Significance
Clinical research and animal models have demonstrated a significant connection between maternal stress during pregnancy and sensitivity to stress in offspring, leading to increased susceptibility to neuropsychiatric disorders later in life. In a unique prenatal cohort that was followed for over four decades, we tested associations between pro- and anti-inflammatory markers in maternal prenatal sera and sex differences in neural responses to negative stress in the offspring in early midlife using functional MRI. Men and women exposed in utero to abnormal levels of pro-inflammatory cytokines and to an imbalance of pro- to anti-inflammatory influences showed dysregulation of stress response circuitry 45 y later, with sex-dependent effects.
Keywords: prenatal immune programming, prenatal stress, stress circuitry, sex, functional brain imaging
Abstract
Stress is associated with numerous chronic diseases, beginning in fetal development with in utero exposures (prenatal stress) impacting offspring’s risk for disorders later in life. In previous studies, we demonstrated adverse maternal in utero immune activity on sex differences in offspring neurodevelopment at age seven and adult risk for major depression and psychoses. Here, we hypothesized that in utero exposure to maternal proinflammatory cytokines has sex-dependent effects on specific brain circuitry regulating stress and immune function in the offspring that are retained across the lifespan. Using a unique prenatal cohort, we tested this hypothesis in 80 adult offspring, equally divided by sex, followed from in utero development to midlife. Functional MRI results showed that exposure to proinflammatory cytokines in utero was significantly associated with sex differences in brain activity and connectivity during response to negative stressful stimuli 45 y later. Lower maternal TNF-α levels were significantly associated with higher hypothalamic activity in both sexes and higher functional connectivity between hypothalamus and anterior cingulate only in men. Higher prenatal levels of IL-6 were significantly associated with higher hippocampal activity in women alone. When examined in relation to the anti-inflammatory effects of IL-10, the ratio TNF-α:IL-10 was associated with sex-dependent effects on hippocampal activity and functional connectivity with the hypothalamus. Collectively, results suggested that adverse levels of maternal in utero proinflammatory cytokines and the balance of pro- to anti-inflammatory cytokines impact brain development of offspring in a sexually dimorphic manner that persists across the lifespan.
Repeated and prolonged adverse responses to negative stress have been associated with increased risk for many chronic diseases, including psychiatric and cardiovascular disorders. In fact, perturbations in the in utero development of the stress response circuitry have played a key role underlying the developmental origins of disease, including what has been termed prenatal-stress models of chronic disease (1–6). These in utero perturbations may also contribute to sex differences in disease risk (2, 4, 6–10), given that the brain circuitry regulating the stress response contains some of the most highly sexually dimorphic regions in the brain (2, 4, 9, 11). That is, they develop differently in the male and female brain in utero, and when developmentally disrupted, have long-lasting effects on sex-dependent disease risk (5).
Brain circuitry involved in the stress response system includes arousal in the hypothalamus (HYPO), amygdala (AMYG), and periaqueductal gray (PAG) and inhibitory control of arousal by the medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and hippocampus (HIPP). These regions not only regulate response to stress but also steroid hormone physiology through the hypothalamic-pituitary-adrenal (HPA) and -gonadal axes. Primary coactivators of the HPA axis are proinflammatory cytokines, that is, tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-6. Receptors for these cytokines are located contiguously with glucocorticoid and gonadal hormone receptors in brain regions that regulate stress response circuitry and are densest in the paraventricular nucleus (PVN) of the HYPO and HIPP.
Thus, the HPA axis is a critical junction for immune factors and steroid hormones, and as a result, the regulation of stress responses (2, 4, 6–8, 10, 12). The release of maternal immune molecules (e.g., TNF-α, IL-1β and IL-6) (4, 5, 13, 14) and glucocorticoids (13, 15, 16) can impact fetal development by stimulating placental production of corticotropin-releasing hormone (CRH) and thereby impact HPA-axis function in the fetus (17). The release of glucocorticoids (i.e., cortisol) has inhibitory effects on cytokine release of TNF-α, IL-1β, and IL-6, with TNF-α being most sensitive to this negative feedback loop (18). This chain of events is thought to have long-term consequences for the offspring’s brain health. Preclinical studies have shown neurologic changes in offspring induced by maternal immune activation that manifest as behavioral deficits in spatial learning and memory (14, 19, 20), increased anxiety and depressive behaviors (21), neurobiologic dysregulation, including hypomyelination and reduced neurogenesis (22, 23), and biochemical dysfunction expressed as decreases in serotonin (24) and alterations in dopaminergic markers (25). Sex differences in offspring outcomes depended on timing of the adverse prenatal maternal immune exposure. Earlier in utero, adverse exposures had greater impact on male offspring and later in utero and postnatal exposure on female offspring (26, 27). Further evidence for this comes from clinical studies of the effects of maternal prenatal immune compromise on offspring risk for sex differences in psychiatric and neurodevelopmental outcomes, for example, from ours (5, 8, 28, 29) and others’ (6, 19, 30).
Despite evidence from preclinical and human studies that maternal immune activity is associated with sex differences in stress-mediated conditions in offspring, it remains to be shown how brain circuitry in adults is impacted by prior exposure to maternal immune activity in utero and the extent to which this impact is sex dependent. Here, we had the unique opportunity to investigate this in the context of a pregnancy cohort with follow-up of offspring in middle adulthood. We investigated whether concentrations of in utero proinflammatory cytokines in maternal sera (drawn at the start of the third trimester) are associated with sex differences in activity in specific brain regions that regulate stress and immune function 45 y later. We predicted that adverse concentrations of proinflammatory cytokines would be associated with hyperactivity in stress-arousal regions (in particular, HYPO) and hypoactivity in inhibitory regions of the stress response (in particular, HIPP), assessed by functional MRI (fMRI). Our rationale was, in part, based on the fact that HIPP has a negative feedback role on HYPO, specifically PVN, and PVN and HIPP are brain regions most dense with receptors for TNF-α, IL-1β, and IL-6 (31, 32).
Results
The sample came from a previous study of offspring followed from prenatal development through their mid-40s, in which we found sex differences in brain activity and connectivity in response to negative stressful stimuli in midlife, with women affected more severely, regardless of diagnosis (33, 34). Here, we extended these findings and tested in the same sample whether adverse in utero maternal immune levels were associated with these sex differences in brain activity and connectivity. A total of 80 offspring ages 40 to 50 (equally divided by sex and comparable within sex for age) from whom we obtained prenatal sera were included in the sample, which consisted of healthy controls and people with major depressive disorder and psychoses. (See Table 1 for a sample description.) They underwent fMRI while viewing negative and neutral valence images adapted from the International Affective Picture System (IAPS; refs. 35–37). Studies from our laboratory and many others have shown that briefly viewing negative images from the IAPS database reliably induces a cortisol response [that physiologically defines a “stress task” (5)], affects subjective feelings of stress and mood, and activates stress response circuitry in the brain (35, 36, 38–42). We analyzed levels of maternal prenatal cytokines at the beginning of the third trimester for IL-6, IL-1β, and TNF-α, and of IL-10 (an anti-inflammatory cytokine used to derive estimates of pro- to anti-inflammatory cytokine imbalance for those cytokines that were significantly associated with brain activity). The sample was enriched for mothers with obstetric complications associated with maternal in utero immune responses (e.g., preeclampsia, infections; see Table 1), in order to ensure variability with regard to maternal cytokine levels during gestation. This was validated in the sample, in particular for preeclampsia with IL-6 exposure (top tertile versus others: OR = 9.00 and P = 0.002) and infections with TNF-α (top tertile versus others: OR = 5.25 and P = 0.03).
Table 1.
Demographic and clinical characteristics of a sample of n = 80 men and women assessed at ages 40 to 50
| Women (n = 40) mean (SD) | Men (n = 40) mean (SD) | |
| Age (in years) | 45.9 (2.3) | 45.8 (2.6) |
| BMI | 29 (6.78) | 29.3 (5.5) |
| Education | ||
| % without completed high school (n) | 5.5% (2) | 15.8% (6) |
| % completed high school (n) | 11.1% (4) | 15.8% (6) |
| % more than high school (n) | 83.3% (30) | 68.4% (26) |
| Ethnicity | ||
| % Caucasian (n) | 87.5% (35) | 89.5% (34) |
| % Other (n) | 12.5% (5) | 10.5% (4) |
| DSM-based diagnosis | ||
| % major depressive disorder (MDD) in remission (n) | 35% (14) | 27.5% (11) |
| % psychosis in remission (n) | 17.5% (7) | 22.5% (9) |
| Psychotropic medication | ||
| % on psychotropic medication (n) | 25% (10) | 27.5% (11) |
| OC during pregnancy | ||
| Preeclampsia | 15.4% (6) | 15.4% (6) |
| Fetal growth restriction | 33.3% (13) | 31.7% (13) |
| Infectious disease | 27.8% (10) | 23.1% (9) |
| Fever during pregnancy | 18.9% (7) | 10% (4) |
| Gestational week of blood draw | 28.8(±3.9) | 29.9 (±6.2) |
BMI, body mass index; OC, obstetric complication. Note that there were only 39 mothers of females due to a sibling pair in our dataset. Comparison by group status was evaluated using nonparametric Wilcoxon rank-sum or χ2 test for continuous and categorical data, respectively. There were no significant differences between men and women on any demographic or clinical characteristics.
Brain activity during fMRI scanning (measured as blood oxygen level–dependent [BOLD] signal changes) and functional connectivity in stress circuitry regions in response to negative compared to neutral images were examined as a function of maternal prenatal cytokine levels (i.e., TNF-α, IL-1β, and IL6 and ratios with IL-10) using general linear models (GLM), controlled for multiple comparisons. (See Materials and Methods for details.) Significant main effects and interactions with sex were identified and effect sizes reported, controlling for psychiatric diagnosis which did not have a significant association with the study outcomes.
Prenatal Maternal Cytokine Exposure by Offspring Sex.
Table 2 shows the non-log-transformed median levels of prenatal maternal cytokines by sex of offspring. Using Wilcoxon rank-sum to test for sex of offspring differences, concentrations of IL-6 and IL-10 (but not TNF-α and TNF-α:IL-10) were significantly different between mothers of male versus female offspring (Wilcoxon rank-sum test: IL-6 M > F, P = 0.03) and IL-10 (M > F, P = 0.01).
Table 2.
Median levels of cytokines in maternal prenatal sera by sex of offspring
| Offspring sex | IL-1β median (25%;75%) | IL-6 median (25%; 75%) | TNF-α median (25%; 75%) | IL-10 median (25%; 75%) | TNF-α:IL-10 median (25%; 75%) |
| Males | 1.04 (0.2; 8.64) | 1.49 (0.45; 14.05) | 3.4 (2.36; 4.48) | 2.38 (1.21; 5.25) | 1.27 (0.77; 2.75) |
| Females | 0.77 (0.2; 2.87) | 0.67 (0.31; 1.7) | 3.1 (1.99; 3.9) | 1.39 (0.79; 2.36) | 1.84 (0.81; 3.19) |
Wilcoxon rank-sum tests were conducted to compare maternal cytokine levels by sex of offspring. Results showed significant differences only in levels of IL-6 (M > F, P = 0.03) and IL-10 (M > F, P = 0.01).
BOLD Responses.
Next, we tested the impact of maternal cytokine concentrations on offspring brain activity in HYPO and HIPP. Analyses of BOLD responses to negative (versus neutral) affective stimuli were restricted to HYPO and HIPP based on our prior hypotheses, primary findings in refs. 33 and 34, and to limit the number of comparisons. To assess the individual and joint associations of the four prenatal cytokines (IL-6, TNF-α, IL-1β, and IL-10) with BOLD responses in the HYPO and HIPP together, we performed multivariate protected F-tests using Wilks’ lambda (43) to determine overall significance. Sex-specific multivariate analyses demonstrated that IL-6, TNF-α, and IL-10 levels (but not IL-1β) were jointly associated with BOLD activity in HYPO and HIPP (Wilks’ lambda = 0.51, F[12, 77] = 1.87, and P = 0.05), particularly in women. This allowed for analyses of individual cytokines on HYPO and HIPP, adjusted for multiple comparisons, that insured lack of spuriousness.
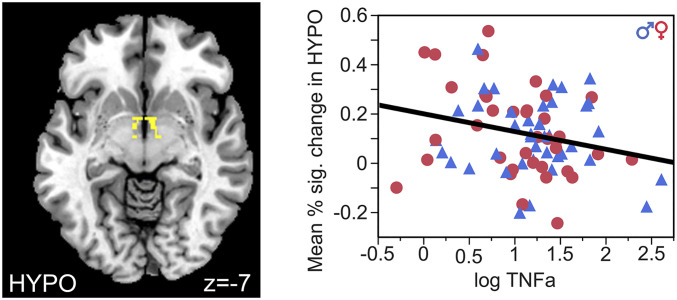
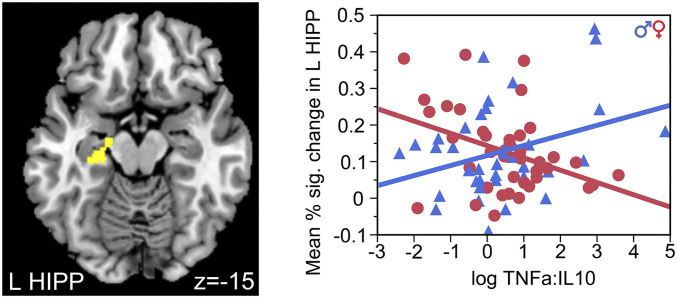
Results of specific cytokines showed concentrations of TNF-α and IL-6 in maternal sera were significantly associated with brain activity in HYPO and HIPP in adult offspring. Further, the ratio of maternal IL-10 with TNF-α (but not with IL-6) was significantly associated with HYPO and HIPP activity. Lower concentration of TNF-α was significantly associated with higher mean percent–signal change in HYPO (β = −0.07, SD = 0.27, effect size = 0.26, F (1,79) = 5.17, and P = 0.03; Fig. 1), independent of sex (F (1,76) = 0.85 and P = 0.36). However, the ratio TNF-α:IL-10 had a significant interaction with sex on BOLD response in HIPP (F (1,79) = 10.97 and P = 0.001; Fig. 2). Post hoc analyses revealed that higher ratios of prenatal TNF-α:IL-10 were associated with lower mean percent–signal change in the left HIPP of women (β = −0.03, SD = 0.27, effect size = −0.11, F (1,39) = 6.69, and P = 0.01) but higher in the left HIPP of men (β = 0.03, SD = 0.09, effect size = 0.33, F (1,39) = 4.54, and P = 0.04). IL-6 also had a significant interaction of sex on HIPP (F (1,79) = 5.93 and P = 0.02). Higher prenatal exposure to IL-6 was associated with lower mean percent–signal change in the right HIPP of women (β = −0.03, SE = 0.09, effect size = −0.33, F (1,39) = 5.81, and P = 0.02) but not men (β = 0.006, SD = 0.07, effect size= 0.09, F (1,39) = 0.59, and P = 0.45). No results changed when controlling for diagnosis, but three-way interactions were underpowered. Thus, findings demonstrated that adverse levels of the in utero proinflammatory cytokines, TNF-α and IL-6, and the balance of the pro- to anti-inflammatory cytokine TNF-α:IL-10, impacted the development of two primary regions of the stress response circuitry (HYPO and HIPP) in a sexually dimorphic manner in the offspring that persisted across the lifespan.
Fig. 1.
Prenatal exposure to lower levels of TNF-α predicted increased BOLD signal in the HYPO in all subjects, independent of sex and diagnosis.
Fig. 2.
Prenatal exposure to increased ratios of TNF-α to IL-10 predicted response in the left HIPP with sex-dependent effects. Increased TNF-α:IL-10 predicted decreased left HIPP-BOLD response in female offspring but increased left HIPP-BOLD response in male offspring.
Task-Related Connectivity Analyses.
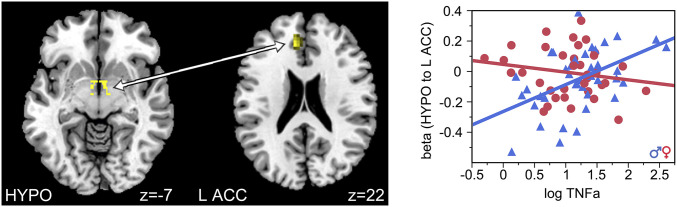
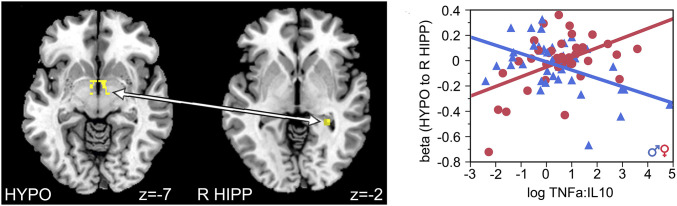
Connectivity analyses using HYPO and HIPP as seeds revealed significant effects of TNF-α and sex on connectivity between HYPO and left ACC (z = 3.8, family-wise error [FWE] corrected for multiple comparisons, P = 0.02; cluster of 91 voxels with peak at x = −9, y = 38, and z = 22; Fig. 3) and TNF-α:IL-10 ratio and sex on connectivity between HYPO and right HIPP (z = 3.99, FWE corrected, P = 0.003; cluster of 12 voxels with peak at x = 30, y = −37, and z = −2; Fig. 4). Post hoc analyses showed that lower prenatal TNF-α exposure was associated with lower connectivity between HYPO and left ACC in men (t (39) = 3.49, P = 0.001, and R2 = 0.24) but not in women (t (35) = −1.05 and P = 0.30). Furthermore, higher prenatal TNF-α:IL-10 exposure was associated with higher connectivity between the HYPO and right HIPP in women (t (38) = 3.24, P = 0.003, and R2 = 0.23) but lower connectivity between the HYPO and right HIPP in men (t (39) = −3.38, P = 0.002, and R2 = 0.23).
Fig. 3.
Functional connectivity between the HYPO and the left ACC increased as a function of prenatal exposure to TNF-α in male, but not female, offspring.
Fig. 4.
Maternal ratio of TNF-α to IL-10 predicted functional connectivity between the HYPO and right HIPP in a sex-dependent manner. Higher prenatal TNF-α:IL-10 was associated with increased connectivity between the HYPO and right HIPP in female offspring but decreased connectivity between the two regions in male offspring.
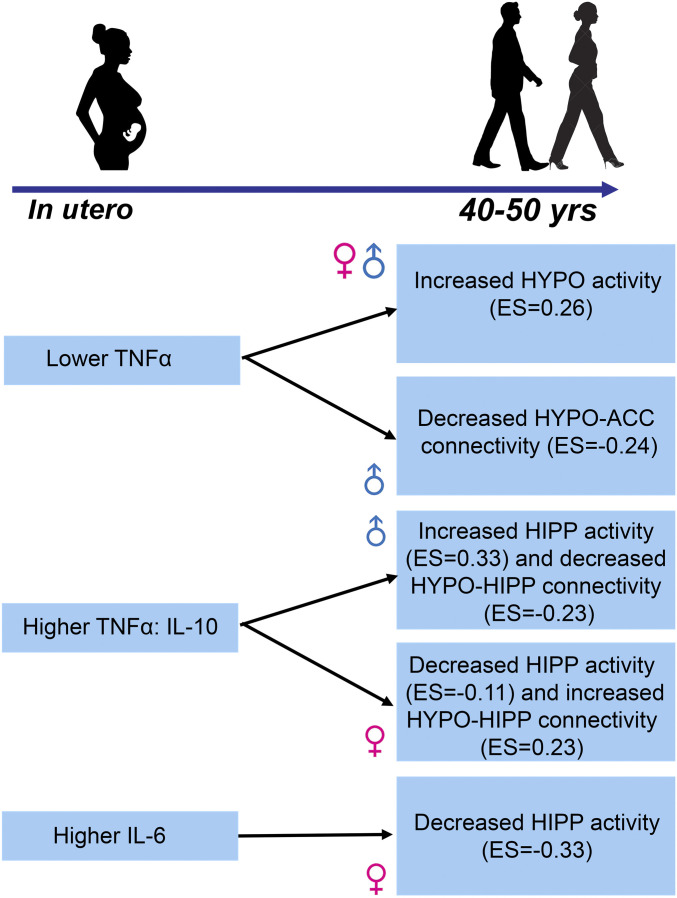
These results remained significant, adjusted for diagnosis, and demonstrated mild-to-moderate effect sizes in tandem with the BOLD responses of the long-term impact of in utero adverse maternal immune perturbations on two critical brain regions in the offspring that regulate response to negative stressful stimuli. Summary of overall findings and effects sizes are illustrated in Fig. 5.
Fig. 5.
This diagram summarizes the main findings of the paper with prenatal immune exposure shown on the left and the effects on stress response circuitry on the right. Effect sizes for activation results are β/SD and for connectivity results are R2.
Discussion
This study investigated the impact of in utero maternal immune activity on sex differences in the adult offspring’s brain circuitry that regulates response to negative stress. Brain regions of interest (HYPO, HIPP, and their connections) are key regulators of the stress response, highly sexually dimorphic, and the densest regions in the brain with cytokines TNF-α and IL-6. The timing of exposure (beginning of the third trimester) was critical for identifying a sex effect, given that this is a key period of the organizational effects of gonadal hormones on sexual differentiation of the brain that persist across the lifespan, demonstrated in preclinical studies (2, 4, 11, 12, 27, 44). Our findings suggest that disrupting this healthy sexual differentiation of the HYPO and HIPP may result in sex differences in lifelong hypersensitivity to negative stressful stimuli in the offspring.
Using a negative stress paradigm shown to induce HPA activation (33, 36, 45), results showed that lower levels of maternal TNF-α were associated with higher hypothalamic activity in response to negative stimuli in both male and female offspring. Sex differences in the impact of TNF-α emerged when maternal prenatal TNF-α concentration was examined in relation to the anti-inflammatory input of IL-10. The release of TNF-α (proinflammatory) can be offset by an increase in anti-inflammatory cytokines, like IL-10. If the ratio of TNF-α:IL-10 is high, it is indicative of immune imbalance and an inadequate anti-inflammatory response. Results showed that higher maternal TNF-α:IL-10 was associated with sex-dependent differences in hippocampal activation and connectivity between HIPP and HYPO. The HIPP provides negative feedback to the HYPO in response to negative stressful stimuli, enabling the HPA axis to inhibit CRH release and regulate arousal due to stress. Thus, findings suggest that adverse in utero immune exposures negatively impacted brain regions that regulate arousal due to negative stressful stimuli in a sex-dependent manner.
With higher TNF-α:IL-10 exposure, men had lower connectivity between HYPO and HIPP and thus less ability to inhibit higher activity of the HYPO by the HIPP. This may have required greater inhibitory control by the ACC, a connectivity result found only in the men and not women. In contrast, women exposed to higher TNF-α:IL-10 exhibited higher connectivity between HIPP and HYPO but lower hippocampal activation and thus, as in men, less ability to down-regulate hypothalamic arousal and potentially CRH release. This is consistent with our previous fMRI study in the original cohort, wherein we demonstrated in response to a negative stress challenge, hypercortisolemia was associated with activation in the HYPO and lower connectivity with the HIPP and cortical inhibitory regions, particularly in women (33, 34). It is also consistent with preclinical studies of prenatal administration of lipopolysaccharide demonstrating adverse microglial and neuronal impacts on offspring hippocampal and cortical structure and function that were sex dependent (46, 47).
Taken together, the results in men and women suggested that prenatal exposure to an imbalance of maternal immune activity at a key period of sex-sensitive brain development resulted in less ability to regulate response to negative stress, neural outcomes that were retained 45 y later, independent of diagnosis. However, the pattern of dysregulation manifested differently in male and female offspring due, in part, to differential maternal in utero anti-inflammatory responses.
During pregnancy, there are high levels of estradiol, especially when the fetus is female, which can affect neuroimmune balance. Consistent with this, high-dose estradiol treatment of female mice was found to reduce proinflammatory cytokines, including TNF-α, and shift the immune system balance toward an anti-inflammatory state (48). Thus, in the context of high estradiol during pregnancy, a maternal TNF-α deficit combined with a higher anti-inflammatory response (e.g., increased levels of IL-10) may have different consequences for male and female fetuses. The TNF-α:IL-10 ratio is typically lower at midgestation, in order to maintain a tolerogenic state that protects against fetal rejection. In our study, a greater T helper type 2 (Th2) bias (IL-10), relative to the strength of the proinflammatory T helper type 1 (Th1) cytokine, TNF-α, was more important than the absolute level of the individual Th2 cytokine (such as IL-10).
We also found lower adverse maternal levels of TNF-α alone had a negative impact on the offspring HYPO, stronger for women. Lower TNF-α was likely associated with co-occurring cortisol released by the adrenal gland in response to HPA-axis activation due to the stress challenge that inhibits TNF-α, creating a negative feedback loop. TNF-α is the HPA coactivator most sensitive to glucocorticoid inhibition (18). Although prenatal cortisol was not assessed in the study presented here, we would argue, and will test in future studies, that lower prenatal maternal TNF-α levels were associated with higher prenatal maternal cortisol in response to the negative stress stimuli. This is consistent with a previous study demonstrating that higher prenatal maternal cortisol was associated with lower prenatal TNF-α in mothers with depression (49). The argument is also consistent with our preclinical work demonstrating sex-dependent effects of excess maternal prenatal glucocorticoids on development of the HIPP and HYPO and mood-related behaviors, particularly in females, at a time of exposure analogous to the timing of the TNF-α exposure assessed here (11, 12, 16, 50, 51). Thus, the impact of maternal in utero glucocorticoid levels in tandem with in utero immune perturbations needs further investigation regarding sex effects on brain circuitry regulating the stress response.
In contrast, higher levels of maternal IL-6 were associated with hypoactivity in the left HIPP, particularly in women, again suggesting less hippocampal ability to inhibit arousal (negative feedback) in the PVN of the HYPO, the key relay station for HPA-axis function. IL-6 is the HPA coactivator least sensitive to the inhibitory effects of glucocorticoids and has previously been implicated in preclinical studies of prenatal lipopolysaccharide challenges on offspring brain and memory (18, 52, 53). Thus, higher levels (rather than lower levels) of IL-6 producing deleterious effects was not surprising.
The validity of these findings is underscored, given that third trimester levels of IL-6 in mothers of male fetuses were significantly higher than in mothers of female fetuses, but the adverse impact at this gestational period was greater for female offspring. This is consistent with preclinical studies of sex differences in microglia and mast cells suggesting that adverse prenatal immune exposures would have greater effects on female fetuses later in gestation/early postnatal and greater effects on male fetuses with early exposure (25–27, 46, 54–56). Thus, we posited that timed adverse–prenatal immune exposures played a key role in shifting the typical sex bias of the fetal brain by disrupting developmentally sensitive neuroimmune interactions (including microglia, mast cells, and neurons) in vulnerable brain regions such as the HYPO and HIPP.
Limitations of this study include the lack of statistical power given the sample size to investigate all brain regions in the stress response circuitry and a larger panel of maternal immune markers. Furthermore, as mentioned, immune activity is coordinated with adrenal hormone activity, and understanding their joint or specific effects on offspring brain development is warranted given findings from previous preclinical and clinical studies (16, 57–61). Finally, further work is needed to investigate whether there is any diagnostic specificity given that there was a lack of power to test for three-way interactions with sex. The strengths of this study outweigh these limitations. The sample is wholly unique in that they have been followed from prenatal development for 45 y to assess brain activity and physiology associated with prenatal maternal serologic exposures. Furthermore, we included potential confounds, stringent controls for multiple comparisons, quality controls for our assessments, and state-of-the-art brain imaging technology.
Findings here suggest a mechanism implicating prenatal stress-immune models of offspring brain development and adult outcomes. Prenatal exposures, either exogenous or endogenous, can result in an adverse maternal inflammatory response, which can produce long-lasting effects on the offspring’s development of specific brain regions involved in the regulation of stress (44). Our findings implicate pregnancy complications, such as preeclampsia and infectious diseases, as potential exogenous prenatal exposures influencing maternal immune responses, although this requires additional investigation. Furthermore, when prenatal exposures occur during critical periods of brain sexual differentiation, the impact will differ by sex in highly sexually dimorphic brain regions including the HYPO and HIPP (2, 5, 7). Numerous preclinical studies (16, 57–61) of dexamethasone and immune prenatal challenges demonstrated lasting consequences on offspring brain and behavior, including specifically, on the HYPO and HIPP.
The study presented here offered a rare opportunity in clinical research to test the impact of in utero immune exposures on offspring neural outcomes retained over 45 y. The mechanisms explaining what is transmitted from mother to offspring to produce brain and associated physiology abnormalities in the offspring needs further work in preclinical studies, which we and others are conducting. Here, we suggest that the prenatal immune impact on offspring brain development may set the stage for producing a hypersensitivity to negative stressful events throughout life, a sex-dependent effect that is quantitative and may contribute to understanding sex differences in disease.
Materials and Methods
Participants.
The study included 80 New England Family Study participants (equally divided by sex and comparable within sex for age) for whom we had data on maternal cytokine concentrations (33). Half the participants had one of three psychiatric diagnoses: major depressive disorder (14 women and 11 men), bipolar disorder (6 women and 3 men), or schizophrenia (1 woman and 6 men), and half had no psychiatric diagnosis. All participants provided written, informed consent and were compensated for their participation. The full study was approved by the Institutional Review Boards of Harvard University, Brown University, and Partners Healthcare system.
Participants’ mothers were an average age of 26 y (SD = 6.1, range 18 to 40) when they were enrolled in the Collaborative Perinatal Project (CPP) in 1959 to 1966 (62), had a mean socioeconomic status (SES) of 5.7 (SD = 1.9, range 1.5 to 9.0), and were 95% White and 5% African American. SES was calculated as a composite score combining parental education (more than high school, high school graduate, less than high school), income relative to the US poverty threshold (>150% of the poverty threshold, 100 to 150% of the poverty threshold, < the poverty threshold), occupation (nonmanual, manual, or unemployed), and family structure (both parents at home, single, or divorced/separated/widowed). The score was 0 to 9 with higher values indicating greater socioeconomic disadvantage (63). There were no significant differences by sex of offspring for any sociodemographic or clinical characteristics. The sample was enriched for obstetric complications (such as preeclampsia), known to be associated with maternal immune responses, in order to ensure variability of the maternal prenatal cytokine levels.
Subjects underwent fMRI scanning during a mild visual stress challenge on a task we have reliably used for >15 y (33–36, 45). Neutral valence/low arousal and negative valence/high arousal images were selected based on ratings from the IAPS, known to reliably invoke stress circuitry (37, 38, 40, 45, 64, 65). Serum cortisol was measured at five timepoints throughout the task. Using an in-scanner baseline, we found a mean peak percent change in cortisol levels in response to the task of ∼23%, demonstrating that the task was indeed a stress reactivity task (5).
Assessment of Maternal Cytokines.
Maternal sera were collected approximately every 2 mo throughout pregnancy and stored at −20 °C at the National Institute of Health (NIH) repository. Previous work using samples stored under similar conditions and for a similar length of time (>40 y) demonstrated the long-term stability of analytes from the CPP samples (66). In addition, studies over several years from our group have demonstrated significant predictive validity of these prenatal maternal immune markers on multiple offspring outcomes in childhood and adulthood (8, 28, 29, 66, 67). Since the primary hypotheses in this study involved sex-dependent risk estimates, we selected the sample drawn closest to the beginning of the third trimester—a period consistent with the timing of the sexual differentiation of the brain (12)—and assessed concentrations of cytokines that are coactivators of HPA circuitry (IL-6, TNF-α, and IL-1β) and the anti-inflammatory cytokine, IL-10.
Serum levels of cytokines were examined using multiplexed, bead-based immunoassays (Milliplex human 5-plex cytokine panel filter plates, including IL-6, TNF-α, IL-1β, IL-10 as well as IL-8; MPXHCYTO-60 K, Millipore) on a Luminex 3D detection platform (68), as previously described (9, 67, 68). Assay detection sensitivities (lower limit of quantitation [LLQ]) ranged from 0.1 to 0.4 pg/mL, within the bounds noted by the kit manufacturer. A total of 25 microliters of each serum sample were diluted 1:1 and run with six serial dilutions (3.2 to 10,000 pg/mL) of cytokine standards, with one high- and one low-quality control sample and a common human reference sample (pooled plasma from healthy donors) on each 96-well plate (69). All samples were run in duplicate and in randomized order across plates. Assays were completed according to the manufacturers’ protocols, with overnight incubation at 4 °C on a shaker prior to detection of the median fluorescence intensity (70) of analyte-specific immunoassay beads by Luminex 3D. Raw data (70) were captured using Luminex xPONENT software (version 4.0.846.0), and concentrations of immune factors in each sample were interpolated from standard curves using a five-parameter, weighted, logistic regression curve equation in Milliplex Analyst (version 3.5.5.0). The intra-assay % coefficient of variation (CV) and the interassay % CV for the two kit-provided QC controls (across all plates) were both <10%. Measurements below the LLQ were excluded (affecting 2.5% of IL-1β values, 1.6% of IL-6 values, 1.5% of TNF-α values, and 0.3% of IL-10; 0.3% of data points). For measurements at or above the upper limit of analyte detection, samples were assayed again at multiple serial dilutions using Assay Buffer to bring concentrations into detectable range. Samples with both wells affected by low bead counts (<50) were rerun without dilution. As distributions of the four cytokines were highly skewed, they were natural log transformed prior to analyses. Reliability and predictive validity of these assay measurements have been demonstrated over the last 10 y in multiple publications (5, 8, 28, 29).
Acquisition of fMRI Data.
fMRI data were acquired on a Siemens Tim Trio 3T MRI scanner with 12-channel head coil. A total of 180 volumes per run were acquired using a spin echo, T2*-weighted sequence (repetition time [TR] = 2,000 ms, echo time [TE] = 40 ms, field of view [FOV] = 200 × 200 mm, matrix 64 × 64, in-plane resolution 3.125 mm, slice thickness 5 mm, 23 contiguous slices aligned to anterior commissure-posterior commissure [AC-PC] plane). The task consisted of presentation of negative valence/high arousal (e.g., snake, car accident, or gun), neutral valence/low arousal (e.g., plant, umbrella, or mushrooms), and fixation images adapted from the IAPS (35, 36, 40, 65). The task lasted 18 min (three runs, 6 min each). Each run contained 72 images ordered in blocks of fixation, negative, and neutral images, consisting of 6 images, each presented for 5 s. The mean normative valence and arousal of the negative valence/high arousal images was 2.19 (SD = 0.48) and 6.35 (SD = 0.52) and the neutral valence/low arousal images was 4.90 (SD = 0.37) and 2.91 (SD = 0.40), respectively. To ensure attention, participants pressed a button when each new image appeared.
Analyses of fMRI Data.
Data were preprocessed and analyzed using SPM8 (Welcome Trust Centre for Neuroimaging). All BOLD (71) signal-change images were motion corrected, realigned, and normalized to the MNI152 brain template in a nonlinear, volume-based method, spatially smoothed with a 6 mm full width at half maximum Gaussian filter and resampled to 3 mm isotropic. Outliers in global mean image–time series (threshold: 3.5 SDs from the mean) and movement (threshold: 0.7 mm, measured as scan-to-scan movement, separately for translation and rotation) were detected using an artifact detection toolbox (ART) (RRID: SCR_005994, https://www.nitrc.org/projects/artifact_detect) and entered as nuisance regressors in the first-level, single-subject GLM. Masks excluding voxels outside the brain were applied to ensure that voxels in regions with high interparticipant variability in signal dropout were not arbitrarily excluded. Comparisons of interest (negative > neutral) from first-level, single-subject analyses were tested using linear contrasts and SPM t-maps. Outputs from first-level, single-subject analyses were submitted to second-level random effects analysis.
BOLD Response.
One-sample t test examined the BOLD response to negative > neutral stimuli across the full sample (n = 80), with whole-brain, voxel-wise FWE-corrected threshold of P < 0.05, providing conservative effects adjusted for multiple comparisons. Next, an intersection analysis, performed using MarsBaR, identified clusters with whole-brain, voxel-wise FWE-corrected P < 0.05 threshold conjointly located within anatomical boundaries of HYPO and HIPP. As mentioned, we focused on HYPO and HIPP, regions densest with cytokine receptors, to avoid attenuating effects with multiple comparisons. Given the small volume and midline location of HYPO, a single region of interest (ROI) combining right and left hemispheres was used for this region. HIPP-ROI masks were bilateral. Percent signal-change values within each ROI were extracted for each participant using REX (72) and exported into JMP (SAS Institute), which was used for all remaining BOLD-response analyses. Activity within each ROI was explored as a function of TNF-α, IL-1β, TNF-α:IL10 ratio, and IL-6 using regression analysis, to identify main effects and interactions with sex, with significance at P < 0.05 level.
To assess the individual and joint associations of the four prenatal cytokines (IL-6, TNF-α, IL-1β, and IL-10) with BOLD responses in the HYPO and HIPP together, we performed multivariate protected F-tests using the PROC REG “mtest” option in SAS 9.4 (SAS Institute). Cytokine levels were evaluated continuously, and right and left HYPO and HIPP BOLD responses were entered into the models as a set of continuous dependent variables. Although power was insufficient to test multivariate interactions by sex, we performed sex-stratified analyses in addition to testing associations in the combined sample of males and females. Wilks’ lambda (43) was used to determine the significance of the protected test and allowed for further analyses of individual cytokines. Finally, regression models were rerun with the inclusion of diagnostic status in the models to explore the potential impact of diagnosis on these relationships.
Functional Connectivity.
Similar to BOLD response analyses above, we assessed task-related connectivity using generalized psychophysiological interaction (gPPI; see ref.73). Time courses from HYPO seed ROI were extracted and added to two additional PPI regressors (interaction of the seed time course with regressors for negative and neutral content) to individual subject-level GLMs. Interaction regressors were orthogonal to task and seed regressors, ensuring that seed ROI activation and PPI connectivity were independent (73). Connectivity was measured at single-subject level by estimating the difference between the interaction of the seed time course with the regressor for negative compared with neutral stimuli. Results of single-subject analysis were entered into second-level random effects analysis to probe group-level changes in connectivity during negative versus neutral conditions. Mirroring BOLD-response analyses, connectivity was explored as a function of TNF-α, TNF-α:IL-10 ratio, IL-1β, and IL-6 and then repeated to detect interactions with sex.
For functional connectivity analyses, we used a small volume correction (SVC) approach in SPM8, which limits voxel-wise analyses to voxels within a priori hypothesized ROIs. Target ROIs (PAG, AMYG, HIPP, OFC, ACC, and mPFC) were defined as anatomical masks and implemented as overlays on the SPM8 canonical brain. These ROIs were chosen based on several years of work identifying stress circuitry activated by our fMRI task, most recently in ∼100 subjects which included the sample here (34). False positives were controlled using conservative FWE correction to control for multiple comparisons. Within an anatomical ROI, results identified using SVC (initial voxel-wise threshold P < 0.05 uncorrected) were reported as significant if they additionally met the peak-level threshold of P < 0.05, FWE corrected. Additionally, average connectivity values (beta weights of PPI regressors) in significant target clusters were extracted using REX (72). Regression models were rerun with the inclusion of diagnostic status to explore whether there was one diagnostic category driving the association between maternal prenatal cytokines, sex, and functional connectivity.
Acknowledgments
This study was supported by National Institute of Mental Health (NIMH) R03 MH105585 and a donor fund from Ms. Gwill York (with some of the original data collected in Office of Research on Women's Health (ORWH)-NIMH P50 MH082679 and NIMH R01 MH56956 Phase III) (J.M.G. Principal Investigator (PI) across studies). J.M.G.’s and J.C.’s time was also, in part, supported by ORWH-NIMH U54 MH118919 (Goldstein/Handa Multi-PIs). The research was also conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH No. UL1 RR025758). For some of K.M.’s time, we would like to thank the European Social Fund and government of Czech Republic who cofinanced Grant No. CZ.1.07/2.3.00/30.0009 and the Marie Curie Intra-European Fellowship for Career Development, funded by the European Union. (Primary work by K.M. on this study was conducted while she was a postdoctoral fellow with J.M.G.) S.E.G.’s contribution was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Finally, we would like to thank Sarah Aroner, ScD., for her data analytic contributions.
Footnotes
Competing interest statement: J.M.G. is on the scientific advisory board for, and has equity interest in, Cala Health (a neuromodulation company). J.M.G.’s interests are managed by Massachusetts General Hospital and Mass General Brigham HealthCare in accordance with their conflict of interest policies. However, the work in the study presented here was conducted prior to that relationship, so there is no conflict of interest.
This article is a PNAS Direct Submission. M.C.-R. is a guest editor invited by the Editorial Board.
Data Availability
Data used for this study can be obtained through a Data Use Agreement with the New England Family Study at: https://sites.google.com/a/brown.edu/nefs/. In addition, all serology analyses results are required to be returned and stored at the NIH repository and are accessible for use by other researchers.
References
- 1.Barker D. J., Intrauterine programming of adult disease. Mol. Med. Today 1, 418–423 (1995). [DOI] [PubMed] [Google Scholar]
- 2.McEwen B. S., Gonadal steroid influences on brain development and sexual differentiation. Int. Rev. Physiol. 27, 99–145 (1983). [PubMed] [Google Scholar]
- 3.Weinstock M., The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 32, 1073–1086 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Bale T. L., Neuroendocrine and immune influences on the CNS: It’s a matter of sex. Neuron 64, 13–16 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Goldstein J. M., Impact of prenatal stress on offspring psychopathology and comorbidity with general medicine later in life. Biol. Psychiatry 85, 94–96 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Sandman C. A., Glynn L. M., Davis E. P., Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J. Psychosom. Res. 75, 327–335 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein J. M., Hale T., Foster S. L., Tobet S. A., Handa R. J., Sex differences in major depression and comorbidity of cardiometabolic disorders: Impact of prenatal stress and immune exposures. Neuropsychopharmacology 44, 59–70 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilman S. E., et al., Prenatal immune programming of the sex-dependent risk for major depression. Transl. Psychiatry 6, e822 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handa R. J., Weiser M. J., Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front. Neuroendocrinol. 35, 197–220 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein J. M., et al., Sex-specific impact of maternal-fetal risk factors on depression and cardiovascular risk 40 years later. J. Dev. Orig. Health Dis. 2, 353–364 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobet S., et al., Brain sex differences and hormone influences: A moving experience? J. Neuroendocrinol. 21, 387–392 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handa R. J., Burgess L. H., Kerr J. E., O’Keefe J. A., Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 28, 464–476 (1994). [DOI] [PubMed] [Google Scholar]
- 13.Mandal M., et al., Maternal immune stimulation during pregnancy shapes the immunological phenotype of offspring. Brain Behav. Immun. 33, 33–45 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Williamson L. L., Sholar P. W., Mistry R. S., Smith S. H., Bilbo S. D., Microglia and memory: Modulation by early-life infection. J. Neurosci. 31, 15511–15521 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besedovsky H. O., et al., Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. J. Steroid Biochem. Mol. Biol. 40, 613–618 (1991). [DOI] [PubMed] [Google Scholar]
- 16.Zuloaga D. G., et al., Perinatal dexamethasone-induced alterations in apoptosis within the hippocampus and paraventricular nucleus of the hypothalamus are influenced by age and sex. J. Neurosci. Res. 90, 1403–1412 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Beijers R., Buitelaar J. K., de Weerth C., Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: Beyond the HPA axis. Eur. Child Adolesc. Psychiatry 23, 943–956 (2014). [DOI] [PubMed] [Google Scholar]
- 18.DeRijk R., et al., Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 beta (IL-1 beta), IL-6, and tumor necrosis factor-alpha (TNF alpha) production in humans: High sensitivity of TNF alpha and resistance of IL-6. J. Clin. Endocrinol. Metab. 82, 2182–2191 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Dozmorov M. G., et al., Associations between maternal cytokine levels during gestation and measures of child cognitive abilities and executive functioning. Brain Behav. Immun. 70, 390–397 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bangasser D. A., Shors T. J., Critical brain circuits at the intersection between stress and learning. Neurosci. Biobehav. Rev. 34, 1223–1233 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y. L., Wang S., Prenatal lipopolysaccharide exposure increases depression-like behaviors and reduces hippocampal neurogenesis in adult rats. Behav. Brain Res. 259, 24–34 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Paintlia M. K., Paintlia A. S., Barbosa E., Singh I., Singh A. K., N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. J. Neurosci. Res. 78, 347–361 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Kumral A., et al., Erythropoietin attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neonatology 92, 269–278 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Winter C., et al., Dopamine and serotonin levels following prenatal viral infection in mouse–implications for psychiatric disorders such as schizophrenia and autism. Eur. Neuropsychopharmacol. 18, 712–716 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling Z., et al., Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp. Neurol. 199, 499–512 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Hanamsagar R., et al., Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 65, 1504–1520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenz K. M., McCarthy M. M., A starring role for microglia in brain sex differences. Neuroscientist 21, 306–321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghassabian A., et al., Gestational cytokine concentrations and neurocognitive development at 7 years. Transl. Psychiatry 8, 64 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein J. M., et al., Prenatal maternal immune disruption and sex-dependent risk for psychoses. Psychol. Med. 44, 3249–3261 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis E. P., Pfaff D., Sexually dimorphic responses to early adversity: Implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology 49, 11–25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arisi G. M., Nervous and immune systems signals and connections: Cytokines in hippocampus physiology and pathology. Epilepsy Behav. 38, 43–47 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Harbuz M. S., Stephanou A., Sarlis N., Lightman S. L., The effects of recombinant human interleukin (IL)-1 alpha, IL-1 beta or IL-6 on hypothalamo-pituitary-adrenal axis activation. J. Endocrinol. 133, 349–355 (1992). [DOI] [PubMed] [Google Scholar]
- 33.Mareckova K., et al., Brain activity and connectivity in response to negative affective stimuli: Impact of dysphoric mood and sex across diagnoses. Hum. Brain Mapp. 37, 3733–3744 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mareckova K., et al., Neural - hormonal responses to negative affective stimuli: Impact of dysphoric mood and sex. J. Affect. Disord. 222, 88–97 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein J. M., Jerram M., Abbs B., Whitfield-Gabrieli S., Makris N., Sex differences in stress response circuitry activation dependent on female hormonal cycle. J. Neurosci. 30, 431–438 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein J. M., et al., Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J. Neurosci. 25, 9309–9316 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang P. J., et al., Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology 35, 199–210 (1998). [PubMed] [Google Scholar]
- 38.Bradley M. M., Codispoti M., Cuthbert B. N., Lang P. J., Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion 1, 276–298 (2001). [PubMed] [Google Scholar]
- 39.Bradley M. M., Codispoti M., Sabatinelli D., Lang P. J., Emotion and motivation II: Sex differences in picture processing. Emotion 1, 300–319 (2001). [PubMed] [Google Scholar]
- 40.Bradley M. M., Cuthbert B. N., Lang P. J., Picture media and emotion: Effects of a sustained affective context. Psychophysiology 33, 662–670 (1996). [DOI] [PubMed] [Google Scholar]
- 41.Briceño E. M., et al., Age and gender modulate the neural circuitry supporting facial emotion processing in adults with major depressive disorder. Am. J. Geriatr. Psychiatry 23, 304–313 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McManis M. H., Bradley M. M., Berg W. K., Cuthbert B. N., Lang P. J., Emotional reactions in children: Verbal, physiological, and behavioral responses to affective pictures. Psychophysiology 38, 222–231 (2001). [PubMed] [Google Scholar]
- 43.Pavur R., Nath R., Exact F tests in an ANOVA procedure for dependent observations. Multivariate Behav. Res. 19, 408–420 (1984). [DOI] [PubMed] [Google Scholar]
- 44.McCormick C. M., Smythe J. W., Sharma S., Meaney M. J., Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res. Dev. Brain Res. 84, 55–61 (1995). [DOI] [PubMed] [Google Scholar]
- 45.Holsen L. M., et al., Stress response circuitry hypoactivation related to hormonal dysfunction in women with major depression. J. Affect. Disord. 131, 379–387 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giovanoli S., et al., Late prenatal immune activation causes hippocampal deficits in the absence of persistent inflammation across aging. J. Neuroinflammation 12, 221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hui C. W., et al., Prenatal immune challenge in mice leads to partly sex-dependent behavioral, microglial, and molecular abnormalities associated with schizophrenia. Front. Mol. Neurosci. 11, 13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Correale J., Arias M., Gilmore W., Steroid hormone regulation of cytokine secretion by proteolipid protein-specific CD4+ T cell clones isolated from multiple sclerosis patients and normal control subjects. J. Immunol. 161, 3365–3374 (1998). [PubMed] [Google Scholar]
- 49.Shelton M. M., Schminkey D. L., Groer M. W., Relationships among prenatal depression, plasma cortisol, and inflammatory cytokines. Biol. Res. Nurs. 17, 295–302 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frahm K. A., Schow M. J., Tobet S. A., The vasculature within the paraventricular nucleus of the hypothalamus in mice varies as a function of development, subnuclear location, and GABA signaling. Horm. Metab. Res. 44, 619–624 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Frahm K. A., Tobet S. A., Development of the blood-brain barrier within the paraventricular nucleus of the hypothalamus: Influence of fetal glucocorticoid excess. Brain Struct. Funct. 220, 2225–2234 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolton J. L., Short A. K., Simeone K. A., Daglian J., Baram T. Z., Programming of stress-sensitive neurons and circuits by early-life experiences. Front. Behav. Neurosci. 13, 30 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brunson K. L., et al., Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 25, 9328–9338 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCarthy M. M., Sex differences in neuroimmunity as an inherent risk factor. Neuropsychopharmacology 44, 38–44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lenz K. M., et al., Mast cells in the developing brain determine adult sexual behavior. J. Neurosci. 38, 8044–8059 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joshi A., et al., Sex differences in the effects of early life stress exposure on mast cells in the developing rat brain. Horm. Behav. 113, 76–84 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Frahm K. A., Handa R. J., Tobet S. A., Embryonic exposure to dexamethasone affects nonneuronal cells in the adult paraventricular nucleus of the hypothalamus. J. Endocr. Soc. 2, 140–153 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bronson S. L., Bale T. L., Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 155, 2635–2646 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mueller B. R., Bale T. L., Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 28, 9055–9065 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mueller B. R., Bale T. L., Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol. Behav. 91, 55–65 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Lenz K. M., Pickett L. A., Wright C. L., Galan A., McCarthy M. M., Prenatal allergen exposure perturbs sexual differentiation and programs lifelong changes in adult social and sexual behavior. Sci. Rep. 9, 4837 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niswander K. R., Gordon M., National Institute of Neurological Diseases and Stroke , The Women and Their Pregnancies; The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke (W.B. Saunders Company, Philadelphia, PA, 1972), pp. 540. [Google Scholar]
- 63.Chin-Lun Hung G., et al., Socioeconomic disadvantage and neural development from infancy through early childhood. Int. J. Epidemiol. 44, 1889–1899 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobs E. G., et al., 17β-estradiol differentially regulates stress circuitry activity in healthy and depressed women. Neuropsychopharmacology 40, 566–576 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holsen L. M., et al., HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience 250, 733–742 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stroud L. R., et al., Long-term stability of maternal prenatal steroid hormones from the national collaborative perinatal Project: Still valid after all these years. Psychoneuroendocrinology 32, 140–150 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilman S. E., et al., Socioeconomic disadvantage, gestational immune activity, and neurodevelopment in early childhood. Proc. Natl. Acad. Sci. U.S.A. 114, 6728–6733 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vignali D. A., Multiplexed particle-based flow cytometric assays. J. Immunol. Methods 243, 243–255 (2000). [DOI] [PubMed] [Google Scholar]
- 69.Martins T. B., Development of internal controls for the Luminex instrument as part of a multiplex seven-analyte viral respiratory antibody profile. Clin. Diagn. Lab. Immunol. 9, 41–45 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Begum G., et al., Maternal undernutrition programs tissue-specific epigenetic changes in the glucocorticoid receptor in adult offspring. Endocrinology 154, 4560–4569 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Hirshfeld D. R., et al., Stable behavioral inhibition and its association with anxiety disorder. J. Am. Acad. Child Adolesc. Psychiatry 31, 103–111 (1992). [DOI] [PubMed] [Google Scholar]
- 72.Duff E. P., Cunnington R., Egan G. F., REX: Response exploration for neuroimaging datasets. Neuroinformatics 5, 223–234 (2007). [DOI] [PubMed] [Google Scholar]
- 73.McLaren D. G., Ries M. L., Xu G., Johnson S. C., A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage 61, 1277–1286 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used for this study can be obtained through a Data Use Agreement with the New England Family Study at: https://sites.google.com/a/brown.edu/nefs/. In addition, all serology analyses results are required to be returned and stored at the NIH repository and are accessible for use by other researchers.