prevalence and longevity of DCWs is essential to elucidate their macroevolutionary importance.
Here we extend the DCW concept by leveraging the global record of fossil occurrences in the Paleobiology Database [PBDB; paleobiodb.org (26)]. We analyze genus richness trends at the order level using a Bayesian change point analysis (27) to identify diversity shifts and thereby isolate the stages which contain the latest significant diversity drop prior to the order’s last appearance datum (LAD). In keeping with the tone of Jablonski’s original terminology (8), we dub this diversity drop without recovery an order’s “death sentence” (DS) (Fig. 1A) to mark the initiation of the DCW pattern. Through this approach, we address three questions: 1) how long do DCWs persist in their low-diversity state, 2) does the occurrence of DCWs’ DS and LADs correlate with the Big Five ME events, and 3) are DCWs disproportionately concentrated within certain phyla or geologic eras?
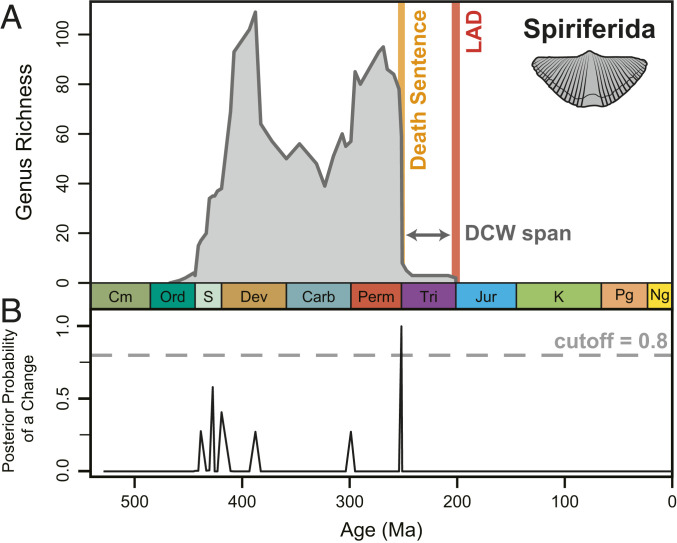
Fig. 1.
The anatomy of a DCW in brachiopod order Spiriferida. (A) Spiriferid range-through genus richness trends. Orange and red bars mark the DS and LAD stages, respectively (Induan–Rhaetian). (B) The posterior probability of a change within the genus richness time series in A, calculated with a Bayesian change point algorithm (Materials and Methods). Probabilities falling below the 0.8 cutoff were excluded as significant change points.
Results
Using global fossil occurrence data from the PBDB and a Bayesian change point algorithm to locate significant shifts in range-through genus richness (Materials and Methods and Fig. 1), we have identified 70 DCWs out of 134 analyzed orders (Fig. 2 and SI Appendix, Fig. S1 and Table S1). Several orders match the originally reported DCW trend, with their DS initiated at a ME and their LAD occurring in an immediate postextinction stage. For example, phacopid trilobites (SI Appendix, Fig. S1A) and agoniatitid cephalopods each experienced a sudden diversity drop during the protracted Late Devonian MEs yet had fossil occurrences into the end-Devonian (Famennian). End-Permian productid brachiopods (SI Appendix, Fig. S1B) similarly survive into the lowermost Triassic before becoming extinct. Contrary to popular belief, however, the majority of DCWs persist at reduced diversity for many millions of years following their DSs (median is 30.4 Myr). For example, several trilobite orders (Agnostida, Ptychopariida, and Olenida) experienced DSs near the Cambrian–Ordovician boundary and did not become extinct until the upper Ordovician (40 Myr). Similarly, eurypterids exhibit a DS preceding the Late Devonian MEs (Eifelian) but ultimately survived >100 Myr into the upper Permian (SI Appendix, Fig. S1D).
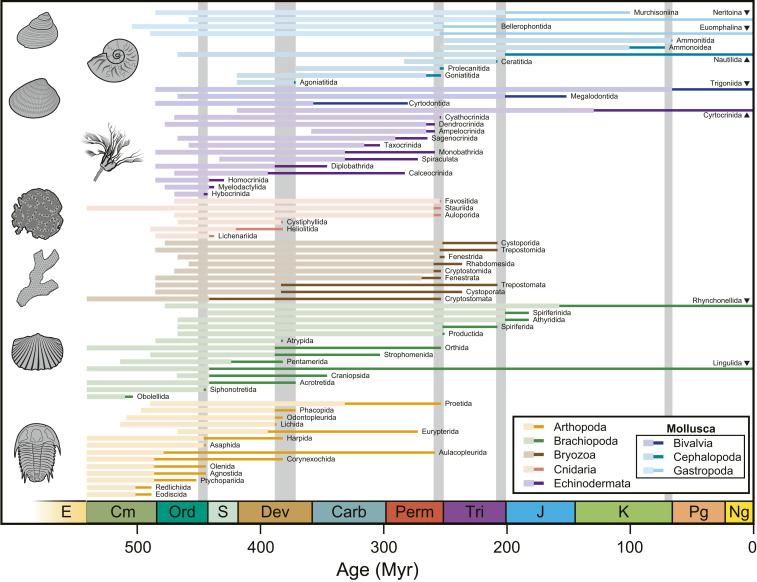
Fig. 2.
Ranges of 70 order-level DCWs. Thick, light lines represent the pre-DS life span of the order; thin, dark lines extend from each clade’s DS to its LAD, each plotted using the lower stage boundary age. Gray bars mark the stages associated with Big Five ME events in this study.
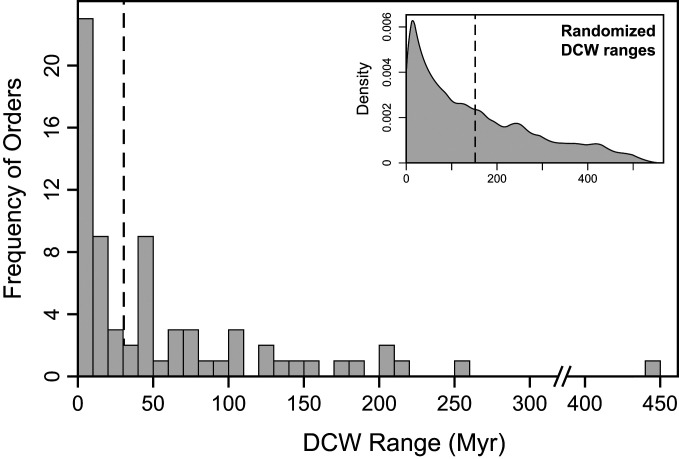
In general, the distribution of orders’ DCW range lengths (Fig. 3) is highly right-skewed, with the majority of ranges persisting for a median of five geologic stages after their DS. One notable outlier, lingulid brachiopods, has survived >440 Myr at low diversity. As a point of comparison, virtual DCWs were simulated with randomly assigned DS and LAD stages. The resulting distribution of randomized DCW ranges (Fig. 3, Inset) has a significantly more pronounced right skew and a higher average span (median is 111 Myr). These distinctions suggest that although there may be random aspects influencing DCW longevity—e.g., stochastic background extinction processes (28, 29) or bias due to unequal stage lengths (30)—the lower average DCW span and infrequency of >300-Myr ranges in the data are likely not artifacts of stochasticity. There is no strong correlative relationship between each order’s longevity as a DCW and their genus or family richness before or after their DS (SI Appendix, Fig. S2), although the orders which experience smaller proportional drops in genus or family richness at their DS tend to persist for a longer DCW span (SI Appendix, Fig. S1 E and F).
Fig. 3.
Histogram of DCW duration following their DS. Dashed line marks the median (30.4 Myr). (Inset) Density plot of randomized DCW span durations (dashed line indicates 111 Myr median range).
DSs in ME vs. Background Stages.
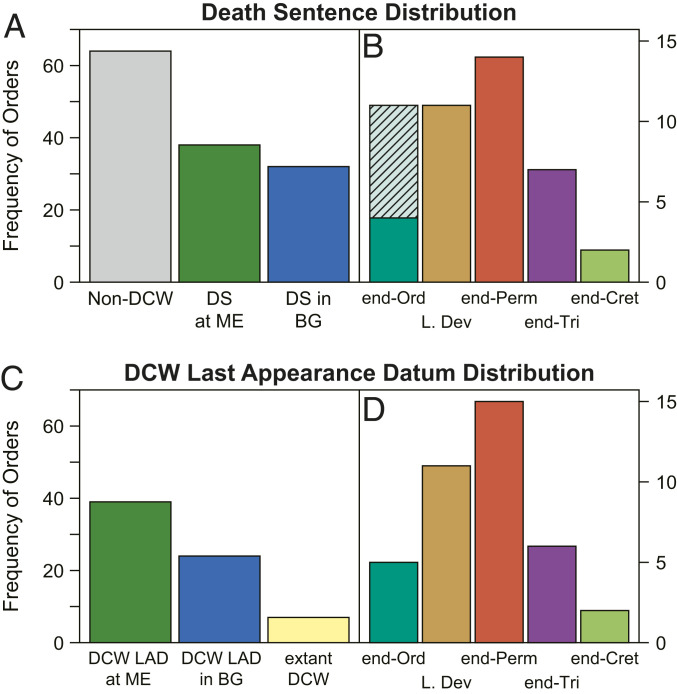
Of the 70 DCW orders identified, 38 experienced a DS associated with one of the Big Five ME events, and 32 had a DS during a background interval (Fig. 4A). The majority of ME-associated DSs crop up at the Late Devonian and end-Permian extinctions (Fig. 4B). Seven DCWs were identified with Aeronian (lower Silurian) DSs which appear to be a continuation of a Hirnantian diversity loss, interrupted by outlier Rhuddanian values potentially related to poor stratigraphic preservation. If these were included, the end-Ordovician DSs become comparable in number to the late Devonian (Fig. 4B). For each ME, the median proportional drops in diversity across DSs were calculated (SI Appendix, Table S2). By this metric, DSs occurring at the end-Cretaceous were most severe (95%), followed by the end-Triassic (72%), whereas the end-Ordovician (52%), Late Devonian, and end-Permian (50% each) drove less severe DSs. Median clade longevity following ME-stage DSs was shortest for the end-Ordovician (0.7 Myr) and end-Permian (5.0 Myr), but spans following the Late Devonian (16 Myr), end-Cretaceous (33 Myr), and end-Triassic (49 Myr) lasted significantly longer.
Fig. 4.
Distribution of the DS and LAD occurrences of DCWs. (A) Number of non-DCW orders compared to DCW orders featuring a DS at either an ME or background stage (BG). (B) Distribution of DS at ME orders throughout the Big Five ME events. Hatched region of the end-Ordovician corresponds to the potentially associated Aeronian DS (DSs in ME vs. Background Stages). (C) Number of DCWs with their LAD associated with either ME or BG stages, compared to extant examples. (D) Distribution of LAD at ME orders throughout the Big Five ME events.
In contrast, 32 of the 72 DCWs experienced a DS in background stages (Fig. 4A), notably, many Paleozoic trilobite and crinoid orders (Fig. 2). Background stage DSs are typified by an intermediate proportional genus richness drop (53%) and the longest lifespan excluding the end-Triassic (40 Myr; SI Appendix, Table S2).
DCW LADs in ME and Background Stages.
The LADs of identified DCWs are mostly clustered at the Big Five ME boundaries (Fig. 4C), with 39 compared to 24 background stage instances (excluding the seven extant DCWs which have no LAD). Paleozoic MEs account for the majority of DCW LADs, particularly the Late Devonian and end-Permian (Fig. 4D). The sustained genus richness of DCWs in the lead-up to their eventual extinctions indicates that the end-Ordovician (median is 12 genera) and end-Permian (median is 7.0) were responsible for the extinction of generally more diverse DCWs, whereas DCW LADs occurring at the Late Devonian and end-Triassic correspond to less diverse groups (6.3 and 3.9 genera, respectively). The median sustained genus richness of DCWs becoming extinct in background stages is 2.0, indicating that more diverse DCWs tend to persist through background stage pressures and minor extinctions.
DCW Trends by Phyla.
Just as DCWs are unevenly distributed in time through the Phanerozoic, certain phyla exhibit a greater prevalence of the survival-without-recovery pattern (Fig. 2 and SI Appendix, Table S3). Of the orders analyzed, identified DCWs are most concentrated in Arthropoda (14 of 14 orders), Bryozoa (9 of 12), and Cnidaria (6 of 9) and less so in Echinodermata (13 of 20) and Brachiopod (14 of 25). Phylum Mollusca comprises 14 DCWs of 53 analyzed orders, but these are disproportionately concentrated in class Cephalopoda (7 of 9) compared to Gastropoda (4 of 17) and Bivalvia (3 of 27). The orders in DCW-rich phyla generally tend toward larger proportional genus richness drops at their DS and subsequently persist for shorter spans of time relative to phyla with a greater proportion of non-DCW orders (SI Appendix, Table S3).
Discussion
Our results demonstrate that rather than representing a rare, ephemeral fossil pattern in the wake of ME events, DCWs comprise over half of the 134 surveyed orders, persist for a median of five stages at low diversity, and are frequently associated with non-Big Five ME stages. These observations are significant not only insofar as they support a broader view of the DCW pattern (8) but because the distribution and cumulative diversity of DCWs over the Phanerozoic (Fig. 2 and SI Appendix, Fig. S3) suggest that this phenomenon reflects a major macroevolutionary pattern.
These findings challenge two paleobiological paradigms. First, the extinction of an animal group is commonly envisioned as a sudden disappearance, particularly when driven by environmental triggers like the climate and impact events at each of the Big Five MEs (3, 31). However, we find that more orders exhibit a prolonged DCW trend of taxonomic attrition rather than a geologically abrupt extinction. Second, studies of taxonomic and ecosystem recovery following a ME often focus on the fossil record for no greater than 10 Myr following the event (32, 33). In contrast, the subset of DCWs experiencing DSs at the Big Five MEs survive a median of 17 Myr before their LAD (Fig. 2 and SI Appendix, Table S2). If the reduced diversity that clades experience at a DS is directly responsible for their inability to recover, then the full taxonomic impact of ME events may not manifest for many stages and thus lag even longer behind the event than previously realized.
Possible Causal Mechanisms.
The ultimate driver of the DCW phenomenon remains ambiguous. The short-term, post-ME DCWs have been previously hypothesized to represent poorly adapted clades which were outcompeted in an inhospitable environment (12, 16, 34). However, many of the long-ranging DCWs we identify survive until or through additional extinction events (SI Appendix, Fig. S1 D, G and H), suggesting a strong resistance to extinction despite an inability to rediversify. A third category are those DCWs whose DS and LAD appear to be contained entirely within the extinction interval, e.g., end-Permian favositid corals, who exhibit a sharp drop without recovery for the stage (Changhsingian) prior to the extinction boundary but do not survive into the lower Triassic. These within-ME patterns likely represent artificial trends or sampling bias (21, 35, 36). Although within-ME DCWs may be explained by the nature of the stratigraphic record, the abundance and longevity of post-ME DCWs and DCWs initiated during background intervals potentially require multiple primary drivers.
The fact that the most taxonomically severe events, the end-Permian and end-Ordovician (3), are associated with a relatively comparable number and taxonomic severity of DSs as the Late Devonian and end-Triassic (Fig. 4 and SI Appendix, Table S2) suggests that the magnitude of the environmental trigger is not an important factor in producing DCWs at ME events. We hypothesize that the Late Devonian and end-Triassic events were uniquely conducive to generating DCWs because they are so-called “mass depletions” rather than true MEs (3, 37), characterized by dramatically reduced origination rates but only moderately elevated extinction rates. The potential for a greater number of survivors across the event could support many more clades to persist as DCWs. Similarly, suppressed origination rates dovetail perfectly with one of the defining traits of DCWs—an absence of rediversification. Although the exact mechanism behind these two mass depletions is unknown, the relative preponderance of DCWs at these events suggests that they are perhaps of greater importance to higher-level taxonomic biodiversity dynamics than is generally thought.
Many long-ranging DCW orders exhibit one or more sharp drops in diversity at ME intervals from which they recovered prior to their eventual DS. For example, the extant brachiopod order Rhynchonellida successfully rediversified following the Late Devonian, end-Permian, and end-Triassic events but ultimately suffered a drop without recovery in the upper Jurassic (SI Appendix, Fig. S1G). Nautilid cephalopods similarly persist into the modern despite a major diversity drop at the end-Permian, from which they recovered, and then an end-Triassic DS (SI Appendix, Fig. S1H). In fact, all seven of the DCWs initiated at the end-Triassic event (e.g., Nautilida; SI Appendix, Fig. S1G) had successfully recovered or rapidly radiated in the aftermath of the end-Permian ME. These examples of how some DCW orders were able to effectively recover in the aftermath of one ME but failed to succeed in a subsequent event suggest that their DS was driven by event-specific environmental triggers rather than an inherent biological factor.
Previous evaluations of selectivity observed that adaptations and traits which promoted clade survival during background stages are often poor predictors of survivors across major ME events (4–6, 38). Despite the difference in selectivity regimes within background and ME stages (4, 38), both feature similar abundances and anatomy of DCW DSs. Nearly as many DCWs experience their DS in a background stage as a ME (32, compared to 38; Fig. 4), and in many cases the genus loss and longevity are comparable (SI Appendix, Fig. S1 C, D, and G and Table S2). This similarity suggests that the global environmental triggers unique to MEs are unlikely to be the sole driver of DSs. Instead, we propose that the temporal ubiquity of DSs demonstrates that it is a pattern likely to be driven by multiple different selective processes.
Distribution of DCWs.
Throughout the surveyed phyla and the Phanerozoic eon, the distribution of DCWs is quite heterogeneous (Fig. 2). DCW orders are most concentrated in the groups Arthropoda, Cephalopoda, and Bryozoa and rarest among Gastropoda and Bivalvia (SI Appendix, Table S3). The distribution of DCW spans (Fig. 2) and their cumulative genus richness through the Phanerozoic (SI Appendix, Fig. S3) have effectively recreated Sepkoski’s evolutionary faunas (figure 5 of ref. 39): the DCW-rich groups correspond to the Cambrian (e.g., trilobites and inarticulate brachiopods) and the Paleozoic faunas (e.g., articulate brachiopods, cephalopods, stenolaemate bryozoans, and crinoids), whereas the DCW-poor groups mainly fall into the Modern (e.g., gastropods, bivalves, and scleractinian corals).
One possible explanation for the observed split in DCW patterns between the Paleozoic and Modern fauna is that some inherent biological factor shared by Paleozoic fauna is key to the survival-without-recovery phenomenon. Extinction rates in Paleozoic taxa were elevated compared to post-Paleozoic taxa (8, 29), and some of the characteristic groups (e.g., trilobites and ammonoids) feature high intrinsic turnover rates which make them particularly volatile and prone to extinction in background stages (29, 40). These aspects may have predisposed certain Paleozoic groups to experiencing diversity drops without recovery, whereas the less volatile orders of the Modern fauna (40) were more prone to stability and rediversification. This hypothesis is supported by the relatively short post-DS lifespan of the numerous cephalopod (2.2 Myr) and arthropod (36 Myr) DCWs, compared to the rare bivalve (66 Myr) and gastropod (155 Myr) DCWs (SI Appendix, Table S3). Another explanation is that the Paleozoic was an environmentally turbulent era: the dense timing of three of the Big Five MEs (Fig. 2) could disproportionately concentrate DCWs.
Alternatively, the DCW phenomenon may be concentrated in the Cambrian and Paleozoic faunas because the pattern is a common eventual feature in the life cycle of an order. Among the 64 non-DCWs of the surveyed orders, only 24 experience a statistically sudden extinction, defined within this study as those which do not exhibit a prolonged survival interval. The remaining 40 are extant. These results indicate that not only are geologically sudden MEs relatively rare at high taxonomic levels, but a large proportion of non-DCW orders cannot be definitively ruled out from experiencing a DS in the future. As the orders associated with the Modern fauna (e.g., Cardiida, Pectinida, and Veneroidei of class Bivalvia and Neogastropoda and Sorbeoconcha of class Gastropoda) continue to diversify into the future (41), they will eventually decline in cumulative diversity and may exhibit the same DCW patterns seen in the Cambrian and Paleozoic faunas. This hypothesis predicts that the DCW phenomenon is inherent to macroevolutionary turnover: as orders age, they are increasingly susceptible to sudden drops in diversity during either background or ME stages but may persist in a vulnerable, low-diversity state until an environmental perturbation or simple taxonomic attrition ultimately finishes them off.
Conclusions
Our results demonstrate that the DCW phenomenon is much more common and long-ranging than previously thought and appears to be a fundamental component of macroevolutionary dynamics that can arise from many different processes. Future research will benefit from coupling our Bayesian approach to identifying change points in taxonomic richness data with complementary methods:
-
1.
Higher taxonomic levels like orders may represent polyphyletic groups and therefore mask a genetic control on DCWs. To that end, fossil richness trends paired with phylogenetic analyses can better constrain a clade’s macroevolution (11).
-
2.
Similarly, assessment of the morphological and ecological variability within DCWs through their DS (11, 19, 25, 42) may help elucidate why the orders fail to recover.
-
3.
Fossil biogeographic reconstructions of DCW occurrences through time will test whether they persist primarily in refugia, having undergone a significant range bottleneck at their DS (12, 16, 43).
Materials and Methods
Fossil data were sourced from the PBDB (26) and constrained to marine occurrences of phyla Arthropoda, Brachiopoda, Bryozoa, Cnidaria, Echinodermata, and Mollusca ranging between the Cambrian and Pliocene (downloaded from paleobiodb.org on February 3, 2021). The majority of PBDB fossil data for these phyla correspond to shallow marine specimens of skeletalized invertebrates, which are generally characterized by greater preservation potential and commonly have the advantage of developed taxonomic classifications and widespread geographic occurrences (44, 45). The entries were vetted to only include occurrences with genus-level identification. Following cleaning, the dataset comprised 699,494 occurrences corresponding to 17,520 unique genera within 185 unique orders. All orders and corresponding subtaxa are classified as cited in the PBDB. Diversity metrics were calculated as the number of unique genera occurring within each stage, assuming that each genus was present in all stages from its first to LAD (range-through genus richness). This approach was intended to reduce sampling bias in stages with relatively poor preservation or documentation and counteract the variable completeness between different taxonomic lineages in the PBDB. These results were compared to shareholder’s quorum subsampling (SQS) standardization of fossil occurrence data (SI Appendix, Fig. S4). The identified DCW orders and their ranges did not significantly differ in either approach, suggesting that the DCW pattern is not an artifact of sampling bias. However, the range-through method was able to identify a greater number of DCWs, including lower-diversity orders with shorter lifespans. First and last appearance data (FAD/LAD) for genera were calculated from the lower and upper ages of the stage bins containing their max_ma and min_ma age fields in the PBDB. When assigning stages to be associated with the Big Five MEs, the two stages on either side of the pertinent boundary were included, with the exception of the prolonged and pulsed Late Devonian (Givetian, Frasnian, and Famennian) and end-Permian (Wuchiapingian, Changhsingian, and Induan). This choice has likely led to an overestimate of the number of DCWs associated with Big Five MEs. Given the potential for misidentified or reworked genera to greatly extend the LAD of an order (20) and correspondingly its DCW range, every order’s last appearance was compared to canonical values derived from Sepkoski’s marine fossil compendium (ref. 46; compiled by strata.geology.wisc.edu/jack/), the Treatise on Invertebrate Paleontology, and other recent literature (SI Appendix, Table S4). PBDB order occurrences which extended past the canonical LAD were excised, whereas orders known to be extant had their ranges extended to the Late Pleistocene in the absence of recent fossil occurrences. DCW ranges were herein defined as the interval between an order’s last significant drop in diversity and its LAD (Fig. 1). The term DS was applied to the former to match the imagery evoked by Jablonski’s DCWs (7, 8) but also to serve as a unique term to distinguish the drop without recovery from other periods of elevated extinction during an order’s history. The DS was calculated as the lower boundary of the stage containing the latest significant drop in diversity prior to the LAD, determined by using the Bayesian change point analysis R package bcp (27). The bcp algorithm calculates a running posterior mean of the range-through genus richness time series for each order and then runs Markov chain Monte Carlo (MCMC) iterations to calculate the posterior probability of the mean changing significantly between two points in time (i.e., the change points). For each analysis, 100,000 MCMC iterations were performed with a burn-in period of 10,000 iterations. Orders whose maximum raw genus richness <5 were excluded from bcp analysis, leaving 134 of the original 185 orders. This step was taken to eliminate orders with low abundance richness trends more likely to be impacted by preservation biases but potentially reinforced the survivorship bias inherent to the fossil record (47); therefore, our approach has likely underestimated the number of DCWs. Due to the reduced variance and time resolution in genus richness trends relative to the target BCP time series (27), we experimented extensively with tuning the hyperparameters p0 and w0 (48) which set the upper range of the prior probability of a change point at each time step and the prior variance of the mean, respectively (SI Appendix, Fig. S5). In order to isolate only the significant diversity shifts as change points, p0 was set to and w0 was allowed to decrease iteratively by orders of magnitude from to until at least one change point was identified. The assigned change points were validated by testing whether stationarity was achieved, i.e., the outputted MCMC results for the probability of a significant change point converged on a single answer. This was evaluated with the Heidelberger and Welch convergence diagnostic which tests for significantly stationary distributions (p 0.05) in MCMCs based on the Cramer-von-Mises statistic using the R package coda (49). Identified change points with posterior probabilities <0.80 (Fig. 1B) were excluded due to inconsistent stationarity.
Supplementary Material
Acknowledgments
We thank M. Patzkowsky for critical discussions on designing the methods for this study; C. Congreve, J. Lamsdell, J. Carr, A. Neely, and the Pennsylvania State University Paleobiology seminar for constructive discourse while developing our ideas; and all contributors to the Paleobiology Database. We also thank two anonymous reviewers and the editor for constructive comments. The authors were supported by the University of Wisconsin-Madison Department of Geoscience and the Pennsylvania State University Department of Geosciences during this research. This is Paleobiology Database Publication 393.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. S.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2019208118/-/DCSupplemental.
Data Availability
All study data are included in the article and SI Appendix.
References
- 1.Raup D. M., Sepkoski J. J., Mass extinctions in the marine fossil record. Science 215, 1501–1503 (1982). [DOI] [PubMed] [Google Scholar]
- 2.Jablonski D., Extinctions in the fossil record. Philos. Trans. R. Soc. Lond. B 344, 11–17 (1994). [Google Scholar]
- 3.Bambach R. K., Phanerozoic biodiversity mass extinctions. Annu. Rev. Earth Planet Sci. 34, 127–155 (2006). [Google Scholar]
- 4.Jablonski D., Background and mass extinctions: The alternation of macroevolutionary regimes. Science 231, 129–133 (1986). [DOI] [PubMed] [Google Scholar]
- 5.Jablonski D., Evolutionary innovations in the fossil record: The intersection of ecology, development, and macroevolution. J. Exp. Zool. 304, 504–519 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Hull P. M., Darroch S. A. F., Erwin D. H., Rarity in mass extinctions and the future of ecosystems. Nature 528, 345–351 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Jablonski D., Lessons from the past: Evolutionary impacts of mass extinctions. Proc. Natl. Acad. Sci. U.S.A. 98, 5393–5398 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jablonski D., Survival without recovery after mass extinctions. Proc. Natl. Acad. Sci. U.S.A. 99, 8139–8144 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rong J. Y., Boucot A. J., Harper D. A. T., Zhan R. B., Neuman R. B., Global analyses of brachiopod faunas through the Ordovician and Silurian transition: Reducing the role of the Lazarus effect. Can. J. Earth Sci. 43, 23–29 (2006). [Google Scholar]
- 10.Stilwell J. D., Håkansson E., “Survival, but…! new tales of ‘dead clade walking’ from austral and boreal post-K-T assemblages” in Earth and Life: Global Biodiversity, Extinction Intervals and Biogeographic Perturbations Through Time, Talent J. A., Ed. (Springer Netherlands, 2012), pp. 795–810. [Google Scholar]
- 11.Lamsdell J. C., Selden P. A., From success to persistence: Identifying an evolutionary regime shift in the diverse Paleozoic aquatic arthropod group Eurypterida, driven by the Devonian biotic crisis. Evolution 71, 95–110 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Salamon M. A., Gorzelak P., Ferré B., Lach R., Roveacrinids (Crinoidea, Echinodermata) survived the Cretaceous-Paleogene (K-pg) extinction event. Geology 38, 883–885 (2010). [Google Scholar]
- 13.Kaim A., Nützel A., Dead bellerophontids walking—The short Mesozoic history of the Bellerophontoidea (Gastropoda). Palaeogeogr. Palaeoclimatol. Palaeoecol. 308, 190–199 (2011). [Google Scholar]
- 14.Vörös A., Kocsis Á., Pálfy J., Demise of the last two spire-bearing brachiopod orders (Spiriferinida and Athyridida) at the Toarcian (Early Jurassic) extinction event. Palaeogeogr. Palaeoclimatol. Palaeoecol. 457, 233–241 (2016). [Google Scholar]
- 15.Chen J., et al. , Size variation of brachiopods from the late Permian through the middle Triassic in South China: Evidence for the Lilliput effect following the Permian-Triassic extinction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 519, 248–257 (2019). [Google Scholar]
- 16.Baarli B. G., Harper D. A. T., Relict Ordovician brachiopod faunas in the lower Silurian of Asker, Oslo region, Norway. Nor. Geol. Tidsskr. 66, 87–98 (1986). [Google Scholar]
- 17.Sepkoski J. J., McKinney F. K., Lidgard S., Competitive displacement among post-Paleozoic cyclostome and cheilostome bryozoans. Paleobiology 26, 7–18 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Aberhan M., Kiessling W., Rebuilding biodiversity of Patagonian marine molluscs after the end-Cretaceous mass extinction. PLoS One 9, e102629 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDougall M. J., Brocklehurst N., Fröbisch J., Species richness and disparity of parareptiles across the end-Permian mass extinction. Proc. R. Soc. B 286, 20182572 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erwin D. H., Droser M. L., Elvis taxa. Palaios 8, 623–624 (1993). [Google Scholar]
- 21.Pospichal J. J., Calcareous nannofossils at the K-T boundary, El Kef: No evidence for stepwise, gradual, or sequential extinctions. Geology 22, 99–102 (1994). [Google Scholar]
- 22.Kowalewski M., Flessa K. W., Improving with age: The fossil record of lingulide brachiopods and the nature of taphonomic megabiases. Geology 24, 977–980 (1996). [Google Scholar]
- 23.Gorzelak P., Salamon M. A, Ferré B., Pelagic crinoids (Roveacrinida, Crinoidea) discovered in the Neogene of Poland. Naturwissenschaften 98, 903–908 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Korn D., Belka Z., Fröhlich S., Rücklin M., Wendt J., The youngest African clymeniids (Ammonoidea, late Devonian)—Failed survivors of the Hangenberg event. Lethaia 37, 307–315 (2004). [Google Scholar]
- 25.Sclafani J. A., Congreve C. R., Krug A. Z., Patzkowsky M. E., Effects of mass extinction and recovery dynamics on long-term evolutionary trends: A morphological study of Strophomenida (Brachiopoda) across the late Ordovician mass extinction. Paleobiology 44, 603–619 (2018). [Google Scholar]
- 26.Peters S. E., McClennan M., The Paleobiology Database application programming interface. Paleobiology 42, 1–7 (2016). [Google Scholar]
- 27.Erdman C., Emerson J. W., bcp: An R package for performing a Bayesian analysis of change point problems. J. Stat. Software 23, 1–13 (2007). [Google Scholar]
- 28.van Valen L., A new evolutionary law. Evol. Theor. 1, 1–30 (1973). [Google Scholar]
- 29.Gilinsky N. L., Volatility and the Phanerozoic decline of background extinction intensity. Paleobiology 20, 445–458 (1994). [Google Scholar]
- 30.Gould S. J., Gilinsky N. L., German R. Z., Asymmetry of lineages and the direction of evolutionary time. Science 236, 1437–1441 (1987). [DOI] [PubMed] [Google Scholar]
- 31.Raup D. M., Gould S. J., Schopf T. J. M., Simberloff D. S., Stochastic models of phylogeny and the evolution of diversity. J. Geol. 81, 8139–8144 (2002). [Google Scholar]
- 32.Kirchner J. W., Well A., Delayed biological recovery from extinctions throughout the fossil record. Nature 404, 177–180 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Chen Z. Q., Benton M. J., The timing and pattern of biotic recovery following the end-Permian mass extinction. Nat. Geosci. 5, 375–383 (2012). [Google Scholar]
- 34.Nützel A., Recovery of gastropods in the early Triassic. C. R. Palevol 4, 501–515 (2005). [Google Scholar]
- 35.Signor P. W., Lipps J. H., Sampling bias, gradual extinction patterns and catastrophes in the fossil record. Spec. Pap. Geol. Soc. Am. 190, 291–296 (1982). [Google Scholar]
- 36.Holland S. M., Patzkowsky M. E., The stratigraphy of mass extinction. Palaeontology 58, 903–924 (2015). [Google Scholar]
- 37.Bambach R. K., Knoll A. H., Wang S. C., Origination, extinction, and mass depletions of marine diversity. Paleobiology 30, 522–542 (2004). [Google Scholar]
- 38.Payne J. L., Finnegan S., The effect of geographic range on extinction risk during background and mass extinction. Proc. Natl. Acad. Sci. U.S.A. 104, 10506–10511 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sepkoski J. J., A factor analytic description of the Phanerozoic marine fossil record. Paleobiology 7, 36–53 (1981). [Google Scholar]
- 40.Stanley S. M., An analysis of the history of marine animal diversity. Paleobiology 33, 1–55 (2007). [Google Scholar]
- 41.Alroy J., The shifting balance of diversity among major marine animal groups. Science 329, 1191–1194 (2010). [DOI] [PubMed] [Google Scholar]
- 42.McGowan A. J., The effect of the Permo-Triassic bottleneck on Triassic ammonoid morphological evolution. Paleobiology 30, 369–395 (2004). [Google Scholar]
- 43.Stanley G. D., Confessions of a displaced reefer. Palaios 11, 1–2 (1996). [Google Scholar]
- 44.Schopf T. J. M., Fossilization potential of an intertidal fauna: Friday Harbor, Washington. Paleobiology 4, 261–270 (1978). [Google Scholar]
- 45.Benton M. J., Mass extinctions among tetrapods and the quality of the fossil record. Philos. Trans. R. Soc. London B 325, 369–386 (1989). [DOI] [PubMed] [Google Scholar]
- 46.Sepkoski J. J., A compendium of fossil marine animal genera. Bull. Am. Paleontol. 363, 1–536 (2002). [Google Scholar]
- 47.Budd G. E., Mann R. P., History is written by the victors: The effect of the push of the past on the fossil record. Evolution 72, 2276–2291 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barry D., Hartigan J. A., A Bayesian analysis for change point problems. J. Am. Stat. Assoc. 88, 309–319 (1993). [Google Scholar]
- 49.Plummer M., Best N., Cowles K., Vines K., CODA: Convergence diagnosis and output analysis for MCMC. R. News 6, 7–11 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.