Significance
Release of oligomeric/fibrillar α-synuclein (αSyn) from damaged neurons contributes to neuronal cell death in Parkinson’s disease and other neurodegenerative disorders in part via microglial activation. Here, we show that αSyn activates the NLRP3 inflammasome in human induced pluripotent stem cell (hiPSC)-derived microglia (hiMG) via dual stimulation involving TLR2 engagement and mitochondrial damage. Oligomerized amyloid-β peptide (Aβ), as found in Alzheimer’s disease brains, exacerbates this neuroinflammation. Importantly, we found that misfolded proteins such as αSyn and Aβ, when bound to antibody and presented to hiMG, result in enhanced proinflammatory response of the NLRP3 inflammasome. These findings may have important implications for antibody therapies aimed at depleting misfolded/aggregated proteins from the human brain, as they may paradoxically trigger neuroinflammation in human microglia.
Keywords: neuroinflammation, Parkinson’s disease, Lewy body dementia, Alzheimer’s disease, antibody therapies
Abstract
Parkinson’s disease is characterized by accumulation of α-synuclein (αSyn). Release of oligomeric/fibrillar αSyn from damaged neurons may potentiate neuronal death in part via microglial activation. Heretofore, it remained unknown if oligomeric/fibrillar αSyn could activate the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) inflammasome in human microglia and whether anti-αSyn antibodies could prevent this effect. Here, we show that αSyn activates the NLRP3 inflammasome in human induced pluripotent stem cell (hiPSC)-derived microglia (hiMG) via dual stimulation involving Toll-like receptor 2 (TLR2) engagement and mitochondrial damage. In vitro, hiMG can be activated by mutant (A53T) αSyn secreted from hiPSC-derived A9-dopaminergic neurons. Surprisingly, αSyn–antibody complexes enhanced rather than suppressed inflammasome-mediated interleukin-1β (IL-1β) secretion, indicating these complexes are neuroinflammatory in a human context. A further increase in inflammation was observed with addition of oligomerized amyloid-β peptide (Aβ) and its cognate antibody. In vivo, engraftment of hiMG with αSyn in humanized mouse brain resulted in caspase-1 activation and neurotoxicity, which was exacerbated by αSyn antibody. These findings may have important implications for antibody therapies aimed at depleting misfolded/aggregated proteins from the human brain, as they may paradoxically trigger inflammation in human microglia.
Parkinson’s disease (PD) is characterized by accumulation of α-synuclein (αSyn; encoded by the SNCA gene) (1). Release of oligomeric/fibrillar αSyn from damaged neurons may potentiate neuronal cell death in part via microglial activation (2, 3). Moreover, misfolded proteins in general are thought to interact with brain microglia, triggering microglial activation that contributes to neurodegenerative disorders, although microglial phagocytosis may also initially clear aberrant proteins to afford some degree of protection (2, 4). Additionally, in Alzheimer’s disease (AD), amyloid-β peptide (Aβ) is thought to trigger similar processes in microglia (5–7); however, the mechanism for this trigger is still poorly understood.
Microglial cells contribute to neuroinflammation, specifically that mediated by the inflammasome. In particular, the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) inflammasome has been associated with several neurodegenerative disorders, although other types of inflammation may also be important in this regard (8). The NLRP3 inflammasome is a multiprotein complex that responds to cell stress and pathogenic stimuli to promote activation of caspase-1, which in turn mediates maturation and release of proinflammatory cytokines, including interleukin-1β (IL-1β) and IL-18 (9–11). NLRP3 inflammasome activation is a two-step process, involving an initial priming step and a secondary trigger. Priming involves a proinflammatory stimulus, such as endotoxin, a ligand for Toll-like receptor 4 (TLR4), that increases the abundance of NLRP3 and promotes de novo synthesis of pro–IL-1β via nuclear factor κB (11). The secondary trigger promotes inflammasome complex assembly and caspase-1 activation that in turn mediates the cleavage of pro–IL-1β and subsequent release of mature IL-1β. There are various secondary triggers, including adenosine triphosphate (ATP), microparticles, and bacterial toxins, all of which somehow lead to mitochondrial damage and release of oxidized mitochondrial DNA (11). Neuroinflammation has been reported in both human PD and AD brains (12–15), and NLRP3 inflammasome activation in particular has been observed in mouse models of PD and AD (7, 16). Importantly, in these PD models, dopaminergic (DA) neurons in the substantia nigra are resistant to damage in NLRP3-deficient mice compared with wild-type (WT) mice (16). Interestingly, a recent report identified an NLRP3 polymorphism that confers decreased risk in PD (17). Several groups have reported that fibrillar αSyn can activate the NLRP3 inflammasome in mice and in human monocytes (18–22), but it remains unknown if human brain microglia can be activated in this manner. Critically, antibodies targeting misfolded proteins are being tested in human clinical trials for several neurodegenerative diseases, including AD and PD; however, it is still unclear how antibodies to αSyn might affect this inflammatory response. In this study, we characterized the response of human induced pluripotent stem cell (hiPSC)-derived microglia (hiMG) to oligomeric/fibrillar αSyn in vitro and in vivo, using engraftment of hiMG in humanized mice. We used these immunocompromised mice because they prevent human cell rejection and express three human genes that support human cell engraftment (23). We show that αSyn and, even more so, αSyn–antibody complexes activate the NLRP3 inflammasome. Moreover, this process is further sensitized by the presence of Aβ and its cognate antibodies. These observations are of heightened interest because recent studies have shown that both misfolded Aβ and αSyn are present in several neurodegenerative disorders such as AD and Lewy body dementia (LBD), a form of dementia that can occur in the setting of PD (24–26).
Results
Generation and Characterization of hiPSC-Derived Microglia-Like Cells.
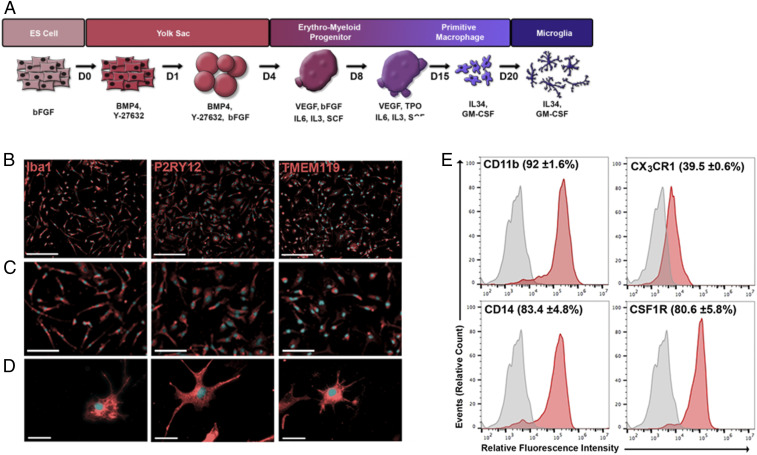
To study neuroinflammatory pathways in molecular detail in a human context, we modeled the normal yolk sac and erythromyeloid embryonic program of brain microglial development to produce a simple 21-d differentiation protocol that generates microglia-like cells from hiPSCs more rapidly than previously reported procedures (27–30). A schematic of the differentiation process is provided in Fig. 1A. Differentiation was initiated with formation of floating embryoid bodies (EBs). Starting from days 3 to 4 of differentiation, cystic EBs were formed and continued to expand in size over the next 4 d (SI Appendix, Fig. S1A). EBs harbored a heterogeneous population of cells, a subset of which displayed coexpression of the yolk sac markers CD41, c-Kit, CD144 (VE-cadherin), and CD235a (glycophorin) (31) (SI Appendix, Fig. S1B).
Fig. 1.
Differentiation of human iPSCs into microglia-like cells. (A) Schematic representation of the differentiation protocol showing media composition and culture conditions. (B) Immunocytochemical characterization of hiMG (red) and Hoechst nuclear staining (blue). (Scale bars, 200 µm.) (C) Higher magnification showing marker expression is evident in virtually every cell. (Scale bars, 100 µm.) (D) Even higher magnification images showing individual cell morphology and marker expression. (Scale bars, 25 µm.) (E) Flow cytometry analysis of cell-surface microglial markers. Representative histograms of CD11b-APC, CX3CR1-PE, CD14-APC, and CSF1R-APC; primary antibody (red) and unstained control (gray). Labels above each histogram indicate the mean percentage of positive cells and SEM (n = 3 biological replicates).
Throughout the differentiation procedure, the cells exhibited temporal patterns of gene expression corresponding to successive stages of microglial embryonic development (SI Appendix, Fig. S1C). For example, at early stages of differentiation (day 4), cells expressed mix paired-like homeobox (MIXL1) (32) and apelin receptor (APLNR) (33). Between days 4 and 11, cells displayed messenger RNA expression of kinase insert domain receptor [KDR; also known as FLK1 (31)], platelet-derived growth factor subunit A (PDGFα), and ETS variant 2 [ETV2; also known as ER71 (33)], and, subsequently, runt-related transcription factor 1 (RUNX1) and TAL bHLH transcription factor 1, erythroid differentiation factor [TAL1; also known as SCL (34)]. Notably, from days 17 to 21, the cells expressed high levels of colony-stimulating factor 1 receptor (CSF1R), interferon regulatory factor 8 (IRF8), crystallin β B1 (CRYBB1), MAF bZIP transcription factor B (MAFB), and CD14, a group of microglia-specific factors that were recently shown to represent mature microglia (35). Interestingly, by day 21, the cells also exhibited decreased levels of CSF1, a phenomenon indicative of microglia maturation (35) (SI Appendix, Fig. S1C). After 21 d of differentiation, the adherent cell population resulted in hiMG exhibiting morphology and markers indistinguishable from human brain-derived microglia (36, 37). They manifested an irregular shape with ramified processes that varied in size and length, and all of the cells expressed the microglial markers transmembrane protein 119 (TMEM119), Iba1, and purinergic receptor P2Y12 (P2RY12) (Fig. 1 B–D) (38–41). TMEM119 and P2RY12 are associated with a homeostatic microglia state, suggesting that our hiMG cells were not activated when cultured and required additional stimulation to acquire a disease-associated microglial phenotype and proinflammatory state (39, 40). Moreover, flow cytometry confirmed the high purity of the hiMG population in our cultures, with >80% of the cells expressing CD11b, CSF1R, and CD14 (Fig. 1E and SI Appendix, Fig. S1D).
To identify genes activated in hiMG cells, we performed RNA sequencing (RNA-seq). In comparison with parental hiPSCs, we identified 2,100 genes with significantly lower and 2,232 genes with significantly higher expression in hiMG (Dataset S1A). As expected, we found lower expression of genes associated with pluripotency in hiMG, including POU5F1 (OCT4), NANOG, SOX2, and DNMT3B. In support of a bona fide microglial identity, genes with elevated expression in hiMG were enriched for functional annotations consistent with cytokine/chemokine function, immune response, Toll receptor signaling, integrin signaling, interleukin signaling, and constituents of lysosomes (SI Appendix, Fig. S2A and Dataset S1B). We analyzed additional RNA-seq data from published human brain microglial cells and other monocytoid cells to offer further support for the identity of our hiMG (SI Appendix, Fig. S2 and Dataset S2).
Next, we examined the function of hiMG in contexts relevant to their activation in the brain. Microglia serve as phagocytic cells and are thus involved in removal of pathogens, isolation of aggregated/misfolded proteins, and pruning of synapses (42). Indeed, indicative of their phagocytic capability, our hiMG engulfed zymosan-pHrodo bioparticles, displaying fluorescent signal only in lysosomes (SI Appendix, Fig. S3A). Another functional characteristic of microglia is proinflammatory activation. Under basal conditions, our hiMG did not produce proinflammatory cytokines and demonstrated gene expression reminiscent of quiescent microglia. Lipopolysaccharide (LPS or endotoxin) stimulation resulted in a dose-dependent increase in secretion of proinflammatory cytokines, including IL-6 and tumor necrosis factor (TNF). Additionally, LPS engendered a dose-dependent increase in mRNA expression of IL-1β (SI Appendix, Fig. S3B) and a concomitant decrease in CSF1R and fractalkine receptor CX3CR1 (SI Appendix, Fig. S3C); CSF1R and CX3CR1 expression are known to negatively correlate with proinflammatory activation (43, 44). Interestingly, LPS also induced dose-dependent decreases in TREM2 and CD33 (SI Appendix, Fig. S3C), whose down-regulation has been shown to be associated with brain inflammation in AD (45). Thus, while our in vitro hiMG may not completely mimic gene expression of human brain microglia in vivo (46, 47), they do provide a platform to study the mechanistic details of inflammatory activation since the expression level of these genes is similar (SI Appendix, Figs. S1 and S2).
Oligomeric/Aggregated αSyn Activates the NLRP3 Inflammasome.
To investigate the effect of soluble αSyn assemblies on NLRP3 inflammasome activation in our hiMG, we generated monomeric αSyn and oligomeric/aggregated αSyn preparations, which are known to contain fibrils (48) (Fig. 2A). The oligomeric preparation exhibited a higher molecular mass than monomers and contained larger particles based on dynamic light scattering (DLS) (Fig. 2A and SI Appendix, Fig. S3 D and E). We then examined NLRP3 inflammasome activation in hiMG using well-established primers and activators, such as LPS and Alum/Nigericin/ATP (11), all of which increased IL-1β secretion (Fig. 2B). Importantly, incubation of hiMG with oligomeric αSyn stimulated IL-1β secretion and caspase-1 activation in a dose-dependent manner, and was far more active than monomeric αSyn (Fig. 2 C and D and SI Appendix, Fig. S3F). This effect was NLRP3-dependent since inhibiting NLRP3 by small interfering RNA knockdown blocked IL-1β release (Fig. 2E and SI Appendix, Fig. S3 G and H). Additionally, MCC950, a pharmacological NLRP3 antagonist (49), attenuated IL-1β release (SI Appendix, Fig. S3I). This finding is consistent with the hypothesis that αSyn oligomers provide both of the necessary triggers for NLRP3 inflammasome priming and activation, and thus does not require a priming signal, consistent with a previous observation in mice (21). After exposure to oligomeric αSyn, we also found increased secretion of TNF and IL-6, further indicating proinflammatory activation (SI Appendix, Fig. S3J). Of note, the minor degree of activation observed with αSyn monomers may well have resulted from incipient oligomer formation, as observed within 2 h of incubation at 37 °C (SI Appendix, Fig. S3E).
Fig. 2.
Oligomeric αSyn provides two signals for NLRP3 inflammasome activation. (A) Dynamic light scattering analysis of the particle size of the two types of αSyn preparations used in this study—αSyn monomers and oligomer/aggregates. Fractions of monomer vs. aggregated αSyn categorized by hydrodynamic diameter (D) on DLS are shown in different shading. Two-way ANOVA followed by Sidak’s multiple-comparisons test revealed a significant difference between the two preparations for monomeric αSyn as well as aggregates >2,000 nm. No significant difference was found for aggregates of 10 to 200 and 200 to 2,000 nm (light gray and dark gray bars). (B) Quantification of IL-1β secretion following inflammasome activation with LPS + ATP/Alum/Nigericin (n = 5 per group). (C) IL-1β secretion following exposure to αSyn monomers and oligomers (750 nM, n = 10 per group). (D) Cleaved caspase-1 (p20) release following exposure to αSyn monomers and oligomer (750 nM, n = 5 to 9). (E) IL-1β secretion following NLRP3 knockdown and exposure to oligomeric αSyn (750 nM, n = 5 per group). (F) Quantification of IL-1β following treatment with neutralizing antibodies against TLR2 and TLR4 or controls (IgA and IgG, respectively) (n = 6 per group). (G) Representative images of MitoSOX fluorescence reflecting mitochondria-generated ROS following vehicle (control) and monomer and oligomer αSyn exposure (750 nM). (Scale bars, 100 µm.) (H) Quantification of MitoSOX fluorescence after exposure to monomeric or oligomeric αSyn (750 nM, n = 9 per group). (I) Representative images of tetramethylrhodamine methylester (TMRM) fluorescence, assessing mitochondrial membrane potential, following control and monomer and oligomer αSyn exposure (750 nM). (Scale bars, 200 µm.) (J) TMRM quantification after exposure to monomeric or oligomeric αSyn (750 nM, n = 5 to 10). (K) mtDNA release of ND5, ND6, and COX1 following exposure to αSyn monomers and oligomers (750 nM, n = 3 to 5). Graphs indicate mean ± SEM. Statistical analysis was performed using two-way ANOVA with Bonferroni post hoc test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
αSyn has been reported to bind to TLR2 and TLR4 (50, 51). To determine which TLR is involved in αSyn-mediated inflammasome activation, we used neutralizing antibodies. We found that neutralizing TLR2 nearly completely inhibited IL-1β release (Fig. 2F). TLR2-neutralizing antibody also reduced IL-6 and TNF release following activation induced by αSyn oligomers (SI Appendix, Fig. S3J). In contrast, neutralizing TLR4 had no effect on IL-1β release (Fig. 2F). This lack of effect was not the result of inefficient neutralization of TLR4 because the TLR4-neutralizing antibody completely blocked LPS-induced IL-6 and TNF release (SI Appendix, Fig. S3K). These results suggest that αSyn binding to TLR2 leads to hiMG release of IL-1β, and may be responsible for priming (the initial step in NLRP3 inflammasome activation).
To determine how αSyn leads to the second signal for NLRP3 inflammasome activation in hiMG, we examined whether oligomeric αSyn induced mitochondrial damage by assessing production of mitochondrial reactive oxygen species (mtROS), maintenance of mitochondrial membrane potential (ΔΨm), and release of mtDNA into the cytosol, the three mitochondrial signals linked to inflammasome activation (11, 52). We found that oligomeric/aggregated αSyn induced a significant increase in mtROS generation (Fig. 2 G and H), a decrease in ΔΨm (Fig. 2 I and J), and an increase in cytosolic mtDNA (Fig. 2K). Previously, αSyn overexpression has been reported to induce mitochondrial damage in neurons (53), and αSyn mutant-initiated cardiolipin exposure has been reported to trigger mitochondrial damage and mitophagy (54). Hence, the mitochondrial damage observed in hiMG in the present study may contribute to NLRP3 inflammasome activation.
Neuronal αSyn and αSyn–Antibody Complexes Induce Inflammasome-Related Cytokine Production by hiMG.
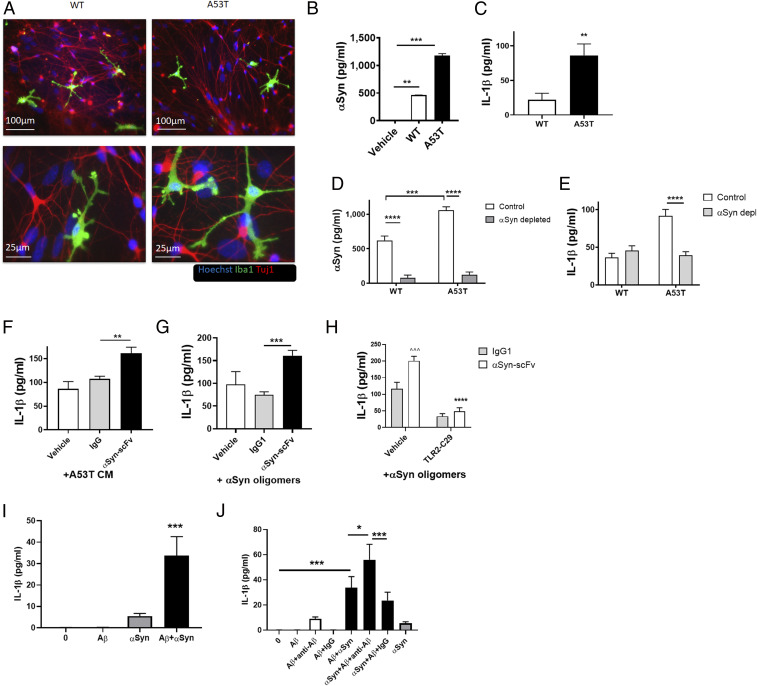
To study the interplay of hiMG and neurons mechanistically, we utilized a “disease-in-a-dish” model of human PD; for this purpose, we cocultured hiMG with hiPSC-derived A9-type DA neurons bearing the A53T αSyn mutation or with gene-corrected isogenic control neurons (WT), each plated on a bed of astrocytes (55, 56). In these cocultures, hiMG developed a highly ramified morphology, reminiscent of microglia in vivo (Fig. 3A). To investigate the effect of endogenous αSyn on the NLRP3 inflammasome in this system, we collected conditioned medium (CM) from control or A53T DA neuronal cultures, which manifest increased production of mutant, aggregated αSyn (Fig. 3B) (57). We found that medium from A53T neurons (A53T CM) induced significantly more IL-1β release from hiMG than CM from isogenic controls (Fig. 3C). To show that the inflammatory effect of A53T neuronal CM was indeed triggered by αSyn, we performed an in vitro immunodepletion experiment on the CM by using anti-αSyn antibody (Ab) coupled to protein-A/G beads. Since this technique should decrease αSyn, it would be expected to decrease inflammasome activation by the CM. We confirmed that αSyn was depleted from the CM by enzyme-linked immunosorbent assay (Fig. 3D). As predicted, this resulted in decreased IL-1β release from hiMG (Fig. 3E). Next, in order to more closely mimic treatment in humans, we added a variety of anti-αSyn Abs to the media without depleting them using beads. Unexpectedly, adding these antibodies, including humanized anti-αSyn single-chain variable fragment (scFv) Ab, known to bind monomeric and oligomeric αSyn (58), further increased IL-1β release rather than preventing it. In contrast, isotype-matched control immunoglobulin G (IgG) manifested no such effect. This deleterious effect of αSyn Abs was observed in the presence of either oligomerized recombinant αSyn or A53T CM (Fig. 3 F and G). Importantly, anti-αSyn Ab alone did not induce IL-1β release (SI Appendix, Fig. S4A), suggesting that this effect was specific to the Ab–αSyn complex and not to a direct effect of antibody. Moreover, scFv lacks the Fc domain that binds to Fc receptors on microglia (59), providing further support for a specific effect of the Ab–αSyn complex. Similar increases in inflammatory cytokines were observed with misfolded protein complexed to antibodies generated in mouse or rabbit (SI Appendix, Fig. S4 B and C). Importantly, this inflammatory effect was not seen in mouse primary microglia with any of the antibodies (e.g., mouse, rabbit, sheep, or goat) (SI Appendix, Fig. S4D). Furthermore, TLR2 inhibition with TLR2-IN-C29, an inhibitor of TLR2/1 and TLR2/6 signaling (60), ameliorated NLRP3 inflammasome activation in hiMG in this setting (Fig. 3H), implying that TLR signaling was involved mechanistically.
Fig. 3.
A53T αSyn mutant CM from hiPSC-derived DA neurons and Ab–αSyn complex activate the inflammasome in hiMG. (A) Representative images of astrocyte/DA neuron/microglia coculture of A53T and isogenic control (WT) neurons. Iba1 (green), Tuj1 (red), and Hoechst (blue). (A, Top) Low magnification. (Scale bars, 100 µm.) (A, Bottom) Higher magnification. (Scale bars, 25 µm.) (B) αSyn levels in CM from WT and A53T CM (n = 4). (C) IL-1β release from hiMG exposed to CM from WT and A53T DA neurons (n = 6 to 8). (D) Secreted αSyn levels in αSyn-immunodepleted CM from isogenic corrected (WT) and A53T DA neurons (n = 4 per group). (E) IL-1β release from hiMG exposed to αSyn-immunodepleted CM from isogenic corrected (WT) and A53T DA neurons (n = 7 to 11). (F) IL-1β release from hiMG exposed to CM from isogenic corrected (WT) and A53T DA neurons pretreated with humanized αSyn scFv or human IgG1 control. Note the increased effect of Ab on CM from A53T over isogenic corrected (WT), consistent with their increased production of αSyn oligomers (n = 6 to 8). (G) Quantification of IL-1β from hiMG exposed to oligomeric αSyn (750 nM) pretreated with humanized αSyn scFv or human IgG1 control (n = 5 to 10). (H) IL-1β secretion from hiMG exposed to oligomeric Aβ (10 µM) and low concentrations of oligomeric αSyn (100 nM) with anti-Aβ Ab, compared with IgG control. ^Comparison with vehicle-IgG. *Comparison with vehicle-scFv (n = 7 to 9). (I) IL-1β release from hiMG following TLR2 inhibition. (n = 7). (J) IL-1β release from hiMG exposed to oligomeric Aβ (10 µM), low concentrations of oligomeric αSyn (100 nM), or a combination, showing a synergistic effect (n = 7 per group). Graphs indicate mean ± SEM. Statistical analysis was performed using a two-way ANOVA with Bonferroni post hoc test (*P < 0.05, **P < 0.01, ^^^P or ***P < 0.001, ****P < 0.0001).
Aβ Oligomers and Aβ–Antibody Complexes Induce Inflammasome-Related Cytokine Production by hiMG.
Next, to generalize this effect to other misfolded proteins and other diseases, we exposed hiMG to oligomeric Aβ. We found that Aβ oligomers alone did not provide the two signals necessary for activation of the inflammasome. Notably, however, the combination of misfolded Aβ with very low concentrations of αSyn, which alone did not induce inflammasome activation, resulted in robust, synergistic inflammasome activation (Fig. 3I). Aβ has been shown to contribute to activation of the NLRP3 inflammasome in a process dependent on TLR4 (61). The synergistic effect of Aβ and αSyn could result from engagement of both TLR2 and TLR4 to induce a stronger immune response. For example, Aβ may provide additional secondary signal for NLRP3 inflammasome activation (62). Moreover, addition of anti-Aβ scFv-Fc Ab increased IL-1β in a similar manner to anti-αSyn Ab, suggesting a generalized effect of antibody treatment on inflammasome activation in hiMG (Fig. 3J). Taken together, these findings are consistent with the notion that the Ab–αSyn complexes are in fact immunoinflammatory by acting on hiMG, and this effect may be unique to human microglia as it was not observed with mouse microglia. Moreover, Aβ and the Aβ–Ab complex may intensify this effect on hiMG, which is relevant given recent findings that Aβ and αSyn aggregates are both often found in the human brain in neurodegenerative diseases such as AD and LBD (24–26).
Antibody–αSyn Complex Induces Inflammation and Neuronal Cell Death in hiMG Engrafted into Mouse Brain.
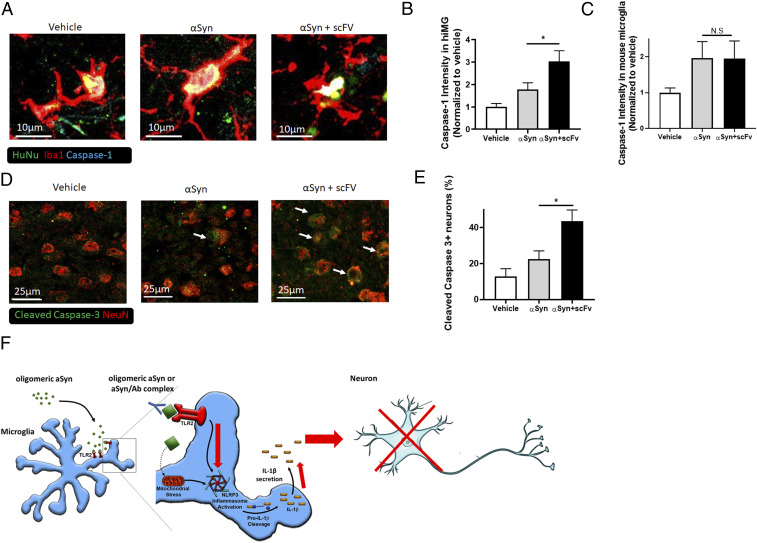
To investigate whether hiMG can also induce inflammasome-like activity in vivo, we engrafted hiMG into the brains of humanized mice; these mice support human cells by expressing three human genes: IL-3, CSF2, and KITLG (23). In this series of experiments, we stereotactically injected hiMG into the ventricles of adult mice at 4 wk of age. We injected either hiMG alone, hiMG together with αSyn oligomers, or hiMG with αSyn oligomers and antibody. We then evaluated the mice 2 wk later for inflammasome activation and neuronal cell death. Engraftment of hiMG with αSyn triggered caspase-1 activity, as indicated by the presence of cleaved caspase-1 in the engrafted hiMG. Notably, αSyn oligomers injected with humanized anti-αSyn scFv Ab induced a significant increase in the caspase-1 response. In contrast, unstimulated hiMG manifested minimal caspase-1 activity after engraftment in the brain (Fig. 4 A and B). Moreover, engrafted hiMG demonstrated the presence of apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (CARD) specks (63), indicating inflammasome activation by the presence of complexes of apoptosis-associated speck-like protein containing a CARD (SI Appendix, Fig. S5A). Remarkably, mouse microglia contiguous to hiMG did not manifest an increase in caspase-1 activation or ASC specks after injection of Ab–αSyn complexes compared with αSyn alone (Fig. 4C), indicating that enhanced inflammation in response to Ab–αSyn is unique to human microglia. These findings are consistent with the notion that αSyn-stimulated hiMG induce inflammasome activation and Ab–αSyn complexes exacerbate this response in vivo, similar to the effect that we had found in vitro.
Fig. 4.
Ab–αSyn complex activates the inflammasome and induces neuronal death in hiMG-engrafted mice. (A) Representative images showing cleaved/activated caspase-1 staining in transplanted hiMG: hiMG only (Left), hiMG with αSyn (Middle), or hiMG with αSyn and Ab scFv (Right). Human nuclear antigen (HuNu; green), Iba1 (red), and activated caspase-1 (cyan). (Scale bars, 10 µm.) (B) Quantification of caspase-1 intensity in engrafted hiMG (Iba1+HuNu+ cells) (n = 6 per group). (C) Quantification of caspase-1 intensity in endogenous mouse microglia (Iba1+/HuNu− cells) (n = 6 per group). (D) Representative images of cleaved/activated caspase-3 staining in endogenous mouse neurons after transplantation of hiMG only (Left), hiMG with αSyn (Middle), or hiMG with αSyn plus scFv (Right). Cleaved caspase-3 (green, indicated by arrows) and NeuN (red). (Scale bars, 25 µm.) (E) Quantification of cleaved caspase-3 in neurons (NeuN+ cells) (n = 6 per group). (F) Schema showing the effect of αSyn on NLRP3 inflammasome activation. Oligomeric/fibrillar αSyn is released from DA neurons, and more so from A53T DA neurons. Oligomeric/fibrillar αSyn activates the NLRP3 inflammasome by providing two triggers: Stimulation of TLR2 provides the priming trigger, and induction of mitochondrial damage and/or ROS production provides the secondary trigger. This leads to the assembly of the NLRP3 inflammasome, which results in secretion of IL-1β and caspase-1, and contributes to caspase-3–associated neuronal cell death. Ab–αSyn complexes enhance inflammasome-mediated IL-1β secretion in a TLR2-dependent manner. Red arrows indicate increased response to the Ab–αSyn complexes. Graphs indicate mean ± SEM. Statistical analysis was performed using a two-way ANOVA with Bonferroni post hoc test (*P < 0.05; N.S., not significant, P > 0.9).
Critically, we also found that neurons in the hiMG- plus αSyn-injected brains underwent increased cell death, as evidenced by the presence of cleaved/active caspase-3 in the neurons, and this effect was further exacerbated with the addition of antibody (αSyn + scFv) (Fig. 4 D and E). Unstimulated (control) hiMG did not induce significant neuronal cell death under similar conditions. As a control for an effect of inflammatory hiMG vs. misfolded αSyn by itself on the mouse brain, when hiMG were preexposed to αSyn aggregates and then extensively washed prior to engraftment, these hiMG also induced neuronal cell death (SI Appendix, Fig. S5 B–E). This finding is consistent with the notion that under these conditions the increase in neurotoxicity was a result of hiMG inflammatory activation rather than merely a direct effect of αSyn on the neurons or other cells in the mouse brain. Collectively, these results provide evidence that αSyn-stimulated hiMG can induce NLRP3 inflammasome activation and neuronal cell death in vivo, and these adverse effects are markedly increased by the presence of Ab–αSyn complexes.
Discussion
The data presented here show that αSyn aggregates, particularly when complexed to cognate antibodies, activate the inflammasome in hiMG. As such, these results have important potential implications for immunotherapies aimed at neurodegenerative disorders because misfolded protein–antibody complexes may trigger a profound microglial inflammatory response in the human context but not in the mouse, where most preclinical experiments have been performed. Along these lines, in prior work on αSyn-overexpressing mouse models of PD, antibodies directed against αSyn were shown to attenuate synaptic and axonal damage, reduce loss of tyrosine hydroxylase fibers, and improve neurobehavioral outcomes, while also decreasing cell-to-cell propagation of αSyn and microglial neuroinflammatory markers such as Iba1 (64). Such findings have led to human clinical trials with antibodies against aberrant misfolded proteins, but we are concerned that our results of increased microglial activation triggered by αSyn protein–antibody (and further enhanced by Aβ–antibody complexes) in a human context with hiMG (Fig. 4F) may not be faithfully reproduced in these mouse models. For example, we suggest that the recent failure of a human phase II clinical trial of αSyn antibodies (65) may, at least in part, have been due to increased inflammation, and human clinical trials with Aβ antibodies may be suffering a similar fate (but see ref. 66). Further along these lines, recent work engrafting human microglia into humanized mice has demonstrated that human microglia manifest distinct transcriptome differences from their mouse counterparts (67). In addition, human microglia have been shown to be more heterogeneous than mouse microglia, displaying a more varied cell repertoire, transcriptome, and proteome (46, 68).
While differences between mouse and human microglia have been reported, another potential explanation of the difference observed here is the difference in the source of the cells, namely primary cells or hiPSC-derived cells. Despite the similarity between hiMG and in vivo microglia, there are some differences. Future studies to test this may use mouse iPSC-derived microglia and human ex vivo microglia. There have been several differentiation protocols for generating mouse iPSC-derived microglia (69, 70), although many of them do not follow the yolk sac pathway used here. Ex vivo human microglia have been isolated in previous reports (46, 71), although they also manifest differences from the in vivo cells, particularly when considering the age of the subjects used as donors.
Our finding of hiMG-induced neurotoxicity is supported by recent reports of inflammasome activation in mouse models of PD expressing the A53T αSyn mutation (21) as well as in genetic AD models (62). The inflammatory side effect mediated by hiMG could potentially be averted if protein–antibody complexes could somehow be removed, or if other immune modulators could be used to prevent the inflammation induced by the complexes. Targeting TLR2 signaling to block inflammasome activation might be beneficial in preventing the adverse effects of antibody treatment, as we show in vitro, because inhibition of TLR2 was recently shown to alleviate some of the harmful effects of αSyn in vivo in the mouse brain (72). Protein–antibody complexes could potentially exacerbate the immune response in several ways, for example by changing the kinetics of fibril formation or by exposing different epitopes of the protein (73). This could result in stronger activation of TLRs, raising the potential of offsetting the adverse effect of antibody treatment by directly inhibiting these TLRs.
In addition to activating the microglial inflammasome, oligomeric/fibrillar αSyn may also initiate an immune cascade via microglial presentation of these peptides to other immune cells such as T cells, which further contribute to the immune pathology of PD (48, 74). Interestingly, human PD patients reportedly have a higher frequency of an IL-1β gene polymorphism that increases IL-1β expression (75); this may result in higher susceptibility to inflammation, and thus contribute to PD pathology via microglial secretion. Collectively, the present study shows that the αSyn- and particularly αSyn/Aβ-driven pathogenesis may manifest a component of neuroinflammation mediated by human microglia that, in contrast to mouse, is enhanced by anti-αSyn or anti-Aβ antibodies.
Materials and Methods
hiPSC Cultures and Reagents.
The use of human cells was approved by the institutional review boards of the Scintillon Institute and The Scripps Research Institute. hiPSCs were generated from normal human fibroblasts (Hs27, ATCC CRL-1634, and Coriell GM02036) using an integration-free reprogramming method (76). hiPSCs were differentiated into hiMG, as described in SI Appendix, Extended Materials and Methods, and subsequently used for characterization and experiments. αSyn oligomers were prepared by shaking in a thermomixer at 1,400 rpm for 6 d at 37 °C, as described in SI Appendix, Extended Materials and Methods.
Detailed information on culture conditions and cell maintenance, reagents, experimental design of in vivo animal studies, analysis of RNA-seq, and immunohistochemistry is given in SI Appendix, Extended Materials and Methods.
Supplementary Material
Acknowledgments
We thank Swagata Ghatak for helpful discussions. This work was supported in part by NIH Grants R01 NS086890, RF1 AG057409, R01 AG056259, R01 DA048882, and DP1 DA041722 (to S.A.L., who holds the Step Family Foundation Endowed Chair), and R01 AI043477 and the Coins for Alzheimer’s Research Trust Foundation (to M.K., who is an American Cancer Research Professor and holds the Ben and Wanda Hildyard Chair for Mitochondrial and Metabolic Diseases). Z.Z. was supported in part by a Cancer Research Institute Irvington Postdoctoral Fellowship, the Prevent Cancer Foundation Board of Directors Research Fund, and the American Association for the Study of Liver Diseases Pinnacle Research Award. Z.Z. is also a Cancer Prevention and Research Institute of Texas (CPRIT) Scholar and is supported by a CPRIT New Investigator Recruitment Award (RR180014). J.W.K. and Y.S.E. were supported in part by NIH Grant R01 DK46335. Y.S.E. was supported by a K99 Pathway to Independence Award from the National Institute on Aging (K99 AG050764). D.T. was supported in part by postdoctoral fellowship grant 11721 from Autism Speaks, Inc.
Footnotes
Competing interest statement: S.A.L. and G.H. are coauthors on a published consensus statement review of cell-death criteria along with several dozen other authors who are authorities in this field [N. M. C. Connolly et al., Cell Death Differ. 25, 542–572 (2018)]. That manuscript was published in order to help nonexperts in the field understand and use criteria for various types of cell death. They also published a similar type of review paper together 10 y ago [G. E. Hardingham, S. A. Lipton, Antioxid. Redox Signal. 14, 1421–1424 (2011)]. However, S.A.L. and G.H. have never formally collaborated or worked together on any laboratory-based scientific project, including the current work.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2025847118/-/DCSupplemental.
Data Availability
The RNA-seq data reported in this article have been deposited in the Gene Expression Omnibus (accession no. GSE169065) (77). Source code used to generate results that are reported in the paper is available in GitHub as “FINALcode.r,”using the following link: https://github.com/dorittrud/hiMG-analysis (78).
References
- 1.Jellinger K. A., A critical evaluation of current staging of α-synuclein pathology in Lewy body disorders. Biochim. Biophys. Acta 1792, 730–740 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Mao X., et al., Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353, aah3374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prinz M., Priller J., Sisodia S. S., Ransohoff R. M., Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 14, 1227–1235 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Krasemann S., et al., The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold M., El Khoury J., β-Amyloid, microglia, and the inflammasome in Alzheimer’s disease. Semin. Immunopathol. 37, 607–611 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammond T. R., Marsh S. E., Stevens B., Immune signaling in neurodegeneration. Immunity 50, 955–974 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heneka M. T., et al., NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenzweig H. L., Planck S. R., Rosenbaum J. T., NLRs in immune privileged sites. Curr. Opin. Pharmacol. 11, 423–428 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halle A., et al., The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heneka M. T., Kummer M. P., Latz E., Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14, 463–477 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Zhong Z., et al., NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164, 896–910 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heneka M. T., et al., Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouchi Y., et al., Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol. 57, 168–175 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Webers A., Heneka M. T., Gleeson P. A., The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 98, 28–41 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Wong Y. C., Krainc D., α-Synuclein toxicity in neurodegeneration: Mechanism and therapeutic strategies. Nat. Med. 23, 1–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan Y., et al., Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73 (2015). [DOI] [PubMed] [Google Scholar]
- 17.von Herrmann K. M., et al., NLRP3 expression in mesencephalic neurons and characterization of a rare NLRP3 polymorphism associated with decreased risk of Parkinson’s disease. NPJ Parkinsons Dis. 4, 24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boza-Serrano A., et al., The role of galectin-3 in α-synuclein-induced microglial activation. Acta Neuropathol. Commun. 2, 156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Codolo G., et al., Triggering of inflammasome by aggregated α-synuclein, an inflammatory response in synucleinopathies. PLoS One 8, e55375 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniele S. G., et al., Activation of MyD88-dependent TLR1/2 signaling by misfolded α-synuclein, a protein linked to neurodegenerative disorders. Sci. Signal. 8, ra45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon R., et al., Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 10, eaah4066 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panicker N., et al., Fyn kinase regulates misfolded α-synuclein uptake and NLRP3 inflammasome activation in microglia. J. Exp. Med. 216, 1411–1430 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wunderlich M., et al., AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia 24, 1785–1788 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassil F., et al., Amyloid-β (Aβ) plaques promote seeding and spreading of α-synuclein and tau in a mouse model of Lewy body disorders with Aβ pathology. Neuron 105, 260–275.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin D. J., Hurtig H. I., The contribution of tau, amyloid-β and α-synuclein pathology to dementia in Lewy body disorders. J. Alzheimers Dis. Parkinsonism 8, 444 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winslow A. R., et al., Convergence of pathology in dementia with Lewy bodies and Alzheimer’s disease: A role for the novel interaction of α-synuclein and presenilin 1 in disease. Brain 137, 1958–1970 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abud E. M., et al., iPSC-derived human microglia-like cells to study neurological diseases. Neuron 94, 278–293.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douvaras P., et al., Directed differentiation of human pluripotent stem cells to microglia. Stem Cell Reports 8, 1516–1524 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muffat J., et al., Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 22, 1358–1367 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandya H., et al., Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat. Neurosci. 20, 753–759 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sturgeon C. M., Ditadi A., Awong G., Kennedy M., Keller G., Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat. Biotechnol. 32, 554–561 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfe A. D., Downs K. M., Mixl1 localizes to putative axial stem cell reservoirs and their posterior descendants in the mouse embryo. Gene Expr. Patterns 15, 8–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uenishi G., et al., Tenascin C promotes hematoendothelial development and T lymphoid commitment from human pluripotent stem cells in chemically defined conditions. Stem Cell Reports 3, 1073–1084 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen T. L., et al., ER71 directs mesodermal fate decisions during embryogenesis. Development 138, 4801–4812 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matcovitch-Natan O., et al., Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, aad8670 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Kreutzberg G. W., Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Torres-Platas S. G., et al., Morphometric characterization of microglial phenotypes in human cerebral cortex. J. Neuroinflammation 11, 12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett M. L., et al., New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. U.S.A. 113, E1738–E1746 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butovsky O., et al., Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butovsky O., Weiner H. L., Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 19, 622–635 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hickman S. E., et al., The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16, 1896–1905 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schafer D. P., et al., Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardona A. E., et al., Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9, 917–924 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Hamilton J. A., Cook A. D., Tak P. P., Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat. Rev. Drug Discov. 16, 53–70 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Hollingworth P.et al.; Alzheimer’s Disease Neuroimaging Initiative; CHARGE Consortium; EADI1 Consortium , Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat. Genet. 43, 429–435 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gosselin D., et al., An environment-dependent transcriptional network specifies human microglia identity. Science 356, eaal3222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olah M., et al., A transcriptomic atlas of aged human microglia. Nat. Commun. 9, 539 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thakur P., et al., Modeling Parkinson’s disease pathology by combination of fibril seeds and α-synuclein overexpression in the rat brain. Proc. Natl. Acad. Sci. U.S.A. 114, E8284–E8293 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coll R. C., et al., A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fellner L., et al., Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia 61, 349–360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim C., et al., Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 4, 1562 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhong Z., et al., New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560, 198–203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie W., Chung K. K., α-Synuclein impairs normal dynamics of mitochondria in cell and animal models of Parkinson’s disease. J. Neurochem. 122, 404–414 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Ryan T., et al., Cardiolipin exposure on the outer mitochondrial membrane modulates α-synuclein. Nat. Commun. 9, 817 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryan S. D., et al., Isogenic human iPSC Parkinson’s model shows nitrosative stress-induced dysfunction in MEF2-PGC1α transcription. Cell 155, 1351–1364 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soldner F., et al., Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell 146, 318–331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lashuel H. A., Overk C. R., Oueslati A., Masliah E., The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 14, 38–48 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emadi S., et al., Inhibiting aggregation of α-synuclein with human single chain antibody fragments. Biochemistry 43, 2871–2878 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Emadi S., Barkhordarian H., Wang M. S., Schulz P., Sierks M. R., Isolation of a human single chain antibody fragment against oligomeric α-synuclein that inhibits aggregation and prevents α-synuclein-induced toxicity. J. Mol. Biol. 368, 1132–1144 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mistry P., et al., Inhibition of TLR2 signaling by small molecule inhibitors targeting a pocket within the TLR2 TIR domain. Proc. Natl. Acad. Sci. U.S.A. 112, 5455–5460 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y., et al., β-Amyloid activates NLRP3 inflammasome via TLR4 in mouse microglia. Neurosci. Lett. 736, 135279 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Venegas C., et al., Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 552, 355–361 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Kuri P., et al., Dynamics of in vivo ASC speck formation. J. Cell Biol. 216, 2891–2909 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Games D., et al., Reducing C-terminal-truncated α-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson’s disease-like models. J. Neurosci. 34, 9441–9454 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mullard A., Parkinson disease setback. Nat. Rev. Drug Discov. 19, 373 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Volc D., et al., Safety and immunogenicity of the α-synuclein active immunotherapeutic PD01A in patients with Parkinson’s disease: A randomised, single-blinded, phase 1 trial. Lancet Neurol. 19, 591–600 (2020). [DOI] [PubMed] [Google Scholar]
- 67.Mancuso R., et al., Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nat. Neurosci. 22, 2111–2116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masuda T., et al., Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019). [DOI] [PubMed] [Google Scholar]
- 69.Quarta A., et al., Murine iPSC-derived microglia and macrophage cell culture models recapitulate distinct phenotypical and functional properties of classical and alternative neuro-immune polarisation. Brain Behav. Immun. 82, 406–421 (2019). [DOI] [PubMed] [Google Scholar]
- 70.Beutner C., Roy K., Linnartz B., Napoli I., Neumann H., Generation of microglial cells from mouse embryonic stem cells. Nat. Protoc. 5, 1481–1494 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Becher B., Antel J. P., Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia 18, 1–10 (1996). [DOI] [PubMed] [Google Scholar]
- 72.Kim C., et al., Immunotherapy targeting Toll-like receptor 2 alleviates neurodegeneration in models of synucleinopathy by modulating α-synuclein transmission and neuroinflammation. Mol. Neurodegener. 13, 43 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Genst E., Messer A., Dobson C. M., Antibodies and protein misfolding: From structural research tools to therapeutic strategies. Biochim. Biophys. Acta 1844, 1907–1919 (2014). [DOI] [PubMed] [Google Scholar]
- 74.Sulzer D., Edwards R. H., The physiological role of α-synuclein and its relationship to Parkinson’s disease. J. Neurochem. 150, 475–486 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schulte T., et al., Polymorphisms in the interleukin-1 α and β genes and the risk for Parkinson’s disease. Neurosci. Lett. 326, 70–72 (2002). [DOI] [PubMed] [Google Scholar]
- 76.Okita K., et al., A more efficient method to generate integration-free human iPS cells. Nat. Methods 8, 409–412 (2011). [DOI] [PubMed] [Google Scholar]
- 77.Trudler D., et al., Analysis of hiPSC-derived microglia compared to other cell types. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE169065. Deposited 17 March 2021.
- 78.Trudler D., et al., hiMG-analysis. GitHub. https://github.com/dorittrud/hiMG-analysis. Deposited 17 March 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data reported in this article have been deposited in the Gene Expression Omnibus (accession no. GSE169065) (77). Source code used to generate results that are reported in the paper is available in GitHub as “FINALcode.r,”using the following link: https://github.com/dorittrud/hiMG-analysis (78).